Thermal and Stability Outcomes of Different Osteotomy Techniques and Implant Macrogeometries in Type IV Bone: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
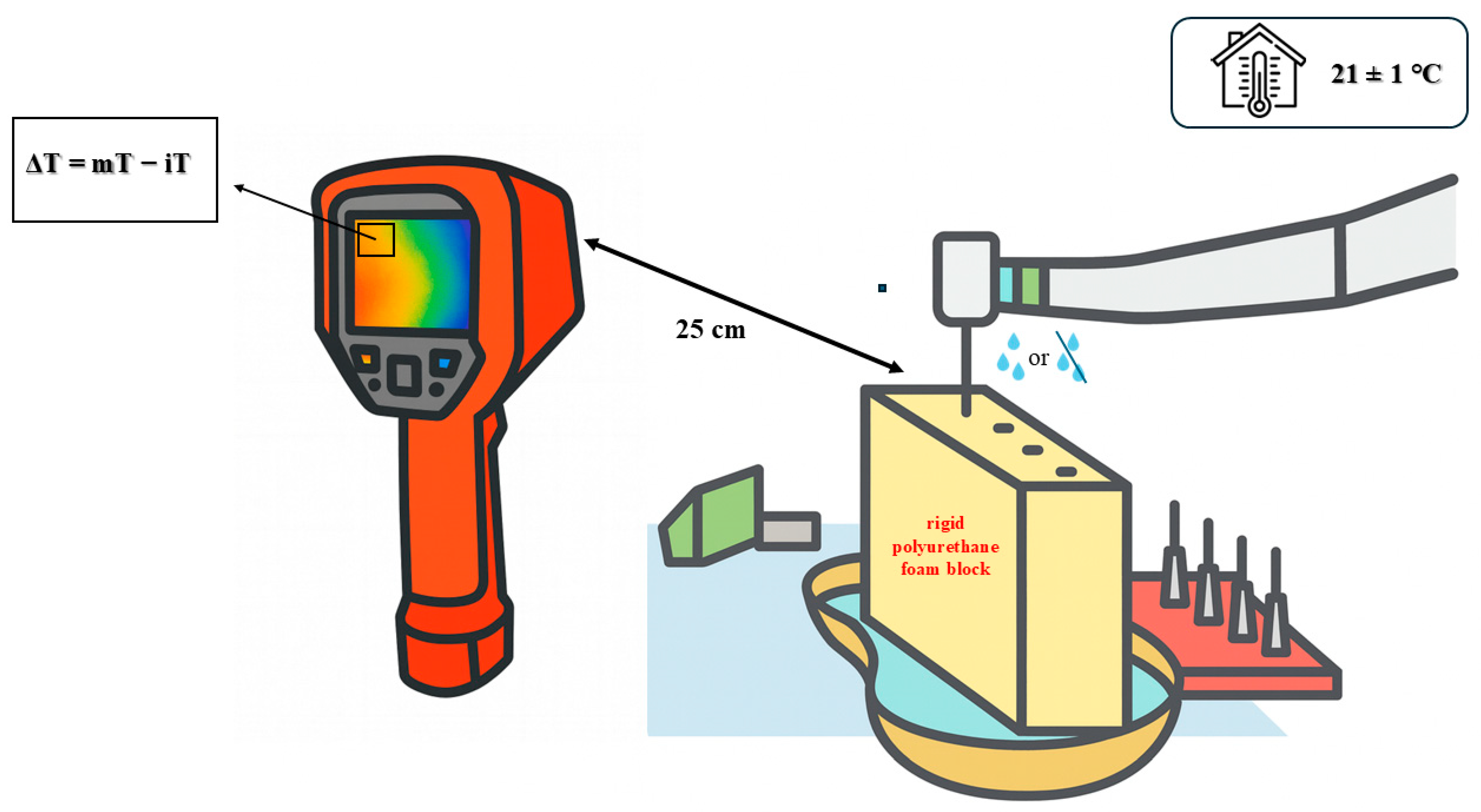
2.2. Bone Model
2.3. Study Groups
2.4. Implant Placement and Primary Stability Assessment
2.5. Thermal Measurements
2.6. Statistical Analysis
3. Results
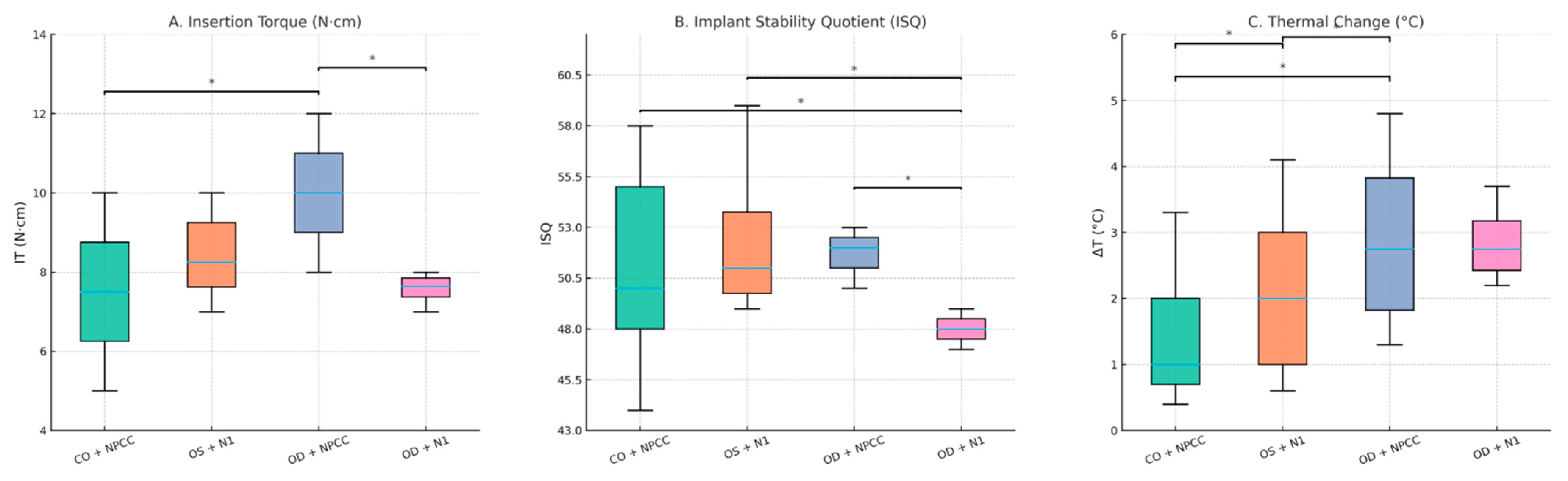
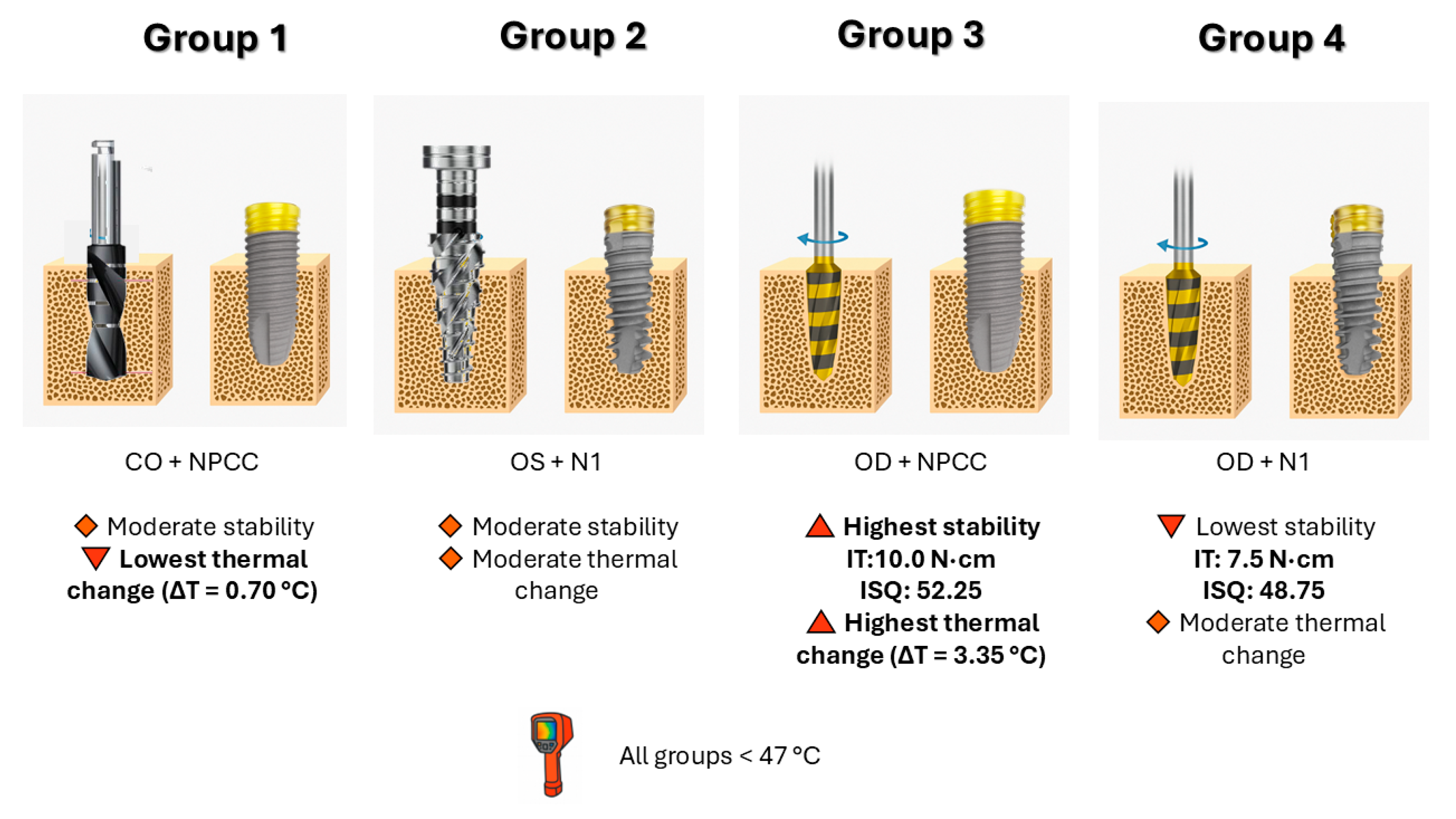
3.1. Insertion Torque (IT)
3.2. Resonance Frequency Analysis (ISQ)
3.3. Thermal Changes (ΔT)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CO | Conventional Osteotomy |
| OD | Osseodensification |
| OS | OsseoShaper |
| NPCC | NobelParallel Conical Connection |
| N1 | Nobel Biocare N1 |
| IT | Insertion Torque |
| ISQ | Implant Stability Quotient |
| ΔT | Thermal Change |
| RFA | Resonance Frequency Analysis |
| SD | Standard Deviation |
| ICC | Intraclass Correlation Coefficient |
| ASTM | American Society for Testing and Materials |
References
- Foletti, J.-M.; Sterba, M.; Maurice, P.; Dibatista, J.-C.; de Gea, R.; Birault, L. Effect of Bone Density on the Survival of 407 Sandblasted and Acid-Etched Dental Implants: A Retrospective Multicenter Study. J. Dent. Res. Dent. Clin. Dent. Prospect. 2023, 17, 112. [Google Scholar] [CrossRef]
- Jaffin, R.A.; Berman, C.L. The Excessive Loss of Brånemark Fixtures in Type IV Bone: A 5-Year Analysis. J. Periodontol. 1991, 62, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Möhlhenrich, S.; Heussen, N.; Elvers, D.; Steiner, T.; Hölzle, F.; Modabber, A. Compensating for Poor Primary Implant Stability in Different Bone Densities by Varying Implant Geometry: A Laboratory Study. Int. J. Oral Maxillofac. Surg. 2015, 44, 1514–1520. [Google Scholar] [CrossRef]
