Analysis of Masseter Muscle Structure in Patients with Mandibular Asymmetry Using Ultrasonic Diagnostic Equipment
Abstract
1. Introduction
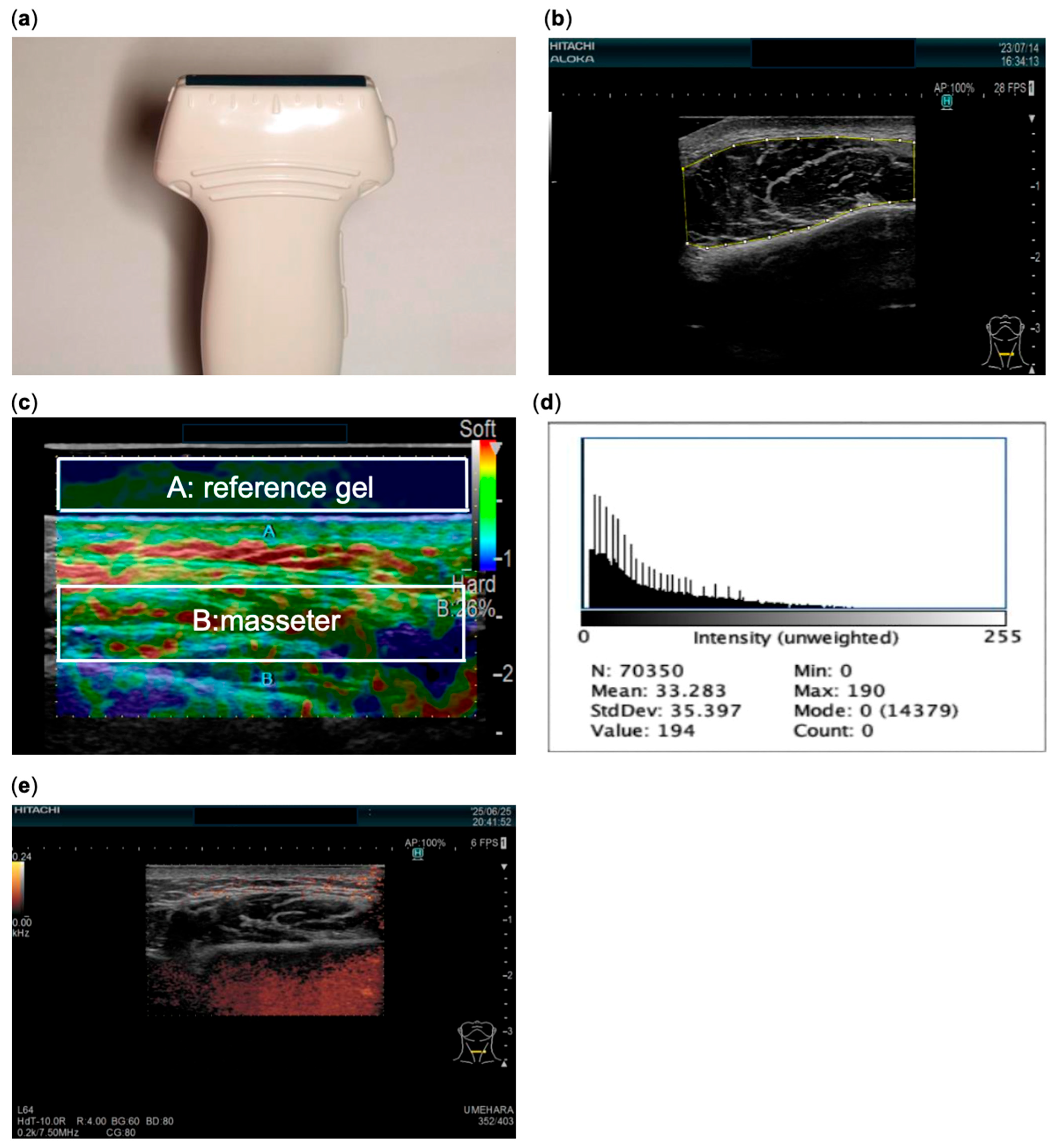
2. Materials and Methods
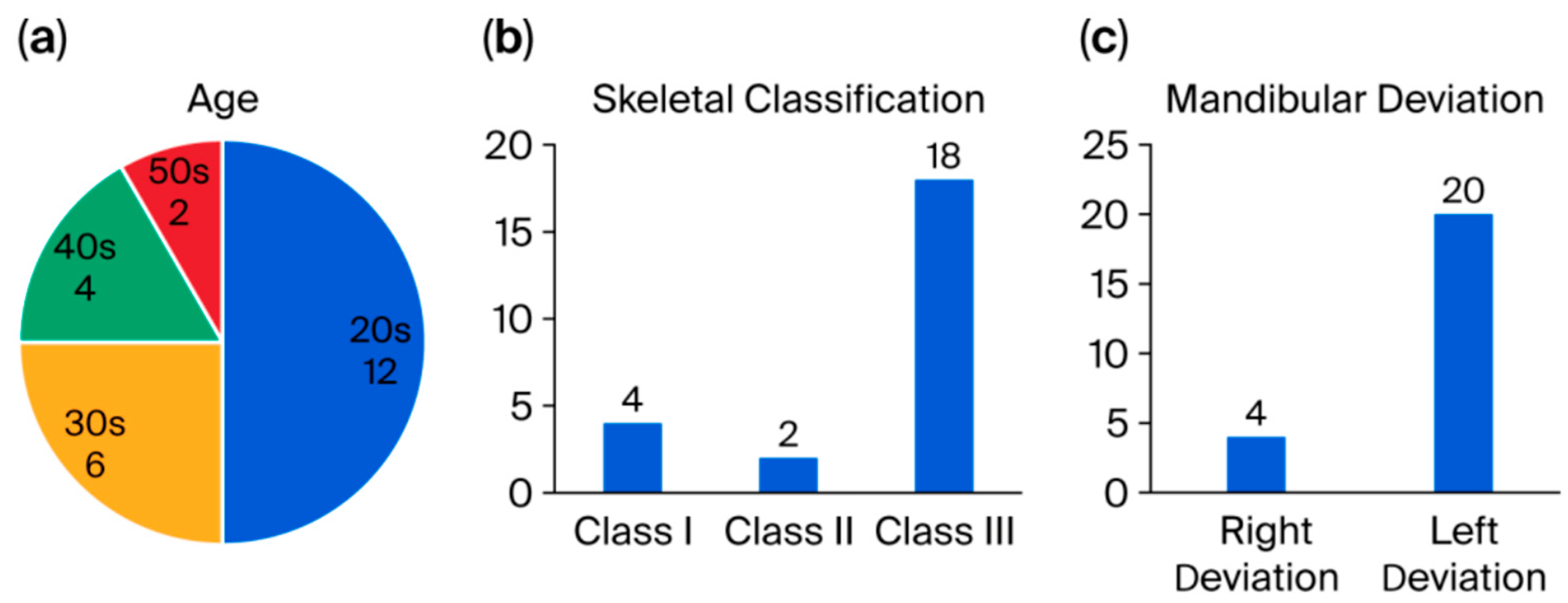
3. Results
4. Discussion
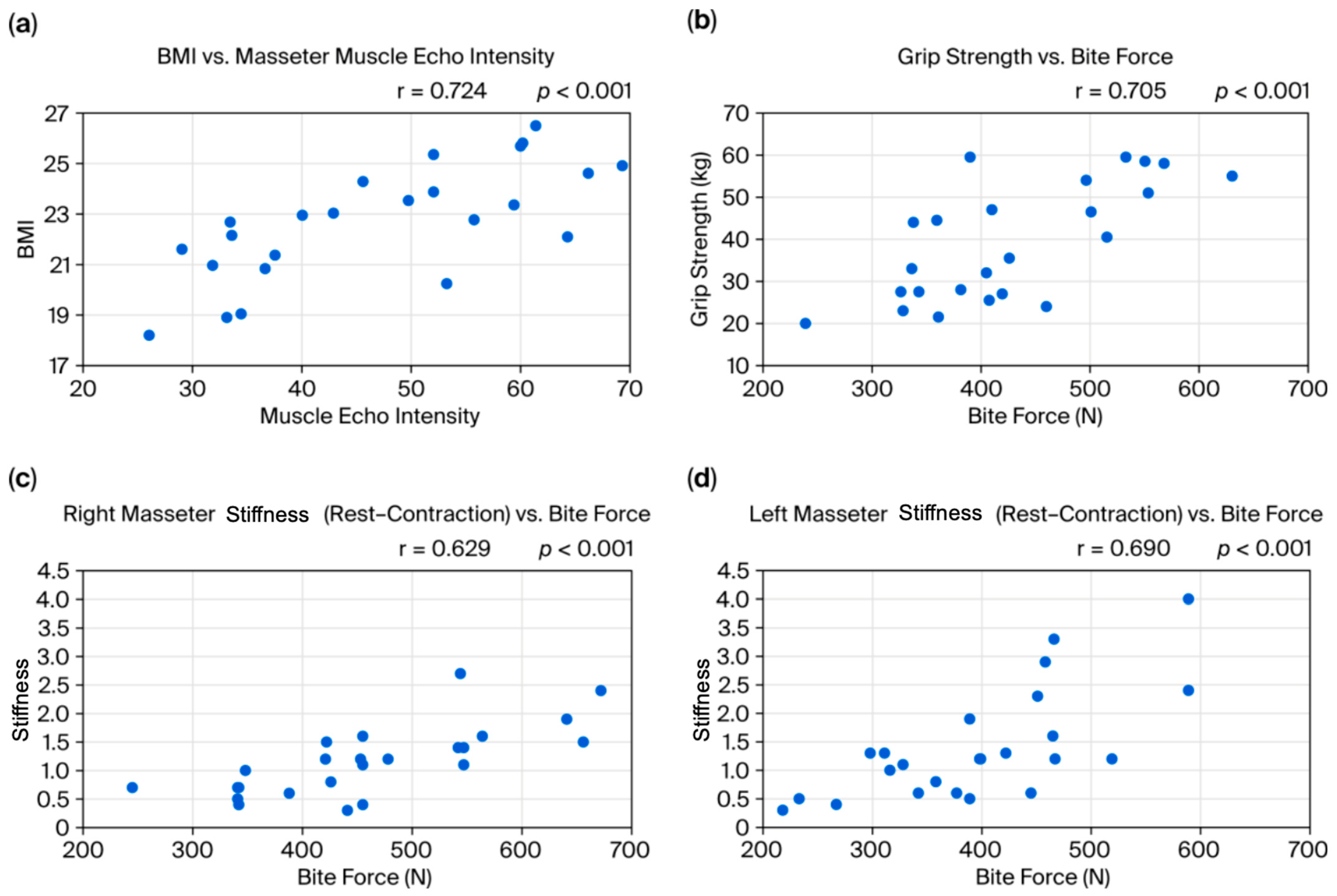
- BMI and masseter echo intensity: The strong positive correlation between BMI and masseter echo intensity suggests that patients with a higher BMI may have increased fat infiltration or fibrosis within the masseter. Previous studies have shown that echo intensity correlates with the proportion of fat or fibrous tissue in muscle [10,11]. Obesity, therefore, may lead not only to increased body weight but also to histological changes in the masticatory muscles, potentially resulting in muscle dysfunction and affecting post-orthognathic surgery stability. However, these findings indicate that BMI may have acted as a confounding factor in our study. Due to the limited sample size, statistical adjustments such as ANCOVA or regression analysis were not feasible. Future studies with larger cohorts should incorporate BMI as a covariate to clarify its influence.
- Muscle function and overall muscle strength: The observed positive correlation between grip strength and bite force, as well as the relationship between the stiffness ratio difference (contraction minus rest) and bite force, indicates synergy between the overall muscle strength and localized masticatory function. Previous studies have used grip strength as an indicator of general muscle strength, finding it to be related to masticatory function and nutritional status [12]. Recent research in children also found a significant correlation between grip strength and tongue pressure [13], supporting a link between overall muscle strength and oral function. Regarding muscle stiffness ratio, when considering factors like aging and contracture, there is little consistent evidence on what stiffness ratio reflects. Our results suggest that, when the masseter is relatively soft at rest, a greater increase in stiffness ratio during contraction is associated with higher force generation.
