Bioengineering Strategies for Corneal Endothelial Cell Injection Therapy: Advances, Challenges, and Clinical Translation
Abstract
1. Introduction
2. Corneal Endothelial Cells Dysfunction and Current Treatments
3. Corneal Endothelial Cells (CECs) Culture
3.1. Popular Culture Supplement for Corneal Endothelial Cells (CECs)
| Supplement | Common Dose Ranges | Function/Benefit | Reported Efficacy |
|---|---|---|---|
| Fetal Bovine Serum (FBS) | 2–10% (v/v); 2–5% for maintenance, 5–10% for proliferation | Provides growth factors and nutrients | Widely used; supports proliferation but may induce fibroblastic changes at high doses [41] |
| Human Serum | 2–10% (v/v); typically 5% | Alternative to FBS; reduces xenogenic risks | Promotes proliferation with better morphology preservation [34] |
| bFGF (Basic Fibroblast Growth Factor) | 5–20 ng/mL (commonly 10 ng/mL) | Stimulates proliferation and survival | Essential for clonal expansion; improves cell yield and risk of EnMT [35,36] |
| EGF (Epidermal Growth Factor) | 5–20 ng/mL (commonly 10 ng/mL) | Enhances proliferation and migration | Supports wound healing and cell expansion; used in combination with other factors [41,53] |
| ROCK Inhibitor (Y-27632) | 3–10 μM (10 μM initially, 5 μM for maintenance) | Inhibits apoptosis and promotes cell adhesion | Dramatically improves survival and proliferation; widely adopted in recent protocols [42,43] |
| Ascorbic Acid (Vitamin C) | 50–200 μM (commonly 100 μM) | Antioxidant; supports collagen synthesis | Enhances cell viability and reduces oxidative stress [45] |
| Insulin-Transferrin-Selenium (ITS) | 0.5×–1× (typically 1×) | Supports metabolic activity and cell growth | Improves proliferation and maintains endothelial phenotype [54] |
| Chondroitin Sulfate | 0.08–0.2% (w/v) | Maintains cell shape and barrier function | Used in organ culture; helps preserve native morphology [41] |
| Stem cell conditioned media | 10–50% (v/v) | contains growth factors, metabolites and ECM proteins secreted by the cells | Maintains stemness and improves regenerative potential in some protocols [55,56] |
| Extracellular Matrix Coatings | Collagen IV (10–50 μg/mL), Laminin (5–20 μg/mL), Fibronectin (2–10 μg/mL) | Collagen IV, VIII, laminin, fibronectin | Critical for CECs adhesion and morphology [57] |
| Nerve growth factor (NGF) | 10–100 ng/mL | may reduce apoptosis and promote regenerative signaling | Enhance CEC proliferation and survival [58,59] |
| Bovine pituitary extract | 30–100 μg/mL | rich source of growth factors (FGF, EGF, etc.) | helps cells overcome senescence and maintain a healthy monolayer [41]. |
| Attribute | Human Serum | Human Platelet Lysate (HPL) |
|---|---|---|
| Source | Serum separated from donated whole blood after clotting | Lysate made from pooled human platelets by freeze–thaw, sonication, or other lysis |
| Key components | Albumin, immunoglobulins, complement proteins, low–moderate growth factor levels | High concentrations of growth factors and cytokines (PDGF; EGF; VEGF; TGF-β) |
| Proliferation support | Moderate cell proliferation support | Strong proliferation support for many cell types, notably MSCs |
| Batch-to-batch variability | Donor-dependent variability; pooling reduces variability | Donor- and process-dependent variability; pooling and standardized production reduce variability |
| Clinical translation | Used in clinical protocols; requires rigorous screening and processing | Widely adopted for clinical-grade expansion; many GMP-produced HPL products available |
| Preparation/processing | Clotting, centrifugation, heat-inactivation commonly required | Platelet collection, lysis (freeze–thaw or mechanical), centrifugation, optional heparin to prevent fibrin gelation |
| Immunogenicity/xenogeneic risk | Human-derived, low xenogeneic risk | Human-derived, avoids animal components and xenogeneic risk |
| Effect on cell phenotype & function | Maintains phenotype; growth factor levels lower than HPL | Maintains phenotype and immunomodulatory functions; can increase VEGF secretion and proliferation |
| Cryopreservation performance | Suitable but sometimes inferior to platelet-derived supplements | Comparable or superior cryopreservation outcomes in some studies |
| Cost & availability | Moderate cost; depends on donor supply | Variable cost; scalable with pooled platelet plasma and commercial GMP HPL products |
3.2. Donor Effect on Cornea Endothelial Cell Culture
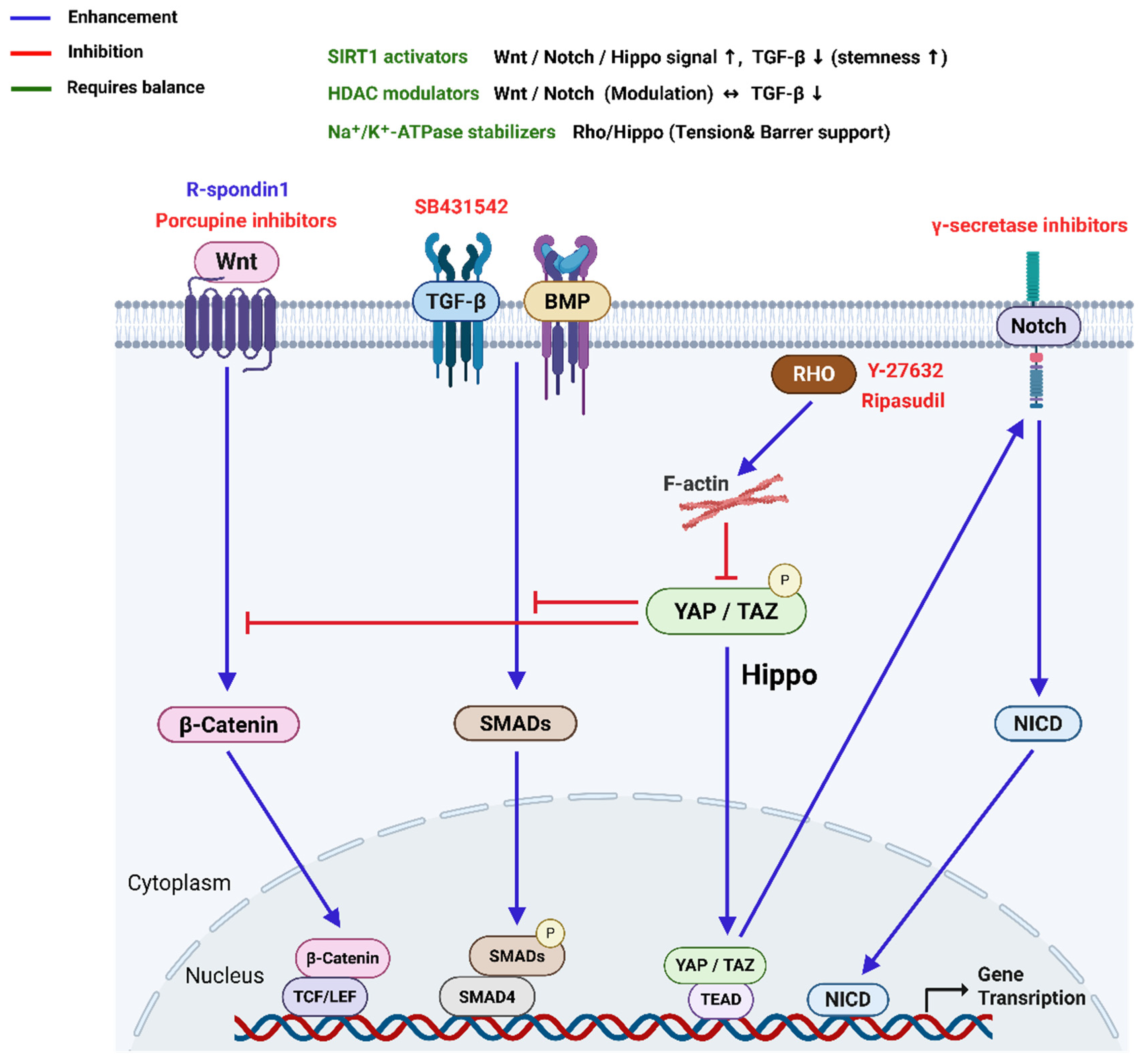
3.3. Signal Pathways to Maintain CECs Phenotype
| Pathway | Role in CECs | Effect on Phenotype | Modulation Strategy | Reference |
|---|---|---|---|---|
| TGF-β | Induce G1 arrest in vivo. Drives EnMT and fibrosis under stress or serum-rich conditions | Loss of polygonal shape, gain of fibroblastic traits | Inhibit with SB431542 or LY2109761 | [68] |
| ROCK | Regulates cytoskeleton, adhesion, and survival | Supports proliferation and phenotype retention | Inhibit with Y27632 or Ripasudil | [14] |
| MAPK (ERK) | Promotes proliferation but may reduce cell density | Mixed effects; can reduce ECD | Use with caution or inhibit selectively | [88] |
| MAPK (p38) | Stress response and senescence regulation | Inhibition increases ECD and preserves phenotype | Inhibit with SB203580 or SB202190 | [89] |
| BMP | Counters TGF-β-induced EnMT | Maintains endothelial morphology | Activate BMP-7 | [90] |
| Notch | Regulates cell fate and EnMT, especially post-injury | Excess activation may cause phenotype loss | Inhibit with γ-secretase inhibitors | [83] |
| Wnt | Stimulates proliferation and regeneration | Promotes recovery and phenotype retention | Activate with Wnt mimetics or ligands | [33,77] |
3.4. Cornea Endothelial Cell Phenotype Markers for Validation of Culture
| Marker | Full Name | Function in CECs | Research/Clinical Relevance | Reference |
|---|---|---|---|---|
| Na+/K+-ATPase | Sodium-Potassium ATPase | Maintains ionic balance and fluid transport across the endothelium | Gold-standard functional marker for CECs identity and pump function | [18] |
| ZO-1 | Zonula Occludens-1 | Tight junction protein that maintains barrier integrity | Used to assess monolayer integrity and hexagonal morphology | [18] |
| CD166 | Activated Leukocyte Cell Adhesion Molecule | Cell adhesion and phenotype maintenance | Marker of non-fibroblastic, functional CECs | [92] |
| sPrdx6 | secreted form of Peroxiredoxin 6 | neutralize reactive oxygen species | Protect CECs from oxidative damage and important marker of functional CECs | [92,96] |
| CD73 | Ecto-5′-nucleotidase | Associated with fibroblastic transformation | High expression indicates mesenchymal-like phenotype; used for negative selection | [91,97] |
