The Role of Hyaluronic Acid in the Treatment of Gingivitis and Periodontitis at Different Stages: A Systematic Review and Meta-Analysis with Short-Term Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Question of Interest and PICO Format
2.3. Study Inclusion and Exclusion Criteria
2.4. Search Strategies
2.5. Data Collection
2.6. Statistical Analysis
2.7. Risk of Bias and GRADE Assessment
3. Results
3.1. Characteristics of the Studies
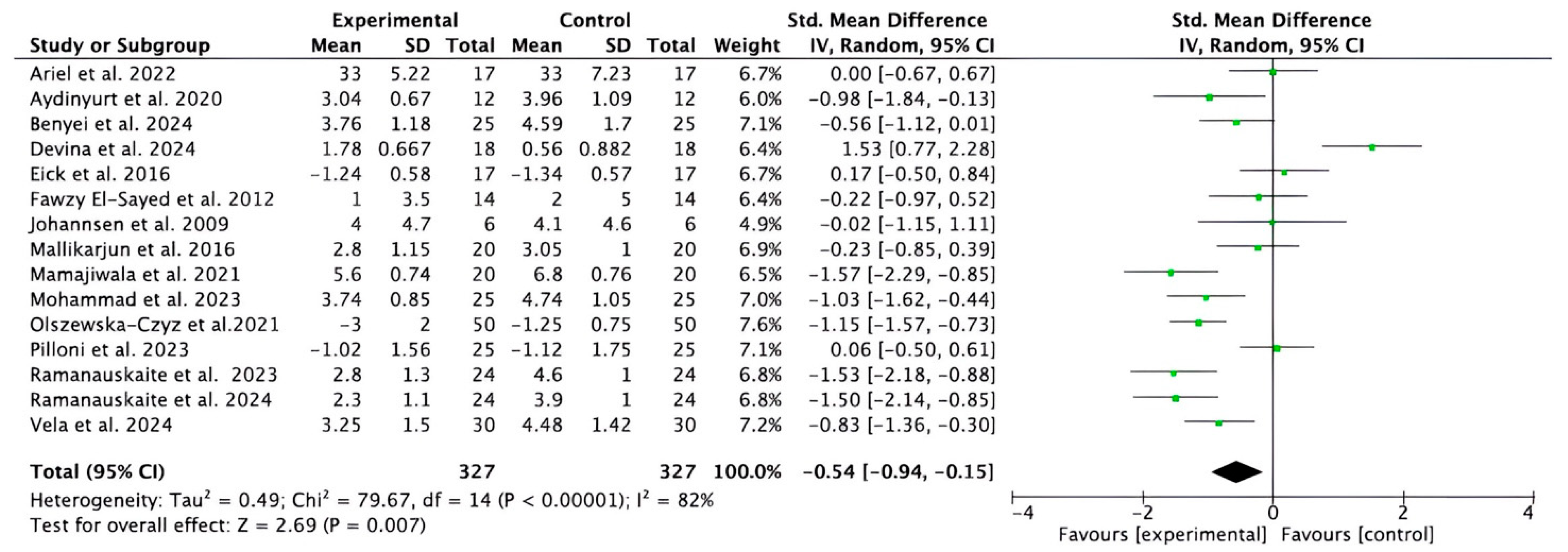
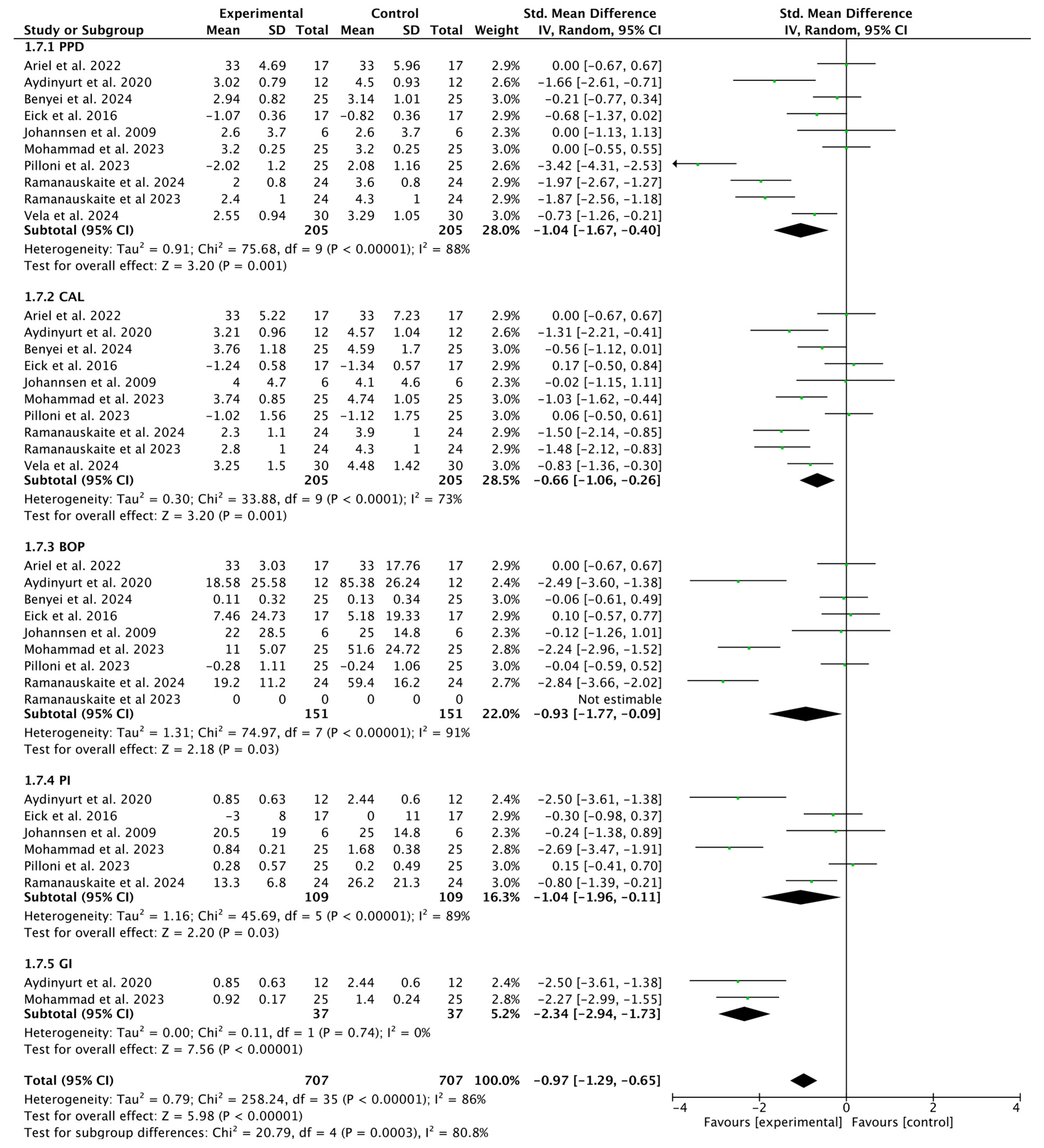
3.2. Overall Meta-Analysis
3.2.1. Meta-Analysis for PPD Parameter
3.2.2. Meta-Analysis for CAL Parameter
3.2.3. Meta-Analysis for BOP Parameter
3.2.4. Meta-Analysis for GI Parameter
3.2.5. Meta-Analysis for PI Parameter
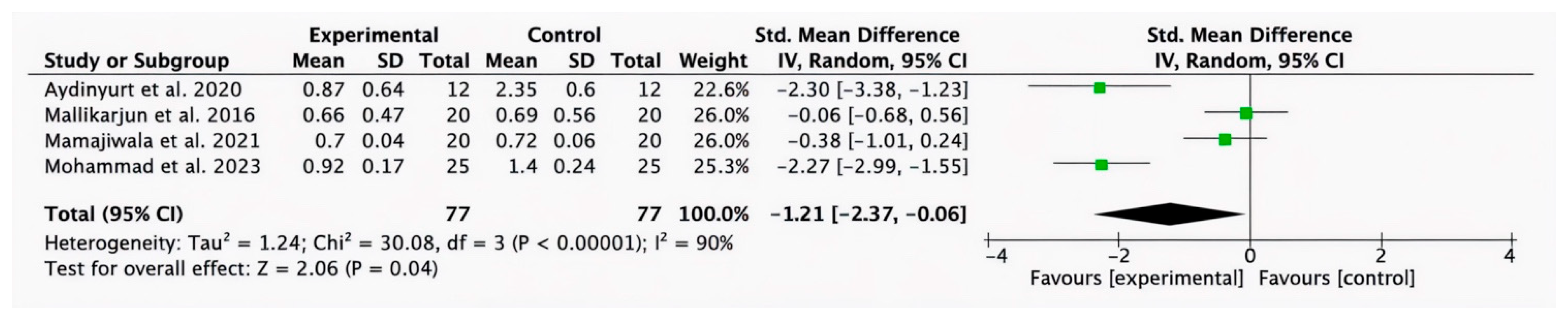
3.2.6. Subgroup Analysis for HA (Test) Compared with SRP Alone
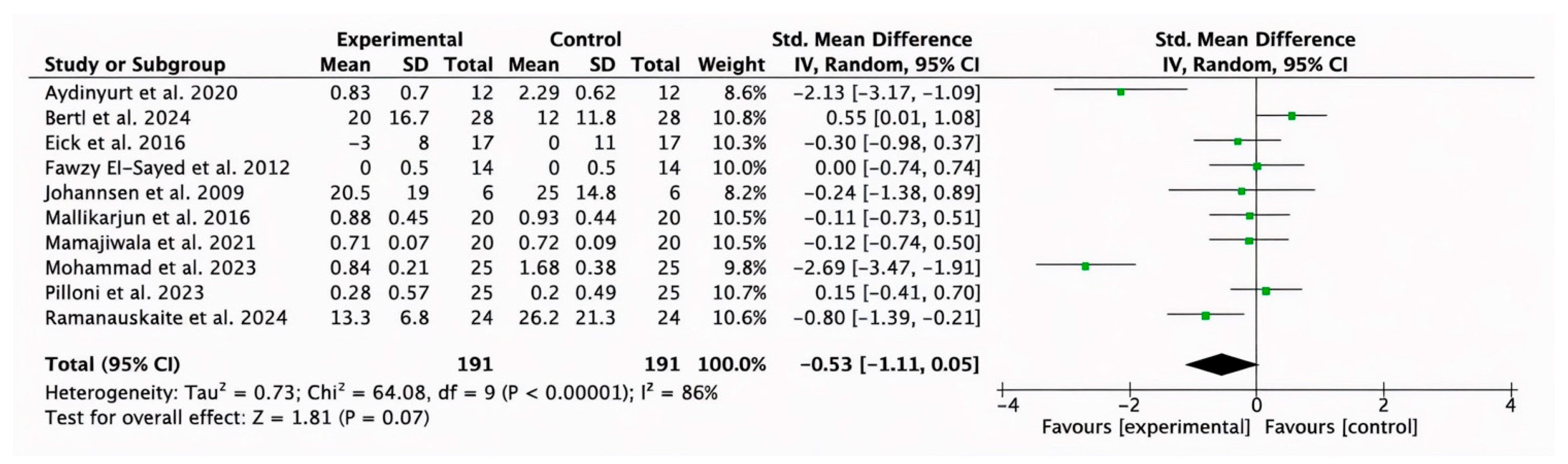
3.2.7. Subgroup Analysis of HA (Test) Compared with Placebo
3.3. Risk of Bias
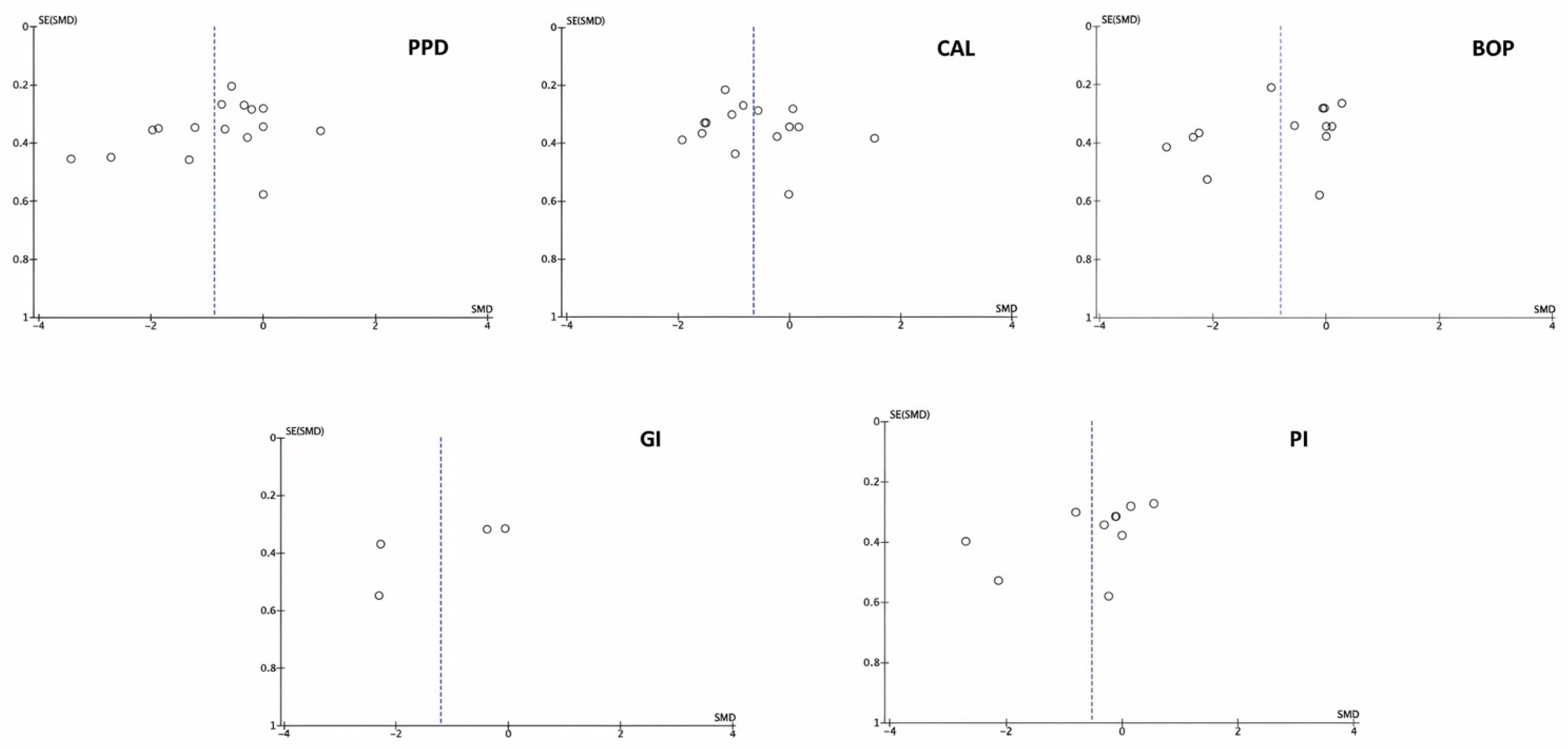
3.4. Publication Bias
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HA | hyaluronic acid |
| PPD | pocket probing depth |
| BOP | bleeding on probing |
| CAL | clinical attachment level |
| PI | plaque index |
| GI | gingival index |
| SMDs | standardized mean differences |
| CI | confidence intervals |
| RCTs | randomized clinical trials |
| MeSH | Medical Subject Headings |
| JBI-MAStARI | Joanna Briggs Institute Meta-Analysis of Statistics Assessment and Review Instrument |
| GR | gingival recession |
References
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef]
- Salamanna, F.; Giavaresi, G.; Parrilli, A.; Martini, L.; Nicoli Aldini, N.; Abatangelo, G.; Frizziero, A.; Fini, M. Effects of intra-articular hyaluronic acid associated to Chitlac (arty-duo®) in a rat knee osteoarthritis model. J. Orthop. Res. 2019, 37, 867–876. [Google Scholar] [CrossRef]
- Tarricone, E.; Elia, R.; Mattiuzzo, E.; Faggian, A.; Pozzuoli, A.; Ruggieri, P.; Brun, P. The viability and anti-inflammatory effects of hyaluronic acid-chitlac-tracimolone acetonide-β-cyclodextrin complex on human chondrocytes. Cartilage 2021, 13 (Suppl. S2), 920S–924S. [Google Scholar] [CrossRef]
- Foley, J.P.; Lam, D.; Jiang, H.; Liao, J.; Cheong, N.; McDevitt, T.M.; Zaman, A.; Wright, J.R.; Savani, R.C. Toll-like receptor 2 (TLR2), transforming growth factor-β, hyaluronan (HA), and receptor for HA-mediated motility (RHAMM) are required for surfactant protein A-stimulated macrophage chemotaxis. J. Biol. Chem. 2012, 287, 37406–37419. [Google Scholar] [CrossRef]
- Tipler, L.S.; Embery, G. Glycosaminoglycan-depolymerizing enzymes produced by anaerobic bacteria isolated from the human mouth. Arch. Oral Biol. 1985, 30, 391–396. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Müller, H.D.; Mueller, A.; Lussi, A.; Sculean, A.; Schmidlin, P.R.; Miron, R.J. In vitro effects of hyaluronic acid on human periodontal ligament cells. BMC Oral Health 2017, 17, 44. [Google Scholar] [CrossRef]
- Polizzi, A.; Leanza, Y.; Belmonte, A.; Grippaudo, C.; Leonardi, R.; Isola, G. Impact of Hyaluronic Acid and Other Re-Epithelializing Agents in Periodontal Regeneration: A Molecular Perspective. Int. J. Mol. Sci. 2024, 25, 12347. [Google Scholar] [CrossRef]
- Chang, W.; Chen, L.; Chen, K. The bioengineering application of hyaluronic acid in tissue regeneration and repair. Int. J. Biol. Macromol. 2024, 270, 132454. [Google Scholar] [CrossRef] [PubMed]
- Miglani, A.; Vishnani, R.; Reche, A.; Buldeo, J.; Wadher, B. Hyaluronic Acid: Exploring Its Versatile Applications in Dentistry. Cureus 2023, 15, e46349. [Google Scholar] [CrossRef] [PubMed]
- Della Sala, F.; Longobardo, G.; Fabozzi, A.; di Gennaro, M.; Borzacchiello, A. Hyaluronic acid-based wound dressing with antimicrobial properties for wound healing application. Appl. Sci. 2022, 12, 3091. [Google Scholar] [CrossRef]
- Pirnazar, P.; Wolinsky, L.; Nachnani, S.; Haake, S.; Pilloni, A.; Bernard, G.W. Bacteriostatic effects of hyaluronic acid. J. Periodontol. 1999, 70, 370–374. [Google Scholar] [CrossRef]
