Intra-Articular Injection of Bone Marrow Concentrate for Patellofemoral Osteoarthritis Treatment: Preliminary Results Using a New Tibial Endplate Sample Under Ultrasound Guidance
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Clinical Assessment
- Persistent, non-traumatic PFOA for more than three months.
- Normal radiographs with MRI evidence of cartilage alterations.
- Age between 30 and 70 years.
- Pregnancy, active smoking, diabetes, or ongoing infection.
- Previous IA treatments (corticosteroids, hyaluronic acid, PRP, MSCs, or BMC).
- Previous surgical treatment of the knee.
- Concomitant tibiofemoral osteoarthritis (assessed by MRI).
- Additional IA or surgical treatments during follow-up.
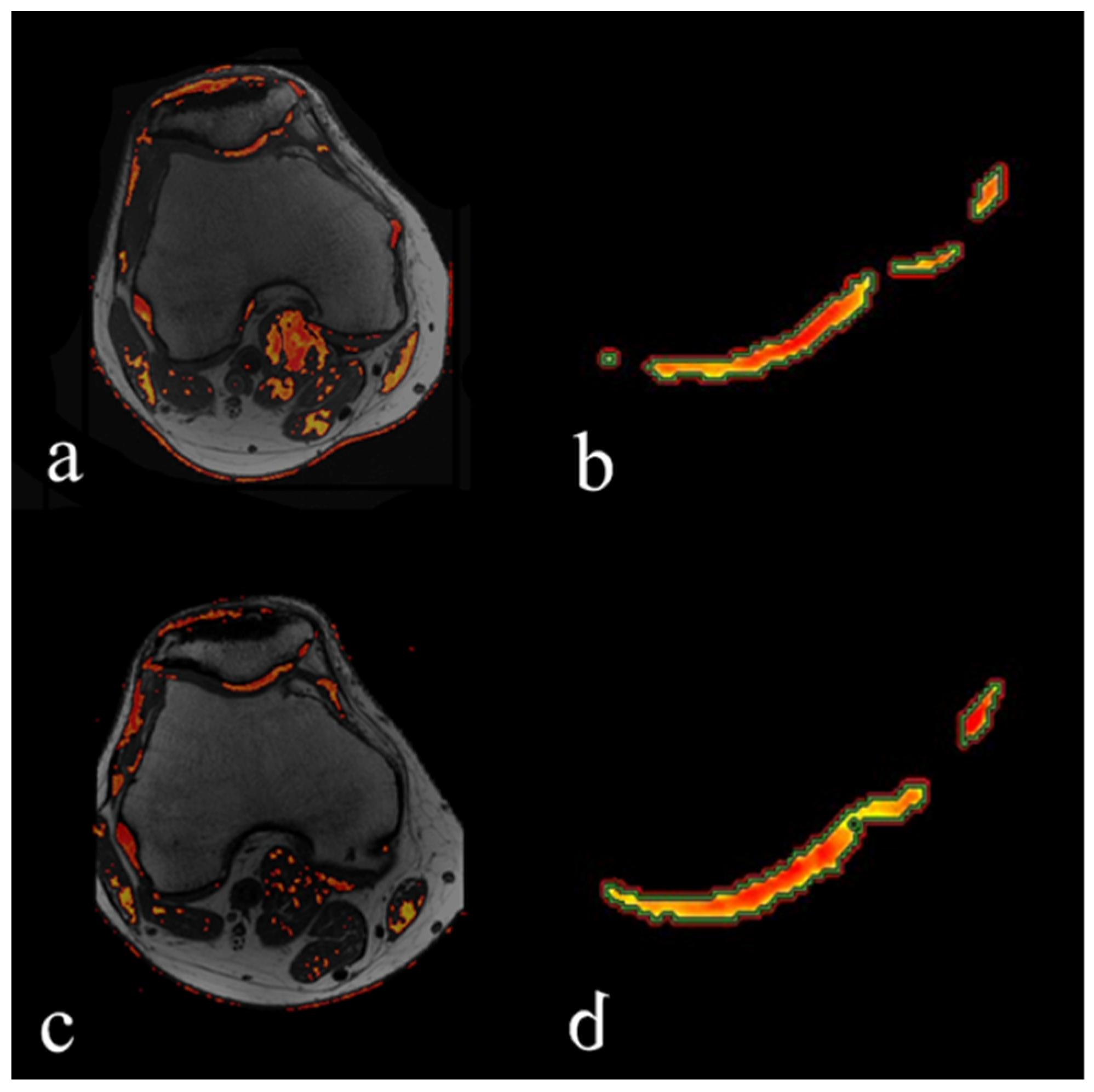
2.2. MRI Evaluation
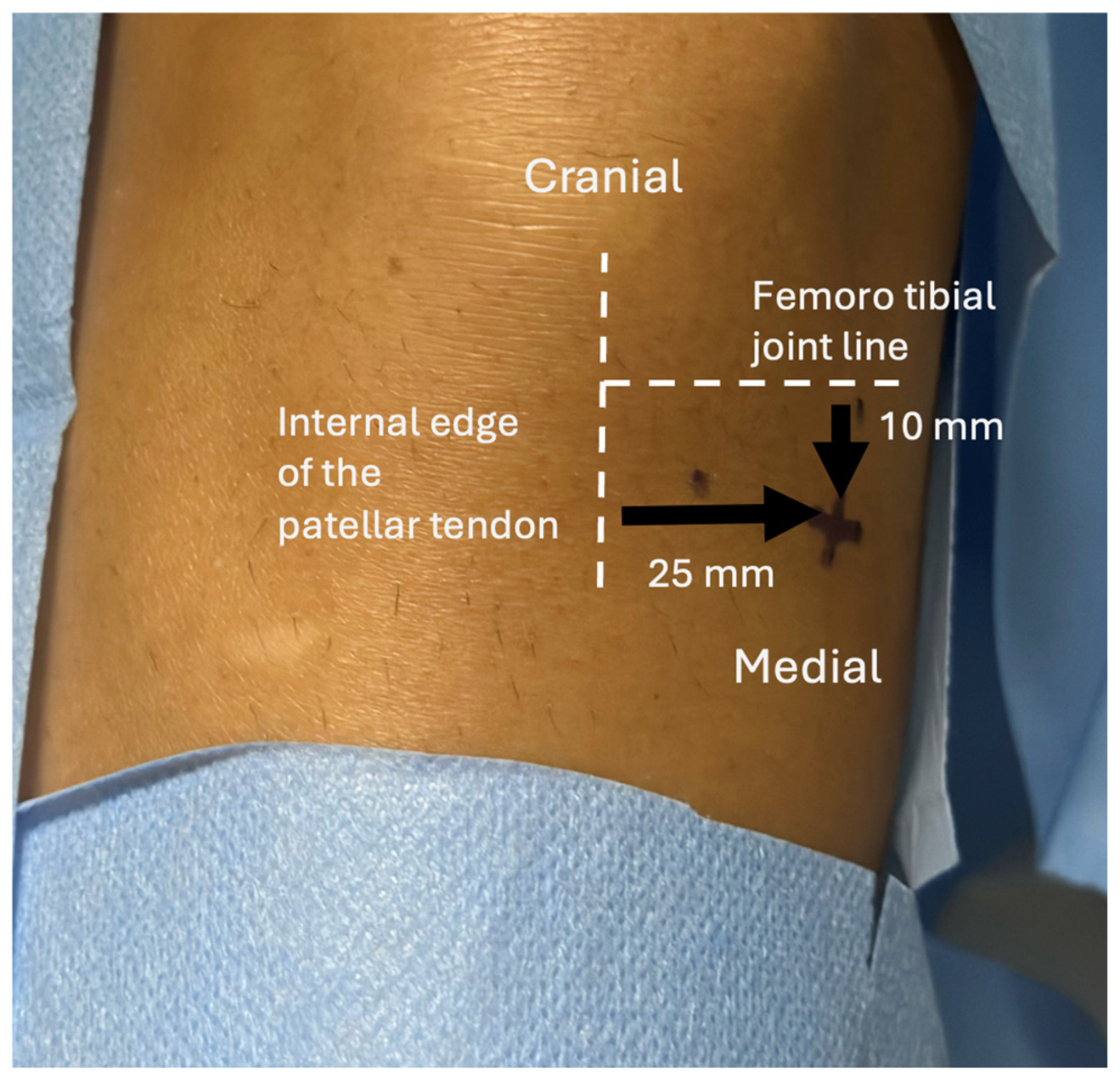
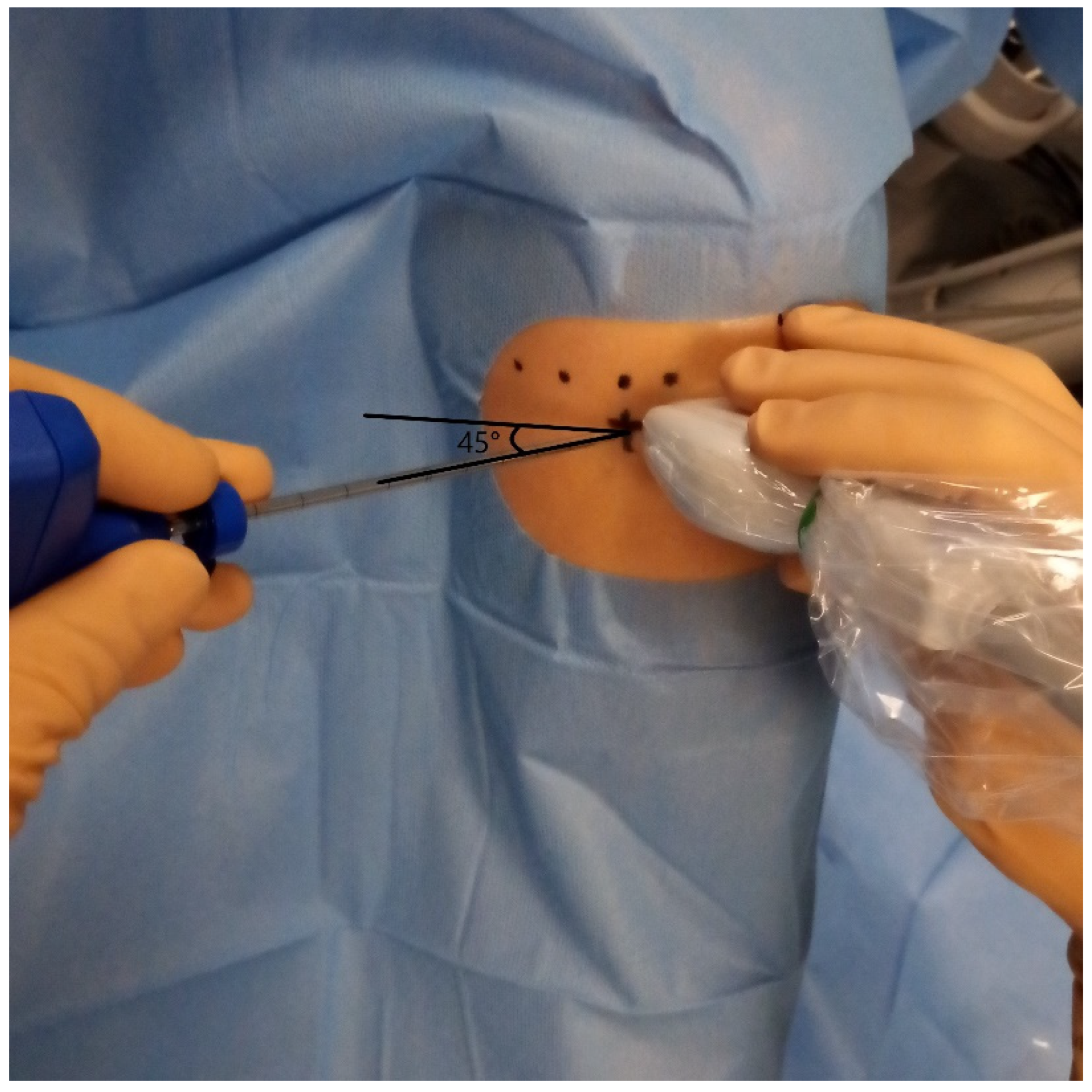