| CD44 | Hyaluronan receptor | Cell adhesion and migration | Elevated in fibroblastic CECs; linked to EnMT | [91] |
| CD49e | Integrin α5 | ECM interaction and cell adhesion | Marker of fibroblastic phenotype | [91] |
| CD98 | 4F2 cell-surface antigen heavy chain | Amino acid transport and cell activation | Elevated in functional, non-fibroblastic CECs | [91] |
| CD340 | HER2/neu | Growth factor receptor | Associated with non-fibroblastic phenotype | [91] |
| N-Cadherin | Cadherin-2 | Cell–cell adhesion and junction stability | Maintains endothelial monolayer structure | [18] |
| SLC4A11 | Solute Carrier Family 4 Member 11 | Ion transport and mitochondrial homeostasis | Mutations linked to endothelial dystrophies; essential for cell survival | [92] |
| Collagen I | Type I Collagen | ECM component | Overexpression indicates fibroblastic transformation | [98] |
| Fibronectin | ECM glycoprotein | Cell adhesion and migration | Marker of mesenchymal phenotype; elevated during EnMT | [98] |
3.5. Evaluating CECs Functionality
4. Bioengineering for Corneal Endothelial Cell
4.1. Enhancing CECs Viability
| Targets | Results |
|---|---|
| E2F2 | Transduction with E2F2 resulted in progression from the G1 to the S phase and increases CECs density [119]. |
| ZONAB | Modulation of contact inhibition by ZO-1/ZONAB gene transfer increases CECs density [120]. |
| ZO-1 | ZO-1 downregulation using ZO-1 shRNA increases CECs proliferation on donor grafts [120]. |
| CKIs and p53 | Stable expression of p53 shRNA enhanced cell survival by approximately 12 population doublings. Combining p53 knockdown with TERT overexpression resulted in the immortalization of CECs [121]. Downregulation of p27KIP1 using siRNA led to a 30% increase in CECs density [122]. |
| p120 Catenin/Kaiso | p120 catenin siRNA increases CECs proliferation by inhibiting Kaiso [123]. |
| SOX2 | SOX2 overexpression increases cell proliferation and viability in CECs [124]. |
| SIRT1 | SIRT1 overexpression increases CECs proliferation and viability [125]. |
4.2. Enhancing CECs Attachment to Recipient Cornea
5. Alternative Source of CECs
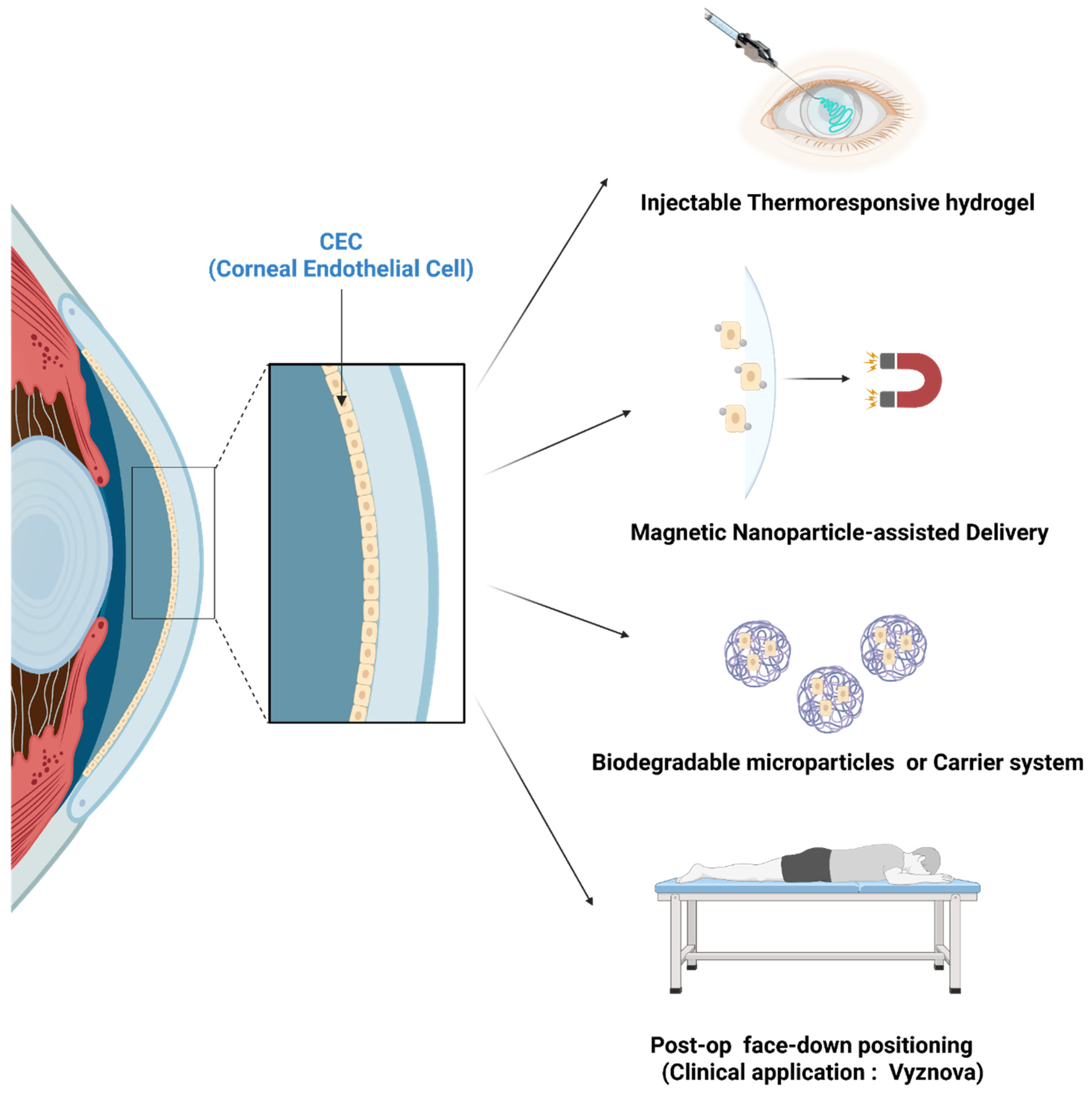
6. Cell Delivery and Engraftment Enhancement
7. Clinical Studies
8. Challenges and Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability statement
Acknowledgments
Conflicts of Interest
References
- Parekh, M.; Ferrari, S.; Sheridan, C.; Kaye, S.; Ahmad, S. Concise Review: An Update on the Culture of Human Corneal Endothelial Cells for Transplantation. Stem Cells Transl. Med. 2016, 5, 258–264. [Google Scholar] [CrossRef]
- Ono, T.; Mori, Y.; Nejima, R.; Iwasaki, T.; Miyai, T.; Miyata, K. Corneal endothelial cell density and morphology in ophthalmologically healthy young individuals in Japan: An observational study of 16842 eyes. Sci. Rep. 2021, 11, 18224. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Duan, H.; Wang, X.; Lin, Z.; Dai, K.; Hu, X.; Zhao, X.; Zhou, Q.; Li, Z.; Xie, L. Assessment of Corneal Endothelial Barrier Function Based on “Y-Junctions”: A Finite Element Analysis. Investig. Ophthalmol. Vis. Sci. 2025, 66, 33. [Google Scholar] [CrossRef]
- Price, M.O.; Mehta, J.S.; Jurkunas, U.V.; Price, F.W., Jr. Corneal endothelial dysfunction: Evolving understanding and treatment options. Prog. Retin. Eye Res. 2021, 82, 100904. [Google Scholar] [CrossRef]
- Joyce, N.C.; Zhu, C.C. Human corneal endothelial cell proliferation: Potential for use in regenerative medicine. Cornea 2004, 23, S8–S19. [Google Scholar] [CrossRef]
- Shimizu, T.; Yamagami, S.; Hayashi, T. The progress and future of corneal endothelial transplantation. Jpn. J. Ophthalmol. 2024, 68, 429–442. [Google Scholar] [CrossRef]
- Hatzfeld, A.S.; Germain, N.; Maboudou, P.; Dhayer, M.; Marchetti, P. Medical and economic impacts of managing corneas from older donors at the tissue bank—A single-center retrospective study spanning over 12 years. Front. Med. 2024, 11, 1415515. [Google Scholar] [CrossRef]
- Alio, J.L.; Montesel, A.; El Sayyad, F.; Barraquer, R.I.; Arnalich-Montiel, F.; Alio Del Barrio, J.L. Corneal graft failure: An update. Br. J. Ophthalmol. 2021, 105, 1049–1058. [Google Scholar] [CrossRef]
- Steger, B.; Kaye, S.B.; Romano, V. Corneal transplantation: The fine line between donor shortage and tissue quality. BMJ Open Ophthalmol. 2022, 7, e001046. [Google Scholar] [CrossRef]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J.; et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 2018, 378, 995–1003. [Google Scholar] [CrossRef]
- Numa, K.; Imai, K.; Ueno, M.; Kitazawa, K.; Tanaka, H.; Bush, J.D.; Teramukai, S.; Okumura, N.; Koizumi, N.; Hamuro, J.; et al. Five-Year Follow-up of First 11 Patients Undergoing Injection of Cultured Corneal Endothelial Cells for Corneal Endothelial Failure. Ophthalmology 2021, 128, 504–514. [Google Scholar] [CrossRef]
- Suanno, G.; Genna, V.G.; Maurizi, E.; Dieh, A.A.; Griffith, M.; Ferrari, G. Cell therapy in the cornea: The emerging role of microenvironment. Prog. Retin. Eye Res. 2024, 102, 101275. [Google Scholar] [CrossRef] [PubMed]
- Futterknecht, S.; Chatzimichail, E.; Gugleta, K.; Panos, G.D.; Gatzioufas, Z. The Role of Rho Kinase Inhibitors in Corneal Diseases. Drug Des. Devel Ther. 2024, 18, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Peh, G.S.; Chng, Z.; Ang, H.P.; Cheng, T.Y.; Adnan, K.; Seah, X.Y.; George, B.L.; Toh, K.P.; Tan, D.T.; Yam, G.H.; et al. Propagation of human corneal endothelial cells: A novel dual media approach. Cell Transplant. 2015, 24, 287–304. [Google Scholar] [CrossRef]
- Joyce, N.C. Proliferative capacity of corneal endothelial cells. Exp. Eye Res. 2012, 95, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Musayeva, A.; Kim, J.; Le Blon, D.; van den Bogerd, B.; Dickman, M.M.; LaPointe, V.L.S.; Ni Dhubhghaill, S.; Oellerich, S. Clinical Applications of Corneal Cells Derived from Induced Pluripotent Stem Cells. Biomolecules 2025, 15, 1139. [Google Scholar] [CrossRef]
- Hatou, S.; Sayano, T.; Higa, K.; Inagaki, E.; Okano, Y.; Sato, Y.; Okano, H.; Tsubota, K.; Shimmura, S. Transplantation of iPSC-derived corneal endothelial substitutes in a monkey corneal edema model. Stem Cell Res. 2021, 55, 102497. [Google Scholar] [CrossRef]
- Hsueh, Y.J.; Chen, H.C.; Pan, Y.Y.; Hsiao, F.C.; Yang, S.J.; Liu, M.C.; Lai, W.Y.; Li, G.; Hui-Kang Ma, D.; James Meir, Y.J. The hiPSC-derived corneal endothelial progenitor-like cell recovers the rabbit model of corneal endothelial dystrophy. J. Adv. Res. 2025, 70, 355–369. [Google Scholar] [CrossRef]
- Zhu, Q.; Hu, M.; Sun, X.M.; Liu, H.; Yang, Z.K.; Dai, X.J.; Hu, Z.L. Experimental study on the rhesus monkey corneal endothelial cells substituted by the allogeneic vascular endothelial cells cultivated in vitro. Zhonghua Yan Ke Za Zhi 2013, 49, 1006–1013. [Google Scholar]