- RomanoÃÄ, C.; Vecchi, E.D.; Bortolin, M.; Morelli, I.; Drago, L. Hyaluronic acid and its composites as a local antimicro- bial/antiadhesive barrier. J. Bone Jt. Infect. 2017, 2, 63–72. [Google Scholar] [CrossRef]
- Zamboni, F.; Okoroafor, C.; Ryan, M.P.; Pembroke, J.T.; Strozyk, M.; Culebras, M.; Collins, M.N. On the bacteriostatic activity of hyaluronic acid composite films. Carbohydr. Polym. 2021, 260, 117803. [Google Scholar] [CrossRef] [PubMed]
- Highfield, J. Diagnosis and classification of periodontal disease. Aust. Dent. J. 2009, 54, S11–S26. [Google Scholar] [CrossRef]
- Talebi Ardakani, M.; Moscowchi, A.; Talebi, A.; Talebi, M.H. Hyaluronic acid efficacy in root coverage procedures: A systematic review and meta-analysis. BMC Oral Health 2025, 25, 119. [Google Scholar] [CrossRef]
- Arpağ, O.F.; Damlar, İ.; Altan, A.; Tatli, U.; Günay, A. To what extent does hyaluronic acid affect healing of xenografts? A histomorphometric study in a rabbit model. J. Appl. Oral Sci. 2018, 26, e20170004. [Google Scholar] [CrossRef]
- Sahayata, V.N.; Bhavsar, N.V.; Brahmbhatt, N.A. An evaluation of 0.2% hyaluronic acid gel (Gengigel®) in the treatment of gingivitis: A clinical and microbiological study. Oral Health Dent. Manag. 2014, 13, 779–785. [Google Scholar]
- Shah, S.A.; Vijayakar, H.N.; Rodrigues, S.V.; Mehta, C.J.; Mitra, D.K.; Shah, R.A. Comparing the effect of local administration of hyaluronic acid as an adjunct to scaling and root planing versus scaling and root planing alone in the treatment of chronic periodontitis. J. Indian. Soc. Periodontol. 2016, 20, 549–556. [Google Scholar] [PubMed]
- Polepalle, T.; Srinivas, M.; Swamy, N.; Aluru, S.; Chakrapani, S.; Chowdary, B.A. Local delivery of hyaluronan 0.8% as an adjunct to scaling and root planing in the treatment of chronic periodontitis: A clinical and microbiological study. J. Indian. Soc. Periodontol. 2015, 19, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Casale, M.; Moffa, A.; Vella, P.; Sabatino, L.; Capuano, F.; Salvinelli, B.; López, M.A.; Carinci, F.; Salvinelli, F. Hyaluronic acid: Perspectives in dentistry. A systematic review. Int. J. Immunopathol. Pharmacol. 2016, 29, 572–582. [Google Scholar] [CrossRef]
- Bhati, A.; Fageeh, H.; Ibraheem, W.; Fageeh, H.; Chopra, H.; Panda, S. Role ofhyaluronic acid in periodontal therapy (Review). Biomed. Rep. 2022, 17, 91. [Google Scholar] [CrossRef] [PubMed]
- Karakostas, P.; Davidopoulou, S.; Kalfas, S. Use of hyaluronic acid in periodontal disease treatment: A systematic review. J. Contemp. Dent. Pract. 2022, 23, 355–370. [Google Scholar]
- Inchingolo, A.D.; Inchingolo, A.M.; Dell’Anna, F.E.; Savino, A. The role of hyaluronic acid in the treatment of periodontal disease: A systematic review. Periodontol. Implant. Res. 2025, 9, 5. [Google Scholar] [CrossRef]
- Bertl, K.; Bruckmann, C.; Isberg, P.E.; Klinge, B.; Gotfredsen, K.; Stavropoulos, A. Hyaluronan in non-surgical and surgical periodontal therapy: A systematic review. J. Clin. Periodontol. 2015, 42, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Shojania, K.G.; Sampson, M.; Ansari, M.T.; Ji, J.; Doucette, S.; Moher, D. How quickly do systematic reviews go out of date? A survival analysis. Ann. Intern. Med. 2007, 147, 224–233. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.T.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.; Wiechula, R.; Court, A.; Lockwood, C. The JBI model of evidence-based healthcare. Int. J. Evid. Based Healthc. 2005, 3, 207–215. [Google Scholar]
- Casagrande, A.; Fabris, F.; Girometti, R. Beyond kappa: An informational index for diagnostic agreement in dichotomous and multivalue ordered-categorical ratings. Med. Biol. Eng. Comput. 2020, 58, 3089–3099. [Google Scholar] [CrossRef]
- Tufanaru, C.; Munn, Z.; Stephenson, M.; Aromataris, E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int. J. Evid. Based Healthc. 2015, 13, 196–207. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Ramanauskaite, E.; Machiulskiene Visockiene, V.; Shirakata, Y.; Friedmann, A.; Pereckaite, L.; Balciunaite, A.; Dvyliene, U.M.; Vitkauskiene, A.; Baseviciene, N.; Sculean, A. Microbiological effects of sodium hypochlorite/-amino acids and cross-linked hyaluronic acid adjunctive to non-surgical periodontal treatment. Oral Health Prev. Dent. 2024, 22, 171–180. [Google Scholar]
