hiPSCGEM01: A Genome-Scale Metabolic Model for Fibroblast-Derived Human iPSCs
Abstract
1. Introduction
2. Materials & Methods
2.1. Constraint-Based Models and Flux-Balance Analysis
2.2. Data Pre-Processing
2.3. Model Reconstruction
2.4. Adaptation to Culture Medium
2.5. Consistency Testing and Model Validation
3. Results
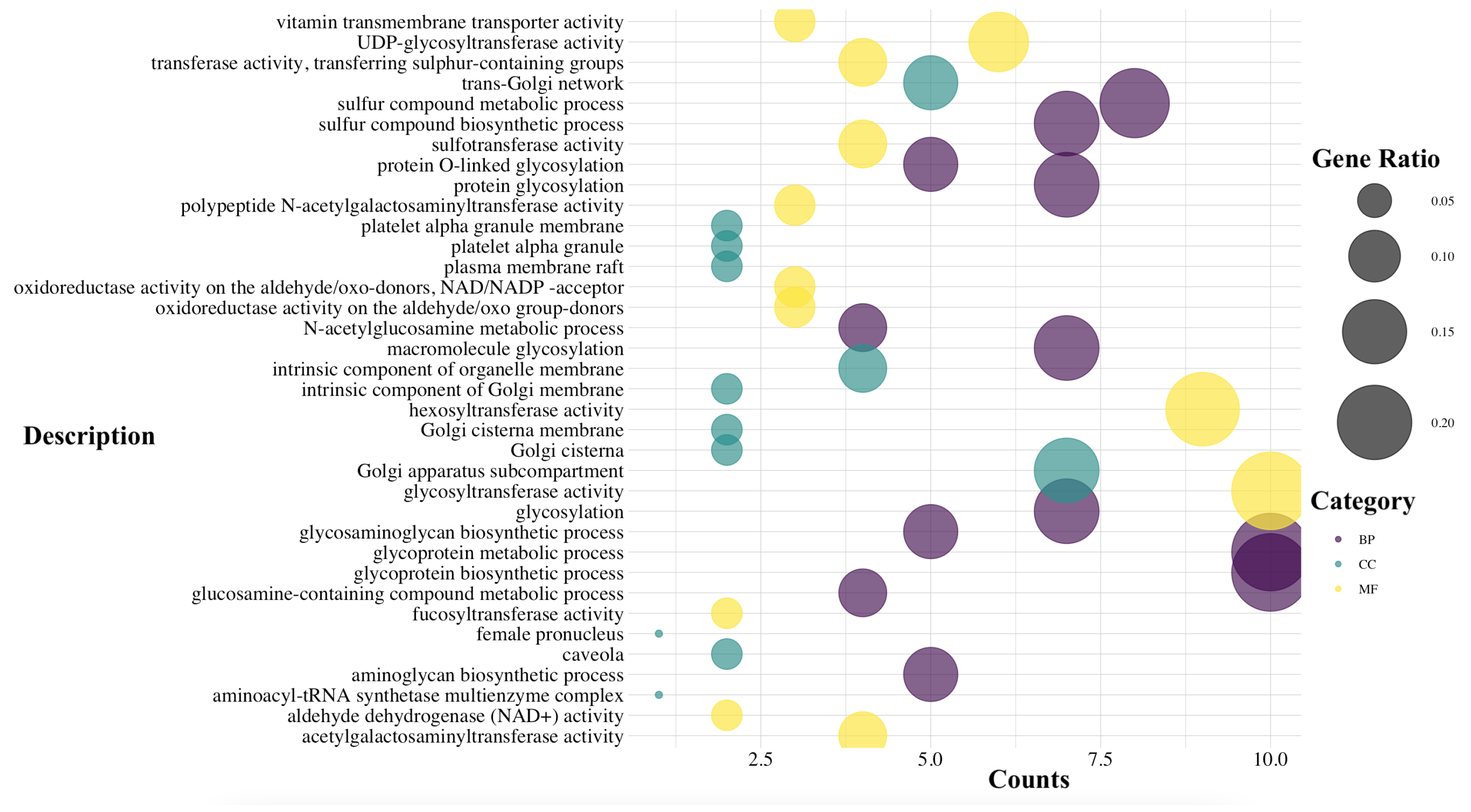
3.1. Analysis and Computation of the Essential Genes
3.2. Analysis and Computation of the Essential Metabolites
4. Discussion
4.1. Central Metabolic Pathways
4.2. Emerging Pathways
4.3. Implication for Regenerative Medicine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tabar, V.; Studer, L. Pluripotent stem cells in regenerative medicine: Challenges and recent progress. Nat. Rev. Genet. 2014, 15, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.; McKee, C.; Bakshi, S.; Walker, K.; Hakman, E.; Halassy, S.; Svinarich, D.; Dodds, R.; Govind, C.K.; Chaudhry, G.R. Mesenchymal stem cells: Cell therapy and regeneration potential. J. Tissue Eng. Regen. Med. 2019, 13, 1738–1755. [Google Scholar] [CrossRef]
- Amabile, G.; Meissner, A. Induced pluripotent stem cells: Current progress and potential for regenerative medicine. Trends Mol. Med. 2009, 15, 59–68. [Google Scholar] [CrossRef]
- Zhang, L.; Fei, Y.Y.; Han, H.T.; Xu, J.; Cheng, L.; Li, X. Stem cell therapy in liver regeneration: Focus on mesenchymal stem cells and induced pluripotent stem cells. Pharmacol. Ther. 2022, 232, 108004. [Google Scholar] [CrossRef]
- de Miguel, M.P.; Prieto, I.; Moratilla, A.; Arias, J.; Aller, M. Mesenchymal stem cells for liver regeneration in liver failure: From experimental models to clinical trials. Stem Cells Int. 2019, 2019, 3945672. [Google Scholar] [CrossRef]
- Nikokiraki, C.; Psaraki, A.; Roubelakis, M.G. The potential clinical use of stem/progenitor cells and organoids in liver diseases. Cells 2022, 11, 1410. [Google Scholar] [CrossRef] [PubMed]
- Sadri, A.R.; Jeschke, M.G.; Amini-Nik, S. Advances in liver regeneration: Revisiting hepatic stem/progenitor cells and their origin. Stem Cells Int. 2016, 2016, 7920897. [Google Scholar] [CrossRef]
- Bria, A.; Marda, J.; Zhou, J.; Sun, X.; Cao, Q.; Petersen, B.E.; Pi, L. Hepatic progenitor cell activation in liver repair. Liver Res. 2017, 1, 81–87. [Google Scholar] [CrossRef]
- Hu, C.; Wu, Z.; Li, L. Mesenchymal stromal cells promote liver regeneration through regulation of immune cells. Int. J. Biol. Sci. 2020, 16, 893. [Google Scholar] [CrossRef]
- Anger, F.; Camara, M.; Ellinger, E.; Germer, C.T.; Schlegel, N.; Otto, C.; Klein, I. Human mesenchymal stromal cell-derived extracellular vesicles improve liver regeneration after ischemia reperfusion injury in mice. Stem Cells Dev. 2019, 28, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Baseer, A.Q.; Mushfiq, S.; Omid, M.T. A Review on Stem Cells: A New Toll in Diseases Therapy. J. Res. Appl. Sci. Biotechnol. 2023, 2, 1–6. [Google Scholar] [CrossRef]
- Okita, K.; Nakagawa, M.; Hyenjong, H.; Ichisaka, T.; Yamanaka, S. Generation of mouse induced pluripotent stem cells without viral vectors. Science 2008, 322, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Kim, G.B.; Kim, W.J.; Kim, H.U.; Lee, S.Y. Current status and applications of genome-scale metabolic models. Genome Biol. 2019, 20, 121. [Google Scholar] [CrossRef] [PubMed]
- Salerno, L.; Cosentino, C.; Merola, A.; Bates, D.G.; Amato, F. Validation of a model of the GAL regulatory system via robustness analysis of its bistability characteristics. BMC Syst. Biol. 2013, 7, 39. [Google Scholar] [CrossRef]
- Cosentino, C.; Salerno, L.; Passanti, A.; Merola, A.; Bates, D.G.; Amato, F. Structural bistability of the GAL regulatory network and characterization of its domains of attraction. J. Comput. Biol. 2012, 19, 148–162. [Google Scholar] [CrossRef]
