VNTR Polymorphisms in the SLC6A3 Gene and Their Impact on Time Perception and EEG Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Genotyping
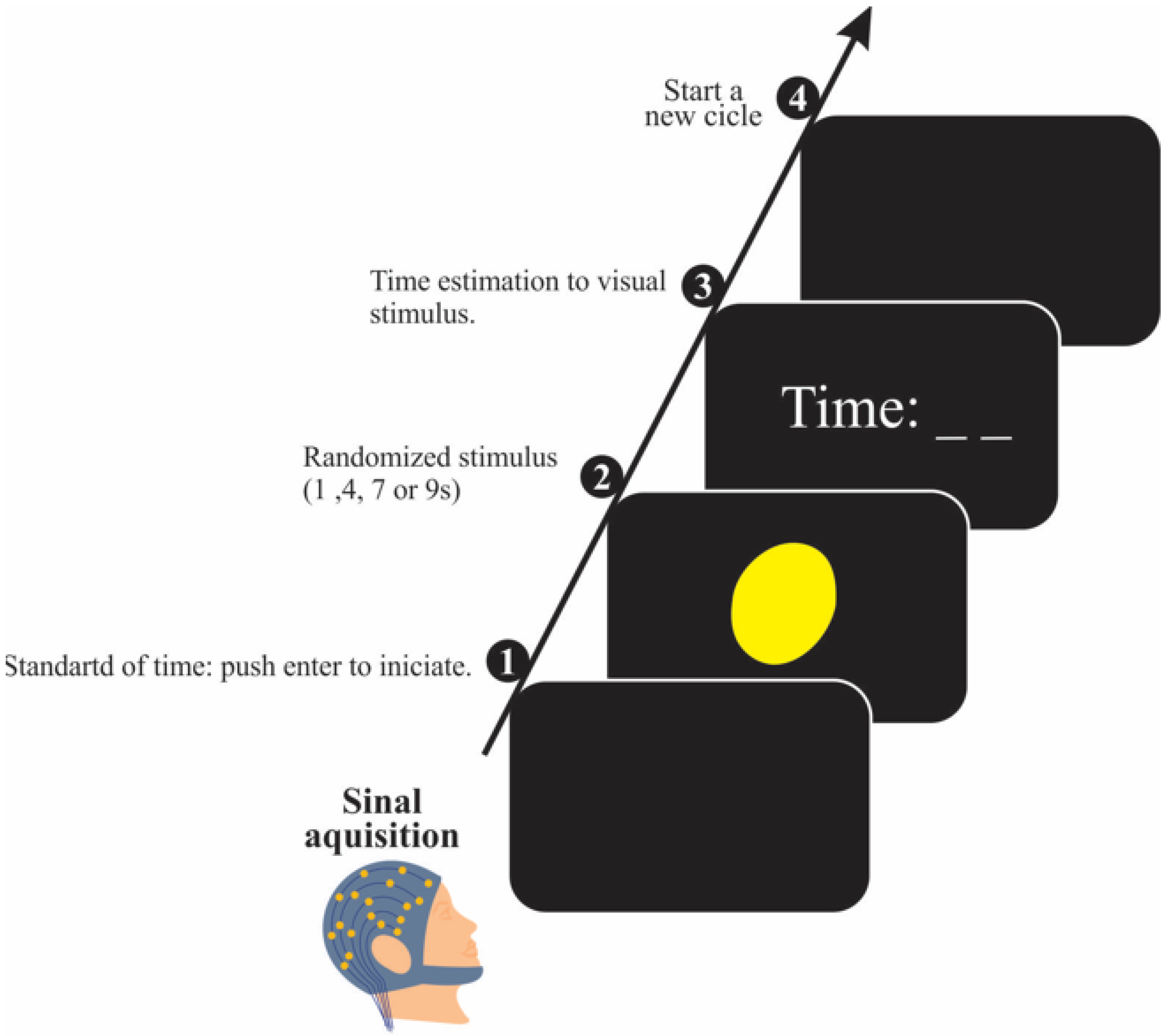
2.3. Time Perception Task
2.4. EEG Recording
2.5. Behavioral Data
2.6. Genetic Data
2.7. EEG Data Processing
2.8. Statistical Analysis
3. Results
3.1. Allele Frequency
3.2. Time Estimation Variables
3.3. EEG Alpha Power During Time Estimation
4. Discussion
4.1. Time Estimation Variables
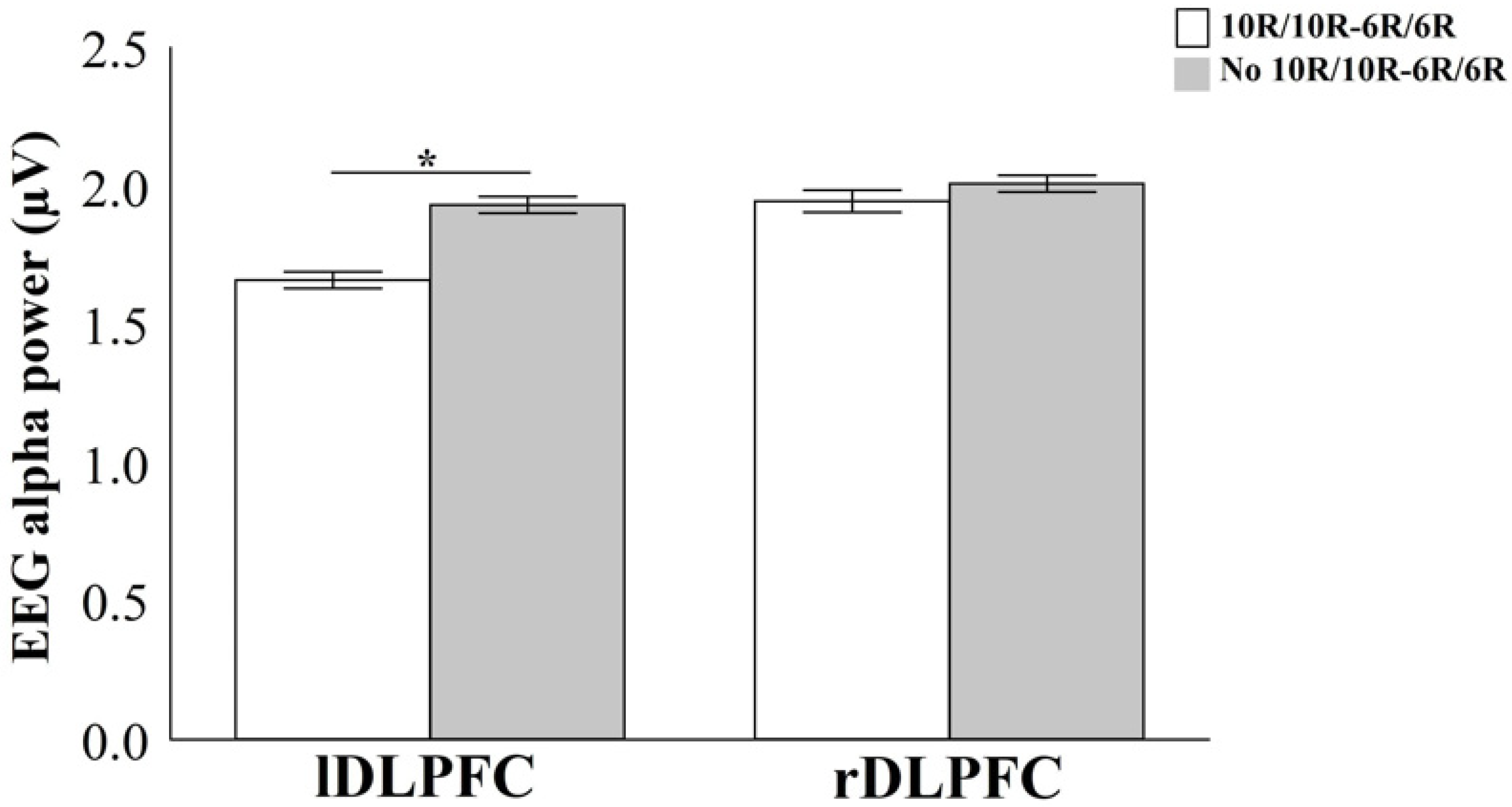
4.2. EEG Alpha Power During Time Estimation
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matthews, W.J.; Meck, W.H. Time perception: The bad news and the good. Wiley Interdiscip. Rev. Cogn. Sci. 2014, 5, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Fontes, R.; Ribeiro, J.; Gupta, D.S.; Machado, D.; Lopes-Júnior, F.; Magalhães, F.; Bastos, V.H.; Rocha, K.; Marinho, V.; Lima, G.; et al. Time Perception Mechanisms at Central Nervous System. Neurol. Int. 2016, 8, 5939. [Google Scholar] [CrossRef]
- Teixeira, S.; Magalhães, F.; Marinho, V.; Velasques, B.; Ribeiro, P. Proposal for Using Time Estimation Training for the Treatment of Parkinson’s Disease. Med. Hypotheses. 2016, 95, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Marinho, V.; Oliveira, T.; Rocha, K.; Ribeiro, J.; Magalhães, F.; Bento, T.; Pinto, G.R.; Velasques, B.; Ribeiro, P.; Di Giorgio, L.; et al. The dopaminergic system dynamic in the time perception: A review of the evidence. Int. J. Neurosci. 2018, 128, 262–282. [Google Scholar] [CrossRef]
- Azari, L.; Mioni, G.; Rousseau, R.; Grondin, S. An analysis of the processing of intramodal and intermodal time intervals. Atten. Percept. Psychophys. 2019, 82, 1473–1487. [Google Scholar] [CrossRef]
- Block, R.A.; Grondin, S. Timing and time perception: A selective review and commentary on recent reviews. Front. Psychol. 2014, 5, 648. [Google Scholar] [CrossRef]
- Allman, M.J.; Meck, W.H. Pathophysiological distortions in time perception and timed performance. Pathophysiological distortions in time perception. Brain 2012, 135, 656–677. [Google Scholar] [CrossRef]
- Merchant, H.; Harrington, D.L.; Meck, W.H. Neural Basis of the Perception and Estimation of Time. Annu. Rev. Neurosci. 2013, 36, 313–336. [Google Scholar] [CrossRef]
- Zhang, L.; Ptáček, L.J.; Fu, Y.H. Diversity of human clock genotypes and consequences. Prog. Mol. Biol. Transl. Sci. 2013, 119, 51–81. [Google Scholar] [PubMed]
- Wittmann, M. The inner sense of time: How the brain creates a representation of duration. Nat. Rev. 2013, 14, 217. [Google Scholar] [CrossRef]
- Bussi, I.L.; Levín, G.; Golombek, D.A.; Agostino, P.V. Involvement of dopamine signaling in the circadian modulation of interval timing. Eur. J. Neurosci. 2014, 40, 2299–2310. [Google Scholar] [CrossRef] [PubMed]