- Vela, O.C.; Boariu, M.; Rusu, D.; Iorio-Siciliano, V.; Ramaglia, L.; Boia, S.; Radulescu, V.; Ilyes, I.; Stratul, S.I. Healing of periodontal suprabony defects following treatment with open flap debridement with or without hyaluronic acid (HA) application. Medicina 2024, 60, 829. [Google Scholar] [CrossRef]
- Axe, A.; Patel, N.; Qaqish, J.; Ling, M.R.; Araga, M.; Parkinson, C.; Goyal, C.R. Efficacy of an experimental toothpaste containing sodium bicarbonate, sodium hyaluronate and sodium fluoride on gingivitis. BMC Oral Health 2024, 24, 209. [Google Scholar] [CrossRef] [PubMed]
- Bertl, K.; Vlachou, S.; Pandis, N.; Zampelis, A.; Stavropoulos, A. Repeated local delivery of hyaluronic acid gel as adjunctive treatment of residual pockets in periodontitis patients undergoing supportive periodontal care: A randomized controlled clinical trial. Clin. Oral Investig. 2024, 28, 158. [Google Scholar] [CrossRef]
- Benyei, L.; Friedmann, A.; Ostermann, T.; Diehl, D. Non-surgical treatment of residual periodontal pockets using sodium hypochlorite/amino acid gel and cross-linked hyaluronic acid—A 9-month pilot randomized controlled clinical trial. Clin. Oral Investig. 2024, 28, 513. [Google Scholar] [CrossRef]
- Devina, A.A.; Halim, F.C.; Meivi, M.; Masulili, S.L.C.; Tadjoedin, E.S.S.; Lessang, R.; Widaryono, A.; Bachtiar, B.M.; Sulijaya, B.; Tadjoedin, F.M.; et al. Effectiveness of 0.2% hyaluronic acid on clinical, biomolecular and microbiological parameters in type 2 diabetes mellitus patients with periodontitis. Eur. J. Dent. 2024, 18, 1090–10100. [Google Scholar] [CrossRef]
- Mohammad, C.A.; Mirza, B.A.; Mahmood, Z.S.; Zardawi, F.M. The effect of hyaluronic acid gel on periodontal parameters, pro-inflammatory cytokines and biochemical markers in periodontitis patients. Gels 2023, 9, 325. [Google Scholar] [CrossRef]
- Ramanauskaite, E.; Machiulskiene, V.; Shirakata, Y.; Dvyliene, U.M.; Nedzelskiene, I.; Sculean, A. Clinical evaluation of sodium hypochlorite/amino acids and cross-linked hyaluronic acid adjunctive to non-surgical periodontal treatment: A randomized controlled clinical trial. Clin. Oral Investig. 2023, 27, 6645–6656. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, A.; Rojas, M.A.; Trezza, C.; Carere, M.; De Filippis, A.; Marsala, R.L.; Marini, L. Clinical effects of the adjunctive use of polynucleotide and hyaluronic acid-based gel in the subgingival re-instrumentation of residual periodontal pockets: A randomized, split-mouth clinical trial. J. Periodontol. 2023, 94, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Ariel, H.; Kahn, A.; Hila, Z.O.; Anton, S.; Natan, G.; Kolerman, R. A thermosensitive gel with an active hyaluronic acid ingredient that contains an octenidine preservation system as an adjunct to scaling and root planning: A randomized prospective clinical study. Clin. Oral Investig. 2022, 26, 3721–3733. [Google Scholar] [CrossRef] [PubMed]
- Mamajiwala, A.S.; Sethi, K.S.; Raut, C.P.; Karde, P.A.; Mamajiwala, B.S. Clinical and radiographic evaluation of 0.8% hyaluronic acid as an adjunct to open flap debridement in the treatment of periodontal intrabony defects: Randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 5257–5271. [Google Scholar]
- Olszewska-Czyz, I.; Kralik, K.; Prpic, J. Biomolecules in Dental Applications: Randomized, Controlled Clinical Trial Evaluating the Influence of Hyaluronic Acid Adjunctive Therapy on Clinical Parameters of Moderate Periodontitis. Biomolecules 2021, 11, 1491. [Google Scholar] [CrossRef] [PubMed]
- Aydinyurt, H.S.; Akbal, D.; Altindal, D.; Bozoglan, A.; Ertugrul, A.S.; Demir, H. Evaluation of biochemical and clinical effects of hyaluronic acid on non-surgical periodontal treatment: A randomized controlled trial. Ir. J. Med. Sci. 2020, 189, 1485–1494. [Google Scholar] [CrossRef]
- Al-Shammari, N.M.; Shafshak, S.M.; Ali, M.S. Effect of 0.8% Hyaluronic Acid in Conventional Treatment of Moderate to Severe Chronic Periodontitis. J. Contemp. Dent. Pract. 2018, 19, 527–534. [Google Scholar] [CrossRef]