- Rathi, S.; Gupta, N.; Hashmi, S.; Abirami, S.; Yusufi, F.N.K. Effect of Densah Burs on Primary and Secondary Stability of Immediately Loaded Implants in Addition to Crestal Bone Loss and Gingival Probing Depth—An Evaluative Study. Indian J. Dent. Res. 2024, 35, 145–148. [Google Scholar] [CrossRef]
- de Carvalho Formiga, M.; da Silva, H.D.P.; Ghiraldini, B.; Siroma, R.S.; Ardelean, L.C.; Piattelli, A.; Shibli, J.A. Effects of Osseodensification on Primary Stability of Cylindrical and Conical Implants—An Ex Vivo Study. J. Clin. Med. 2023, 12, 3736. [Google Scholar] [CrossRef]
- Vaddamanu, S.K.; Saini, R.S.; Vyas, R.; Kanji, M.A.; Alshadidi, A.A.F.; Hafedh, S.; Cicciù, M.; Minervini, G. A Comparative Study on Bone Density Before and After Implant Placement Using Osseodensification Technique: A Clinical Evaluation. Int. J. Implant Dent. 2024, 10, 56. [Google Scholar] [CrossRef]
- Fabbri, G.; Staas, T.; Urban, I. A Retrospective Observational Study Assessing the Clinical Outcomes of a Novel Implant System with Low-Speed Site Preparation Protocol and Tri-Oval Implant Geometry. J. Clin. Med. 2022, 11, 4859. [Google Scholar] [CrossRef] [PubMed]
- Musskopf, M.L.; Finger Stadler, A.; Fiorini, T.; Ramos, U.D.; de Sousa Rabelo, M.; de Castro Pinto, R.N.; Susin, C. Performance of a New Implant System and Drilling Protocol—A Minipig Intraoral Dental Implant Model Study. Clin. Oral Implants Res. 2024, 35, 40–51. [Google Scholar] [CrossRef]