- Salerno, L.; Cosentino, C.; Morrone, G.; Amato, F. Computational modeling of a transcriptional switch underlying B-lymphocyte lineage commitment of hematopoietic multipotent cells. PLoS ONE 2015, 10, e0132208. [Google Scholar] [CrossRef]
- Montefusco, F.; Procopio, A.; Bates, D.G.; Amato, F.; Cosentino, C. Scalable reverse-engineering of gene regulatory networks from time-course measurements. Int. J. Robust Nonlinear Control 2023, 33, 5023–5038. [Google Scholar] [CrossRef]
- Procopio, A.; De Rosa, S.; Covello, C.; Merola, A.; Sabatino, J.; De Luca, A.; Indolfi, C.; Amato, F.; Cosentino, C. A model of cardiac troponin T release in patient with acute myocardial infarction. In Proceedings of the 2017 IEEE 56th Annual Conference on Decision and Control (CDC), Melbourne, VIC, Australia, 12–15 December 2017; pp. 435–440. [Google Scholar]
- Procopio, A.; De Rosa, S.; Covello, C.; Merola, A.; Sabatino, J.; De Luca, A.; Indolfi, C.; Amato, F.; Cosentino, C. Mathematical Model of the Release of the cTnT and CK-MB cardiac biomarkers in patients with acute myocardial infarction. In Proceedings of the 2019 18th European Control Conference (ECC), Naples, Italy, 25–28 June 2019; pp. 1653–1658. [Google Scholar]
- Glauche, I.; Herberg, M.; Roeder, I. Nanog variability and pluripotency regulation of embryonic stem cells-insights from a mathematical model analysis. PLoS ONE 2010, 5, e11238. [Google Scholar] [CrossRef]
- Morris, R.; Sancho-Martinez, I.; Sharpee, T.O.; Izpisua Belmonte, J.C. Mathematical approaches to modeling development and reprogramming. Proc. Natl. Acad. Sci. USA 2014, 111, 5076–5082. [Google Scholar] [CrossRef]
- Heinken, A.; Hertel, J.; Acharya, G.; Ravcheev, D.A.; Nyga, M.; Okpala, O.E.; Hogan, M.; Magnúsdóttir, S.; Martinelli, F.; Nap, B.; et al. Genome-scale metabolic reconstruction of 7,302 human microorganisms for personalized medicine. Nat. Biotechnol. 2023, 41, 1320–1331. [Google Scholar] [CrossRef]
- Gunter, J.H.; Kruithof-de Julio, M.; Zoni, E. Personalized medicine for urological cancers: Targeting cancer metabolism. Front. Oncol. 2022, 12, 862811. [Google Scholar] [CrossRef]
- Basile, A.; Heinken, A.; Hertel, J.; Smarr, L.; Li, W.; Treu, L.; Valle, G.; Campanaro, S.; Thiele, I. Longitudinal flux balance analyses of a patient with episodic colonic inflammation reveals microbiome metabolic dynamics. Gut Microbes 2023, 15, 2226921. [Google Scholar] [CrossRef]
- Kumar, M.; Ji, B.; Zengler, K.; Nielsen, J. Modelling approaches for studying the microbiome. Nat. Microbiol. 2019, 4, 1253–1267. [Google Scholar] [CrossRef]
- Saifuddin, M.; Bhatnagar, J.M.; Segrè, D.; Finzi, A.C. Microbial carbon use efficiency predicted from genome-scale metabolic models. Nat. Commun. 2019, 10, 3568. [Google Scholar] [CrossRef] [PubMed]
- Amaradio, M.N.; Jansen, G.; Ojha, V.; Costanza, J.; Di Fatta, G.; Nicosia, G. Inferring Pathological Metabolic Patterns in Breast Cancer Tissue from Genome-Scale Models. In Proceedings of the International Conference on Machine Learning, Optimization, and Data Science, Castiglione della Pescaia, Italy, 19–22 September 2022; Springer: Cham, Switzerland, 2022; Volume 13810, pp. 596–612. [Google Scholar]
- Granata, I.; Troiano, E.; Sangiovanni, M.; Guarracino, M.R. Integration of transcriptomic data in a genome-scale metabolic model to investigate the link between obesity and breast cancer. BMC Bioinform. 2019, 20, 162. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.T.; Lai, J.M.; Chang, P.M.H.; Hong, Y.R.; Huang, C.Y.F.; Wang, F.S. Identifying essential genes in genome-scale metabolic models of consensus molecular subtypes of colorectal cancer. PLoS ONE 2023, 18, e0286032. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.E.; Abdel-Haleem, A.M. The evolution of genome-scale models of cancer metabolism. Front. Physiol. 2013, 4, 237. [Google Scholar] [CrossRef]