- Cirulli, E.T.; Kasperaviciūte, D.; Attix, D.K.; Need, A.C.; Ge, D.; Gibson, G.; Goldstein, D.B. Common genetic variation and performance on standardized cognitive tests. Eur. J. Hum. Genet. 2010, 18, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Meck, W.H. Neuropharmacology of timing and time perception. Brain Res. Cogn. Brain Res. 1996, 3, 227–242. [Google Scholar] [CrossRef]
- Green, A.E.; Munafò, M.R.; DeYoung, C.G.; Fossella, J.A.; Fan, J.; Gray, J.R. Using genetic data in cognitive neuroscience: From growing pains to genuine insights. Nat. Rev. Neurosci. 2008, 9, 710–720. [Google Scholar] [CrossRef]
- Meck, W.H.; Cheng, R.K.; MacDonald, C.J.; Gainetdinov, R.R.; Caron, M.G.; Cevik, M.Ö. Gene-dose dependent effects of methamphetamine on interval timing in dopamine-transporter knockout mice. Neuropharmacology 2012, 62, 1221–1229. [Google Scholar] [CrossRef]
- Bartholomew, A.J.; Meck, W.H.; Cirulli, E.T. Analysis of Genetic and Non-Genetic Factors Influencing Timing and Time Perception. PLoS ONE 2015, 10, e0143873. [Google Scholar] [CrossRef] [PubMed]
- Marinho, F.V.C.; Pinto, G.R.; Oliveira, T.; Gomes, A.; Lima, V.; Ferreira-Fernandes, H.; Rocha, K.; Magalhães, F.; Velasques, B.; Ribeiro, P.; et al. The SLC6A3 3’-UTR VNTR and intron 8 VNTR polymorphisms association in the time estimation. Brain Struct. Funct. 2019, 224, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Coull, J.T.; Hwang, H.J.; Leyton, M.; Dagher, A. Dopamine precursor depletion impairs timing in healthy volunteers by attenuating activity in putamen and supplementary motor area. J. Neurosci. 2012, 32, 16704–16715. [Google Scholar] [CrossRef]
- Wiener, M.; Lee, Y.S.; Lohoff, F.W.; Coslett, H.B. Individual differences in the morphometry and activation of time perception networks are influenced by dopamine genotype. Neuroimage 2014, 89, 10–22. [Google Scholar] [CrossRef]
- Marinho, V.; Oliveira, T.; Bandeira, J.; Pinto, G.R.; Gomes, A.; Lima, V.; Magalhães, F.; Rocha, K.; Ayres, C.; Carvalho, V.; et al. Genetic influence alters the brain synchronism in perception and timing. J. Biomed. Sci. 2018, 25, 61. [Google Scholar] [CrossRef]
- Tong, J.H.; Cummins, T.D.; Johnson, B.P.; McKinley, L.A.; Pickering, H.E.; Fanning, P.; Stefanac, N.R.; Newman, D.P.; Hawi, Z.; Bellgrove, M.A. An association between a dopamine transporter gene (SLC6A3) haplotype and ADHD symptom measures in nonclinical adults. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2015, 168B, 89–96. [Google Scholar] [CrossRef]