- Gökçe Uçkun, G.; Saygılı, S.; Çakır, M.; Geçkili, O. Effect of Osteotomy Strategy on Primary Stability and Intraosseous Temperature Rise: An Ex-Vivo Study. BMC Oral Health 2025, 25, 830. [Google Scholar] [CrossRef]
- Popovski, J.; Mikic, M.; Tasevski, D.; Dabic, S.; Mladenovic, R. Comparing Implant Macrodesigns and Their Impact on Stability: A Year-Long Clinical Study. Medicina 2024, 60, 1546. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, R.; Albrektsson, T. The Effect of Heat on Bone Regeneration: An Experimental Study in the Rabbit Using the Bone Growth Chamber. J. Oral Maxillofac. Surg. 1984, 42, 705–711. [Google Scholar] [CrossRef]
- Islam, M.A.; Kamarrudin, N.S.; Daud, R.; Mohd Noor, S.N.F.; Azmi, A.I.; Razlan, Z.M. A Review of Surgical Bone Drilling and Drill Bit Heat Generation for Implantation. Metals 2022, 12, 1900. [Google Scholar] [CrossRef]
- Az, Z.A.A.; Ak, G. Comparison of Temperature Changes during Implant Osteotomy: Conventional, Single, and Osseodensification Drilling. Int. J. Med. Sci. 2025, 22, 1237. [Google Scholar] [CrossRef]
- Rugova, S.; Abboud, M. Comparison of One-Drill Protocol to Sequential Drilling In Vitro and In Vivo. Bioengineering 2025, 12, 51. [Google Scholar] [CrossRef]
- Bandela, V.; Shetty, N.; Munagapati, B.; Basany, R.B.; Kanaparthi, S. Comparative Evaluation of Osseodensification versus Conventional Osteotomy Technique on Dental Implant Primary Stability: An Ex Vivo Study. Cureus 2022, 14, e30843. [Google Scholar] [CrossRef]
- Orth, C.; Haas, A.N.; Peruzzo, D.C.; Carvahlo da Silva, R.; Mesquita de Carvahlo, P.; de Barros Carrilho, G.P.; Joly, J.C. Primary Stability of Dental Implants Installed Using Osseodensification or Bone Expansion Drilling Systems: A Comparative Clinical Study. J. Int. Acad. Periodontol. 2022, 24, 165–174. [Google Scholar]