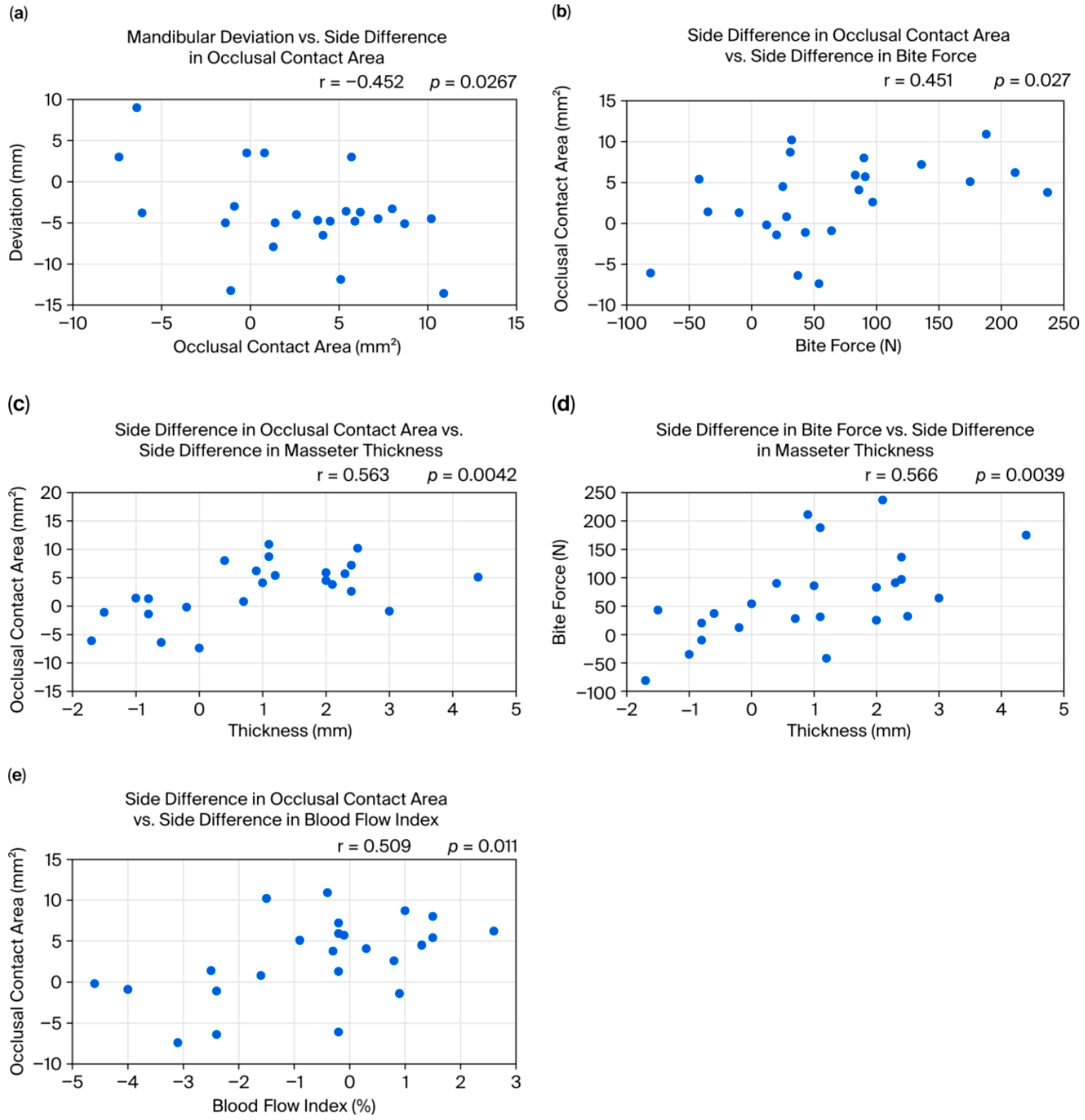
- Mandibular asymmetry and occlusion–masseter relationships: Previous studies have not reached a consensus on the relationship between mandibular asymmetry and masticatory muscle activity or conditions. For example, some have reported that, in mandibular asymmetry cases, the masseter on the deviated side is thinner and less active [3]. Matsuda et al. observed that, during clenching, the temporalis on the deviated side and the masseter on the non-deviated side tended to be dominant [14], and Beltrami et al. reported that, in crossbite patients, the masseter on the crossbite (deviated) side was thinner than on the non-crossbite side [5]. Other studies have found conflicting results, including that the masseter is thicker on the deviated side, thicker on the non-deviated side, or shows no side difference, leading to confusion. In the present study, masseter thickness was more strongly associated with the occlusal contact area than with the direction of mandibular deviation. Specifically, patients who had a greater occlusal contact area on one side tended to have increased masseter thickness on that side, regardless of the deviation direction; that is, patients who habitually chew on one side (the side with more occlusal contact) tend to develop a stronger bite force and masseter hypertrophy on that side, where it is known that habitual unilateral chewing leads to masseter hypertrophy on the favored side [15]. Nakano et al. found that shifting the mandible laterally in a rat model changed the shape and trabecular bone of the mandible and condyle prior to changes in the masseter muscle [16]. These findings suggest that daily masticatory load may have a greater effect on masseter thickness than the mandible’s skeletal deviation direction. However, the causal relationship between mandibular asymmetry and occlusal deviation remains unclear: it is not certain whether a skeletal shift causes occlusal deviation or whether an asymmetric occlusion leads to or worsens mandibular asymmetry. This issue was not directly tested in the present study and remains a subject for future research.
