Enhancing Anaerobic Digestion of Agricultural By-Products: Insights and Future Directions in Microaeration
Abstract
1. Introduction
2. Overview of Anaerobic Digestion Systems
2.1. Operational Scales of Anaerobic Digestion
2.2. Batch Systems
2.3. Semi-Continuous Systems
2.4. Continuous Systems
2.5. Operating Temperatures in Anaerobic Digestion
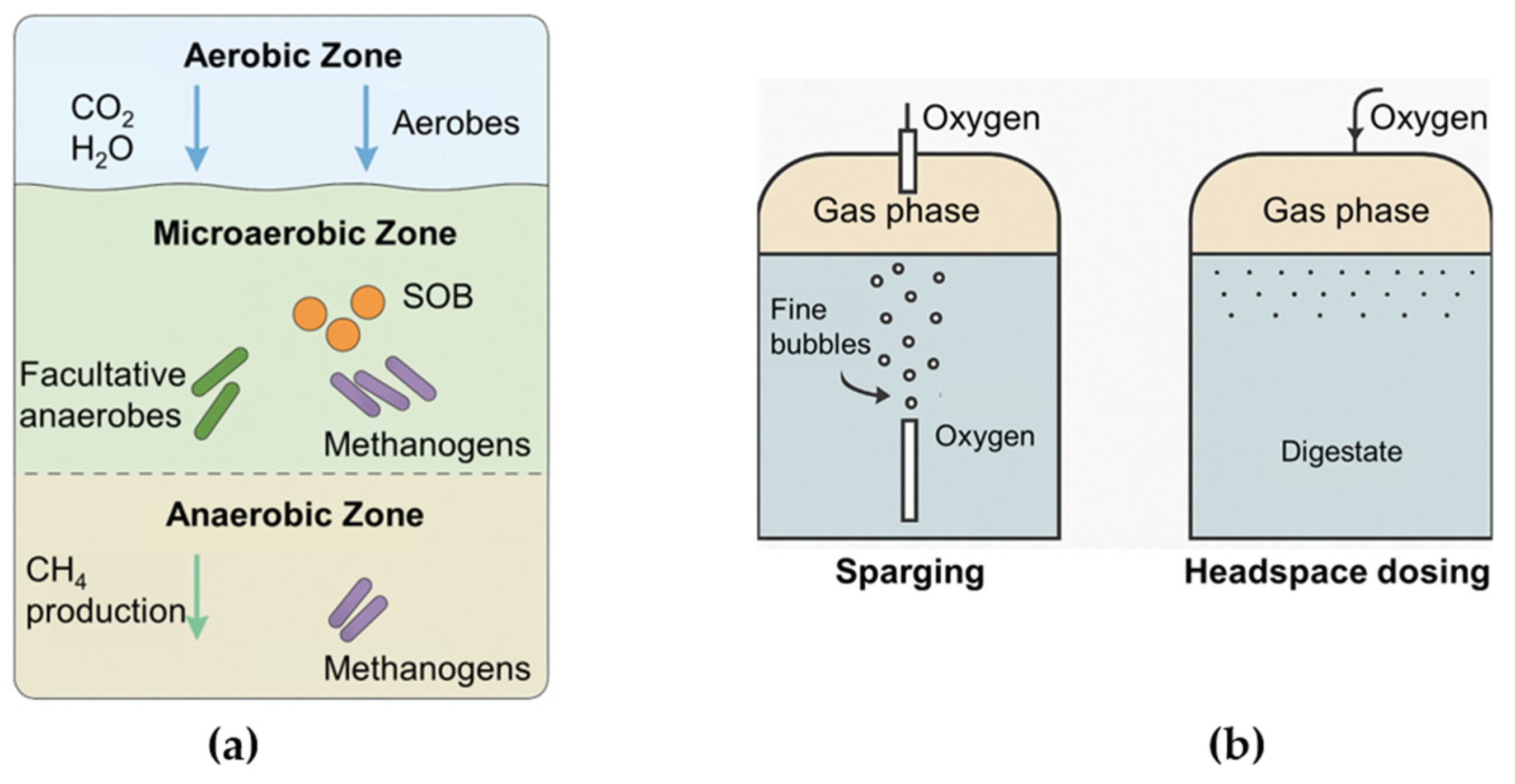
3. Defining Microaeration and the Microaerobic Environment
3.1. Definition and Historical Context
3.2. Characteristics of the Microaerobic Zone
4. Oxygen Dynamics and System Design Considerations
4.1. Oxygen Tolerance in Anaerobic Consortia
4.2. Oxygen Transfer Methods
4.3. Process Controls and Monitoring
5. Microbial Community Responses
5.1. Impacts on Hydrolytic and Fermentative Bacteria
5.2. Sulfur-Oxidizing Bacteria and Their Roles
5.3. Methanogens Under Microaerobic Stress
5.4. Microbial Community Shifts and Syntrophic Partnerships
6. Effects on Biogas Yield and Digester Performance
6.1. Effects on Methane Yield
| Reactor Type | Materials | Digestion Temperature | Working Volume | Aeration Rate | Effect on CH4 | Reference |
|---|---|---|---|---|---|---|
| Batch | Olive mill wastewater | 38 °C | 2 L | 0.65 L air/L/min for 5–7 days | 31.58–78.95% increase | [76] |
| Batch | Corn straw | 55 °C | 200 mL | 2.5–20 mL air/day | 1.6–16.1% increase | [20] |
| Batch | Municipal solid waste | 35 ± 1 °C | --- | 0.5 L air/kg/min for 4–12 days | 33% decrease-24.9% increase | [77] |
| Batch | Synthetic food waste | 35 ± 1 °C | 1 L | 5 L air/h for 24 h | 8.75% decrease-45.62% increase | [85] |
| Batch | wastewater and straw | 55 °C | 200 mL | 5–15 mL O2/gVS | 12.7% decrease-7.2% increase | [78] |
| Batch | Rice straw | 25–45 °C | 10 L | 0.5 L/min/kg for 2–8 days | 24.89% decrease-16.04% increase | [67] |
| Batch | Orange peel | 35 ± 0.5 °C | --- | 400 L air/kg/h for 24 h | 1.01–4.91% increase | [68] |
| Batch | Sludge | 35 ± 1 °C | 2.5 mL | 1–6 air volume/gTS/min | 3.5–17.8% increase | [79] |