- Rommelse, N.N.; Arias-Vásquez, A.; Altink, M.E.; Buschgens, C.J.; Fliers, E.; Asherson, P.; Faraone, S.V.; Buitelaar, J.K.; Sergeant, J.A.; Oosterlaan, J.; et al. Neuropsychological endophenotype approach to genome-wide linkage analysis identifies susceptibility loci for ADHD on 2q21.1 and 13q12.11. Am. J. Hum. Genet. 2008, 83, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Ebstein, R.; Tse, C.Y.; Monakhov, M.; Lai, P.S.; Dinges, D.F.; Kwok, K. Dopaminergic polymorphisms associated with time-on-task declines and fatigue in the Psychomotor Vigilance Test. PLoS ONE 2012, 7, e33767. [Google Scholar] [CrossRef]
- Zilles, D.; Meyer, J.; Schneider-Axmann, T.; Ekawardhani, S.; Gruber, E.; Falkai, P.; Gruber, O. Genetic polymorphisms of 5-HTT and DAT but not COMT differentially affect verbal and visuospatial working memory functioning. Eur. Arch. Psychiatry Clin. Neurosci. 2012, 262, 667–676. [Google Scholar] [CrossRef]
- Katz, A.C.; Sarapas, C.; Bishop, J.R.; Patel, S.R.; Shankman, S.A. The mediating effect of prefrontal asymmetry on the relationship between the COMT Val(158)Met SNP and trait consummatory positive affect. Cogn. Emot. 2015, 29, 867–881. [Google Scholar] [CrossRef]
- Balcı, F.; Wiener, M.; Cavdaroğlu, B.; Branch Coslett, H. Epistasis effects of dopamine genes on interval timing and reward magnitude in humans. Neuropsychologia 2013, 51, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Fallgatter, A.J.; Herrmann, M.J.; Roemmler, J.; Ehlis, A.C.; Wagener, A.; Heidrich, A.; Ortega, G.; Zeng, Y.; Lesch, K.P. Allelic variation of serotonin transporter function modulates the brain electrical response for error processing. Neuropsychopharmacology 2004, 29, 1506–1511. [Google Scholar] [CrossRef]
- Schirmer, A.; Meck, W.H.; Penney, T.B. The Socio-Temporal Brain: Connecting People in Time. Trends Cogn. Sci. 2016, 20, 760–772. [Google Scholar] [CrossRef]
- Marinho, V.; Pinto, G.R.; Figueiredo, R.; Ayres, C.; Bandeira, J.; Teixeira, S. The BDNF Val66Met Polymorphism Promotes Changes in the Neuronal Integrity and Alters the Time Perception. J. Mol. Neurosci. 2019, 67, 82–88. [Google Scholar] [CrossRef]
- Davidson, R.J. What does the prefrontal cortex “do” in affect: Perspectives on frontal EEG asymmetry research. Biol. Psychol. 2004, 67, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Tomer, R.; Goldstein, R.Z.; Wang, G.J.; Wong, C.; Volkow, N.D. Incentive motivation is associated with striatal dopamine asymmetry. Biol. Psychol. 2008, 77, 98–101. [Google Scholar] [CrossRef]
- Laakso, A.; Vilkman, H.; Alakare, B.; Haaparanta, M.; Bergman, J.; Solin, O.; Peurasaari, J.; Räkköläinen, V.; Syvälahti, E.; Hietala, J. Striatal dopamine transporter binding in neuroleptic-naive patients with schizophrenia studied with positron emission tomography. Am. J. Psychiatry. 2000, 157, 269–271. [Google Scholar] [CrossRef]