- Mallikarjun, S.; Neelakanti, A.; Babu, H.; Pai, S.B.; Shinde, S.V.; Krishnan, S. Neutrophil elastase levels in the gingival crevicular fluid following hyaluronan gel application in the treatment of chronic periodontitis: A randomized split-mouth study. Indian. J. Dent. Res. 2016, 27, 397–404. [Google Scholar]
- Eick, S.; Renatus, A.; Heinicke, M.; Pfister, W.; Stratul, S.I.; Jentsch, H. Hyaluronic Acid as an adjunct after scaling and root planing: A prospective randomized clinical trial. J. Periodontol. 2013, 84, 941–949. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Dahaba, M.A.; Aboul-Ela, S.; Darhous, M.S. Local application of hyaluronan gel in conjunction with periodontal surgery: A randomized controlled trial. Clin. Oral Investig. 2012, 16, 1229–1236. [Google Scholar] [CrossRef]
- Johannsen, A.; Tellefsen, M.; Wikesjö, U.; Johannsen, G. Local delivery of hyaluronan as an adjunct to scaling and root planing in the treatment of chronic periodontitis. J. Periodontol. 2009, 80, 1493–1497. [Google Scholar] [CrossRef]
- Murakami, S.; Mealey, B.L.; Mariotti, A.; Chapple, I.L.C. Dental plaque-induced gingival conditions. J. Clin. Periodontol. 2018, 45, S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, S.; Drízhal, I. Hyaluronic acid and periodontitis. Acta Medica 2007, 50, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Diehl, D.; Friedmann, A.; Liedloff, P.; Jung, R.M.; Sculean, A.; Bilhan, H. Adjunctive Application of Hyaluronic Acid in Combination with a Sodium Hypochlorite Gel for Non-Surgical Treatment of Residual Pockets Reduces the Need for Periodontal Surgery-Retrospective Analysis of a Clinical Case Series. Materials 2022, 15, 6508. [Google Scholar] [CrossRef]
- Ramanauskaite, E.; Machiulskiene, V.; Dvyliene, U.M.; Eliezer, M.; Sculean, A. Clinical Evaluation of a Novel Combination of Sodium Hypochlorite/Amino Acid and Cross-linked Hyaluronic Acid Adjunctive to Non-surgical Periodontal Treatment: A Case Series. Oral Health Prev. Dent. 2023, 21, 279–284. [Google Scholar] [PubMed]
- Iorio-Siciliano, V.; Blasi, A.; Mauriello, L.; Salvi, G.E.; Ramaglia, L.; Sculean, A. Non-Surgical Treatment of Moderate Periodontal Intrabony Defects with Adjunctive Cross-Linked Hyaluronic Acid: A Single-Blinded Randomized Controlled Clinical Trial. J. Clin. Periodontol. 2025, 52, 310–322. [Google Scholar] [CrossRef]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar]
- Reynolds, M.A.; Kao, R.T.; Camargo, P.M.; Caton, J.G.; Clem, D.S.; Fiorellini, J.P.; Geisinger, M.L.; Mills, M.P.; Nares, S.; Nevins, M.L. Periodontal regeneration—Intrabony defects: A consensus report from the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S105–S107. [Google Scholar] [CrossRef]
- Sharma, R.; Hegde, V.; Siddharth, M.; Hegde, R.; Manchanda, G.; Agarwal, P. Endodontic-periodontal microsurgery for combined endodontic-periodontal lesions: An overview. J. Conserv. Dent. 2014, 17, 510–516. [Google Scholar] [CrossRef]
- Mohammed, A.I.; Celentano, A.; Paolini, R.; Low, J.T.; Silke, J.; O’Reilly, L.A.; McCullough, M.; Cirillo, N. High molecular weight hyaluronic acid drastically reduces chemotherapy-induced mucositis and apoptotic cell death. Cell Death Dis. 2023, 14, 453. [Google Scholar] [CrossRef]
- Eliezer, M.; Imber, J.C.; Sculean, A.; Pandis, N.; Teich, S. Hyaluronic acid as adjunctive to non-surgical and surgical periodontal therapy: A systematic review and meta-analysis. Clin. Oral Investig. 2019, 23, 3423–3435. [Google Scholar] [CrossRef]
- Iorio-Siciliano, V.; Ramaglia, L.; Isola, G.; Blasi, A.; Salvi, G.E.; Sculean, A. Changes in clinical parameters following adjunctive local sodium hypochlorite gel in minimally invasive nonsurgical therapy (MINST) of periodontal pockets: A 6-month randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 5331–5340. [Google Scholar] [CrossRef]
- Jurczyk, K.; Nietzsche, S.; Ender, C.; Sculean, A.; Eick, S. In-vitro activity of sodium-hypochlorite gel on bacteria associated with periodontitis. Clin. Oral Investig. 2016, 20, 2165–2173. [Google Scholar] [CrossRef]