- Raškevičius, V.; Mikalayeva, V.; Antanavičiūtė, I.; Ceslevičienė, I.; Skeberdis, V.A.; Kairys, V.; Bordel, S. Genome scale metabolic models as tools for drug design and personalized medicine. PLoS ONE 2018, 13, e0190636. [Google Scholar] [CrossRef]
- Larsson, I.; Uhlén, M.; Zhang, C.; Mardinoglu, A. Genome-scale metabolic modeling of glioblastoma reveals promising targets for drug development. Front. Genet. 2020, 11, 381. [Google Scholar] [CrossRef]
- Procopio, A.; Cesarelli, G.; Donisi, L.; Merola, A.; Amato, F.; Cosentino, C. Combined mechanistic modeling and machine-learning approaches in systems biology—A systematic literature review. Comput. Methods Programs Biomed. 2023, 240, 107681. [Google Scholar] [CrossRef]
- Magazzù, G.; Zampieri, G.; Angione, C. Clinical stratification improves the diagnostic accuracy of small omics datasets within machine learning and genome-scale metabolic modelling methods. Comput. Biol. Med. 2022, 151, 106244. [Google Scholar] [CrossRef] [PubMed]
- Moolamalla, S.; Vinod, P. Genome-scale metabolic modelling predicts biomarkers and therapeutic targets for neuropsychiatric disorders. Comput. Biol. Med. 2020, 125, 103994. [Google Scholar] [CrossRef] [PubMed]
- Barata, T.; Vieira, V.; Rodrigues, R.; das Neves, R.P.; Rocha, M. Reconstruction of tissue-specific genome-scale metabolic models for human cancer stem cells. Comput. Biol. Med. 2022, 142, 105177. [Google Scholar] [CrossRef]
- Bordbar, A.; Monk, J.M.; King, Z.A.; Palsson, B.O. Constraint-based models predict metabolic and associated cellular functions. Nat. Rev. Genet. 2014, 15, 107–120. [Google Scholar] [CrossRef]
- Becker, S.A.; Feist, A.M.; Mo, M.L.; Hannum, G.; Palsson, B.Ø.; Herrgard, M.J. Quantitative prediction of cellular metabolism with constraint-based models: The COBRA Toolbox. Nat. Protoc. 2007, 2, 727–738. [Google Scholar] [CrossRef]
- Mendoza, S.N.; Olivier, B.G.; Molenaar, D.; Teusink, B. A systematic assessment of current genome-scale metabolic reconstruction tools. Genome Biol. 2019, 20, 158. [Google Scholar] [CrossRef]
- Thiele, I.; Palsson, B.Ø. A protocol for generating a high-quality genome-scale metabolic reconstruction. Nat. Protoc. 2010, 5, 93–121. [Google Scholar] [CrossRef]
- Correia, S.; Costa, B.; Rocha, M. Reconstruction of consensus tissue-specific metabolic models. bioRxiv 2018, 327262. [Google Scholar] [CrossRef]
- Lee, J.M.; Gianchandani, E.P.; Papin, J.A. Flux balance analysis in the era of metabolomics. Briefings Bioinform. 2006, 7, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Orth, J.D.; Thiele, I.; Palsson, B.Ø. What is flux balance analysis? Nat. Biotechnol. 2010, 28, 245–248. [Google Scholar] [CrossRef]
- Feist, A.M.; Palsson, B.O. The biomass objective function. Curr. Opin. Microbiol. 2010, 13, 344–349. [Google Scholar] [CrossRef]
- Causton, H.; Quackenbush, J.; Brazma, A. Microarray Gene Expression Data Analysis: A Beginner’s Guide; Wiley-Blackwell: Hoboken, NJ, USA, 2009. [Google Scholar]
- Soldner, F.; Hockemeyer, D.; Beard, C.; Gao, Q.; Bell, G.W.; Cook, E.G.; Hargus, G.; Blak, A.; Cooper, O.; Mitalipova, M.; et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell 2009, 136, 964–977. [Google Scholar] [CrossRef]
- Lowry, W.; Richter, L.; Yachechko, R.; Pyle, A.; Tchieu, J.; Sridharan, R.; Clark, A.; Plath, K. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc. Natl. Acad. Sci. USA 2008, 105, 2883–2888. [Google Scholar] [CrossRef]