| Batch | Chicken manure | 37 ± 1 °C | 120 mL | 7–50 mL air/gVS | 4.7–76.3% increase | [72] |
| Batch | Buffalo manure | 35 ± 2 °C | 600 mL | 7.3 mL O2/gVS | 32% increase | [10] |
| Batch | Corn straw | 37 °C | 200 mL | 0.05–1.6 mL air/gVS/day | 11.2% decrease-7.8% increase | [70] |
| Batch | Cow manure | 35 ± 1 °C | 0.8 L | 12.5–62.5 mL air/L/min | 5.7–13.1% increase | [14] |
| Batch | Sludge and food waste | 35 ± 1 °C | 50 mL | 7.5–120 mL air/gCOD | 0.4–5.2% increase | [80] |
| Semi-continuous | Blackwater | 22 °C | 1.5 L | 5–150 mg O2/L | 39.6–50.7% increase | [81] |
| Semi-continuous | Swine wastewater | 35 ± 1 °C | 4 L | 33.3 mL/L/min for 1–120 min | 20.5% decrease-7.9% increase | [73] |
| Semi-continuous | Corn straw | 37 ± 1 °C | 2 L | 0.2 mL air/gVS | 6–10% increase | [71] |
| Semi-continuous | Food waste | 35 ± 1 °C | 0.7 L | 5 mL air/day | 13.2% increase | [69] |
| Semi-continuous | Sludge and food waste | 35 ± 1 °C | 8 L | 29–82 mL O2/L/day | 13.1–21.1% increase | [82] |
| Continuous | Grass and cattle manure | 35 ± 1 °C | 2 L | 1 mL O2/min for 24 h | 11.76–22.55% increase | [74] |
| Continuous | Straw and poultry litter | 37 °C | 500 mL | 375 mL air/day | 21.5% increase | [75] |
6.2. Effect on Hydrogen Sulfide Production
| Digester Type | Materials | Digestion Temperature | Working Volume | Aeration Rate | Effect on H2S | Reference |
|---|---|---|---|---|---|---|
| Batch | Sludge | 37 °C | 2.7 L | 1 L air/day | 73–99% removal | [86] |
| Batch | Sludge | 30 ± 1 °C | 1 L | 0.2 mg air/L | 35–80% removal | [87] |
| Batch | Chicken manure | 37 ± 1 °C | 120 mL | 7–50 mL air/gVS | 28–58% removal | [72] |
| Batch | Swine wastewater | 35 ± 1 °C | 4 L | 66.7 mL air/L/min | <70.2% removal | [96] |
| Semi-continuous | Sludge | 35 ± 0.2 °C | 50 L | 0.14 mL O2/s | 58–99% reduction | [36] |
| Continuous | Sludge | 35 ± 1 °C | 200 L | 0.2–0.25 Nm3 O2/m3 | >99% removal | [95] |
| Continuous | Dairy manure | 35 °C | 338 m3 | 20 m3 air/day | 68.2% reduction | [97] |
| Continuous | Sludge | 35 °C | 50 L | 4.4–6.2 NL air/m3/day | 90% removal | [92] |