- Liu, J.X.; Werner, J.; Kirsch, T.; Zuckerman, J.D.; Virk, M.S. Cytotoxicity evaluation of chlorhexidine gluconate on human fibroblasts, myoblasts, and osteoblasts. J. Bone Jt. Infect. 2018, 3, 165–172. [Google Scholar] [CrossRef]
- Balloni, S.; Locci, P.; Lumare, A.; Marinucci, L. Cytotoxicity of three commercial mouthrinses on extracellular matrix metabolism and human gingival cell behaviour. Toxicol. Vitr. 2016, 34, 88–96. [Google Scholar] [CrossRef]
- Munar-Bestard, M.; Vargas-Alfredo, N.; Ramis, J.M.; Monjo, M. Mangostanin hyaluronic acid hydrogel as an effective biocompatible alternative to chlorhexidine. Int. J. Biol. Macromol. 2024, 279 Pt 1, 135187. [Google Scholar] [CrossRef]
- Shirakata, Y.; Imafuji, T.; Nakamura, T.; Shinohara, Y.; Iwata, M.; Setoguchi, F.; Noguchi, K.; Sculean, A. Cross-linked hyaluronic acid gel with or without a collagen matrix in the treatment of class III furcation defects: A histologic and histomorphometric study in dogs. J. Clin. Periodontol. 2022, 49, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Shirakata, Y.; Imafuji, T.; Nakamura, T.; Kawakami, Y.; Shinohara, Y.; Noguchi, K.; Pilloni, A.; Sculean, A. Periodontal wound healing/regeneration of two-wall intrabony defects following reconstructive surgery with cross-linked hyaluronic acid-gel with or without a collagen matrix: A preclinical study in dogs. Quintessence Int. 2021, 52, 308–316. [Google Scholar]
- Pilloni, A.; Schmidlin, P.R.; Sahrmann, P.; Sculean, A.; Rojas, M.A. Effectiveness of adjunctive hyaluronic acid application in coronally advanced flap in Miller class I single gingival recession sites: A randomized controlled clinical trial. Clin. Oral Investig. 2019, 23, 1133–1141. [Google Scholar] [CrossRef]
- Butera, A.; Folini, E.; Cosola, S.; Russo, G.; Scribante, A.; Gallo, S.; Stablum, G.; Menchini Fabris, G.B.; Covani, U.; Genovesi, A. Evaluation of the Efficacy of Probiotics Domiciliary Protocols for the Management of Periodontal Disease, in Adjunction of Non-Surgical Periodontal Therapy (NSPT): A Systematic Literature Review. Appl. Sci. 2023, 13, 663. [Google Scholar] [CrossRef]
- Ramanauskaite, E.; Sakalauskaite, U.M.; Machiulskiene, V. The Efficacy of Adjunctive Aids in Periodontal Maintenance Therapy: A Systematic Literature Review and Meta-analysis. Oral Health Prev. Dent. 2020, 18, 889–910. [Google Scholar]
- Chen, L.F.; Vander Weg, M.W.; Hofmann, D.A.; Reisinger, H.S. The Hawthorne Effect in Infection Prevention and Epidemiology. Infect. Control Hosp. Epidemiol. 2015, 36, 1444–1450. [Google Scholar] [CrossRef]




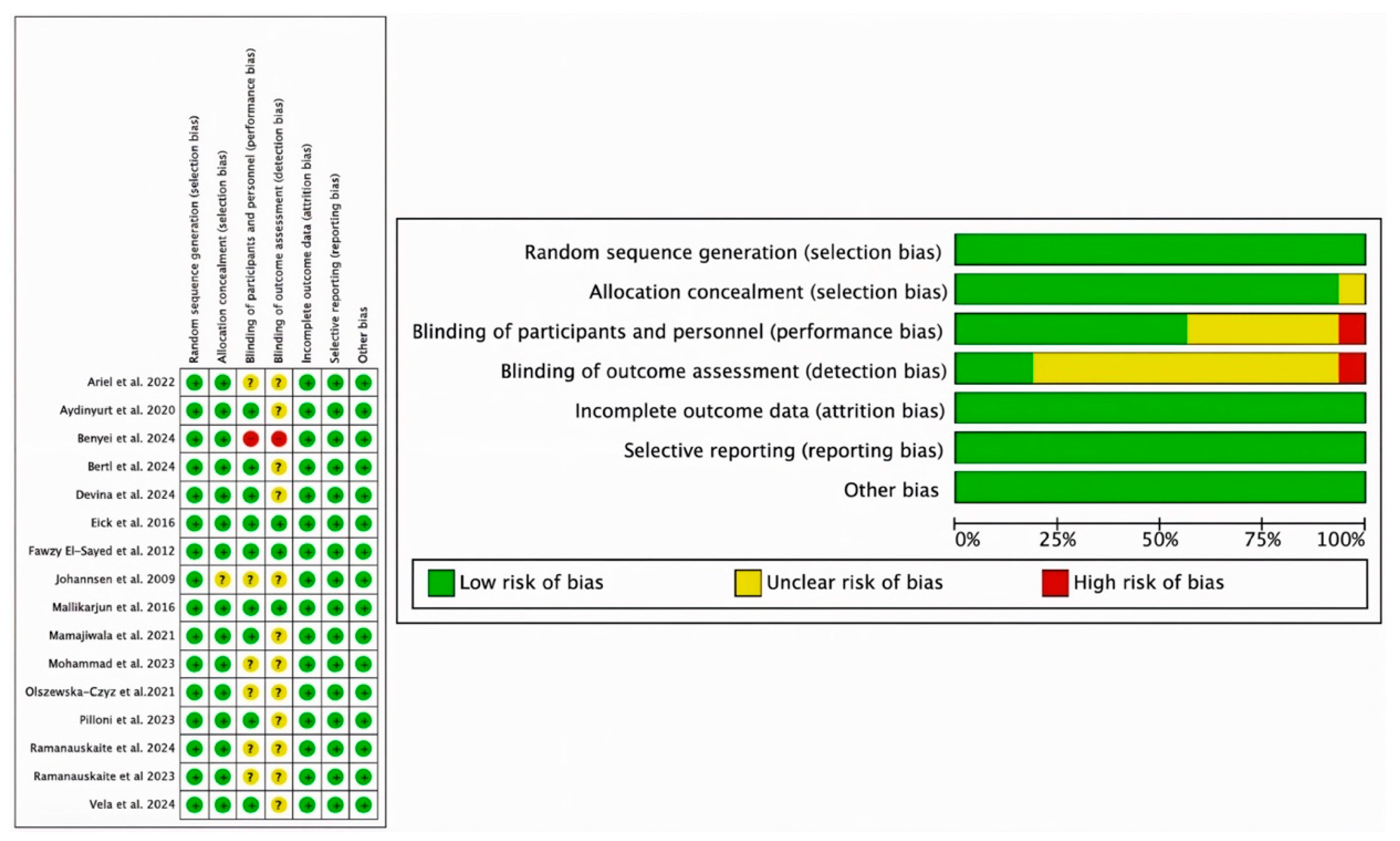
| Population | Adult subjects suffering from gingivitis or periodontitis |
| Intervention | HA, either as a single or adjuvant treatment |
| Comparisons | Conventional or placebo treatment |
| Outcomes | Observe the effects of treatment on clinical parameters indicative of gingivitis/periodontitis (Δ PPD; Δ BOP; Δ CAL; Δ PI; Δ GI) |
| Study design | RCTs |
| Databases | Search Details |
|---|---|
| PubMed via Medline | “hyaluronic acid” [MeSH Terms] OR “hyaluronic” [All Fields] AND “acid” [All Fields] AND “Humans” [MeSH terms]. “hyaluronic acid” [MeSH Terms] OR “hyaluronic” [All Fields] AND “acid” [All Fields] AND “gingivitis” [MeSH Terms] OR “periodontitis” [All Fields] OR “periodontal diseases” [MeSH Terms]. |
| Embase | “gingivitis” OR “periodontitis” [Title/Abstract] AND “hyaluronic acid” [Title/Abstract]. “gingivitis” [MeSH Terms] OR “periodontitis” OR “periodontal diseases” [MeSH Terms] AND “hyaluronic acid” [MeSH Terms]. |
| Cochrane Central | “hyaluronic acid” AND “gingivitis” OR “periodontitis” OR “periodontal diseases” AND “Humans”. |
| Web of Science | “hyaluronan” OR “hyaluronic acid” AND “gingivitis” OR “periodontitis” OR “periodontal diseases” AND “Humans”. |
| Scopus | “hyaluronic acid” AND “gingivitis treatment” OR “periodontitis treatment” |
| Boolean operators | AND and OR |
| Study, Year | Type of Study | Subjects Number | Pathology | Diagnostic Criteria | HA Type and Concentration | Control Treatment | Periodontal Parameters Involved | Follow-Up | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Ramanauskaite et al. 2024 [32] | Randomized, controlled, parallel study | 48 | Generalized Periodontitis (stages II–III) | NR | High-molecular-weight HA | Subgingival debridement | PPD, CAL, BOP, PI | 6 months | The PPD and CAL were statistically significant in the test group (p < 0.001). |