- Politi, I.; Honari, B.; Winning, L.; Polyzois, I. The Effect of Osseodensification on Implant Stability and Marginal Bone Levels: A Randomized Control Clinical Trial. Clin. Exp. Dent. Res. 2025, 11, e70126. [Google Scholar] [CrossRef] [PubMed]
- Saqr, A.M.; Ayilavarapu, S.; Gandhi, K.; Lee, C.T.; Stylianou, P. Ridge Dimensional Changes and Implant Stability Utilizing the Osseodensification Protocol: A Randomized Clinical Trial. J. Periodontol. 2025, 96, 739–747. [Google Scholar] [CrossRef]
- Antonelli, A.; Barone, S.; Attanasio, F.; Salviati, M.; Cerra, M.G.; Calabria, E.; Bennardo, F.; Giudice, A. Effect of Implant Macro-Design and Magnetodynamic Surgical Preparation on Primary Implant Stability: An In Vitro Investigation. Dent. J. 2023, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Yang, J.; Ma, T.; Chen, M.; An, Q.; Yu, D. Optimizing Osseodensification Drilling for Dental Implant Placement: An In Vitro Study. Clin. Exp. Dent. Res. 2025, 11, e70155. [Google Scholar] [CrossRef]
- Comuzzi, L.; Tumedei, M.; Pontes, A.E.; Piattelli, A.; Iezzi, G. Primary Stability of Dental Implants in Low-Density (10 and 20 pcf) Polyurethane Foam Blocks: Conical vs Cylindrical Implants. Int. J. Environ. Res. Public Health 2020, 17, 2617. [Google Scholar] [CrossRef]
- ASTM F1839-08; Standard Specification for Rigid Polyurethane Foam for Use as a Standard Material for Testing Orthopaedic Devices and Instruments. ASTM International: West Conshohocken, PA, USA, 2008.