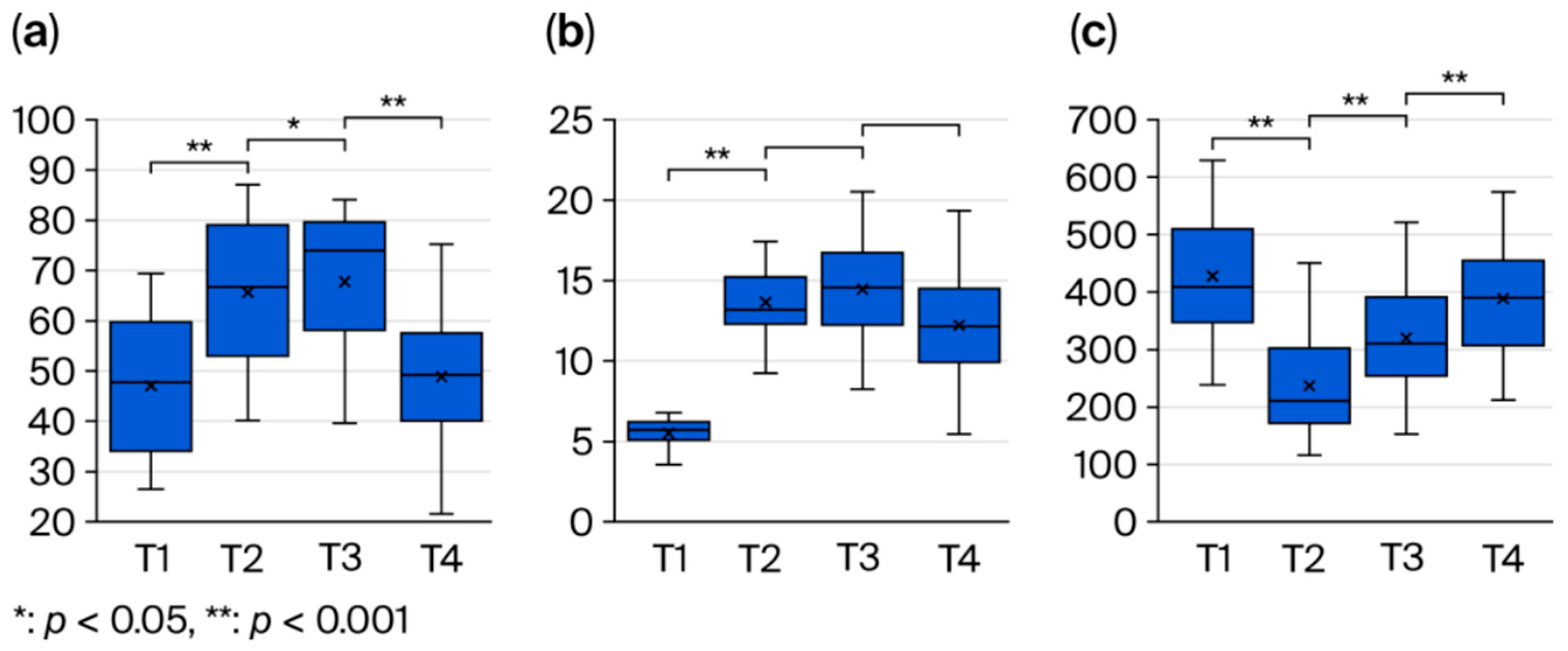
- Postoperative changes in the masseter: The observed increase in echo intensity at 1 month post-operation (T2) is likely due to inflammation, edema, or fibrosis, and its subsequent recovery over time reflects tissue repair processes. A similar pattern of echogenic change has been reported in prior studies on muscle healing [17]. The early increase in blood flow is consistent with the circulatory response and healing process after surgical trauma, as reported by Salmi et al. [18]. Bite force exhibited an early postoperative decline followed by recovery by 6 months, likely reflecting a temporary reduction in muscle function due to surgical trauma and the avoidance of chewing due to pain, followed by muscle remodeling and functional recovery. Previous reports have similarly shown a transient decrease in bite force after surgery, with gradual improvement over time [19], which is consistent with our findings. Bite force recovery may be influenced by postoperative diet and rehabilitation and is an important indicator of long-term stability [20].