- Dong, C.; Zou, D.; Duan, H.; Hu, X.; Zhou, Q.; Shi, W.; Li, Z. Ex vivo cultivated retinal pigment epithelial cell transplantation for the treatment of rabbit corneal endothelial dysfunction. Eye Vis. 2023, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, J.L.; Cai, W.Y.; Wang, G.Y.; Li, Y.Z.; Wang, J.S.; Xie, H.T.; Zhang, M.C. Progress in Transdifferentiation of Autologous Alternative Cell Sources into Corneal Epithelial Cells. Stem Cell Rev. Rep. 2025, 21, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Khan, S.Y.; Gottsch, J.D.; Hutchinson, E.K.; Khan, A.; Riazuddin, S.A. Pluripotent stem cell-derived corneal endothelial cells as an alternative to donor corneal endothelium in keratoplasty. Stem Cell Rep. 2021, 16, 2320–2335. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Berdahl, J.P.; Chan, C.C.; Rocha, K.M.; Yeu, E.; Ayres, B.; Farid, M.; Lee, W.B.; Beckman, K.A.; Kim, T.; et al. The corneal endothelium: Clinical review of endothelial cell health and function. J. Cataract. Refract. Surg. 2021, 47, 1218–1226. [Google Scholar] [CrossRef]
- Feizi, S. Corneal endothelial cell dysfunction: Etiologies and management. Ther. Adv. Ophthalmol. 2018, 10, 2515841418815802. [Google Scholar] [CrossRef]
- Altamirano, F.; Ortiz-Morales, G.; O’Connor-Cordova, M.A.; Sancen-Herrera, J.P.; Zavala, J.; Valdez-Garcia, J.E. Fuchs endothelial corneal dystrophy: An updated review. Int. Ophthalmol. 2024, 44, 61. [Google Scholar] [CrossRef]
- Narayanan, R.; Gaster, R.N.; Kenney, M.C. Pseudophakic corneal edema: A review of mechanisms and treatments. Cornea 2006, 25, 993–1004. [Google Scholar] [CrossRef]
- Anshu, A.; Li, L.; Htoon, H.M.; de Benito-Llopis, L.; Shuang, L.S.; Singh, M.J.; Tiang Hwee, T.D. Long-Term Review of Penetrating Keratoplasty: A 20-Year Review in Asian Eyes. Am. J. Ophthalmol. 2021, 224, 254–266. [Google Scholar] [CrossRef]
- Wilhelm, T.I.; Gauche, L.; Bohringer, D.; Maier, P.; Heinzelmann, S.; Glegola, M.; Kammrath Betancor, P.; Reinhard, T. Ten-year outcomes after DMEK, DSAEK, and PK: Insights on graft survival, endothelial cell density loss, rejection and visual acuity. Sci. Rep. 2025, 15, 1249. [Google Scholar] [CrossRef]
- Singh, P.; Sinha, A.; Nagpal, R.; Chaurasia, S. Descemet membrane endothelial keratoplasty: Update on preoperative considerations, surgical techniques, and outcomes. Indian. J. Ophthalmol. 2022, 70, 3222–3238. [Google Scholar] [CrossRef]
- Kinoshita, S.; Ueno, M.; Toda, M.; Imai, K.; Tomioka, Y.; Numa, K.; Tanaka, H.; Inatomi, T.; Kameda, T.; Tsujikawa, A.; et al. Long-term Corneal Rejuvenation after Transplantation of Cultured Human Corneal Endothelial Cells. Ophthalmology 2025, 132, 1105–1113. [Google Scholar] [CrossRef]
- Hirayama, M.; Hatou, S.; Nomura, M.; Hokama, R.; Hirayama, O.I.; Inagaki, E.; Aso, K.; Sayano, T.; Dohi, H.; Hanatani, T.; et al. A first-in-human clinical study of an allogenic iPSC-derived corneal endothelial cell substitute transplantation for bullous keratopathy. Cell Rep. Med. 2025, 6, 101847. [Google Scholar] [CrossRef]
- Okumura, N.; Nakamura, T.; Kay, E.P.; Nakahara, M.; Kinoshita, S.; Koizumi, N. R-spondin1 regulates cell proliferation of corneal endothelial cells via the Wnt3a/beta-catenin pathway. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6861–6869. [Google Scholar] [CrossRef]
- Vianna, L.M.; Kallay, L.; Toyono, T.; Belfort, R., Jr.; Holiman, J.D.; Jun, A.S. Use of human serum for human corneal endothelial cell culture. Br. J. Ophthalmol. 2015, 99, 267–271. [Google Scholar] [CrossRef]
- Lee, J.G.; Kay, E.P. FGF-2-mediated signal transduction during endothelial mesenchymal transformation in corneal endothelial cells. Exp. Eye Res. 2006, 83, 1309–1316. [Google Scholar] [CrossRef]
- Lee, J.G.; Jung, E.; Heur, M. Fibroblast growth factor 2 induces proliferation and fibrosis via SNAI1-mediated activation of CDK2 and ZEB1 in corneal endothelium. J. Biol. Chem. 2018, 293, 3758–3769. [Google Scholar] [CrossRef] [PubMed]
- Merra, A.; Maurizi, E.; Pellegrini, G. Impact of culture media on primary human corneal endothelial cells derived from old donors. Exp. Eye Res. 2024, 240, 109815. [Google Scholar] [CrossRef] [PubMed]
- Song, E.S.; Park, J.H.; Ha, S.S.; Cha, P.H.; Kang, J.T.; Park, C.Y.; Park, K. Novel Corneal Endothelial Cell Carrier Couples a Biodegradable Polymer and a Mesenchymal Stem Cell-Derived Extracellular Matrix. ACS Appl. Mater. Interfaces 2022, 14, 12116–12129. [Google Scholar] [CrossRef] [PubMed]
- Nevo, Z.; Gonzalez, R.; Gospodarowicz, D. Extracellular matrix (ECM) proteoglycans produced by cultured bovine corneal endothelial cells. Connect. Tissue Res. 1984, 13, 45–57. [Google Scholar] [CrossRef]
- Mitsumoto, T.; Nishimura, T.; Toda, S.; Okinami, S.; Oono, S.; Sugihara, H. Combined effect of extracellular matrices and growth factors on bovine corneal endothelial cells in culture. Jpn. J. Ophthalmol. 2001, 45, 115–124. [Google Scholar] [CrossRef]
- Zhu, C.; Joyce, N.C. Proliferative response of corneal endothelial cells from young and older donors. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1743–1751. [Google Scholar] [CrossRef]
- Okumura, N.; Koizumi, N.; Ueno, M.; Sakamoto, Y.; Takahashi, H.; Tsuchiya, H.; Hamuro, J.; Kinoshita, S. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. Am. J. Pathol. 2012, 181, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Syed, Z.A.; Rapuano, C.J. Rho kinase (ROCK) inhibitors in the management of corneal endothelial disease. Curr. Opin. Ophthalmol. 2021, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Kay, E.P.; Nakahara, M.; Hamuro, J.; Kinoshita, S.; Koizumi, N. Inhibition of TGF-beta signaling enables human corneal endothelial cell expansion in vitro for use in regenerative medicine. PLoS ONE 2013, 8, e58000. [Google Scholar] [CrossRef] [PubMed]
- Shima, N.; Kimoto, M.; Yamaguchi, M.; Yamagami, S. Increased proliferation and replicative lifespan of isolated human corneal endothelial cells with L-ascorbic acid 2-phosphate. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8711–8717. [Google Scholar] [CrossRef]
- Cela, E.; Patterson, E.K.; Gill, S.E.; Cepinskas, G.; Fraser, D.D. Application of Human Plasma/Serum to Cell Culture In Vitro: A Translational Research Approach to Better Define Disease Mechanisms. Clin. Transl. Sci. 2025, 18, e70161. [Google Scholar] [CrossRef]
- Alonso-Alonso, S.; Vazquez, N.; Chacon, M.; Caballero-Sanchez, N.; Del Olmo-Aguado, S.; Suarez, C.; Alfonso-Bartolozzi, B.; Fernandez-Vega-Cueto, L.; Nagy, L.; Merayo-Lloves, J.; et al. An effective method for culturing functional human corneal endothelial cells using a xenogeneic free culture medium. Sci. Rep. 2023, 13, 19492. [Google Scholar] [CrossRef]