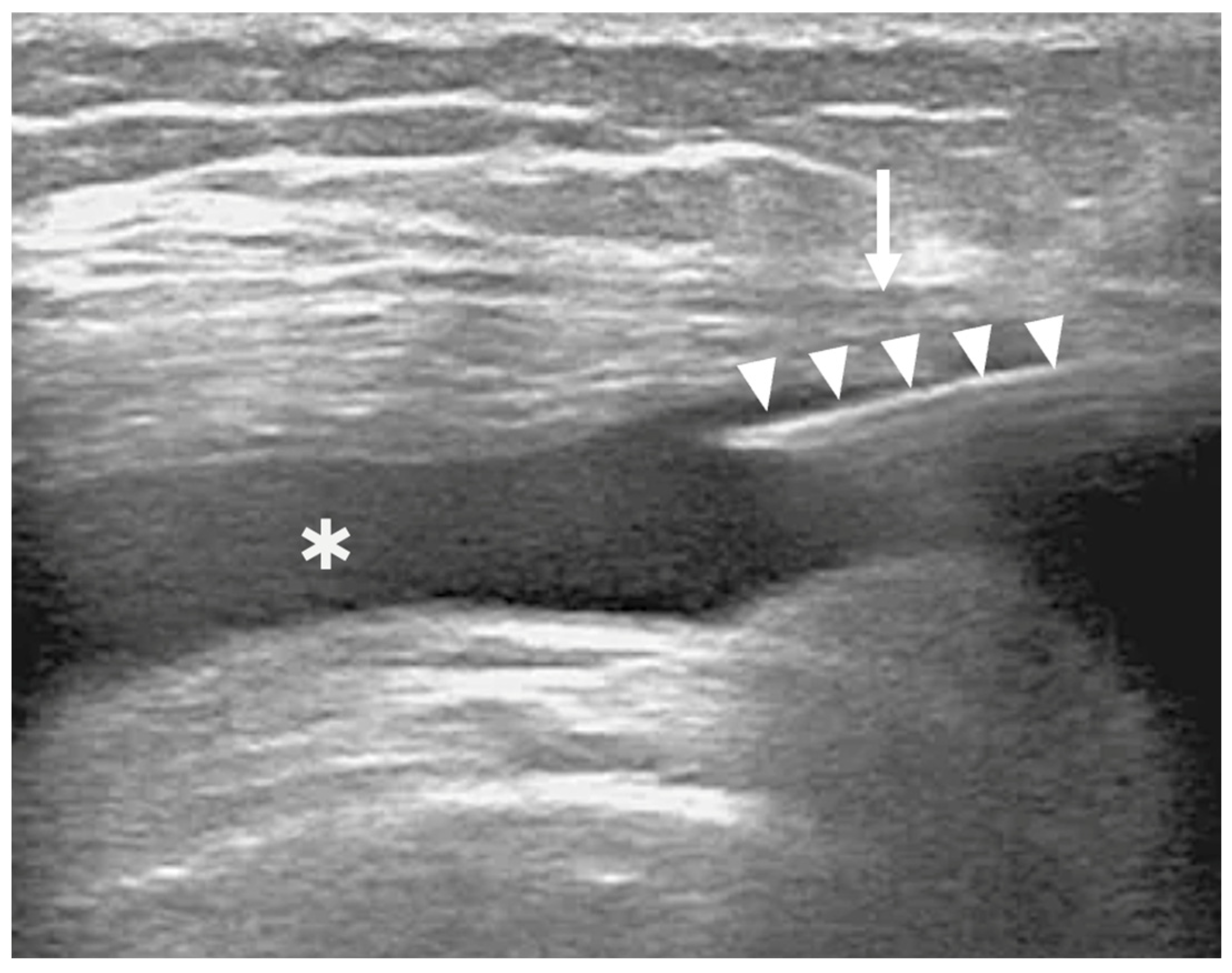
2.3. BMC Sampling, Preparation, and Injection
2.4. Cell Counting and Colony-Forming Unit Fibroblast (CFU-F) Assay
2.5. BMC IA Injection
2.6. Twelve-Month Clinical and MR Follow-Up
2.7. Statistical Analysis
3. Results
3.1. BMA and BMC Cell Counts