- Bender, S.; Rellum, T.; Freitag, C.; Resch, F.; Rietschel, M.; Treutlein, J.; Jennen-Steinmetz, C.; Brandeis, D.; Banaschewski, T.; Laucht, M. Dopamine inactivation efficacy related to functional DAT1 and COMT variants influences motor response evaluation. PLoS ONE 2012, 7, e37814. [Google Scholar] [CrossRef]
- Lustig, C.; Meck, W.H. Modality differences in timing and temporal memory throughout the lifespan. Brain Cogn. 2011, 77, 298–303. [Google Scholar] [CrossRef]
- van Rijn, H.; Gu, B.M.; Meck, W.H. Dedicated clock/timing-circuit theories of time perception and timed performance. Adv. Exp. Med. Biol. 2014, 829, 75–99. [Google Scholar]
- Snyder, A.C.; Foxe, J.J. Anticipatory attentional suppression of visual features indexed by oscillatory alpha-band power increases: A high-density electrical mapping study. J. Neurosci. 2010, 30, 4024–4032. [Google Scholar] [CrossRef] [PubMed]
- Nenert, R.; Viswanathan, S.; Dubuc, D.M.; Visscher, K.M. Modulations of ongoing alpha oscillations predict successful short-term visual memory encoding. Front. Hum. Neurosci. 2012, 6, 127. [Google Scholar] [CrossRef]
- Horr, N.K.; Wimber, M.; Di Luca, M. Perceived time and temporal structure: Neural entrainment to isochronous stimulation increases duration estimates. Neuroimage 2016, 132, 148–156. [Google Scholar] [CrossRef]
- Farias, T.L.; Marinho, V.; Carvalho, V.; Rocha, K.; da Silva, P.R.A.; Silva, F.; Teles, A.S.; Gupta, D.; Ribeiro, P.; Velasques, B.; et al. Methylphenidate modifies activity in the prefrontal and parietal cortex accelerating the time judgment. Neurol. Sci. 2019, 40, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Jozefowiez, J.; Polack, C.W.; Machado, A.; Miller, R.R. Trial Frequency Effects in Human Temporal Bisection: Implications for Theories of Timing. Behav. Processes. 2014, 101, 81–88. [Google Scholar] [CrossRef]
- Brown, S.W. Time perception and attention: The effects of prospective versus retrospective paradigms and task demands on perceived duration. Percept. Psychophys. 1985, 38, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Mioni, G.; Stablum, F.; McClintock, S.M.; Grondin, S. Different methods for reproducing time, different results. Atten. Percept. Psychophys. 2014, 76, 675–681. [Google Scholar] [CrossRef]
- Reed, G.F.; Lynn, F.; Meade, B.D. Use of coefficient of variation in assessing variability of quantitative assays. Clin. Diagn. Lab. Immunol. 2002, 9, 1235–1239. [Google Scholar] [CrossRef]
- Vasconcelos, A.C.; Neto Ede, S.; Pinto, G.R.; Yoshioka, F.K.; Motta, F.J.; Vasconcelos, D.F.; Canalle, R. Association study of the SLC6A3 VNTR (DAT) and DRD2/ANKK1 Taq1A polymorphisms with alcohol dependence in a population from northeastern Brazil. Alcohol. Clin. Exp. Res. 2015, 39, 205–211. [Google Scholar] [CrossRef]