- Wang, Y.; Eddy, J.A.; Price, N.D. Reconstruction of genome-scale metabolic models for 126 human tissues using mCADRE. BMC Syst. Biol. 2012, 6, 153. [Google Scholar] [CrossRef]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef]
- Heirendt, L.; Arreckx, S.; Pfau, T.; Mendoza, S.N.; Richelle, A.; Heinken, A.; Haraldsdóttir, H.S.; Wachowiak, J.; Keating, S.M.; Vlasov, V.; et al. Creation and analysis of biochemical constraint-based models using the COBRA Toolbox v. 3.0. Nat. Protoc. 2019, 14, 639–702. [Google Scholar] [CrossRef]
- Brunk, E.; Sahoo, S.; Zielinski, D.C.; Altunkaya, A.; Dräger, A.; Mih, N.; Gatto, F.; Nilsson, A.; Preciat Gonzalez, G.A.; Aurich, M.K.; et al. Recon3D enables a three-dimensional view of gene variation in human metabolism. Nat. Biotechnol. 2018, 36, 272–281. [Google Scholar] [CrossRef]
- Ludwig, T.E.; Bergendahl, V.; Levenstein, M.E.; Yu, J.; Probasco, M.D.; Thomson, J.A. Feeder-independent culture of human embryonic stem cells. Nat. Methods 2006, 3, 637–646. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Midford, P.E.; Ong, Q.; Ong, W.K.; et al. The MetaCyc database of metabolic pathways and enzymes. Nucleic Acids Res. 2018, 46, D633–D639. [Google Scholar] [CrossRef]
- Kanehisa, M. The KEGG database. In Proceedings of the ‘In Silico’ Simulation of Biological Processes: Novartis Foundation Symposium 247, London, UK, 27–29 November 2001; Wiley Online Library: Hoboken, NJ, USA, 2002; Volume 247, pp. 91–103. [Google Scholar]
- Noronha, A.; Modamio, J.; Jarosz, Y.; Guerard, E.; Sompairac, N.; Preciat, G.; Daníelsdóttir, A.D.; Krecke, M.; Merten, D.; Haraldsdóttir, H.S.; et al. The Virtual Metabolic Human database: Integrating human and gut microbiome metabolism with nutrition and disease. Nucleic Acids Res. 2019, 47, D614–D624. [Google Scholar] [CrossRef]
- Fabregat, A.; Jupe, S.; Matthews, L.; Sidiropoulos, K.; Gillespie, M.; Garapati, P.; Haw, R.; Jassal, B.; Korninger, F.; May, B.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2018, 46, D649–D655. [Google Scholar] [CrossRef]
- Robinson, J.L.; Kocabaş, P.; Wang, H.; Cholley, P.E.; Cook, D.; Nilsson, A.; Anton, M.; Ferreira, R.; Domenzain, I.; Billa, V.; et al. An atlas of human metabolism. Sci. Signal. 2020, 13, eaaz1482. [Google Scholar] [CrossRef]
- Schellenberger, J.; Que, R.; Fleming, R.M.; Thiele, I.; Orth, J.D.; Feist, A.M.; Zielinski, D.C.; Bordbar, A.; Lewis, N.E.; Rahmanian, S.; et al. Quantitative prediction of cellular metabolism with constraint-based models: The COBRA Toolbox v2. 0. Nat. Protoc. 2011, 6, 1290–1307. [Google Scholar] [CrossRef]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The human metabolome database. Nucleic Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef]
- Gevorgyan, A.; Poolman, M.G.; Fell, D.A. Detection of stoichiometric inconsistencies in biomolecular models. Bioinformatics 2008, 24, 2245–2251. [Google Scholar] [CrossRef]
- Mackie, A.; Keseler, I.M.; Nolan, L.; Karp, P.D.; Paulsen, I.T. Dead end metabolites-defining the known unknowns of the E. coli metabolic network. PLoS ONE 2013, 8, e75210. [Google Scholar] [CrossRef]
- Vlassis, N.; Pacheco, M.P.; Sauter, T. Fast reconstruction of compact context-specific metabolic network models. PLoS Comput. Biol. 2014, 10, e1003424. [Google Scholar] [CrossRef]
- Gudmundsson, S.; Thiele, I. Computationally efficient flux variability analysis. BMC Bioinform. 2010, 11, 489. [Google Scholar] [CrossRef]
- Lieven, C.; Beber, M.E.; Olivier, B.G.; Bergmann, F.T.; Ataman, M.; Babaei, P.; Bartell, J.A.; Blank, L.M.; Chauhan, S.; Correia, K.; et al. MEMOTE for standardized genome-scale metabolic model testing. Nat. Biotechnol. 2020, 38, 272–276. [Google Scholar]
- Bartha, I.; Di Iulio, J.; Venter, J.C.; Telenti, A. Human gene essentiality. Nat. Rev. Genet. 2018, 19, 51–62. [Google Scholar]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar]