- Petsoglou, C.; Wen, L.; Hoque, M.; Zhu, M.; Valtink, M.; Sutton, G.; You, J. Effects of human platelet lysate on the growth of cultured human corneal endothelial cells. Exp. Eye Res. 2021, 208, 108613. [Google Scholar] [CrossRef]
- Widyaningrum, R.; Burnouf, T.; Nebie, O.; Delila, L.; Wang, T.J. A purified human platelet pellet lysate rich in neurotrophic factors and antioxidants repairs and protects corneal endothelial cells from oxidative stress. Biomed. Pharmacother. 2021, 142, 112046. [Google Scholar] [CrossRef]
- Mishan, M.A.; Balagholi, S.; Chamani, T.; Feizi, S.; Soheili, Z.S.; Kanavi, M.R. Potential Effect of Human Platelet Lysate on in vitro Expansion of Human Corneal Endothelial Cells Compared with Y-27632 ROCK Inhibitor. J. Ophthalmic Vis. Res. 2021, 16, 349–356. [Google Scholar] [CrossRef]
- Thieme, D.; Reuland, L.; Lindl, T.; Kruse, F.; Fuchsluger, T. Optimized human platelet lysate as novel basis for a serum-, xeno-, and additive-free corneal endothelial cell and tissue culture. J. Tissue Eng. Regen. Med. 2018, 12, 557–564. [Google Scholar] [CrossRef]
- Talpan, D.; Salla, S.; Meusel, L.; Walter, P.; Kuo, C.C.; Franzen, J.; Fuest, M. Cytoprotective Effects of Human Platelet Lysate during the Xeno-Free Culture of Human Donor Corneas. Int. J. Mol. Sci. 2023, 24, 2882. [Google Scholar] [CrossRef]
- Senoo, T.; Joyce, N.C. Cell cycle kinetics in corneal endothelium from old and young donors. Investig. Ophthalmol. Vis. Sci. 2000, 41, 660–667. [Google Scholar]
- Woost, P.G.; Jumblatt, M.M.; Eiferman, R.A.; Schultz, G.S. Growth factors and corneal endothelial cells: I. Stimulation of bovine corneal endothelial cell DNA synthesis by defined growth factors. Cornea 1992, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, M.; Okumura, N.; Kay, E.P.; Hagiya, M.; Imagawa, K.; Hosoda, Y.; Kinoshita, S.; Koizumi, N. Corneal endothelial expansion promoted by human bone marrow mesenchymal stem cell-derived conditioned medium. PLoS ONE 2013, 8, e69009. [Google Scholar] [CrossRef] [PubMed]
- Sha, X.; Liu, Z.; Song, L.; Wang, Z.; Liang, X. Human amniotic epithelial cell niche enhances the functional properties of human corneal endothelial cells via inhibiting P53-survivin-mitochondria axis. Exp. Eye Res. 2013, 116, 36–46. [Google Scholar] [CrossRef]
- Petrela, R.B.; Patel, S.P. The soil and the seed: The relationship between Descemet’s membrane and the corneal endothelium. Exp. Eye Res. 2023, 227, 109376. [Google Scholar] [CrossRef]
- Sornelli, F.; Lambiase, A.; Mantelli, F.; Aloe, L. NGF and NGF-receptor expression of cultured immortalized human corneal endothelial cells. Mol. Vis. 2010, 16, 1439–1447. [Google Scholar]
- Li, X.; Li, Z.; Qiu, L.; Zhao, C.; Hu, Z. Nerve growth factor modulate proliferation of cultured rabbit corneal endothelial cells and epithelial cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2005, 25, 575–577. [Google Scholar] [CrossRef]
- Shehab, A.; Gram, N.; Ivarsen, A.; Hjortdal, J. The importance of donor characteristics, post-mortem time and preservation time for use and efficacy of donated corneas for posterior lamellar keratoplasty. Acta Ophthalmol. 2022, 100, 269–276. [Google Scholar] [CrossRef]
- Romano, V.; Passaro, M.L.; Ruzza, A.; Parekh, M.; Airaldi, M.; Levis, H.J.; Ferrari, S.; Costagliola, C.; Semeraro, F.; Ponzin, D. Quality assurance in corneal transplants: Donor cornea assessment and oversight. Surv. Ophthalmol. 2024, 69, 465–482. [Google Scholar] [CrossRef]
- Krohn, J.; Hovding, G. The influence of donor age and cause of death on corneal endothelial cell density. Acta Ophthalmol. Scand. 2005, 83, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Petithomme, R.; Karmakar, R.; Lohmeier, J.; Terrin, S.; Koo, E.H.; Eghrari, A.O. Comparison of corneal endothelial cell density and morphology with Optisol GS and Life4C storage media in the eye bank: A 5-year retrospective analysis. Eye Bank. Corneal Transpl. 2023, 2, e0019. [Google Scholar] [CrossRef] [PubMed]
- Bertolin, M.; Ruzza, A.; Barbaro, V.; Zanetti, E.; Ponzin, D.; Ferrari, S. Factors Affecting the Density of Corneal Endothelial Cells Cultured from Donor Corneas. Int. J. Mol. Sci. 2024, 25, 11884. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, K.; Toda, M.; Ueno, M.; Uehara, A.; Sotozono, C.; Kinoshita, S. The Biologic Character of Donor Corneal Endothelial Cells Influences Endothelial Cell Density Post Successful Corneal Transplantation. Ophthalmol. Sci. 2023, 3, 100239. [Google Scholar] [CrossRef]
- Parekh, M.; Ahmad, S.; Ruzza, A.; Ferrari, S. Human Corneal Endothelial Cell Cultivation from Old Donor Corneas with Forced Attachment. Sci. Rep. 2017, 7, 142. [Google Scholar] [CrossRef]
- Trouvain, A.M.; Szurman, P.; Wahl, S.; Siegel, R.; Boden, K.T.; Seitz, B.; Fries, F.N.; Rickmann, A. Impact of Previous Cataract Surgery in Corneal Donors on the Outcome of Descemet Membrane Endothelial Keratoplasty. Cornea 2024, 43, 844–852. [Google Scholar] [CrossRef]
- Aouimeur, I.; Sagnial, T.; Coulomb, L.; Maurin, C.; Thomas, J.; Forestier, P.; Ninotta, S.; Perrache, C.; Forest, F.; Gain, P.; et al. Investigating the Role of TGF-beta Signaling Pathways in Human Corneal Endothelial Cell Primary Culture. Cells 2023, 12, 1624. [Google Scholar] [CrossRef]
- Funaki, T.; Nakao, A.; Ebihara, N.; Setoguchi, Y.; Fukuchi, Y.; Okumura, K.; Ra, C.; Ogawa, H.; Kanai, A. Smad7 suppresses the inhibitory effect of TGF-beta2 on corneal endothelial cell proliferation and accelerates corneal endothelial wound closure in vitro. Cornea 2003, 22, 153–159. [Google Scholar] [CrossRef]
- Funaki, T.; Ebihara, N.; Murakami, A.; Nakao, A. Ex vivo transfer of Smad7 decreases damage to the corneal endothelium after penetrating keratoplasty. Jpn. J. Ophthalmol. 2008, 52, 204–210. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, R.; Ling, J.; Xu, C.; Ma, M.; Dong, X.; Wu, J.; Huang, T. SIRT1 Activation Suppresses Corneal Endothelial-Mesenchymal Transition via the TGF-beta/Smad2/3 Pathway. Curr. Issues Mol. Biol. 2024, 46, 13846–13859. [Google Scholar] [CrossRef]
- Ryu, Y.; Son, H.J.; Hwang, J.S.; Noh, K.B.; Oh, S.H.; Choi, E.K.; Shin, Y.J. AMF30a promotes survival and function of human corneal endothelial cells by regulating TGF-beta/ROCK/HIPPO pathway. Sci. Rep. 2025, 15, 28271. [Google Scholar] [CrossRef]
- Joko, T.; Shiraishi, A.; Akune, Y.; Tokumaru, S.; Kobayashi, T.; Miyata, K.; Ohashi, Y. Involvement of P38MAPK in human corneal endothelial cell migration induced by TGF-beta(2). Exp. Eye Res. 2013, 108, 23–32. [Google Scholar] [CrossRef]
- Khoramjoo, S.M.; Kazemifard, N.; Baradaran Ghavami, S.; Farmani, M.; Shahrokh, S.; Asadzadeh Aghdaei, H.; Sherkat, G.; Zali, M.R. Overview of Three Proliferation Pathways (Wnt, Notch, and Hippo) in Intestine and Immune System and Their Role in Inflammatory Bowel Diseases (IBDs). Front. Med. 2022, 9, 865131. [Google Scholar] [CrossRef]