- Jung, T.P.; Makeig, S.; Humphries, C.; Lee, T.W.; Mckeown, M.J.; Iragui, V.; Sejnowski, T.J. Removing electroencephalographic artifacts by blind source separation. Psychophysiology 2000, 37, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Martins ESilva, D.C.; Marinho, V.; Teixeira, S.; Teles, G.; Marques, J.; Escórcio, A.; Fernandes, T.; Freitas, A.C.; Nunes, M.; Ayres, M.; et al. Non-immersive 3D virtual stimulus alter the time production task performance and increase the EEG theta power in dorsolateral prefrontal cortex. Int. J. Neurosci. 2020, 179, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Neuper, C.; Pfurtscheller, G. Event-related dynamics of cortical rhythms: Frequency-specific features and functional correlates. Int. J. Psychophysiol. 2001, 43, 41–58. [Google Scholar] [CrossRef]
- Rocha, K.; Marinho, V.; Magalhães, F.; Ribeiro, J.; Oliveira, T.; Gupta, D.S.; Chaves, F.; Velasques, B.; Ribeiro, P.; Cagy, M.; et al. Low-frequency rTMS stimulation over superior parietal cortex medially improves time reproduction and increases the right dorsolateral prefrontal cortex predominance. Int. J. Neurosci. 2019, 129, 523–533. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge Academic: New York, NY, USA, 1998. [Google Scholar]
- Addyman, C.; Rocha, S.; Mareschal, D. Mapping the Origins of Time: Scalar Errors in Infant Time Estimation. Dev. Psychol. 2014, 30, a0037108. [Google Scholar] [CrossRef]
- Hancock, P.A.; Rausch, R. The effects of sex, age, and interval duration on the perception of time. Acta Psychol. 2010, 133, 170–179. [Google Scholar] [CrossRef]
- Finnerty, G.T.; Shadlen, M.N.; Jazayeri, M.; Nobre, A.C.; Buonomano, D.V. Time in Cortical Circuits. J. Neurosci. 2015, 35, 13912–13916. [Google Scholar] [CrossRef]
- Buhusi, C.V.; Meck, W.H. What makes us tick? Functional and neural mechanisms of interval timing. Nat. Rev. Neurosci. 2005, 6, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Matell, M.S.; Meck, W.H. Cortico-striatal circuits and interval timing: Coincidence detection of oscillatory processes. Brain Res. Cogn. Brain Res. 2004, 21, 139–170. [Google Scholar] [CrossRef]
- Gu, B.M.; Cheng, R.K.; Yin, B.; Meck, W.H. Quinpirole-induced sensitization to noisy/sparse periodic input: Temporal synchronization as a component of obsessive-compulsive disorder. Neuroscience 2011, 179, 143–150. [Google Scholar] [CrossRef]
- Coull, J.T.; Cheng, R.K.; Meck, W.H. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology 2011, 36, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Kononowicz, T.W. Dopamine-dependent oscillations in frontal cortex index “start-gun” signal in interval timing. Front. Hum. Neurosci. 2015, 9, 331. [Google Scholar] [CrossRef] [PubMed]
- Harrington, D.L.; Jahanshahi, M. Reconfiguration of striatal connectivity for timing and action. Curr. Opin. Behav. Sci. 2016, 8, 78–84. [Google Scholar] [CrossRef]