| Vela et al. 2024 [33] | Randomized, double-arm, multicentric clinical trial | 100 | Periodontitis (stages III and IV) | NR | HA gel NR | Debridement only | CAL, PPD, GR | 12 months | PPD and CAL were significant at 12 months (p < 0.0001). |
| Axe et al. 2024 [34] | Randomized, clinical study | 110 | Moderate gingivitis | Modified Gingival Index | High-molecular-weight HA | Fluoride toothpaste | GI, BOP; PI | 1, 2 and 6 weeks | No difference was observed between the toothpaste with HA and the control toothpaste (without HA). Both reduced gingival bleeding. |
| Bertl et al. 2024 [35] | Randomized controlled clinical trial | 56 | Chronic periodontitis patients (stage III and IV) | World Workshop classification (2017) | HA gel 0.3% | Placebo | CAL, PPD, PI | 12 months | Supragingival and subgingival HA gel resulted in fewer sites requiring further intervention. |
| Benyei et al. 2024 [36] | Pilot randomized controlled clinical trial | 52 | Residual periodontal pockets | NR | HA gel NR | SRP | BOP, CAL, PPD | 3 and 9 months | HA in the experimental group showed an improvement in CAL (p = 0.001) and a significant reduction in PPD (p = 0.001). |
| Devina et al. 2024 [37] | Double-blind randomized clinical trial | 32 | Periodontitis. NR stage | European Federation of Periodontology criteria | HA gel 0.2% | Placebo | BOP, CAL, PPD | 4 weeks | The groups showed significant reductions in all clinical parameters (p ≤ 0.05), except for PPD and CAL in the placebo group. |
| Mohammad et al. 2023 [38] | Clinical comparative study | 75 | Periodontitis. NR stage | NR | HA gel 0.8% | SRP | PI, GI, BOP, PPD, CAL | 2 months | Clinical parameters decreased significantly in the experimental group (p ≤ 0.001). |
| Ramanauskaite et al. 2023 [39] | Randomized controlled clinical trial | 48 | Periodontitis (stages II–III) | NR | HA gel NR | SRP | BOP, PI, PPD, CAL | 3 and 6 months | Significant reduction in PPD and BOP in the test group compared to the control group (p < 0.001). |
| Pilloni et al. 2023 [40] | Randomized, split-mouth, single-blind, clinical trial | 50 | Residual periodontal pockets | NR | HA gel NR | SRP | BOP, PI, PPD, CAL | 6, 8, 24, 36, and 48 weeks | The test sites showed a higher percentage of pockets with a PPD ≤ 4 mm. Bleeding decreased in both groups. At sites with baseline PPD values ≥ 6 mm, statistically significant differences were observed between the groups (p = 0.004). |
| Ariel et al. 2022 [41] | Randomized prospective clinical study | 34 | Periodontitis (stage III) | World Workshop classification (2017) | HA gel NR | SRP | PI, BOP, PPD, CAL | 3 and 6 months | PI, PPD, and CAL scores at baseline and follow-up showed a significant reduction in both the test and control groups (p < 0.01 for PI and p < 0.0001 for PPD and CAL). BOP values were reduced in the test group (p < 0.001). |
| Mamajiwala et al. 2021 [42] | Randomized controlled clinical trial | 20 | Chronic periodontitis (stage II or III) | Classification of periodontal diseases (1999) | HA gel 0.8% | Debridement + placebo | PI, PPD, CAL, GI, GR | 12 months | The test group showed a significantly greater gain in CAL compared to the control group (p < 0.001). PPD was significantly reduced in the test group (p < 0.05). |
| Olszewska-Czyz et al. 2021 [43] | Randomized, Controlled Clinical Trial | 100 | Moderate periodontitis. NR stage | Clinical and radiological examination. 2017 World Workshop on the Classification of Periodontal and Peri-Implant Disease (2017) | HA gel NR | Debridement only | CAL, PPD, BOP | 12 weeks | Significant differences between the groups in terms of BOP and CAL in favor of the HA group |
| Aydinyurt et al. 2020 [44] | Randomized controlled trial | 96 | Periodontitis. NR stage | NR | HA gel NR | SRP + placebo | BOP, GI, PPD, CAL | 4 weeks | BOP, CAL, and PPD were significantly reduced in the HA group (p < 0.05). |