- Ward, D.; Montes Olivas, S.; Fletcher, A.; Homer, M.; Marucci, L. Cross-talk between Hippo and Wnt signalling pathways in intestinal crypts: Insights from an agent-based model. Comput. Struct. Biotechnol. J. 2020, 18, 230–240. [Google Scholar] [CrossRef]
- Gao, J.; Fan, L.; Zhao, L.; Su, Y. The interaction of Notch and Wnt signaling pathways in vertebrate regeneration. Cell Regen. 2021, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Hirata-Tominaga, K.; Nakamura, T.; Okumura, N.; Kawasaki, S.; Kay, E.P.; Barrandon, Y.; Koizumi, N.; Kinoshita, S. Corneal endothelial cell fate is maintained by LGR5 through the regulation of hedgehog and Wnt pathway. Stem Cells 2013, 31, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.T.; Chen, H.C.; Chen, S.Y.; Tseng, S.C. Nuclear p120 catenin unlocks mitotic block of contact-inhibited human corneal endothelial monolayers without disrupting adherent junctions. J. Cell Sci. 2012, 125, 3636–3648. [Google Scholar] [CrossRef]
- Lee, J.G.; Heur, M. Interleukin-1beta-induced Wnt5a enhances human corneal endothelial cell migration through regulation of Cdc42 and RhoA. Mol. Cell. Biol. 2014, 34, 3535–3545. [Google Scholar] [CrossRef]
- Maurizi, E.; Schiroli, D.; Zini, R.; Limongelli, A.; Misto, R.; Macaluso, C.; Pellegrini, G. A fine-tuned beta-catenin regulation during proliferation of corneal endothelial cells revealed using proteomics analysis. Sci. Rep. 2020, 10, 13841. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Fu, Y.; Fan, X. The signaling pathway involved in the proliferation of corneal endothelial cells. J. Recept. Signal Transduct. Res. 2015, 35, 585–591. [Google Scholar] [CrossRef]
- Collu, G.M.; Hidalgo-Sastre, A.; Brennan, K. Wnt-Notch signalling crosstalk in development and disease. Cell Mol. Life Sci. 2014, 71, 3553–3567. [Google Scholar] [CrossRef]
- Li, C.; Dong, F.; Jia, Y.; Du, H.; Dong, N.; Xu, Y.; Wang, S.; Wu, H.; Liu, Z.; Li, W. Notch signal regulates corneal endothelial-to-mesenchymal transition. Am. J. Pathol. 2013, 183, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Catala, P.; Vivensang, F.; van Beek, D.; Adriaens, M.E.; Dickman, M.M.; LaPointe, V.L.S.; Kutmon, M. Elucidating the Corneal Endothelial Cell Proliferation Capacity through an Interspecies Transcriptome Comparison. Adv. Biol. 2023, 7, e2300065. [Google Scholar] [CrossRef] [PubMed]
- Hall, A. Rho GTPases and the actin cytoskeleton. Science 1998, 279, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Cannon, R.D.; Coates, D.E.; Mei, L. Effect of the Rho-Kinase/ROCK Signaling Pathway on Cytoskeleton Components. Genes 2023, 14, 272. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Sahu, S.K. Rho-kinase inhibitors: Role in corneal endothelial disorders. Semin. Ophthalmol. 2023, 38, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Lin, C.T.; Li, J.W.; Hu, F.R.; Chen, C.C. ERK1/2 activation regulates the wound healing process of rabbit corneal endothelial cells. Curr. Eye Res. 2009, 34, 103–111. [Google Scholar] [CrossRef]
- Nakahara, M.; Okumura, N.; Nakano, S.; Koizumi, N. Effect of a p38 Mitogen-Activated Protein Kinase Inhibitor on Corneal Endothelial Cell Proliferation. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4218–4227. [Google Scholar] [CrossRef]
- Ge, W.; Mi, Y.; Xu, S.; Li, T.; Lu, Y.; Jiang, J. rhBMP-7 suppresses TGF-beta1-induced endothelial to mesenchymal transition in circulating endothelial cells by regulating Smad5. Mol. Med. Rep. 2020, 21, 478–484. [Google Scholar] [CrossRef]
- Okumura, N.; Hirano, H.; Numata, R.; Nakahara, M.; Ueno, M.; Hamuro, J.; Kinoshita, S.; Koizumi, N. Cell surface markers of functional phenotypic corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7610–7618. [Google Scholar] [CrossRef]
- Catala, P.; Groen, N.; LaPointe, V.L.S.; Dickman, M.M. A single-cell RNA-seq analysis unravels the heterogeneity of primary cultured human corneal endothelial cells. Sci. Rep. 2023, 13, 9361. [Google Scholar] [CrossRef]
- Ding, V.; Chin, A.; Peh, G.; Mehta, J.S.; Choo, A. Generation of novel monoclonal antibodies for the enrichment and characterization of human corneal endothelial cells (hCENC) necessary for the treatment of corneal endothelial blindness. MAbs 2014, 6, 1439–1452. [Google Scholar] [CrossRef]
- Dorfmueller, S.; Tan, H.C.; Ngoh, Z.X.; Toh, K.Y.; Peh, G.; Ang, H.P.; Seah, X.Y.; Chin, A.; Choo, A.; Mehta, J.S.; et al. Isolation of a recombinant antibody specific for a surface marker of the corneal endothelium by phage display. Sci. Rep. 2016, 6, 21661. [Google Scholar] [CrossRef] [PubMed]
- Hamuro, J.; Ueno, M.; Toda, M.; Sotozono, C.; Montoya, M.; Kinoshita, S. Cultured Human Corneal Endothelial Cell Aneuploidy Dependence on the Presence of Heterogeneous Subpopulations with Distinct Differentiation Phenotypes. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4385–4392. [Google Scholar] [CrossRef] [PubMed]
- Ng, X.Y.; Peh, G.S.L.; Yam, G.H.; Tay, H.G.; Mehta, J.S. Corneal Endothelial-like Cells Derived from Induced Pluripotent Stem Cells for Cell Therapy. Int. J. Mol. Sci. 2023, 24, 12433. [Google Scholar] [CrossRef] [PubMed]
- Bartakova, A.; Alvarez-Delfin, K.; Weisman, A.D.; Salero, E.; Raffa, G.A.; Merkhofer, R.M., Jr.; Kunzevitzky, N.J.; Goldberg, J.L. Novel Identity and Functional Markers for Human Corneal Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2749–2762. [Google Scholar] [CrossRef]
- Sun, F.; Xi, L.W.Q.; Luu, W.; Enkhbat, M.; Neo, D.; Mehta, J.S.; Peh, G.S.L.; Yim, E.K.F. Preclinical Models for Studying Fuchs Endothelial Corneal Dystrophy. Cells 2025, 14, 505. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Takezawa, T. Fabrication of a Corneal Model Composed of Corneal Epithelial and Endothelial Cells via a Collagen Vitrigel Membrane Functioned as an Acellular Stroma and Its Application to the Corneal Permeability Test of Chemicals. Drug Metab. Dispos. 2018, 46, 1684–1691. [Google Scholar] [CrossRef]
- Bonanno, J.A. Molecular mechanisms underlying the corneal endothelial pump. Exp. Eye Res. 2012, 95, 2–7. [Google Scholar] [CrossRef]
- Tsai, M.C.; Kureshi, A.; Daniels, J.T. Establishment of an Ex Vivo Human Corneal Endothelium Wound Model. Transl. Vis. Sci. Technol. 2025, 14, 24. [Google Scholar] [CrossRef]
- Yu, Z.; Hao, R.; Chen, X.; Ma, L.; Zhang, Y.; Yang, H. Protocol to develop a microfluidic human corneal barrier-on-a-chip to evaluate the corneal epithelial wound repair process. STAR Protoc. 2023, 4, 102122. [Google Scholar] [CrossRef]
- Mimura, T.; Yamagami, S.; Yokoo, S.; Usui, T.; Tanaka, K.; Hattori, S.; Irie, S.; Miyata, K.; Araie, M.; Amano, S. Cultured human corneal endothelial cell transplantation with a collagen sheet in a rabbit model. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2992–2997. [Google Scholar] [CrossRef] [PubMed]