| Continuous | Sludge | --- | --- | 0.28–6.00 m3 air/h | 73.8–99.1% removal | [46] |
| Continuous | Sludge | 40 °C | 120 L | 2–400 mL air/min | 87–97.5% removal | [90] |
| Continuous | Sludge | 55 °C | <500m3 | 0.09–0.77 kNm3 air/day | 75–100% removal | [94] |
| Continuous | Wastewater | 20–22 °C | 3.8 L | 10–20 mL air/min | 60.6–100% removal | [91] |
| Continuous | Sludge | 33–37.5 °C | 2450–3300 m3 | 2.5–14 m3 air/h | 50–87% removal | [88] |
| Continuous | Wastewater | 15–25 °C | 24 m3 | 8.2–32.7 m3 air/day | >99% removal | [93] |
| Continuous | Sludge | 35 °C | 6965 m3 | 2.5–5 m3 air/h for 49 days | 13–20% removal | [89] |
6.3. Process Stability and Digestion Kinetics
7. Process Modeling and Predictive Tools
8. Cost–Benefit Analysis of Microaeration Systems
9. Future Perspectives
- Microbial mechanisms and community shiftsMore research is needed to clarify how low oxygen levels influence microbial community structure, particularly the activity of SOB and facultative organisms during long-term operation.
- Oxygen transfer and dosing optimizationStudies should focus on quantifying oxygen mass transfer rates in different reactor types and under varying conditions to improve dosing strategies and prevent over-aeration.
- Impacts on digestate quality and emissionsThe long-term effects of microaeration on digestate composition, nutrient availability, and emissions of nitrous oxide (N2O) require further study to ensure environmental compliance.
- Emerging contaminantsRecent findings indicate that anaerobic digestion can transform certain emerging contaminants, such as tetrabromobisphenol A, through partial reductive debromination to bisphenol A [128], which highlights the need for more research regarding the removal of emerging contaminants.
- Integration with automation and monitoring systemsResearch is needed on how to incorporate real-time monitoring and control systems for precise oxygen delivery, especially in large-scale or continuously fed digesters.