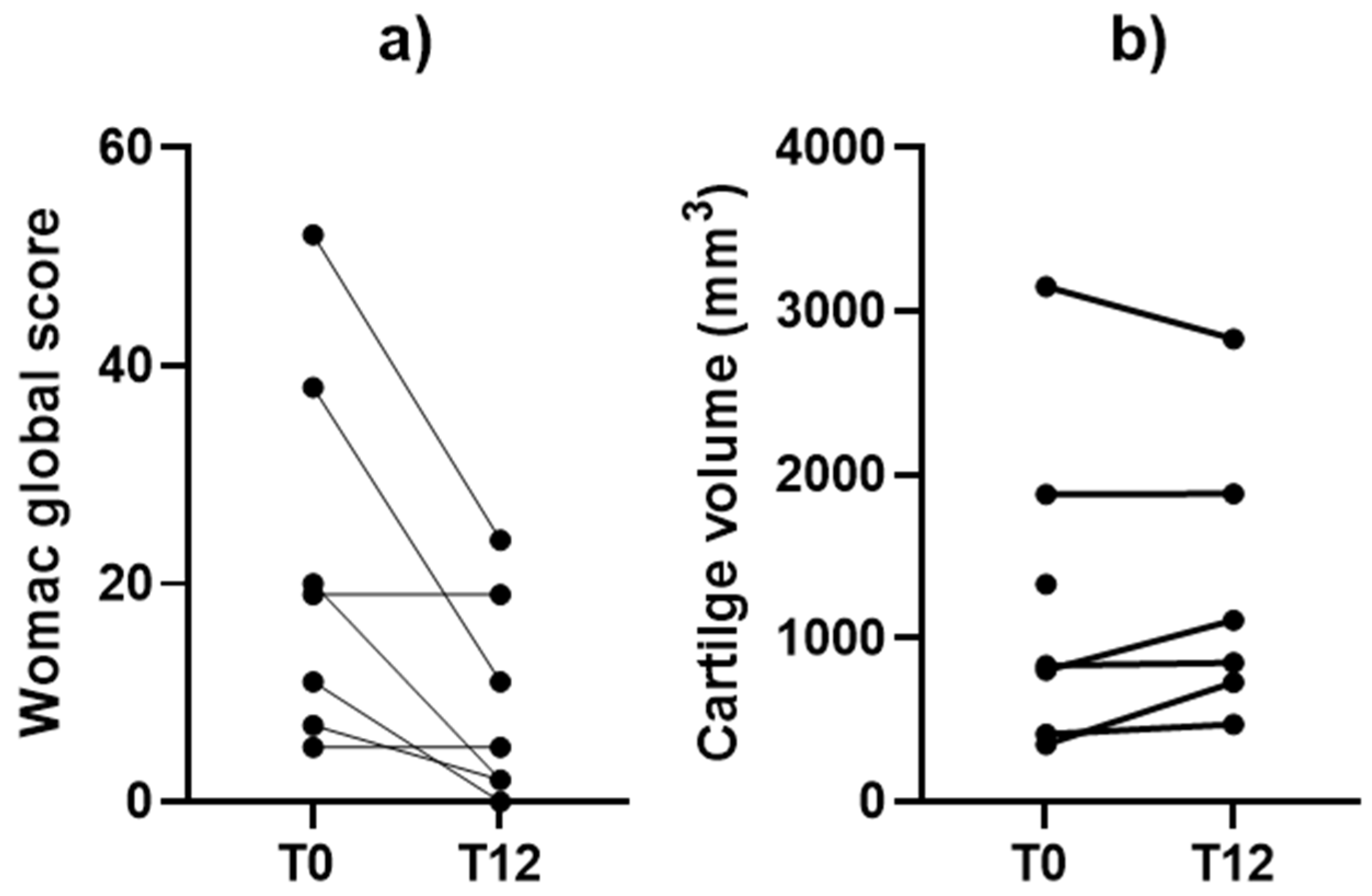
3.2. Clinical Outcomes
3.3. Safety, Adverse Events, and Twelve-Month Clinical/MRI Follow-Up
- Pain > 3/10 within the first 7 days after the procedure.
- Symptomatic hematoma at the puncture site within 2 weeks.
- Knee or periarticular infection within 3 months.