- Hatou, S.; Higa, K.; Inagaki, E.; Yoshida, S.; Kimura, E.; Hayashi, R.; Tsujikawa, M.; Tsubota, K.; Nishida, K.; Shimmura, S. Validation of Na,K-ATPase pump function of corneal endothelial cells for corneal regenerative medicine. Tissue Eng. Part. C Methods 2013, 19, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Choi, K.; Lin, A.; Kim, J. Current and Future Cornea Chip Models for Advancing Ophthalmic Research and Therapeutics. Adv. Biol. 2025, 9, e2400571. [Google Scholar] [CrossRef] [PubMed]
- Kerk, Y.J.; Jameel, A.; Xing, X.H.; Zhang, C. Recent advances of integrated microfluidic suspension cell culture system. Eng. Biol. 2021, 5, 103–119. [Google Scholar] [CrossRef]
- Enkhbat, M.; Mehta, J.S.; Peh, G.S.L.; Yim, E.K.F. Biomaterial-based strategies for primary human corneal endothelial cells for therapeutic applications: From cell expansion to transplantable carrier. Biomater. Sci. 2025, 13, 1114–1130. [Google Scholar] [CrossRef]
- Huang, A.J.; Shui, Y.B.; Han, Y.P.; Bai, F.; Siegfried, C.J.; Beebe, D.C. Impact of Corneal Endothelial Dysfunctions on Intraocular Oxygen Levels in Human Eyes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6483–6488. [Google Scholar] [CrossRef]
- Cheng, Q.; Nguyen, T.; Song, H.; Bonanno, J. Hypoxia protects human corneal endothelium from tertiary butyl hydroperoxide and paraquat-induced cell death in vitro. Exp. Biol. Med. 2007, 232, 445–453. [Google Scholar]
- Patel, S.P.; Calle Gonzalez, B.; Paone, N.; Mueller, C.; Floss, J.C.; Sousa, M.E.; Shi, M.Y. Effect of Physiological Oxygen on Primary Human Corneal Endothelial Cell Cultures. Transl. Vis. Sci. Technol. 2022, 11, 33. [Google Scholar] [CrossRef]
- Ma, D.J.; Hwang, J.S.; Noh, K.B.; Oh, S.H.; Kim, K.W.; Shin, Y.J. Role of NADPH Oxidase 4 in Corneal Endothelial Cells Is Mediated by Endoplasmic Reticulum Stress and Autophagy. Antioxidants 2023, 12, 1228. [Google Scholar] [CrossRef]
- Fautsch, M.P.; Wieben, E.D.; Baratz, K.H.; Bhattacharyya, N.; Sadan, A.N.; Hafford-Tear, N.J.; Tuft, S.J.; Davidson, A.E. TCF4-mediated Fuchs endothelial corneal dystrophy: Insights into a common trinucleotide repeat-associated disease. Prog. Retin. Eye Res. 2021, 81, 100883. [Google Scholar] [CrossRef]
- Yan, J.; Mehta, S.; Patel, K.; Dhupar, N.; Little, N.; Ong Tone, S. Transcription factor 4 promotes increased corneal endothelial cellular migration by altering microtubules in Fuchs endothelial corneal dystrophy. Sci. Rep. 2024, 14, 10276. [Google Scholar] [CrossRef] [PubMed]
- Fuchsluger, T.A.; Jurkunas, U.; Kazlauskas, A.; Dana, R. Corneal endothelial cells are protected from apoptosis by gene therapy. Hum. Gene Ther. 2011, 22, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, C.; Skotheim, J.M.; de Bruin, R.A. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell. Biol. 2013, 14, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Pellarin, I.; Dall’Acqua, A.; Favero, A.; Segatto, I.; Rossi, V.; Crestan, N.; Karimbayli, J.; Belletti, B.; Baldassarre, G. Cyclin-dependent protein kinases and cell cycle regulation in biology and disease. Signal Transduct. Target. Ther. 2025, 10, 11. [Google Scholar] [CrossRef]
- Suryadinata, R.; Sadowski, M.; Sarcevic, B. Control of cell cycle progression by phosphorylation of cyclin-dependent kinase (CDK) substrates. Biosci. Rep. 2010, 30, 243–255. [Google Scholar] [CrossRef]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef]
- McAlister, J.C.; Joyce, N.C.; Harris, D.L.; Ali, R.R.; Larkin, D.F. Induction of replication in human corneal endothelial cells by E2F2 transcription factor cDNA transfer. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3597–3603. [Google Scholar] [CrossRef]
- Kampik, D.; Basche, M.; Georgiadis, A.; Luhmann, U.F.O.; Larkin, D.F.; Smith, A.J.; Ali, R.R. Modulation of Contact Inhibition by ZO-1/ZONAB Gene Transfer-A New Strategy to Increase the Endothelial Cell Density of Corneal Grafts. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3170–3177. [Google Scholar] [CrossRef]
- Sheerin, A.N.; Smith, S.K.; Jennert-Burston, K.; Brook, A.J.; Allen, M.C.; Ibrahim, B.; Jones, D.; Wallis, C.; Engelmann, K.; Rhys-Williams, W.; et al. Characterization of cellular senescence mechanisms in human corneal endothelial cells. Aging Cell 2012, 11, 234–240. [Google Scholar] [CrossRef]
- Kikuchi, M.; Zhu, C.; Senoo, T.; Obara, Y.; Joyce, N.C. p27kip1 siRNA induces proliferation in corneal endothelial cells from young but not older donors. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4803–4809. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Han, B.; Li, F.; Chen, S.Y.; Tighe, S.; Zhang, S.; Tseng, S.C. Knockdown of both p120 catenin and Kaiso promotes expansion of human corneal endothelial monolayers via RhoA-ROCK-noncanonical BMP-NFkappaB pathway. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1509–1518. [Google Scholar] [CrossRef]
- Chang, Y.K.; Hwang, J.S.; Chung, T.Y.; Shin, Y.J. SOX2 Activation Using CRISPR/dCas9 Promotes Wound Healing in Corneal Endothelial Cells. Stem Cells 2018, 36, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.J.; Ma, D.J.; Hwang, J.S.; Shin, Y.J. SIRT1 Activation Using CRISPR/dCas9 Promotes Regeneration of Human Corneal Endothelial Cells through Inhibiting Senescence. Antioxidants 2020, 9, 1085. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, M.; Hariri, S.; Westphal, K.C.G.; Janssen, M.J.; Oost, M.J.; Bongiovanni, L.; van den Heuvel, L.P.; de Bruin, A.; Hilbrands, L.B.; Masereeuw, R. Safety evaluation of conditionally immortalized cells for renal replacement therapy. Oncotarget 2019, 10, 5332–5348. [Google Scholar] [CrossRef] [PubMed]
- Chehelgerdi, M.; Chehelgerdi, M.; Khorramian-Ghahfarokhi, M.; Shafieizadeh, M.; Mahmoudi, E.; Eskandari, F.; Rashidi, M.; Arshi, A.; Mokhtari-Farsani, A. Comprehensive review of CRISPR-based gene editing: Mechanisms, challenges, and applications in cancer therapy. Mol. Cancer 2024, 23, 9. [Google Scholar] [CrossRef]
- Wilson, S.E.; Lloyd, S.A.; He, Y.G.; McCash, C.S. Extended life of human corneal endothelial cells transfected with the SV40 large T antigen. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2112–2123. [Google Scholar]
- Oshikawa, K.; Matsumoto, M.; Kodama, M.; Shimizu, H.; Nakayama, K.I. A fail-safe system to prevent oncogenesis by senescence is targeted by SV40 small T antigen. Oncogene 2020, 39, 2170–2186. [Google Scholar] [CrossRef]
- Bednarz, J.; Teifel, M.; Friedl, P.; Engelmann, K. Immortalization of human corneal endothelial cells using electroporation protocol optimized for human corneal endothelial and human retinal pigment epithelial cells. Acta Ophthalmol. Scand. 2000, 78, 130–136. [Google Scholar] [CrossRef]