- Economic and life cycle assessmentsComprehensive cost–benefit analyses and life cycle assessments across different substrates and regions are essential to determine the economic viability and environmental sustainability of microaeration.
- Application across understudied substratesMore data is needed for substrates such as industrial organic waste, agricultural residues beyond manure, and high-fat or protein-rich waste streams to understand the broader applicability of microaeration.
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Anaerobic digestion |
| ADM1 | Anaerobic digestion model no. 1 |
| ADM1-SO | Anaerobic digestion model no 1: sulfur oxidation |
| COD | Chemical oxygen demand |
| DO | Dissolved oxygen |
| HRT | Hydraulic retention time |
| IWA | International water association |
| ORP | Oxidation-reduction potential |
| ppm | Parts per million |
| SOB | Sulfur oxidizing bacteria |
| TS | Total solids |
| UASB | Upflow anaerobic sludge blanket |
| VFA | Volatile fatty acid |
| VS | Volatile solids |
References
- Karthick, C.; Nanthagopal, K.; Ashok, B.; Saravanan, S.V. Influence of alcohol and gaseous fuels on NOx reduction in IC engines. In NOx Emission Control Technologies in Stationary and Automotive Internal Combustion Engines; Elsevier: Amsterdam, The Netherlands, 2022; pp. 347–385. [Google Scholar] [CrossRef]
- Swaminaathan, P.; Saravanan, A.; Thamarai, P. Utilization of bioresources for high-value bioproducts production: Sustainability and perspectives in circular bioeconomy. Sustain. Energy Technol. Assess. 2024, 63, 103672. [Google Scholar] [CrossRef]
- Hu, Y.; Bassi, A.; Xu, C. Energy from biomass. In Future Energy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 447–471. [Google Scholar] [CrossRef]
- Dykstra, C.M.; Pavlostathis, S.G. Hydrogen sulfide affects the performance of a methanogenic bioelectrochemical system used for biogas upgrading. Water Res. 2021, 200, 117268. [Google Scholar] [CrossRef]
- Vu, H.P.; Nguyen, L.N.; Wang, Q.; Ngo, H.H.; Liu, Q.; Zhang, X.; Nghiem, L.D. Hydrogen sulphide management in anaerobic digestion: A critical review on input control, process regulation, and post-treatment. Bioresour. Technol. 2022, 346, 126634. [Google Scholar] [CrossRef]
- Zhou, Z.; Ming, Q.; An, Y.; Ruan, D.; Chen, G.; Wei, H.; Wang, M.; Wu, Z. Performance and microbial community analysis of anaerobic sludge digestion enhanced by in-situ microaeration. J. Water Process Eng. 2021, 42, 102171. [Google Scholar] [CrossRef]
- Nguyen, D.; Khanal, S.K. A little breath of fresh air into an anaerobic system: How microaeration facilitates anaerobic digestion process. Biotechnol. Adv. 2018, 36, 1971–1983. [Google Scholar] [CrossRef]
- Fu, S.; Lian, S.; Angelidaki, I.; Guo, R. Micro-aeration: An attractive strategy to facilitate anaerobic digestion. Trends Biotechnol. 2023, 41, 714–726. [Google Scholar] [CrossRef]
- Wei, C.H.; Wang, Z.W.; Dai, J.H.; Xiao, K.; Yu, H.R.; Qu, F.S.; Rong, H.W.; He, J.G.; Ngo, H.H. Enhanced anaerobic digestion performance and sludge filterability by membrane microaeration for anaerobic membrane bioreactor application. Bioresour. Technol. 2024, 402, 130787. [Google Scholar] [CrossRef]