| Al-Shammari et al. 2018 [45] | 24 | Moderate and Severe Chronic Periodontitis | Classification of periodontal diseases (1999) | HA gel 0.8% | SRP | PI, GI, CAL, PPD | 6 and 12 weeks | Statistically significant differences in PPD between the control and test groups (p = 0.041 and p = 0.02, respectively). | |
| Mallikarjun et al. 2016 [46] | Randomized split-mouth study | 20 | Chronic periodontitis | NR | HA gel 0.2% | Mechanical debridement | PI, GI, PPD, CAL | 6 weeks | The difference in PI, GI, PPD and CAL scores of the control and experimental groups was statistically highly significant (p < 0.001). |
| Eick et al. 2016 [47] | Prospective Randomized Clinical Trial | 42 | Chronic periodontitis | NR | HA gel 0.8% | SRP | PPD, CAL, BOP, PI | 3 and 6 months | The number of sites with PPD ≥ 5 mm decreased more in the test group than in the control group. No differences were observed in CAL, BOP, and PI. |
| Fawzy El-Sayed et al. 2012 [48] | Randomized controlled trial | 28 | Chronic periodontitis | NR | HA gel NR | Gel placebo | CAL, BOP, PPD, GR, PI | 3 and 6 months | Statistically significant differences in CAL (p < 0.05) between the test and control areas. |
| Johannsen et al. 2009 [49] | Split mouth | 12 | Chronic periodontitis | NR | HA gel NR | SRP | BOP, CAL, PPD, PI | 12 weeks | Significant reduction in PI in the test (p < 0.01) and control (p < 0.01) groups. PPD was also significantly reduced in the test group (p < 0.05). |
| Study | Follow-Up | Stage of Periodontitis | Results for CAL Gain | Results for PPD Reduction |
|---|---|---|---|---|
| Vela et al. 2024 [33] | 12 months | Stages III and IV | 3.06 ± 1.13 mm (test group) vs. 1.44 ± 1.07 mm (control group); p < 0.001 * | 3.28 ± 1.14 mm (test group) vs. 2.61 ± 1.22 mm (control group); p = 0.032 * |
| Bertl et al. 2024 [35] | 12 months | Stages III and IV | NR | 4.2 ± 0.9 mm (test group) vs. 4.5 ± 0.8 mm (control group); p = 0.007 |
| Pilloni et al. 2023 [40] | 12 months | Residual periodontal pockets after treatment of aggressive periodontitis | (test: −0.50 ± 1.85 mm vs. control: −0.36 ± 1.80 mm). CAL gain was comparable between groups | −2.08 ± 1.24 mm ± 1.24 (test group) vs. −1.94 ± 1.19 ( control group); p < 0.0001 * |
| Mamajiwala et al. 2021 [42] | 12 months | Stage III | 4.0 ± 0.56 mm (test group) vs. 5.4 ± 0.82 mm (control group); p < 0.001 * | 3.1 ± 0.58 mm (test group) vs. 4.3 ± 0.47 mm (control group); p < 0.0018 * |
| Study | Country | Journal | Age Range | Sex | Tobacco Smokers | Study Registration | Statistical Tests | Dropouts | Adverse Events |
|---|---|---|---|---|---|---|---|---|---|
| Ramanauskaite et al. 2024 [32] | Lithuanian | Oral Health Prev Dent | 30 to 72 years | NR | Non-smokers | ClinicalTrials.gov, NCT04662216. | Mann–Whitney, McNemar, Wilcoxon | No dropouts | NR |
| Vela et al. 2024 [33] | Romania | Medicina (Kaunas) | 30 to 60 years | 26 females and 34 males | Non-smokers | Clinical Trials (NCT05073575) | Kolmogorov–Smirnov, Chi-square, Fisher’s | No dropouts | NR |
| Axe et al. 2024 [34] | Canada | BMC Oral Health | 18 to 65 years | 66 females and 44 males | Non-smokers | International Council for Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice | ANCOVA | No dropouts | Reported adverse events |
| Bertl et al. 2024 [35] | Sweden | Clin Oral Investig | 35 to 75 years | 20 females and 36 males | Non-smokers | Clinicaltrials.gov (NCT04792541) | Chi-squared, Mann–Whitney-U, Shapiro–Wilk | No dropouts | No Adverse events |
| Benyei et al. 2024 [36] | Germany | Clin Oral Investig | 49.3 ± 11.2 47.3 ± 10.7 | 35 females and 13 males | Non-smokers | ClinicalTrials.gov, NCT04662216 | Shapiro–Wilk, Wilcoxon, Mann–Whitney | No dropouts | No Adverse events |
| Devina et al. 2024 [37] | Indonesia | Eur J Dent | 31–71 years | 24 females and 26 males | 9 (≤10 cigarettes/day) | (ClinicalTrials.gov- NCT05210686) | Shapiro–Wilk | No dropouts | No Adverse events |
| Mohammad et al. 2023 [38] | Irak | Gels | 29–78 years | 21 males and 13 females | Smoking less than 10 cigarettes/day | Israeli Ministry of Health (0034–17-MHMC). | NR | No dropouts | Reported adverse events |