- Scheffner, M.; Werness, B.A.; Huibregtse, J.M.; Levine, A.J.; Howley, P.M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 1990, 63, 1129–1136. [Google Scholar] [CrossRef]
- Huh, K.; Zhou, X.; Hayakawa, H.; Cho, J.Y.; Libermann, T.A.; Jin, J.; Harper, J.W.; Munger, K. Human papillomavirus type 16 E7 oncoprotein associates with the cullin 2 ubiquitin ligase complex, which contributes to degradation of the retinoblastoma tumor suppressor. J. Virol. 2007, 81, 9737–9747. [Google Scholar] [CrossRef]
- Yokoi, T.; Seko, Y.; Yokoi, T.; Makino, H.; Hatou, S.; Yamada, M.; Kiyono, T.; Umezawa, A.; Nishina, H.; Azuma, N. Establishment of functioning human corneal endothelial cell line with high growth potential. PLoS ONE 2012, 7, e29677. [Google Scholar] [CrossRef] [PubMed]
- Yik, M.Y.; Azlan, A.; Rajasegaran, Y.; Rosli, A.; Yusoff, N.M.; Moses, E.J. Mechanism of Human Telomerase Reverse Transcriptase (hTERT) Regulation and Clinical Impacts in Leukemia. Genes 2021, 12, 1188. [Google Scholar] [CrossRef]
- Liu, Z.; Zhuang, J.; Li, C.; Wan, P.; Li, N.; Zhou, Q.; Zhou, C.; Huang, Z.; Wang, Z. Long-term cultivation of human corneal endothelial cells by telomerase expression. Exp. Eye Res. 2012, 100, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Agrahari, V.; Agrahari, V. Extracellular Vesicles as Nanomedicine: Hopes and Hurdles in Clinical Translation. Int. J. Nanomed. 2019, 14, 8847–8859. [Google Scholar] [CrossRef] [PubMed]
- Colao, I.L.; Corteling, R.; Bracewell, D.; Wall, I. Manufacturing Exosomes: A Promising Therapeutic Platform. Trends Mol. Med. 2018, 24, 242–256. [Google Scholar] [CrossRef]
- Ryu, Y.; Hwang, J.S.; Bo Noh, K.; Park, S.H.; Seo, J.H.; Shin, Y.J. Adipose Mesenchymal Stem Cell-Derived Exosomes Promote the Regeneration of Corneal Endothelium Through Ameliorating Senescence. Investig. Ophthalmol. Vis. Sci. 2023, 64, 29. [Google Scholar] [CrossRef]
- Yamashita, T.; Asada, K.; Ueno, M.; Hiramoto, N.; Fujita, T.; Toda, M.; Sotozono, C.; Kinoshita, S.; Hamuro, J. Cellular Interplay Through Extracellular Vesicle miR-184 Alleviates Corneal Endothelium Degeneration. Ophthalmol. Sci. 2022, 2, 100212. [Google Scholar] [CrossRef]
- Park, S.H.; Hwang, J.S.; Oh, S.H.; Shin, Y.J. MiR-302a Regenerates Human Corneal Endothelial Cells against IFN-gamma-Induced Cell Death. Cells 2022, 12, 36. [Google Scholar] [CrossRef]
- Parekh, M.; Ramos, T.; Ferrari, S.; Ahmad, S. Inhibiting miR-195-5p Induces Proliferation of Human Corneal Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 11490. [Google Scholar] [CrossRef]
- Xia, X.; Atkins, M.; Dalal, R.; Kuzmenko, O.; Chang, K.C.; Sun, C.B.; Benatti, C.A.; Rak, D.J.; Nahmou, M.; Kunzevitzky, N.J.; et al. Magnetic Human Corneal Endothelial Cell Transplant: Delivery, Retention, and Short-Term Efficacy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2438–2448. [Google Scholar] [CrossRef] [PubMed]
- Moysidis, S.N.; Alvarez-Delfin, K.; Peschansky, V.J.; Salero, E.; Weisman, A.D.; Bartakova, A.; Raffa, G.A.; Merkhofer, R.M., Jr.; Kador, K.E.; Kunzevitzky, N.J.; et al. Magnetic field-guided cell delivery with nanoparticle-loaded human corneal endothelial cells. Nanomedicine 2015, 11, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, K.; Park, C.Y. Effect of Magnetic Microparticles on Cultivated Human Corneal Endothelial Cells. Transl. Vis. Sci. Technol. 2023, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Yamagami, S.; Usui, T.; Ishii, Y.; Ono, K.; Yokoo, S.; Funatsu, H.; Araie, M.; Amano, S. Long-term outcome of iron-endocytosing cultured corneal endothelial cell transplantation with magnetic attraction. Exp. Eye Res. 2005, 80, 149–157. [Google Scholar] [CrossRef]
- Zhao, S.; Hou, S.; Li, D.; Li, L.; Ding, X.; Huang, Y.; Li, Y.; Ji, J.; Wang, L.; Fan, Y. Injectable magnetic hyaluronic acid gel for corneal endothelial cells efficient delivery and retention. Appl. Mater. 2024, 37, 102090. [Google Scholar]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef]
- Teng, L.; Mundell, N.A.; Frist, A.Y.; Wang, Q.; Labosky, P.A. Requirement for Foxd3 in the maintenance of neural crest progenitors. Development 2008, 135, 1615–1624. [Google Scholar] [CrossRef]
- Zhao, J.J.; Afshari, N.A. Generation of Human Corneal Endothelial Cells via In Vitro Ocular Lineage Restriction of Pluripotent Stem Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6878–6884. [Google Scholar] [CrossRef]
- Gronroos, P.; Ilmarinen, T.; Skottman, H. Directed Differentiation of Human Pluripotent Stem Cells towards Corneal Endothelial-Like Cells under Defined Conditions. Cells 2021, 10, 331. [Google Scholar] [CrossRef]
- Maurizi, E.; Merra, A.; Macaluso, C.; Schiroli, D.; Pellegrini, G. GSK-3 inhibition reverts mesenchymal transition in primary human corneal endothelial cells. Eur. J. Cell Biol. 2023, 102, 151302. [Google Scholar] [CrossRef]
- Song, Q.; Yuan, S.; An, Q.; Chen, Y.; Mao, F.F.; Liu, Y.; Liu, Q.; Fan, G. Directed differentiation of human embryonic stem cells to corneal endothelial cell-like cells: A transcriptomic analysis. Exp. Eye Res. 2016, 151, 107–114. [Google Scholar] [CrossRef] [PubMed]
- McCabe, K.L.; Kunzevitzky, N.J.; Chiswell, B.P.; Xia, X.; Goldberg, J.L.; Lanza, R. Efficient Generation of Human Embryonic Stem Cell-Derived Corneal Endothelial Cells by Directed Differentiation. PLoS ONE 2015, 10, e0145266. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yu, N.; Tian, Y.; Fang, Y.; An, B.; Feng, G.; Wu, J.; Wang, L.; Hao, J.; Wang, L.; et al. Safety and efficacy of human ESC-derived corneal endothelial cells for corneal endothelial dysfunction. Cell Biosci. 2023, 13, 201. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Hu, Z.; Sun, X.; Hu, M.; Liu, H.; Yang, Z. [Transplanted vascular endothelial cells to replace corneal endothelial cells by improved anterior chamber injection]. Zhonghua Yan Ke Za Zhi 2014, 50, 277–284. [Google Scholar]
- Pan, S.H.; Zhao, N.; Feng, X.; Jie, Y.; Jin, Z.B. Conversion of mouse embryonic fibroblasts into neural crest cells and functional corneal endothelia by defined small molecules. Sci. Adv. 2021, 7, eabg5749. [Google Scholar] [CrossRef]
- Ali, M.; Raghunathan, V.; Li, J.Y.; Murphy, C.J.; Thomasy, S.M. Biomechanical relationships between the corneal endothelium and Descemet’s membrane. Exp. Eye Res. 2016, 152, 57–70. [Google Scholar] [CrossRef]
- Muhammad, R.; Peh, G.S.; Adnan, K.; Law, J.B.; Mehta, J.S.; Yim, E.K. Micro- and nano-topography to enhance proliferation and sustain functional markers of donor-derived primary human corneal endothelial cells. Acta Biomater. 2015, 19, 138–148. [Google Scholar] [CrossRef]