- Seo, D.-J.; Moon, S.-Y.; You, J.-S.; Lee, W.-P.; Oh, J.-S. The Effect of Under-Drilling and Osseodensification Drilling on Low-Density Bone: A Comparative Ex Vivo Study. Appl. Sci. 2022, 12, 1163. [Google Scholar] [CrossRef]
- Costa, J.A.; Mendes, J.M.; Salazar, F.; Pacheco, J.J.; Rompante, P.; Moreira, J.F.; Mesquita, J.D.; Adubeiro, N.; da Câmara, M.I. Osseodensification vs. Conventional Osteotomy: A Case Series with Cone Beam Computed Tomography. J. Clin. Med. 2024, 13, 1568. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Xhajanka, E.; Romeo, D.M.; Romeo, M.; Zappone, C.M.F.; Malcangi, G.; Scarano, A.; Lorusso, F.; et al. The Effectiveness of Osseodensification Drilling Protocol for Implant Site Osteotomy: A Systematic Review of the Literature and Meta-Analysis. Materials 2021, 14, 1147. [Google Scholar] [CrossRef]
- Pai, U.Y.; Rodrigues, S.J.; Talreja, K.S.; Mundathaje, M. Osseodensification—A Novel Approach in Implant Dentistry. J. Indian Prosthodont. Soc. 2018, 18, 196–200. [Google Scholar] [CrossRef]
- Salman, R.D.; Bede, S.Y. The Use of Osseodensification for Ridge Expansion and Dental Implant Placement in Narrow Alveolar Ridges: A Prospective Observational Clinical Study. J. Craniofac. Surg. 2022, 33, 2114–2117. [Google Scholar] [CrossRef] [PubMed]
- Fontes Pereira, J.; Costa, R.; Nunes Vasques, M.; Salazar, F.; Mendes, J.M.; Infante da Câmara, M. Osseodensification: An Alternative to Conventional Osteotomy in Implant Site Preparation: A Systematic Review. J. Clin. Med. 2023, 12, 7046. [Google Scholar] [CrossRef]
- Abboud, M.; Delgado-Ruiz, R.A.; Kucine, A.; Rugova, S.; Balanta, J.; Calvo-Guirado, J.L. Multistepped Drill Design for Single-Stage Implant Site Preparation: Experimental Study in Type 2 Bone. Clin. Implant Dent. Relat. Res. 2015, 17, e472–e485. [Google Scholar] [CrossRef]
- Wu, H.-C.; Huang, H.-L.; Fuh, L.-J.; Tsai, M.-T.; Hsu, J.-T. Influence of Implant Length and Insertion Depth on Primary Stability of Short Dental Implants: An In Vitro Study of a Novel Mandibular Artificial Bone Model. J. Dent. Sci. 2024, 19, 139–147. [Google Scholar] [CrossRef]