- Tibial endplate fracture during aspiration.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crossley, K.M.; Stefanik, J.J.; Selfe, J.; Collins, N.J.; Davis, I.S.; Powers, C.M.; McConnell, J.; Vicenzino, B.; Bazett-Jones, D.M.; Esculier, J.-F.; et al. Patellofemoral pain consensus statement from the 4th International Patellofemoral Pain Research Retreat, Manchester. Part 1: Terminology, definitions, clinical examination, natural history, patellofemoral osteoarthritis and patient-reported outcome measures. Br. J. Sports Med. 2016, 50, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Roos, E.M.; Arden, N.K. Strategies for the prevention of knee osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 92–101. [Google Scholar] [CrossRef]
- Centeno, C.J.; Al-Sayegh, H.; Bashir, J.; Goodyear, S.; Freeman, M.D. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. J. Pain Res. 2015, 8, 269–276. [Google Scholar] [CrossRef]
- Dulic, O.; Rasovic, P.; Lalic, I.; Kecojevic, V.; Gavrilovic, G.; Abazovic, D.; Maric, D.; Miskulin, M.; Bumbasirevic, M. Bone marrow aspirate concentrate versus platelet rich plasma or hyaluronic acid for the treatment of knee osteoarthritis. Medicina 2021, 57, 1193. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, A.; Bise, S.; Delavigne, C.; Merle, F.; Caudron, S.; Pesquer, L.; Lintingre, P.-F.; Dallaudière, B. Intra-articular injection of bone marrow concentrate for treatment of patellofemoral osteoarthritis: Preliminary results utilizing an ultrasound-guided marrow harvesting technique. J. Vasc. Interv. Radiol. 2023, 34, 71–78.e1. [Google Scholar] [CrossRef]
- Hernigou, P.; Homma, Y.; Lachaniette, C.H.F.; Poignard, A.; Allain, J.; Chevallier, N.; Rouard, H. Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int. Orthop. 2013, 37, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Narbona-Carceles, J.; Vaquero, J.; Suárez-Sancho, S.; Forriol, F.; Fernández-Santos, M.E. Bone marrow mesenchymal stem cell aspirates from alternative sources: Is the knee as good as the iliac crest? Injury 2014, 45 (Suppl. 4), S42–S47. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, C.; Boffa, A.; de Girolamo, L.; Merli, G.; Kon, E.; Cattini, L.; Santo, E.; Grigolo, B.; Filardo, G. Bone marrow aspirate concentrate quality is affected by age and harvest site. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 2140–2151. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Santiago, F.; Orellana González, C.; Moraleda Cabrera, B.; Láinez Ramos-Bossini, A.J. Ultrasound guided procedures in the musculoskeletal system: A narrative review with illustrative examples. Quant. Imaging Med. Surg. 2024, 14, 8028–8049. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, M.; Rios, P.A.G.M.; Ruiz, M.; Noronha, J.C.; Lopes Terra, D.; Lana, J.F. Patellofemoral osteoarthritis: Treatment with autologous bone marrow mononuclear cells and arthroscopic surgery, a prospective study. Stem Cell Regen. Med. 2018, 2, 1–6. [Google Scholar] [CrossRef]
- McConnell, S.; Kolopack, P.; Davis, A.M. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): A review of its utility and measurement properties. Arthritis Rheum. 2001, 45, 453–461. [Google Scholar] [CrossRef]
- Nelson, T.R.; Tung, S.M. Temperature dependence of proton relaxation times in vitro. Magn. Reson. Imaging 1987, 5, 189–199. [Google Scholar] [CrossRef]
- David-Vaudey, E.; Ghosh, S.; Ries, M.; Majumdar, S. T2 relaxation time measurements in osteoarthritis. Magn. Reson. Imaging 2004, 22, 673–682. [Google Scholar] [CrossRef]