- Chi, J.; Wang, S.; Ju, R.; Li, S.; Liu, C.; Zou, M.; Xu, T.; Wang, Y.; Jiang, Z.; Yang, C.; et al. Repair effects of thermosensitive hydrogels combined with iPSC-derived corneal endothelial cells on rabbit corneal endothelial dysfunction. Acta Biomater. 2025, 191, 216–232. [Google Scholar] [CrossRef]
- Borden, B.A.; Yockman, J.; Kim, S.W. Thermoresponsive hydrogel as a delivery scaffold for transfected rat mesenchymal stem cells. Mol. Pharm. 2010, 7, 963–968. [Google Scholar] [CrossRef]
- Sherstneva, A.A.; Demina, T.S.; Monteiro, A.P.F.; Akopova, T.A.; Grandfils, C.; Ilangala, A.B. Biodegradable Microparticles for Regenerative Medicine: A State of the Art and Trends to Clinical Application. Polymers 2022, 14, 1314. [Google Scholar] [CrossRef]
- Lee, S.; Dohlman, T.H.; Dana, R. Immunology in corneal transplantation-From homeostasis to graft rejection. Transpl. Rev. 2025, 39, 100909. [Google Scholar] [CrossRef]


| Factor | Specific Details & Findings | Influence on Culture Success | Reference |
|---|---|---|---|
| Donor Age | Optimal age range typically under 60 years; younger donors (≤50 years) tend to have higher endothelial cell viability and proliferative capacity. | Significantly higher success rates with younger donors. | [41] |
| Donor Health Status | Good systemic health, absence of diabetes, hypertension, ocular diseases like Fuchs endothelial dystrophy, or previous ocular surgeries. | Better endothelial cell quality and culture outcomes. | [61] |
| Cause of Death | Non-traumatic causes (e.g., natural causes) linked with better cell quality; traumatic deaths may cause cell damage. | Traumatic death reduces culturability and cell viability. | [61,62] |
| Death-to-Preservation Interval | Recommendations suggest within 6–12 h; shorter intervals improve cell viability. | Longer intervals (>12 h) negatively impact success. | [60] |
| Preservation Medium and Conditions | Use of preservative media like Optisol-GS, stored at 4 °C; delays in preservation decrease cell viability. | Proper preservation is critical to successful culture. | [63] |
| Endothelial Cell Density | Higher initial cell density (preferably > 2500 cells/mm2) correlates with better proliferation potential. | Low initial density (<2000 cells/mm2) reduces success. | [64] |
| Donor Sex | No consistent evidence suggesting sex significantly impacts endothelial cell culture outcomes. | Typically negligible effect. | [64,65] |
| Donor Age-Related Changes | Age-related decrease in cell proliferative capacity and wound healing ability. | Older donors (>60 years) have lower success rates. | [64,66] |
| Prior Ocular Surgeries or Treatments | Previous surgeries (e.g., cataract surgery) may induce endothelial cell loss or damage. | May decrease regenerative capacity, reducing success. | [67] |
| Viral Oncogene | Details |
|---|---|
| SV40 Large T-antigen | Stimulates cell proliferation by inhibiting p53 and disrupting the Rb-E2F complex. Increases expression of CDK1, CDK2, and CDK4, and upregulates cyclin A and D [118]. Decreases cell cycle inhibitors p27KIP1 and p21CIP1. Results in increased proliferation rate and extended survival of CECs [128]. |
| SV40 Small T-antigen | Increases cell proliferation by binding with protein phosphatase 2A and inhibiting heterochromatin protein 1-binding protein 3 [129]. Contributes to SV40 large T-antigen-mediated cell transformation. Expression of both large and small T-antigens results in similar proliferation effect on CECs as large T-antigen alone [130]. |
| HPV-16 E6/E7 | E6 oncoprotein increases cell proliferation by degrading p53 [131]. E7 induces ubiquitination of Rb proteins [132]. Stable expression of both E6 and E7 results in immortalization of CECs. Cells exhibit cobblestone-like polygonal morphology and are mostly diploid [133]. |
| Trial | Goal | Design | Product Used | Primary Outcome Measure | Study Start/Completion | Results |
|---|---|---|---|---|---|---|
| CLARA trial (NCT06041256) | To compare different doses of AURN001 in patients with cornea edema secondary to corneal endothelial dysfunction | Phase 1/2, multicenter, randomized, double masked, prospective, parallel arm study | AURN001: combination of CEC and Y27632 | BCVA 15 letters (3-lines) or more improvement at 6 months | 18 October 2023/25 October 2024 | The high-dose AURN001 group achieved the primary endpoint in 50% of participants, compared to just 14.3% in the group treated with Y27632 alone |
| EMME-001 (NCT04894110) | To evaluate the safety and tolerability of 3 doses of EO2002 with or without endothelial brushing or Descemet stripping in cornea edema secondary to corneal endothelial dysfunction with | Group 1: Phase 1, prospective, multi-center, open-label, dose escalation study Group 2: prospective, multi-center, double-masked study | EO2002: magnetic human corneal endothelial cells | Incidence of treatment-emergent adverse events at 26 weeks | 22 June 2021/3 October 2024 | Patients receiving 150,000 cells showed a mean 11-letter BCVA gain at six months, with 38% improving by at least 15 letters. All cohorts improved in BCVA and central corneal thickness |
| A first-in-human clinical study (doi.org/10.1016/j.xcrm.2024.101847) | To evaluate the safety and efficacy of an iPSC-derived corneal endothelial cell substitute. | Phase 1: first human trial for one patient with severe bullous keratopathy recurring after prior corneal transplantation | CLS001: iPSC derived corneal endothelial cells | Any adverse event | 1 August 2022/30 March 2023 | Over a 52-week follow-up, no tumor formation or severe inflammation was observed, and patients showed improved visual acuity with a trend toward restored corneal transparency |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Jung, M.-Y.; Han, E.; Park, C.Y. Bioengineering Strategies for Corneal Endothelial Cell Injection Therapy: Advances, Challenges, and Clinical Translation. Bioengineering 2025, 12, 1162. https://doi.org/10.3390/bioengineering12111162
Choi Y, Jung M-Y, Han E, Park CY. Bioengineering Strategies for Corneal Endothelial Cell Injection Therapy: Advances, Challenges, and Clinical Translation. Bioengineering. 2025; 12(11):1162. https://doi.org/10.3390/bioengineering12111162
Chicago/Turabian StyleChoi, Yura, Mi-Young Jung, Eunsun Han, and Choul Yong Park. 2025. "Bioengineering Strategies for Corneal Endothelial Cell Injection Therapy: Advances, Challenges, and Clinical Translation" Bioengineering 12, no. 11: 1162. https://doi.org/10.3390/bioengineering12111162
APA StyleChoi, Y., Jung, M.-Y., Han, E., & Park, C. Y. (2025). Bioengineering Strategies for Corneal Endothelial Cell Injection Therapy: Advances, Challenges, and Clinical Translation. Bioengineering, 12(11), 1162. https://doi.org/10.3390/bioengineering12111162