- Bhargava, N.; Perrotti, V.; Caponio, V.C.A.; Matsubara, V.H.; Patalwala, D.; Quaranta, A. Comparison of Heat Production and Bone Architecture Changes in the Implant Site Preparation with Compressive Osteotomes, Osseodensification Technique, Piezoelectric Devices, and Standard Drills: An Ex Vivo Study on Porcine Ribs. Odontology 2023, 111, 142–153. [Google Scholar] [CrossRef]
- Bettach, R.; Boukhris, G.; De Aza, P.N.; da Costa, E.M.; Scarano, A.; Fernandes, G.V.O.; Gehrke, S.A. New Strategy for Osseodensification during Osteotomy in Low-Density Bone: An In Vitro Experimental Study. Sci. Rep. 2023, 13, 11924. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E. Contemporary Implant Dentistry, 3rd ed.; Mosby: St. Louis, MO, USA, 2008; p. 1061. [Google Scholar]
- Friberg, B.; Sennerby, L.; Roos, J.; Johansson, P.; Strid, C.; Lekholm, U. Evaluation of Bone Density Using Cutting Resistance Measurements and Microradiography. An In Vitro Study in Pig Ribs. Clin. Oral Implants Res. 1995, 6, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Möhlhenrich, S.C.; Heussen, N.; Modabber, A.; Kniha, K.; Hölzle, F.; Wilmes, B.; Danesh, G.; Szalma, J. Influence of Bone Density, Screw Size and Surgical Procedure on Orthodontic Mini-Implant Placement—Part A: Temperature Development. Int. J. Oral Maxillofac. Surg. 2021, 50, 555–564. [Google Scholar] [CrossRef]
- Derks, J.; Tomasi, C. Peri-Implant Health and Disease. A Systematic Review of Current Epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S158–S171. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Salvi, G.E. Peri-Implant Mucositis. J. Clin. Periodontol. 2018, 45, S237–S245. [Google Scholar] [CrossRef]
- Javed, F.; Romanos, G.E. The Role of Primary Stability for Successful Immediate Loading of Dental Implants. A Literature Review. J. Dent. 2010, 38, 612–620. [Google Scholar] [CrossRef]
- Meredith, N. Assessment of Implant Stability as a Prognostic Determinant. Int. J. Prosthodont. 1998, 11, 491–501. [Google Scholar] [PubMed]
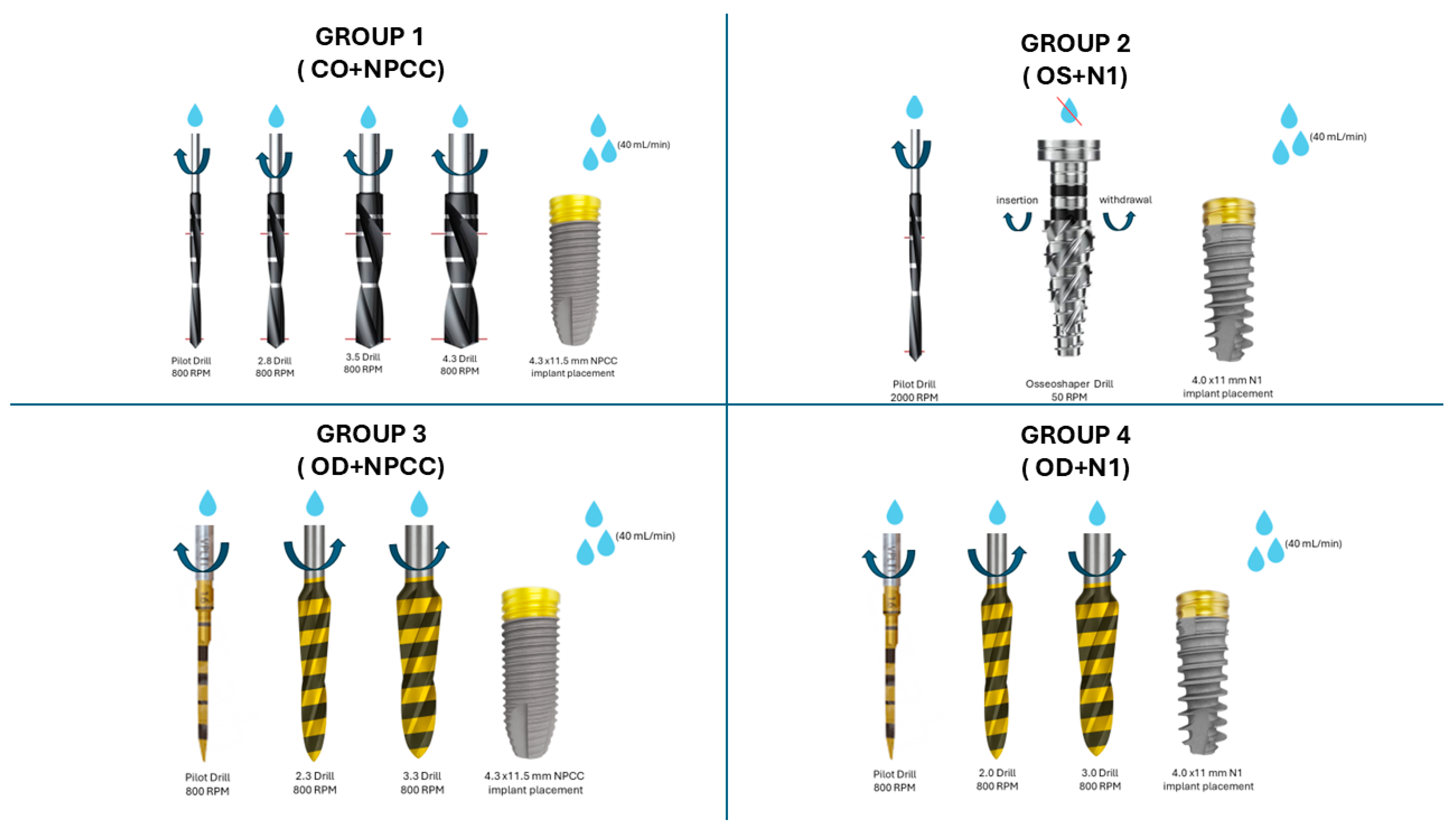