- Masseter-side difference progression: In this study, no significant changes were observed in the side differences in masseter thickness, stiffness ratio, echo intensity, or blood flow from before surgery to 6 months post-operation. This is consistent with previous reports suggesting that muscle plasticity and functional adaptation may continue over a period of 1 year to 4 years after surgery [6]. Our study is ongoing, and future follow-up will include data beyond 6 months to comprehensively evaluate these long-term changes.
- Relation to relapse amount: The relapse rate in this study was 25% (6 of 24 patients), which is similar to the proportion reported by Yang et al. [21]. The number of relapse cases at 6 months was correlated with the preoperative mandibular deviation amount; that is, cases with larger initial deviation (requiring larger surgical movements) tended to have a higher risk of relapse, which is consistent with the findings from Lai et al. [22].
Limitations
- This study included a relatively small sample (n = 24, with only six relapse cases), which limits statistical power; small-to-moderate effects may therefore have gone undetected. The low number of relapse events also precluded robust multivariable modeling to identify independent predictors. Accordingly, the findings of this study should be regarded as preliminary evidence that provides a foundation for future large-scale investigations.
- Because the primary aim of this study was to evaluate longitudinal changes in patients with mandibular asymmetry, a healthy symmetric control group was not included. This absence represents a limitation, as it prevents the establishment of normative baseline values and may restrict the clinical interpretation of our findings.
- To avoid overcomplicating the analysis, relapse in this study was defined solely as lateral mandibular displacement. This restricted definition meant that vertical and anteroposterior relapse were not assessed, which represents one of the limitations of this study.
- Although ultrasonography is a safe, real-time, and noninvasive method for evaluating the masseter, it has inherent limitations compared with MRI or CT. Previous studies have demonstrated that ultrasound can provide results comparable to MRI and CT under standardized conditions [23,24,25], but inter-operator variability and probe positioning remain challenges. Recent innovations, such as AI-assisted ultrasound analysis and 3D/AR elastography, have been reported to improve reproducibility and enhance the accuracy of anatomical localization, even for less experienced operators. Yan et al. summarized advances in AI-assisted ultrasound diagnosis [26], Costa et al. presented an AR-based ultrasound guidance system for breast biopsy [27], and Ng et al. developed a portable mixed-reality 3D ultrasound tracking and reconstruction system [28]. In line with these developments, our findings may further broaden the clinical utility of ultrasound in postoperative evaluation and relapse prediction when combined with such cutting-edge technologies.
- Postoperative rehabilitation and physiotherapy may also influence the recovery and adaptation of the masticatory muscles. Previous studies have suggested that targeted rehabilitation programs can facilitate functional recovery after orthognathic surgery [29,30]. However, because standardized rehabilitation data were not collected in our study, this represents another limitation.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inchingolo, A.M.; Patano, A.; Piras, F.; de Ruvo, E.; Ferrante, L.; Di Noia, A.; Dongiovanni, L.; Palermo, A.; Inchingolo, F.; Inchingolo, A.D.; et al. Orthognathic surgery and relapse: A systematic review. Bioengineering 2023, 10, 1071. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, K.; Wang, X.; Wu, G. Assessment of masseter volume and postoperative stability after orthognathic surgery in patients with skeletal class III malocclusion with facial asymmetry. J. Craniofac. Surg. 2024, 35, 1249–1252. [Google Scholar] [CrossRef]