- Sysoeva, O.V.; Tonevitsky, A.G.; Wackermann, J. Genetic determinants of time perception mediated by the serotonergic system. PLoS ONE 2010, 5, e12650. [Google Scholar] [CrossRef]
- Droit-Volet, S.; Meck, W.H. How emotions colour our perception of time. Trends Cogn. Sci. 2007, 11, 504–513. [Google Scholar] [CrossRef]
- Balci, F.; Ludvig, E.A.; Abner, R.; Zhuang, X.; Poon, P.; Brunner, D. Motivational effects on interval timing in dopamine transporter (DAT) knockdown mice. Brain Res. 2010, 1325, 89–99. [Google Scholar] [CrossRef]
- Rammsayer, T.H. On dopaminergic modulation of temporal information processing. Biol. Psychol. 1993, 36, 209–222. [Google Scholar] [CrossRef]
- Minkwitz, J.; Trenner, M.U.; Sander, C.; Olbrich, S.; Sheldrick, A.J.; Hegerl, U.; Himmerich, H. Time perception at different EEG-vigilance levels. Behav. Brain Funct. 2012, 8, 50. [Google Scholar] [CrossRef]
- Wiener, M.; Lohoff, F.W.; Coslett, H.B. Double dissociation of dopamine genes and timing in humans. J. Cogn. Neurosci. 2011, 23, 2811–2821. [Google Scholar] [CrossRef]
- Drew, M.R.; Fairhurst, S.; Malapani, C.; Horvitz, J.C.; Balsam, P.D. Effects of dopamine antagonists on the timing of two intervals. Pharmacol. Biochem. Behav. 2003, 75, 9–15. [Google Scholar] [CrossRef]
- Klimesch, W. α-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Coull, J.T.; Vidal, F.; Nazarian, B.; Macar, F. Functional anatomy of the attentional modulation of time estimation. Science 2004, 5, 1506–1508. [Google Scholar] [CrossRef] [PubMed]
- Meck, W.H. Neuroanatomical localization of an internal clock: A functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Res. 2009, 1109, 93–107. [Google Scholar] [CrossRef]
- Lewis, P.A.; Miall, R.C. Remembering the time: A continuous clock. Trends Cogn. Sci. 2006, 10, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Mazaheri, A. Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Front. Hum. Neurosci. 2010, 4, 186. [Google Scholar] [CrossRef]
- Bonnefond, M.; Jensen, O. Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Curr. Biol. 2012, 22, 1969–1974. [Google Scholar] [CrossRef]
- Samaha, J.; Postle, B.R. The Speed of Alpha-Band Oscillations Predicts the Temporal Resolution of Visual Perception. Curr. Biol. 2015, 25, 2985–2990. [Google Scholar] [CrossRef] [PubMed]
- Palva, S.; Palva, J.M. Functional roles of alpha-band phase synchronization in local and large-scale cortical networks. Front. Psychol. 2011, 2, 204. [Google Scholar] [CrossRef] [PubMed]
- Plomin, R.; Haworth, C.M.A.; Meaburn, E.L.; Price, T.S. Wellcome Trust Case Control Consortium, Davis OSP. Common DNA markers can account for more than half of the genetic influence on cognitive abilities. Psychol. Sci. 2013, 24, 562–568. [Google Scholar] [CrossRef]