- Goebel, J.C.; Watrin-Pinzano, A.; Bettembourg-Brault, I.; Odille, F.; Felblinger, J.; Chary-Valckenaere, I.; Netter, P.; Blum, A.; Gillet, P.; Loeuille, D. Age-related quantitative MRI changes in healthy cartilage: Preliminary results. Biorheology 2006, 43, 547–551. [Google Scholar] [CrossRef]
- White, L.M.; Sussman, M.S.; Hurtig, M.; Probyn, L.; Tomlinson, G.; Kandel, R. Cartilage T2 assessment: Differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology 2006, 241, 407–414. [Google Scholar] [CrossRef]
- Castro-Malaspina, H.; Gay, R.E.; Resnick, G.; Kapoor, N.; Meyers, P.; Chiarieri, D.; McKenzie, S.; Broxmeyer, H.E.; Moore, M.A.S. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood 1980, 56, 289–301. [Google Scholar] [CrossRef]
- Schäfer, R.; DeBaun, M.R.; Fleck, E.; Centeno, C.J.; Kraft, D.; Leibacher, J.; Bieback, K.; Seifried, E.; Dragoo, J.L. Quantitation of progenitor cell populations and growth factors after bone marrow aspirate concentration. J. Transl. Med. 2019, 17, 115. [Google Scholar] [CrossRef]
- Wong, K.L.; Lee, K.B.; Tai, B.C.; Law, P.; Lee, E.H.; Hui, J.H. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: A prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy 2013, 29, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Goncars, V.; Kalnberzs, K.; Jakobsons, E.; Enģele, I.; Briede, I.; Blums, K.; Erglis, K.; Erglis, M.; Patetko, L.; Muiznieks, I.; et al. Treatment of knee osteoarthritis with bone marrow-derived mononuclear cell injection: 12-month follow-up. Cartilage 2019, 10, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Hannila, I.; Lammentausta, E.; Tervonen, O.; Nieminen, M.T. The repeatability of T2 relaxation time measurement of human knee articular cartilage. Magn. Reson. Mater. Phys. Biol. Med. 2015, 28, 547–553. [Google Scholar] [CrossRef]
- Shapiro, S.A.; Arthurs, J.R. Bone marrow aspiration for regenerative orthopedic intervention: Technique with ultrasound guidance for needle placement. Regen. Med. 2017, 12, 917–928. [Google Scholar] [CrossRef]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentís, J.; Sánchez, A.; García-Sancho, J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: A pilot study. Transplantation 2013, 95, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; Martín-Ferrero, M.A.; Del Canto, F.; Alberca, M.; García, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.J.; Huguet, M.; et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: A randomized controlled trial. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Wluka, A.E.; Forbes, A.; Wang, Y.; Hanna, F.; Jones, G.; Cicuttini, F.M. Knee cartilage loss in symptomatic knee osteoarthritis over 4.5 years. Arthritis Res. Ther. 2006, 8, R90. [Google Scholar] [CrossRef]
- Centeno, C.J.; Al-Sayegh, H.; Bashir, J.; Goodyear, S.; Freeman, M.D. A dose response analysis of a specific bone marrow concentrate treatment protocol for knee osteoarthritis. BMC Musculoskelet. Disord. 2015, 16, 258. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Koh, H.S.; Choi, Y.H.; Park, I. Bone marrow aspirate concentrate (BMAC) for knee osteoarthritis: A narrative review of clinical efficacy and future directions. Medicina 2025, 61, 853. [Google Scholar] [CrossRef]
- Kunselman, A.R. A brief overview of pilot studies and their sample size justification. Fertil. Steril. 2024, 121, 899–901. [Google Scholar] [CrossRef]
| Sequences | Parameters |
|---|---|
| Sagittal T1-weighted | TR = 588 ms, TE minimum, Nex = 1, FOV 16 cm, thickness 3 mm, spacing 0.5 mm, 24 slices, anteroposterior direction, duration 2 min 17 s |