- Goto, T.K.; Nishida, S.; Yahagi, M.; Langenbach, G.E.J.; Nakamura, Y.; Tokumori, K.; Sakai, S.; Yabuuchi, H.; Yoshiura, K. Size and orientation of masticatory muscles in patients with mandibular laterognathism. J. Dent. Res. 2006, 85, 552–556. [Google Scholar] [CrossRef]
- Kwon, T.G.; Lee, K.H.; Park, H.S.; Ryoo, H.M.; Kim, H.J.; Lee, S.H. Relationship between the masticatory muscles and mandibular skeleton in mandibular prognathism with and without asymmetry. J. Oral. Maxillofac. Surg. 2007, 65, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, F.; Kiliaridis, S.; Antonarakis, G.S. Masseter muscle thickness before and after the correction of unilateral functional posterior crossbite in growing individuals: A prospective controlled clinical trial. Eur. J. Orthod. 2024, 47, cjae078. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Yu, H.S. Masseter muscle changes following orthognathic surgery: A long-term three-dimensional computed tomography follow-up. Angle Orthod. 2012, 82, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Aktürk, E.S.; Eren, H.; Görürgöz, C.; Orhan, K.; Karasu, H.A.; Akat, B.; Memikoğlu, T.U.T. Electromyographic, ultrasonographic, and ultrasound elastographic evaluation of the masseter muscle in Class III patients before and after orthognathic surgery. J. Craniofac. Surg. 2020, 31, 2049–2053. [Google Scholar] [CrossRef]
- Akbulut, N.; Gökçe, E.; Akbulut, S.; Şen, E.; Altan, A.; Balel, Y. Ultrasonographic evaluation of changes in the thickness, width, elasticity index and echogenic pattern of the masseter muscle after mandibular set-back surgery. J. Stomatol. Oral. Maxillofac. Surg. 2023, 124 (6 Suppl. 2), 101567. [Google Scholar] [CrossRef]
- Kobayashi, R.; Haga, S.; Umehara, A.; Takakaze, M.; Akatsuka, K.; Nakano, H. Quantitative and qualitative evaluation of the masseter muscle by ultrasonography and correlation with whole body health status. J. Phys. Ther. Sci. 2024, 36, 136–141. [Google Scholar] [CrossRef]
- Pillen, S.; Tak, R.O.; Zwarts, M.J.; Lammens, M.M.; Verrijp, K.N.; Arts, I.M.; van der Laak, J.A.; Hoogerbrugge, P.M.; van Engelen, B.G.; Verrips, A. Skeletal muscle ultrasound: Correlation between fibrous tissue and echo intensity. Ultrasound Med. Biol. 2009, 35, 443–446. [Google Scholar] [CrossRef]
- Reimers, K.; Reimers, C.D.; Wagner, S.; Paetzke, I.; Pongratz, D.E. Skeletal muscle sonography: A correlative study of echogenicity and morphology. J. Ultrasound Med. 1993, 12, 73–77. [Google Scholar] [CrossRef]
- Ling, C.H.; Taekema, D.; de Craen, A.J.; Gussekloo, J.; Westendorp, R.G.; Maier, A.B. Handgrip strength and mortality in the oldest old population: The Leiden 85-plus study. CMAJ 2010, 182, 429–435. [Google Scholar] [CrossRef]
- Akatsuka, K.; Haga, S.; Nagahama, R.; Maki, K. Investigation of tongue hardness using ultrasound elastography in children during growth period. Clin. Investig. Orthod. 2023, 82, 98–107. [Google Scholar] [CrossRef]
- Matsuda, N.; Hasegawa, S.; Shinoda, K.; Tamura, Y. Influence of mandibular deviation on masticatory muscle asymmetry index in children. Jpn. J. Pediatr. Dent. 1997, 35, 706–714. [Google Scholar]
- Wang, J.C. Masseter Muscle Hypertrophy: Symptoms, Causes and Treatment Options. USC Ostrow News 2022. Available online: https://ostrowonline.usc.edu/what-is-the-masseter-muscle/ (accessed on 30 August 2025).
- Nakano, H.; Maki, K.; Shibasaki, Y.; Miller, A.J. Three-dimensional changes in the condyle during development of an asymmetrical mandible in a rat: A microcomputed tomography study. Am. J. Orthod. Dentofac. Orthop. 2004, 126, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.F.; Chen, C.P.; Tsai, W.C.; Hu, L.L.; Hsu, C.C.; Tseng, S.T.; Shau, Y.W. Quantification of skeletal muscle fibrosis at different healing stages using sonography: A morphologic and histologic study in an animal model. J. Ultrasound Med. 2012, 31, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Salmi, A.M.; Tierala, E.K.; Tukiainen, E.J.; Asko-Seljavaara, S.L. Blood flow in free muscle flaps measured by color Doppler ultrasonography. Microsurgery 1995, 16, 666–672. [Google Scholar] [CrossRef]
- Islam, I.; Lim, A.A.T.; Wong, R.C.W. Changes in bite force after orthognathic surgical correction of mandibular prognathism: A systematic review. Int. J. Oral. Maxillofac. Surg. 2017, 46, 746–755. [Google Scholar] [CrossRef]