- Narayanan, N.S.; Rodnitzky, R.L.; Uc, E.Y. Prefrontal dopamine signaling and cognitive symptoms of Parkinson’s disease. Rev. Neurosci. 2013, 24, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Cools, R.; D’Esposito, M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry 2011, 69, e113–e125. [Google Scholar] [CrossRef]
- Papousek, I.; Reiser, E.M.; Schulter, G.; Fink, A.; Holmes, E.A.; Niederstätter, H.; Nagl, S.; Parson, W.; Weiss, E.M. Serotonin transporter genotype (5-HTTLPR) and electrocortical responses indicating the sensitivity to negative emotional cues. Emotion 2013, 13, 1173–1181. [Google Scholar] [CrossRef]
- Gupta, D.S. Processing of sub- and supra-second intervals in the primate brain results from the calibration of neuronal oscillators via sensory, motor, and feedback processes. Front. Psychol. 2014, 5, 816. [Google Scholar] [CrossRef]
- Kononowicz, T.W.; van Rijn, H. Decoupling interval timing and climbing neural activity: A dissociation between CNV and N1P2 amplitudes. J. Neurosci. 2014, 34, 2931–2939. [Google Scholar] [CrossRef]
- Block, R.A.; Zakay, D.; Hancock, P.A. Human aging and duration judgments: A meta-analytic review. Psychol. Aging 2000, 15, 3–16. [Google Scholar] [CrossRef]
- Pouthas, V.; Perbal, S. Time perception depends on accurate clock mechanisms as well as unimpaired attention and memory processes. Acta Neurobiol. Exp. 2004, 64, 367–385. [Google Scholar] [CrossRef] [PubMed]
- Glicksohn, J.; Hadad, Y. Sex differences in time production revisited. J. Individ. Differ. 2012, 33, 35–42. [Google Scholar] [CrossRef]

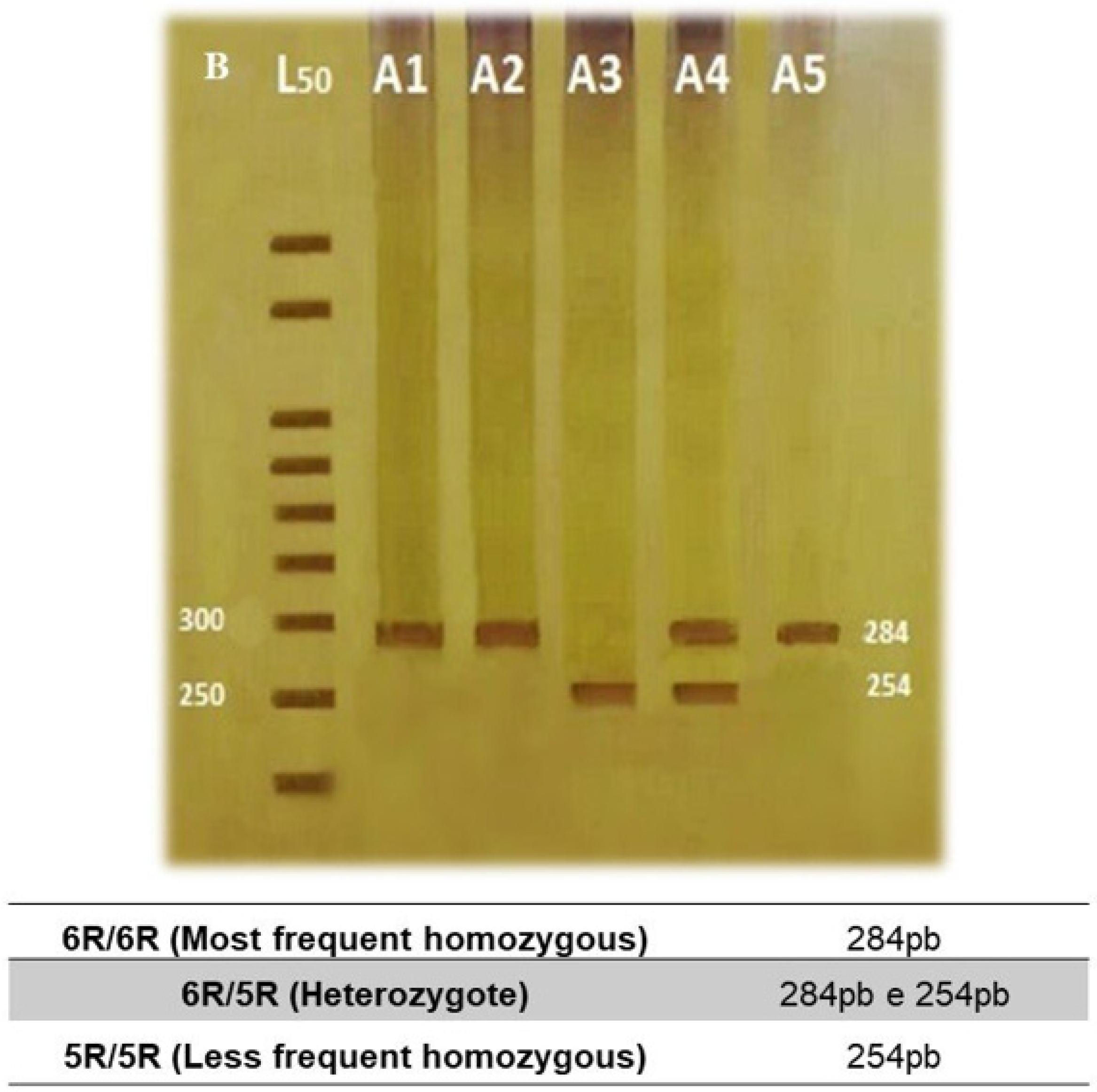


| Polymorphisms | Frequencies | Hardy–Weinberg Equilibrium |
|---|---|---|
| SLC6A3 3′-UTR VNTR | (n = 174) | p = 0.230 |
| 10R/10R | 93 (53.5%) | |
| 10R/9R | 64 (36.8%) | |
| 9R/9R | 17 (9.77%) | |
| Alleles | ||
| 10R | 218 (76.7%) | |
| 9R | 66 (23.3%) | |
| SLC6A3 intron 8 VNTR | (n = 178) | p = 0.705 |
| 6R/6R | 103 (57.8%) | |
| 6R/5R | 66 (37.1%) | |
| 5R/5R | 9 (5.1%) | |
| Alleles | ||
| 6R | 239 (82.4%) | |
| 5R | 51 (17.6%) |
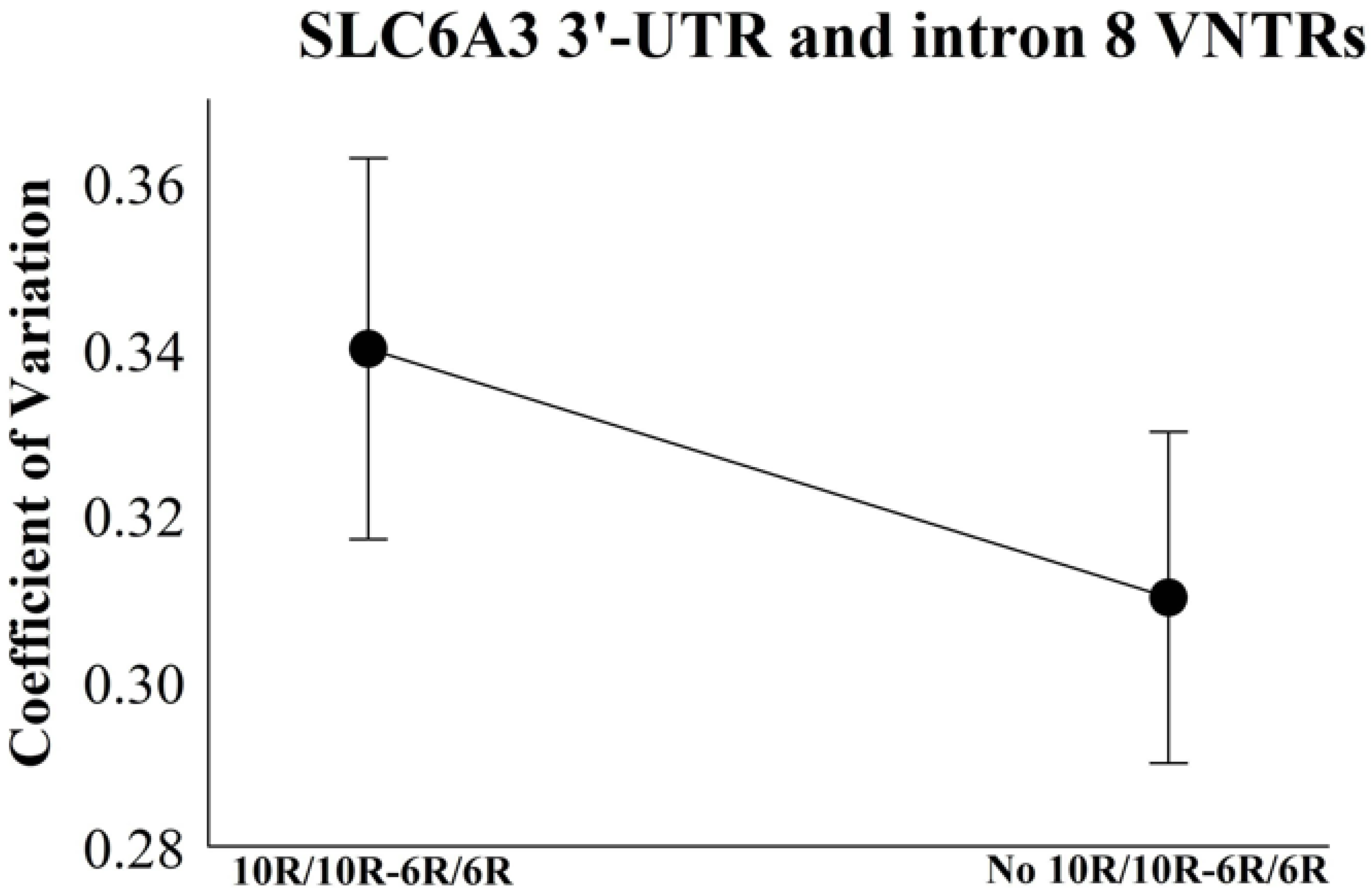
| Coefficient of Variation—SLC6A3 3′-UTR and Intron 8 VNTRs | ||||||
|---|---|---|---|---|---|---|
| Groups | Mean | SD | Median | Pseudo-Sigma | 95% CI | |
| Lower | High | |||||
| 10R/10R-6R/6R | 0.34 | 0.25 | 0.25 | 0.23 | 0.31 | 0.34 |
| No 10R/10R-6R/6R | 0.31 | 0.19 | 0.26 | 0.18 | 0.30 | 0.33 |
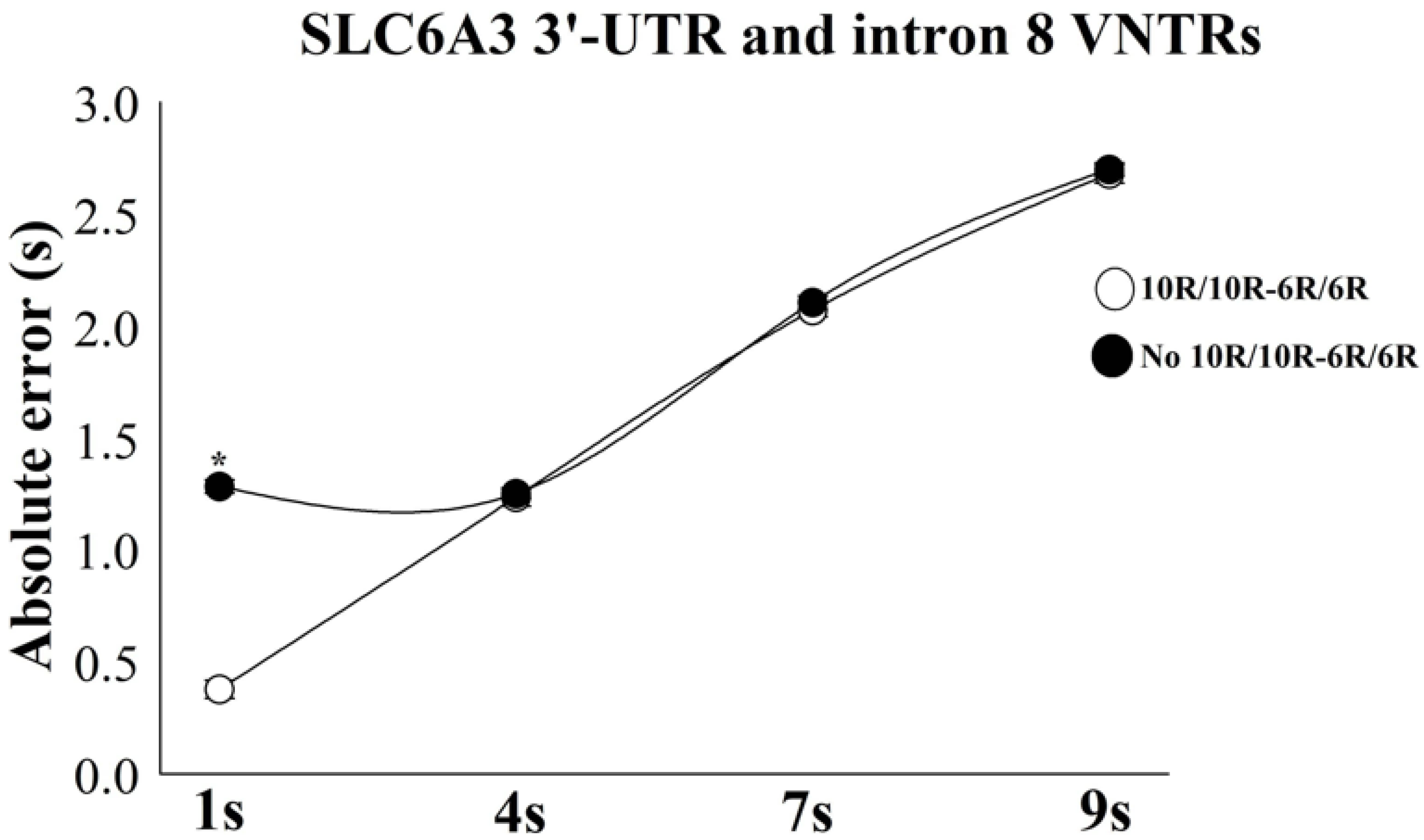
| Variables | B | S.E | Wald | df | p-Value | Odds Ratio | 95%CI for Odds Ratio | |
|---|---|---|---|---|---|---|---|---|
| Lower | High | |||||||
| AE 1 s | 1.79 | 0.04 | 7.47 | 1 | 0.035 * | 1.07 | 0.87 | 0.92 |