- Selvarasu, S.; Karimi, I.A.; Ghim, G.H.; Lee, D.Y. Genome-scale modeling and in silico analysis of mouse cell metabolic network. Mol. Biosyst. 2009, 6, 152–161. [Google Scholar] [CrossRef]
- Thirumurugan, D.; Cholarajan, A.; Raja, S.; Vijayakumar, R. An introductory chapter: Secondary metabolites. Second. Metab. Sources Appl. 2018, 1, 3–21. [Google Scholar]
- Kim, H.U.; Kim, S.Y.; Jeong, H.; Kim, T.Y.; Kim, J.J.; Choy, H.E.; Yi, K.Y.; Rhee, J.H.; Lee, S.Y. Integrative genome-scale metabolic analysis of Vibrio vulnificus for drug targeting and discovery. Mol. Syst. Biol. 2011, 7, 460. [Google Scholar]
- Kim, P.J.; Lee, D.Y.; Kim, T.Y.; Lee, K.H.; Jeong, H.; Lee, S.Y.; Park, S. Metabolite essentiality elucidates robustness of Escherichia coli metabolism. Proc. Natl. Acad. Sci. USA 2007, 104, 13638–13642. [Google Scholar]
- Kim, E.Y.; Ashlock, D.; Yoon, S.H. Identification of critical connectors in the directed reaction-centric graphs of microbial metabolic networks. BMC Bioinform. 2019, 20, 328. [Google Scholar] [CrossRef]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef]
- Cornacchia, D.; Zhang, C.; Zimmer, B.; Chung, S.Y.; Fan, Y.; Soliman, M.A.; Tchieu, J.; Chambers, S.M.; Shah, H.; Paull, D.; et al. Lipid deprivation induces a stable, naive-to-primed intermediate state of pluripotency in human PSCs. Cell Stem Cell 2019, 25, 120–136. [Google Scholar] [CrossRef]
- Yi, M.; Li, J.; Chen, S.; Cai, J.; Ban, Y.; Peng, Q.; Zhou, Y.; Zeng, Z.; Peng, S.; Li, X.; et al. Emerging role of lipid metabolism alterations in Cancer stem cells. J. Exp. Clin. Cancer Res. 2018, 37, 118. [Google Scholar]
- Abu-Dawud, R.; Graffmann, N.; Ferber, S.; Wruck, W.; Adjaye, J. Pluripotent stem cells: Induction and self-renewal. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170213. [Google Scholar]
- Horikoshi, Y.; Yan, Y.; Terashvili, M.; Wells, C.; Horikoshi, H.; Fujita, S.; Bosnjak, Z.J.; Bai, X. Fatty acid-treated induced pluripotent stem cell-derived human cardiomyocytes exhibit adult cardiomyocyte-like energy metabolism phenotypes. Cells 2019, 8, 1095. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.; Wang, L.; Cai, Y.; Zhong, X.; He, X.; Hu, L.; Tian, S.; Wu, M.; Hui, L.; et al. Fatty acid synthesis is critical for stem cell pluripotency via promoting mitochondrial fission. EMBO J. 2017, 36, 1330–1347. [Google Scholar] [CrossRef]
- Tanosaki, S.; Tohyama, S.; Fujita, J.; Someya, S.; Hishiki, T.; Matsuura, T.; Nakanishi, H.; Ohto-Nakanishi, T.; Akiyama, T.; Morita, Y.; et al. Fatty acid synthesis is indispensable for survival of human pluripotent stem cells. iScience 2020, 23, 101535. [Google Scholar] [CrossRef]
- Diamante, L.; Martello, G. Metabolic regulation in pluripotent stem cells. Curr. Opin. Genet. Dev. 2022, 75, 101923. [Google Scholar] [CrossRef]
- Hosseini, V.; Kalantary-Charvadeh, A.; Hajikarami, M.; Fayyazpour, P.; Rahbarghazi, R.; Totonchi, M.; Darabi, M. A small molecule modulating monounsaturated fatty acids and Wnt signaling confers maintenance to induced pluripotent stem cells against endodermal differentiation. Stem Cell Res. Ther. 2021, 12, 550. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, Y.; Zhong, L.; Shi, B.; Liang, H.; Han, J. Slc25a36 modulates pluripotency of mouse embryonic stem cells by regulating mitochondrial function and glutathione level. Biochem. J. 2019, 476, 1585–1604. [Google Scholar] [CrossRef]
- Ivanova, J.S.; Lyublinskaya, O.G. Redox homeostasis and regulation in pluripotent stem cells: Uniqueness or versatility? Int. J. Mol. Sci. 2021, 22, 10946. [Google Scholar] [CrossRef]