| Ramanauskaite et al. 2023 [39] | Lithuanian | Clin Oral Investig | 34 to 51 years | 11 males and 9 females | Non-smokers | Institutional Ethical Committee Maharashtra University of Health Sciences, Nashik (MGV/KBHC/786/2016-17) | Shapiro–Wilk, Student’s t, Mantel–Haenszel χ2 | No dropouts | NR |
| Pilloni et al. 2023 [40] | Italy | J Periodontol | 25 to 65 years | 51% women | Non-smokers (for a minimum of 5 years) | Jagiellonian University Ethics Committee (122.6120.132.2015) | Mann–Whitney U, Shapiro–Wilk | No dropouts. | No Adverse events |
| Ariel et al. 2022 [41] | Israel | Clin Oral Investig | 18 to 55 years | 40 males, 56 females | Non-smokers | ClinicalTrials.gov.tr (NCT03754010). | Kruskal–Wallis, Turkey, Kramer, Bonferroni | No dropouts | NR |
| Mamajiwala et al. 2021 [42] | India | Clin Oral Investig | 24 to 57 years | 14 females, 10 males | Non-smokers | Riyadh Elm University. (RC/IRB/2016/478) | Shapiro, Mann–Whitney U, Wilcoxon | 2 dropped out | |
| Olszewska-Czyz et al. 2021 [43] | Croatia | Biomolecules | 20–60 years | 11 males and nine females | Non-smokers | Institutional Ethics Committee, Department of Periodontics, Dayananda Sagar College of Dental Sciences, Bengaluru, India | Student’s t, Pearson’s correlation | No dropouts | NR |
| Aydinyurt et al. 2020 [44] | Turkey | Irish Journal of Medical Science. | 41 to 72 years | 18 males and 24 females | Ethics Commission (#121 in 2006) of the University of Leipzig Medical Faculty | Wilcoxon, U-test | 8 dropped out | No Adverse events | |
| Al-Shammari et al. 2018 [45] | Saudi Arabia | J Contemp Dent Pract. | NR | NR | Non-smokers | Ethical Committee at Cairo University Hospital, Cairo, Egypt. | Shapiro–Wilk, Wilcoxon, McNemar, Friedman, Cochran | 2 dropped out | NR |
| Mallikarjun et al. 2016 [46] | India | Indian J Dent Res. | 42 to 63 years | 7 males and 5 women | Two were smokers, and one used snuff | Ethics Committee at Huddinge University Hospital, Huddinge, Sweden | Wilcoxon | No dropouts | NR |
| Eick et al. 2016 [47] | Switzerland | J Periodontol | 41 to 72 years | 18 males and 24 females | Ethics Commission (#121 in 2006) of the University of Leipzig Medical Faculty | Wilcoxon, U-test | 8 dropped out | No Adverse events | |
| Fawzy El-Sayed et al. 2012 [48] | Egypt | Clin Oral Investig. | NR | NR | Non-smokers | Ethical Committee at Cairo University Hospital, Cairo, Egypt. | Shapiro–Wilk, Wilcoxon, McNemar, Friedman, Cochran | No dropouts | NR |
| Johannsen et al. 2009 [49] | Sweden | J Periodontol. | 42 to 63 years | 7 males and 5 women | Two were smokers, and one used snuff | Ethics Committee at Huddinge University Hospital, Huddinge, Sweden | Wilcoxon | No dropouts | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Valverde, N.; Quispe-López, N.; Flores Fraile, J.; López-Valverde, A.; Macedo de Sousa, B.; Rueda, J.A.B. The Role of Hyaluronic Acid in the Treatment of Gingivitis and Periodontitis at Different Stages: A Systematic Review and Meta-Analysis with Short-Term Follow-Up. Bioengineering 2025, 12, 1135. https://doi.org/10.3390/bioengineering12111135
López-Valverde N, Quispe-López N, Flores Fraile J, López-Valverde A, Macedo de Sousa B, Rueda JAB. The Role of Hyaluronic Acid in the Treatment of Gingivitis and Periodontitis at Different Stages: A Systematic Review and Meta-Analysis with Short-Term Follow-Up. Bioengineering. 2025; 12(11):1135. https://doi.org/10.3390/bioengineering12111135
Chicago/Turabian StyleLópez-Valverde, Nansi, Norberto Quispe-López, Javier Flores Fraile, Antonio López-Valverde, Bruno Macedo de Sousa, and José Antonio Blanco Rueda. 2025. "The Role of Hyaluronic Acid in the Treatment of Gingivitis and Periodontitis at Different Stages: A Systematic Review and Meta-Analysis with Short-Term Follow-Up" Bioengineering 12, no. 11: 1135. https://doi.org/10.3390/bioengineering12111135
APA StyleLópez-Valverde, N., Quispe-López, N., Flores Fraile, J., López-Valverde, A., Macedo de Sousa, B., & Rueda, J. A. B. (2025). The Role of Hyaluronic Acid in the Treatment of Gingivitis and Periodontitis at Different Stages: A Systematic Review and Meta-Analysis with Short-Term Follow-Up. Bioengineering, 12(11), 1135. https://doi.org/10.3390/bioengineering12111135