- Van der Bilt, A. Assessment of mastication with implications for oral rehabilitation: A review. J. Oral. Rehabil. 2011, 38, 754–780. [Google Scholar] [CrossRef]
- Yang, H.J.; Hwang, S.J. Evaluation of postoperative stability after BSSRO to correct facial asymmetry depending on the amount of bone contact between the proximal and distal segment. J. Cranio-Maxillofac. Surg. 2014, 42, e165–e170. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Yamada, K.; Hanada, K.; Ali, I.M.; Takagi, R.; Kobayashi, T.; Hayashi, T. Postoperative mandibular stability after orthognathic surgery in patients with mandibular protrusion and mandibular deviation. Int. J. Adult Orthod. Orthognath. Surg. 2002, 17, 13–22. [Google Scholar]
- Rogulski, M.; Pałac, M.; Wolny, T.; Linek, P. Assessment of Reliability, Agreement, and Accuracy of Masseter Muscle Ultrasound Thickness Measurement Using a New Standardized Protocol. Diagnostics 2024, 14, 1771. [Google Scholar] [CrossRef] [PubMed]
- Raadsheer, M.C.; Van Eijden, T.M.; Van Spronsen, P.H.; Van Ginkel, F.C.; Kiliaridis, S.; Prahl-Andersen, B. A comparison of human masseter muscle thickness measured by ultrasonography and magnetic resonance imaging. Arch. Oral. Biol. 1994, 39, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Chun, Y.H.; Bae, H.; Lee, J.W.; Kim, H.J. Comparison of ultrasonography-based masticatory muscle thickness between temporomandibular disorders bruxers and temporomandibular disorders non-bruxers. Sci. Rep. 2024, 14, 6923. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Li, Q.; Fu, K.; Zhou, X.; Zhang, K. Progress in the Application of Artificial Intelligence in Ultrasound-Assisted Medical Diagnosis. Bioengineering 2025, 12, 288. [Google Scholar] [CrossRef]
- Costa, N.; Ferreira, L.; de Araújo, A.R.V.F.; Oliveira, B.; Torres, H.R.; Morais, P.; Alves, V.; Vilaça, J.L. Augmented Reality-Assisted Ultrasound Breast Biopsy. Sensors 2023, 23, 1838. [Google Scholar] [CrossRef]
- Ng, K.W.; Gao, Y.; Furqan, S.; Al-Shaikhli, S.; Meiburger, K.M.; Elson, D.S. HoloPOCUS: Portable mixed-reality 3D ultrasound tracking, reconstruction and overlay. In International Workshop on Advances in Simplifying Medical Ultrasound; Springer Nature: Cham, Switzerland, 2023. [Google Scholar]
- Kawai, N.; Shibata, M.; Watanabe, M.; Horiuchi, S.; Fushima, K.; Tanaka, E. Effects of functional training after orthognathic surgery on masticatory function in patients with mandibular prognathism. J. Dent. Sci. 2020, 15, 419–425. [Google Scholar] [CrossRef]
- Florjanski, W.; Malysa, A.; Orzeszek, S.; Smardz, J.; Olchowy, A.; Paradowska-Stolarz, A.; Wieckiewicz, M. Evaluation of Biofeedback Usefulness in Masticatory Muscle Activity Management-A Systematic Review. J. Clin. Med. 2019, 8, 766. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umehara, A.; Haga, S.; Takakaze, M.; Kobayashi, R.; Akatsuka, K.; Yamashiro, M.; Tatsuta, S.; Nakano, H. Analysis of Masseter Muscle Structure in Patients with Mandibular Asymmetry Using Ultrasonic Diagnostic Equipment. Bioengineering 2025, 12, 1159. https://doi.org/10.3390/bioengineering12111159
Umehara A, Haga S, Takakaze M, Kobayashi R, Akatsuka K, Yamashiro M, Tatsuta S, Nakano H. Analysis of Masseter Muscle Structure in Patients with Mandibular Asymmetry Using Ultrasonic Diagnostic Equipment. Bioengineering. 2025; 12(11):1159. https://doi.org/10.3390/bioengineering12111159
Chicago/Turabian StyleUmehara, Akito, Shugo Haga, Momoko Takakaze, Rika Kobayashi, Kanako Akatsuka, Misaki Yamashiro, Shiina Tatsuta, and Haruhisa Nakano. 2025. "Analysis of Masseter Muscle Structure in Patients with Mandibular Asymmetry Using Ultrasonic Diagnostic Equipment" Bioengineering 12, no. 11: 1159. https://doi.org/10.3390/bioengineering12111159
APA StyleUmehara, A., Haga, S., Takakaze, M., Kobayashi, R., Akatsuka, K., Yamashiro, M., Tatsuta, S., & Nakano, H. (2025). Analysis of Masseter Muscle Structure in Patients with Mandibular Asymmetry Using Ultrasonic Diagnostic Equipment. Bioengineering, 12(11), 1159. https://doi.org/10.3390/bioengineering12111159