- Gray, L.R.; Tompkins, S.C.; Taylor, E.B. Regulation of pyruvate metabolism and human disease. Cell. Mol. Life Sci. 2014, 71, 2577–2604. [Google Scholar] [CrossRef]
- Odenwelder, D.C.; Lu, X.; Harcum, S.W. Induced pluripotent stem cells can utilize lactate as a metabolic substrate to support proliferation. Biotechnol. Prog. 2021, 37, e3090. [Google Scholar] [CrossRef]
- Cluntun, A.A.; Rutter, J. The secret life of lactate: A novel cell-cycle regulatory mechanism. Cell Metab. 2023, 35, 730–732. [Google Scholar] [CrossRef]
- Varum, S.; Rodrigues, A.S.; Moura, M.B.; Momcilovic, O.; Easley IV, C.A.; Ramalho-Santos, J.; Van Houten, B.; Schatten, G. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS ONE 2011, 6, e20914. [Google Scholar] [CrossRef]
- Traut, T.W. Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 1994, 140, 1–22. [Google Scholar] [CrossRef]
- Emwas, A.H.; Szczepski, K.; Al-Younis, I.; Lachowicz, J.I.; Jaremko, M. Fluxomics-new metabolomics approaches to monitor metabolic pathways. Front. Pharmacol. 2022, 13, 805782. [Google Scholar] [CrossRef]
- Beltrán, B.; Mathur, A.; Duchen, M.R.; Erusalimsky, J.D.; Moncada, S. The effect of nitric oxide on cell respiration: A key to understanding its role in cell survival or death. Proc. Natl. Acad. Sci. USA 2000, 97, 14602–14607. [Google Scholar] [CrossRef]
- Armstrong, L.; Tilgner, K.; Saretzki, G.; Atkinson, S.P.; Stojkovic, M.; Moreno, R.; Przyborski, S.; Lako, M. Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem Cells 2010, 28, 661–673. [Google Scholar] [CrossRef]
- Sasaki, K.; Inoue, M.; Machida, M.; Kawasaki, T.; Tsuruta, S.; Uchida, H.; Sakamoto, S.; Kasahara, M.; Umezawa, A.; Akutsu, H. Human pluripotent stem cell-derived organoids as a model of intestinal xenobiotic metabolism. StemJournal 2021, 3, 1–10. [Google Scholar] [CrossRef]
- Carcamo-Orive, I.; Hoffman, G.E.; Cundiff, P.; Beckmann, N.D.; D’Souza, S.L.; Knowles, J.W.; Patel, A.; Hendry, C.; Papatsenko, D.; Abbasi, F.; et al. Analysis of transcriptional variability in a large human iPSC library reveals genetic and non-genetic determinants of heterogeneity. Cell Stem Cell 2017, 20, 518–532. [Google Scholar] [CrossRef]
- Kuriya, Y.; Araki, M. Dynamic flux balance analysis to evaluate the strain production performance on shikimic acid production in Escherichia coli. Metabolites 2020, 10, 198. [Google Scholar] [CrossRef]
- Jamshidi, N.; Palsson, B.Ø. Formulating genome-scale kinetic models in the post-genome era. Mol. Syst. Biol. 2008, 4, 171. [Google Scholar] [CrossRef]



| GEO ID | Authors | Description | Series |
|---|---|---|---|
| GSE14711 | Soldner, F. et al. [46] | hiPSCs were generated by reprogramming fibroblasts derived from 5 Parkinson’s disease patients. | GSM367219 GSM367240 GSM367241 GSM367242 GSM367243 GSM367244 GSM367245 GSM367258 |
| GSE9865 | Lowry, W. E. et al. [47] | hiPSC were generated through the reprogramming of dermal fibroblasts using various methodologies. | GSM249028 GSM249095 GSM249096 GSM249137 |
| Pathway | Essential Metabolites List |
|---|---|
| Fatty Acid Elongation In Mitochondria | NADP, Palmitic acid, NADPH, NAD, Octanoyl-CoA, Acetyl-CoA, Coenzyme A, NADH, Water, (2E)-Dodecenoyl-CoA, (2E)-Decenoyl-CoA, Decanoyl-CoA (n-C10:0CoA), Hydrogen Ion |
| Fatty Acid Metabolism | Adenosine monophosphate, L-Carnitine, Palmitic acid, Pyrophosphate, Adenosine triphosphate, NAD, Octanoyl-CoA, Acetyl-CoA, Coenzyme A, NADH, Carbon dioxide, Water, (2E)-Decenoyl-CoA, Decanoyl-CoA (n-C10:0CoA), Hydrogen Ion |
| Alpha Linolenic Acid and Linoleic Acid Metabolism | Linoleic acid, Arachidonic acid, Alpha-Linolenic acid, Eicosapentaenoic acid, Cis-(8,11,14,17)-Eicosatetraenoic acid, Docosahexaenoic acid, Adrenic acid, (8,11,14)-Eicosatrienoic acid, Gamma-Linolenic acid, Docosapentaenoic acid, Stearidonic acid |