| Sagittal PDW | TR/TE, 2.362/45 ms; Nex = 2, FOV 16 cm, 3 mm thickness, 0.5 spacing, 24 slices, duration 3 min 14 s |
| Coronal PDW | TR/TE, 2.000/45 ms; Nex = 2, FOV 16 cm, 3 mm thickness, 0.5 spacing, 20 slices, duration 2 min 44 |
| Axial PDW | TR/TE, 2.257/45 ms; Nex = 2, FOV 16 cm, 3 mm thickness, 0.5 spacing, 24 slices, duration 3 min 05 s |
| Axial T2 mapping sequence for patellofemoral joint | TR = 1000 ms, TE = 6.1, 14.1, 22.1, 30.1, 38.1,46.1, 54.1, and 62.1 ms; Nex = 2; FOV 16 cm; 256 × 192 matrix; 9 slices; thickness = 3 mm with 0.6-mm spacing; duration 5 min 09 s |
| Sex | Age (Years) | Weight (kg) | Size (Meters) | BMI | BMA Volume (mL) | BMC Volume (mL) | Total Cells Injected (×106) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNCs | Platelets | Leukocytes | Granulocytes | Lymphocytes | Erythrocytes | CFU-F | |||||||
| M | 51 | 51 | 1.70 | 17.0 | 20 | 6.0 | 3.36 | 1284 | 71.7 | 44.88 | 20.34 | 44.76 | 215 |
| M | 53 | 76 | 1.76 | 24.5 | 20 | 6.0 | 0.84 | 408 | 19.38 | 12.6 | 5.46 | 2.22 | 19 |
| M | 37 | 85 | 1.76 | 27.4 | 20 | 10 | 3.00 | 360 | 47.0 | 31.0 | 11.6 | 1.70 | 94 |
| F | 68 | 68 | 1.60 | 26.6 | 20 | 12 | 5.04 | 2040 | 231.5 | 186 | 38.9 | 25.0 | 1158 |
| M | 40 | 88 | 1.88 | 24.9 | 20 | 10 | 1.10 | 210 | 33.5 | 27.0 | 5.00 | 0.90 | 235 |
| F | 52 | 69 | 1.79 | 21.5 | 20 | 9.0 | 1.10 | 144 | 22.5 | 13.3 | 7.70 | 0.70 | 23 |
| F | 50 | 56 | 1.62 | 21.3 | 20 | 6.0 | 0.70 | 126 | 20.0 | 16.0 | 3.70 | 0.50 | 40 |
| Mean ± SD | 50.1 ± 10.1 | 70.4 ± 13.8 | 1.73 ± 0.10 | 23.3 ± 3.6 | 20.0 ± 0.0 | 8.4 ± 2.4 | 2.16 ± 1.66 | 653.1 ± 730.4 | 63.7 ± 76.4 | 47.3 ± 62.3 | 13.2 ± 12.7 | 10.8 ± 17.4 | 254.9 ± 408.1 |
| Median [IQR] | 51 [47.5–55.0] | 69 [60.5–79.0] | 1.76 [1.65–1.76] | 24.5 [21.1–25.4] | 20 [20–20] | 9 [6–10] | 1.10 [0.95–3.16] | 360 [144–813] | 33.5 [21.3–59.4] | 27.0 [13.8–37.1] | 7.7 [5.0–15.7] | 1.7 [0.8–13.6] | 94 [23–235] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silvestre, A.; Caudron, S.; Rouchaud, A.; Borodetsky, V.; Pesquer, L.; González-Adrio, C.F.; Dallaudière, B. Intra-Articular Injection of Bone Marrow Concentrate for Patellofemoral Osteoarthritis Treatment: Preliminary Results Using a New Tibial Endplate Sample Under Ultrasound Guidance. Bioengineering 2025, 12, 1150. https://doi.org/10.3390/bioengineering12111150
Silvestre A, Caudron S, Rouchaud A, Borodetsky V, Pesquer L, González-Adrio CF, Dallaudière B. Intra-Articular Injection of Bone Marrow Concentrate for Patellofemoral Osteoarthritis Treatment: Preliminary Results Using a New Tibial Endplate Sample Under Ultrasound Guidance. Bioengineering. 2025; 12(11):1150. https://doi.org/10.3390/bioengineering12111150
Chicago/Turabian StyleSilvestre, Alain, Sébastien Caudron, Aymeric Rouchaud, Vladimir Borodetsky, Lionel Pesquer, Carlos Ferrer González-Adrio, and Benjamin Dallaudière. 2025. "Intra-Articular Injection of Bone Marrow Concentrate for Patellofemoral Osteoarthritis Treatment: Preliminary Results Using a New Tibial Endplate Sample Under Ultrasound Guidance" Bioengineering 12, no. 11: 1150. https://doi.org/10.3390/bioengineering12111150
APA StyleSilvestre, A., Caudron, S., Rouchaud, A., Borodetsky, V., Pesquer, L., González-Adrio, C. F., & Dallaudière, B. (2025). Intra-Articular Injection of Bone Marrow Concentrate for Patellofemoral Osteoarthritis Treatment: Preliminary Results Using a New Tibial Endplate Sample Under Ultrasound Guidance. Bioengineering, 12(11), 1150. https://doi.org/10.3390/bioengineering12111150