| AE 4 s | 0.74 | 0.03 | 2.13 | 1 | 0.144 | 0.94 | 1.01 | 1.15 |
| AE 7 s | 0.51 | 0.09 | 0.56 | 1 | 0.454 | 1.01 | 1.04 | 1.19 |
| AE 9 s | 0.35 | 0.01 | 0.72 | 1 | 0.394 | 0.98 | 1.11 | 1.22 |
| Ratio 1 s | 1.51 | 0.05 | 18.45 | 1 | 0.004 * | 1.09 | 0.81 | 0.96 |
| Ratio 7 s | −0.14 | 0.14 | 0.51 | 1 | 0.831 | 0.92 | 0.74 | 1.12 |
| Ratio 9 s | −0.44 | 0.13 | 0.73 | 1 | 0.639 | 0.64 | 0.49 | 1.83 |
| Constant | 0.66 | 0.15 | 11.72 | 1 | 0.0001 | 0.87 | - | - |
| Variables | B | S.E | Wald | df | p-Value | Odds Ratio | 95%CI for Odds Ratio | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| lDLPFC 1 s | 0.32 | 0.07 | 3.42 | 1 | 0.054 | 1.14 | 0.99 | 1.32 |
| lDLPFC 4 s | 0.16 | 0.04 | 0.62 | 1 | 0.806 | 1.01 | 0.89 | 1.15 |
| lDLPFC 7 s | 0.32 | 0.05 | 1.06 | 1 | 0.441 | 0.92 | 0.90 | 1.12 |
| lDLPFC 9 s | 0.71 | 0.04 | 0.73 | 1 | 0.145 | 0.98 | 1.11 | 1.22 |
| rDLPFC 1 s | 0.55 | 0.06 | 0.95 | 1 | 0.210 | 1.01 | 0.87 | 1.12 |
| rDLPFC 4 s | 0.40 | 0.05 | 1.02 | 1 | 0.439 | 0.99 | 0.72 | 1.14 |
| rDLPFC 7 s | 0.44 | 0.04 | 2.03 | 1 | 0.111 | 1.04 | 0.85 | 1.06 |
| rDLPFC 9 s | 0.80 | 0.03 | 0.72 | 0.543 | 0.96 | 0.88 | 1.11 | |
| Constant | 0.62 | 0.05 | 1.98 | 1 | 0.042 | 1.07 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinho, F.V.C.; Teixeira, S.S.; Rebouças Pinto, G.; de Oliveira, T.; Yoshioka, F.K.N.; Fernandes, H.; Miranda, A.; Velasques, B.B.; de Souza e Silva, A.P.R.; Cagy, M.; et al. VNTR Polymorphisms in the SLC6A3 Gene and Their Impact on Time Perception and EEG Activity. Bioengineering 2025, 12, 1118. https://doi.org/10.3390/bioengineering12101118
Marinho FVC, Teixeira SS, Rebouças Pinto G, de Oliveira T, Yoshioka FKN, Fernandes H, Miranda A, Velasques BB, de Souza e Silva APR, Cagy M, et al. VNTR Polymorphisms in the SLC6A3 Gene and Their Impact on Time Perception and EEG Activity. Bioengineering. 2025; 12(10):1118. https://doi.org/10.3390/bioengineering12101118
Chicago/Turabian StyleMarinho, Francisco Victor Costa, Silmar Silva Teixeira, Giovanny Rebouças Pinto, Thomaz de Oliveira, France Keiko Nascimento Yoshioka, Hygor Fernandes, Aline Miranda, Bruna Brandão Velasques, Alair Pedro Ribeiro de Souza e Silva, Maurício Cagy, and et al. 2025. "VNTR Polymorphisms in the SLC6A3 Gene and Their Impact on Time Perception and EEG Activity" Bioengineering 12, no. 10: 1118. https://doi.org/10.3390/bioengineering12101118
APA StyleMarinho, F. V. C., Teixeira, S. S., Rebouças Pinto, G., de Oliveira, T., Yoshioka, F. K. N., Fernandes, H., Miranda, A., Velasques, B. B., de Souza e Silva, A. P. R., Cagy, M., & Bastos, V. H. d. V. (2025). VNTR Polymorphisms in the SLC6A3 Gene and Their Impact on Time Perception and EEG Activity. Bioengineering, 12(10), 1118. https://doi.org/10.3390/bioengineering12101118