| Group | Osteotomy Technique | Implant Design |
|---|---|---|
| 1 | CO | NPCC (4.3 × 11.5 mm) |
| 2 | OS | N1 (4.0 × 11 mm) |
| 3 | OD | NPCC (4.3 × 11.5 mm) |
| 4 | OD | N1 (4.0 × 11 mm) |
| Group | IT Median (N·cm) (Min–Max) | ΔT Median (°C) (Min–Max) | ISQ Median (Min–Max) |
|---|---|---|---|
| Group 1 | 8.0 (5.0–10.0) | 0.70 (0.40–3.30) | 52.5 (44.0–58.5) |
| Group 2 | 8.0 (7.0–10.0) | 1.75 (0.60–4.10) | 50.0 (49.5–59.0) |
| Group 3 | 10.0 (8.0–12.0) | 3.35 (1.30–4.80) | 52.25 (50.0–53.5) |
| Group 4 | 7.5 (7.0–8.0) | 2.60 (2.20–3.70) | 48.75 (47.0–49.5) |
| p values | 0.001 | 0.001 | 0.001 |
| Comparison | IT (Ncm) | ΔT (°C) | ISQ |
|---|---|---|---|
| Group 1/Group 3 | 0.040 | 0.004 | 1.000 |
| Group 2/Group 4 | 0.090 | 1.000 | 0.002 |
| Group 1/Group 4 | 1.000 | 0.290 | 0.040 |
| Group 2/Group 3 | 0.160 | 0.010 | 0.010 |
| Group 1/Group 2 | 1.000 | 0.040 | 0.120 |
| Group 3/Group 4 | 0.002 | 0.720 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çanakçi, F.G.; Çakır, M.; Yalcin-Ülker, G.M.; Duygu, G. Thermal and Stability Outcomes of Different Osteotomy Techniques and Implant Macrogeometries in Type IV Bone: An In Vitro Study. Bioengineering 2025, 12, 1155. https://doi.org/10.3390/bioengineering12111155
Çanakçi FG, Çakır M, Yalcin-Ülker GM, Duygu G. Thermal and Stability Outcomes of Different Osteotomy Techniques and Implant Macrogeometries in Type IV Bone: An In Vitro Study. Bioengineering. 2025; 12(11):1155. https://doi.org/10.3390/bioengineering12111155
Chicago/Turabian StyleÇanakçi, F. Gülfeşan, Merve Çakır, Gül Merve Yalcin-Ülker, and Gonca Duygu. 2025. "Thermal and Stability Outcomes of Different Osteotomy Techniques and Implant Macrogeometries in Type IV Bone: An In Vitro Study" Bioengineering 12, no. 11: 1155. https://doi.org/10.3390/bioengineering12111155
APA StyleÇanakçi, F. G., Çakır, M., Yalcin-Ülker, G. M., & Duygu, G. (2025). Thermal and Stability Outcomes of Different Osteotomy Techniques and Implant Macrogeometries in Type IV Bone: An In Vitro Study. Bioengineering, 12(11), 1155. https://doi.org/10.3390/bioengineering12111155