| Transfer of Acetyl Groups into Mitochondria | NADP, NADPH, Adenosine triphosphate, NAD, Acetyl-CoA, ADP, Coenzyme A, NADH, Carbon dioxide, Water, Hydrogen Ion |
| Glycerolipid Metabolism | Glycerol 3-phosphate, NADP, Palmitic acid, NADPH, Adenosine triphosphate, NAD, ADP, Coenzyme A, NADH, Water |
| Plasmalogen Synthesis | Cytidine monophosphate, NADP, NADPH, Stearic acid, NAD, Stearoyl-CoA, Oxygen, Citicoline, Coenzyme A, NADH, Water, Hydrogen peroxide |
| Glucose-Alanine Cycle | L-Glutamic acid, L-Alanine, NADP, NADPH, NAD, NADH, Water, Hydrogen Ion |
| Mitochondrial Beta-Oxidation of Short Chain Saturated Fatty Acids | Adenosine monophosphate, L-Carnitine, Pyrophosphate, Adenosine triphosphate, NAD, Octanoyl-CoA, Acetyl-CoA, Coenzyme A, NADH, Water, Hydrogen Ion |
| Urea Cycle | Adenosine monophosphate, L-Glutamic acid, L-Alanine, L-Aspartic acid, Pyrophosphate, L-Arginine, Adenosine triphosphate, L-Glutamine, NAD, ADP, NADH, Carbon dioxide, Water |
| Pyruvate Metabolism | Adenosine monophosphate, NADP, NADPH, Pyrophosphate, Adenosine triphosphate, NAD, Malonyl-CoA, Acetyl-CoA, Guanosine triphosphate, ADP, Coenzyme A, NADH, Carbon dioxide, Water, Hydrogen Ion |
| Glutamate Metabolism | Adenosine monophosphate, Glycine, L-Glutamic acid, L-Alanine, L-Aspartic acid, NADP, NADPH, Pyrophosphate, Adenosine triphosphate, L-Cysteine, L-Glutamine, NAD, Acetyl-CoA, ADP, Coenzyme A, NADH, Carbon dioxide, Water, Hydrogen Ion |
| Ammonia Recycling | Adenosine monophosphate, Glycine, L-Glutamic acid, L-Asparagine, L-Histidine, L-Serine, L-Aspartic acid, Pyrophosphate, Adenosine triphosphate, L-Glutamine, NAD, ADP, NADH, Carbon dioxide, Water |
| Glycine and Serine Metabolism | Adenosine monophosphate, Glycine, L-Glutamic acid, L-Alanine, L-Threonine, L-Serine, Pyrophosphate, L-Arginine, Adenosine triphosphate, L-Cysteine, L-Methionine, NAD, Acetyl-CoA, ADP, Oxygen, Coenzyme A, NADH, Carbon dioxide, Water, Hydrogen peroxide |
| Pyrimidine Metabolism | Deoxycytidine, Cytidine triphosphate, Cytidine monophosphate, NADP, NADPH, Pyrophosphate, Uridine triphosphate, Uridine -monophosphate, Uridine -diphosphate, Adenosine triphosphate, L-Glutamine, dCTP, dCMP, dCDP, dTDP, ADP, Thymidine -triphosphate, Carbon dioxide, Water |
| Glutathione Metabolism | Glycine, L-Glutamic acid, L-Alanine, NADP, NADPH, Adenosine triphosphate, L-Cysteine, ADP, Water, Hydrogen peroxide |
| Pantothenate and CoA Biosynthesis | Adenosine monophosphate, Cytidine triphosphate, Cytidine monophosphate, Pyrophosphate, Adenosine triphosphate, L-Cysteine, ADP, Coenzyme A, Carbon dioxide, Water |
| Phytanic Acid Peroxisomal Oxidation | NADP, NADPH, Pyrophosphate, Adenosine triphosphate, NAD, Acetyl-CoA, ADP, Oxygen, Coenzyme A, NADH, Carbon dioxide, Water |
| Mitochondrial Beta-Oxidation of Medium Chain Saturated Fatty Acids | Adenosine monophosphate, Pyrophosphate, Adenosine triphosphate, Dodecanoic acid, NAD, Octanoyl-CoA, Acetyl-CoA, Coenzyme A, NADH, Water, (2E)-Dodecenoyl-CoA, (2E)-Decenoyl-CoA, Decanoyl-CoA (n-C10:0CoA), Hydrogen Ion |
| Cardiolipin Biosynthesis | Cytidine triphosphate, Cytidine monophosphate, Glycerol 3-phosphate, Pyrophosphate, NAD, Coenzyme A, NADH, Water, Hydrogen Ion |
| Mitochondrial Beta-Oxidation of Long Chain Saturated Fatty Acids | Adenosine monophosphate, L-Carnitine, Pyrophosphate, Adenosine triphosphate, Stearic acid, NAD, Stearoyl-CoA, Acetyl-CoA, Coenzyme A, NADH, Water, Hydrogen Ion |
| Ethanol Degradation | Adenosine monophosphate, NADP, NADPH, Pyrophosphate, Adenosine triphosphate, NAD, Acetyl-CoA, Oxygen, Coenzyme A, NADH, Water, Hydrogen peroxide, Hydrogen Ion |
| Arginine and Proline Metabolism | Adenosine monophosphate, Glycine, L-Glutamic acid, L-Proline, L-Aspartic acid, NADP, NADPH, Pyrophosphate, L-Arginine, Adenosine triphosphate, NAD, ADP, Oxygen, NADH, Carbon dioxide, Water, Hydrogen peroxide, Hydrogen Ion |
| Nicotinate and Nicotinamide Metabolism | Adenosine monophosphate, L-Glutamic acid, NADP, NADPH, Pyrophosphate, Adenosine triphosphate, L-Glutamine, NAD, ADP, Oxygen, NADH, Carbon dioxide, Water, Hydrogen peroxide, Hydrogen Ion |
| Beta-Alanine Metabolism | L-Glutamic acid, L-Histidine, L-Aspartic acid, NADP, NADPH, NAD, Acetyl-CoA, Oxygen, Coenzyme A, NADH, Carbon dioxide, Water, Hydrogen peroxide, Hydrogen Ion |
| Purine Metabolism | Adenosine monophosphate, Glycine, L-Glutamic acid, L-Aspartic acid, NADP, NADPH, Pyrophosphate, Adenosine triphosphate, L-Glutamine, NAD, Guanosine triphosphate, ADP, Oxygen, dGTP, NADH, dADP, Deoxyadenosine triphosphate, Carbon dioxide, Water, Hydrogen peroxide |
| Histidine Metabolism | Adenosine monophosphate, L-Glutamic acid, L-Histidine, NADP, NADPH, Pyrophosphate, Adenosine triphosphate, NAD, ADP, Oxygen, NADH, Carbon dioxide, Water, Hydrogen peroxide, Hydrogen Ion |
| Phosphatidylethanolamine Biosynthesis | Cytidine triphosphate, Cytidine monophosphate, L-Serine, Pyrophosphate, Adenosine triphosphate, ADP, Carbon dioxide, Hydrogen Ion |
| Aspartate Metabolism | Adenosine monophosphate, L-Glutamic acid, L-Asparagine, L-Aspartic acid, Pyrophosphate, L-Arginine, Adenosine triphosphate, L-Glutamine, Guanosine triphosphate, Oxygen, Carbon dioxide, Water, Hydrogen peroxide |
| Lysine Degradation | L-Glutamic acid, L-Lysine, NADP, NADPH, NAD, Acetyl-CoA, Oxygen, Coenzyme A, NADH, Carbon dioxide, Water, Hydrogen peroxide |
| Propanoate Metabolism | Adenosine monophosphate, L-Glutamic acid, Pyrophosphate, Adenosine triphosphate, L-Valine, NAD, Malonyl-CoA, Acetyl-CoA, ADP, Coenzyme A, NADH, Carbon dioxide, Water, Hydrogen Ion |
| Phosphatidylcholine Biosynthesis | Cytidine triphosphate, Cytidine monophosphate, Pyrophosphate, Adenosine triphosphate, ADP, Phosphorylcholine, Carbon dioxide, Hydrogen Ion |
| Nucleotide Sugars Metabolism | Pyrophosphate, Uridine triphosphate, Adenosine triphosphate, NAD, ADP, Glucose 6-phosphate, NADH, Carbon dioxide, Water |
| Methionine Metabolism | Adenosine monophosphate, Glycine, L-Serine, Pyrophosphate, Adenosine triphosphate, L-Cysteine, L-Methionine, NAD, Oxygen, NADH, Carbon dioxide, Water, Hydrogen peroxide |
| Cysteine Metabolism | Adenosine monophosphate, L-Glutamic acid, Pyrophosphate, Adenosine triphosphate, L-Cysteine, NAD, ADP, Oxygen, NADH, Water |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Procopio, A.; Parrotta, E.I.; Scalise, S.; Zaffino, P.; Granata, R.; Amato, F.; Cuda, G.; Cosentino, C. hiPSCGEM01: A Genome-Scale Metabolic Model for Fibroblast-Derived Human iPSCs. Bioengineering 2025, 12, 1128. https://doi.org/10.3390/bioengineering12101128
Procopio A, Parrotta EI, Scalise S, Zaffino P, Granata R, Amato F, Cuda G, Cosentino C. hiPSCGEM01: A Genome-Scale Metabolic Model for Fibroblast-Derived Human iPSCs. Bioengineering. 2025; 12(10):1128. https://doi.org/10.3390/bioengineering12101128
Chicago/Turabian StyleProcopio, Anna, Elvira Immacolata Parrotta, Stefania Scalise, Paolo Zaffino, Rita Granata, Francesco Amato, Giovanni Cuda, and Carlo Cosentino. 2025. "hiPSCGEM01: A Genome-Scale Metabolic Model for Fibroblast-Derived Human iPSCs" Bioengineering 12, no. 10: 1128. https://doi.org/10.3390/bioengineering12101128
APA StyleProcopio, A., Parrotta, E. I., Scalise, S., Zaffino, P., Granata, R., Amato, F., Cuda, G., & Cosentino, C. (2025). hiPSCGEM01: A Genome-Scale Metabolic Model for Fibroblast-Derived Human iPSCs. Bioengineering, 12(10), 1128. https://doi.org/10.3390/bioengineering12101128