- Zeb, I.; Yousaf, S.; Ali, M.; Yasmeen, A.; Khan, A.Z.; Tariq, J.A.; Zhao, Q.; Abbasi, A.M.; Ahmad, R.; Khalil, T.M.; et al. In-situ microaeration of anaerobic digester treating buffalo manure for enhanced biogas yield. Renew. Energy. 2022, 181, 843–850. [Google Scholar] [CrossRef]
- Oliveros-Muñoz, J.M.; Martínez-Villalba, J.A.; Jiménez-Islas, H.; Luna-Porres, M.Y.; Escamilla-Alvarado, C.; Ríos-Fránquez, F.J. Luus-Jaakola method and ADM1 based optimization of hydrogen sulfide in anaerobic digestion of cow manure. Biochem. Eng. J. 2021, 171, 108012. [Google Scholar] [CrossRef]
- Di, Y.; Hou, W.; Bian, C.; Zheng, T.; Xiao, B.; Li, L. Enhancing anaerobic digestion of swine manure using microbial electrolysis cell and microaeration. Chem. Eng. J. 2025, 514, 163319. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Hua, D.; Zhao, Y.; Chen, L.; Zhou, L.; Chen, G. Effect of microaerobic microbial pretreatment on anaerobic digestion of a lignocellulosic substrate under controlled pH conditions. Bioresour. Technol. 2021, 328, 124852. [Google Scholar] [CrossRef]
- Li, X.; Deng, L.; Li, F.; Zheng, D.; Yang, H. Effect of air mixing on high-solids anaerobic digestion of cow manure: Performance and mechanism. Bioresour. Technol. 2023, 370, 128545. [Google Scholar] [CrossRef] [PubMed]
- Morais, B.P.; Magalhaes, C.P.; Martins, G.; Pereira, M.A.; Cavaleiro, A.J. Effect of micro-aeration on syntrophic and methanogenic activity in anaerobic sludge. Appl. Microbiol. Biotechnol. 2024, 108, 192. [Google Scholar] [CrossRef] [PubMed]
- Wellinger, A.; Lindberg, A. Biogas Upgrading and Utilisation. IEA Bioenergy Task 24. 2000. Available online: https://www.researchgate.net/publication/245587554_Biogas_Upgrading_and_Utilisation (accessed on 17 October 2025).
- Sun, Z.; Liu, Q.; Li, Y.; Mazarji, M.; Feng, L.; Pan, J. Deciphering the Impact of Lignin on Anaerobic Digestion: Focus on Inhibition Mechanisms and Methods for Alleviating Inhibition. ACS Omega 2024, 9, 44033–44041. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; He, Y.; Wang, Y.; Wang, S.; Zheng, Z.; Wang, S.; Xu, J.; Cai, Y.; Ying, H. A Comprehensive Review of the Strategies to Improve Anaerobic Digestion: Their Mechanism and Digestion Performance. Methane 2024, 3, 227–256. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; Zhang, H.; Rahman, S.U.; Zhang, R.; Liu, G.; Chen, C. Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express. 2017, 7, 72. [Google Scholar] [CrossRef]
- Fu, S.-F.; Wang, F.; Shi, X.-S.; Guo, R.-B. Impacts of microaeration on the anaerobic digestion of corn straw and the microbial community structure. Chem. Eng. J. 2016, 287, 523–528. [Google Scholar] [CrossRef]
- Muller, C.; Guevarra, K.; Summers, A.; Pierce, L.; Shahbaz, P.; Zemke, P.E.; Woodland, K.; Hollingsworth, V.; Nakhla, G.; Bell, K.; et al. A review of the practical application of micro-aeration and oxygenation for hydrogen sulfide management in anaerobic digesters. Process Saf. Environ. Prot. 2022, 165, 126–137. [Google Scholar] [CrossRef]
- Yang, Z.; Larsen, O.C.; Muhayodin, F.; Hu, J.; Xue, B.; Rotter, V.S. Review of anaerobic digestion models for organic solid waste treatment with a focus on the fates of C, N, and P. Energy Ecol. Environ. 2024, 10, 1–14. [Google Scholar] [CrossRef]
- Mosquera, J.; Rangel, C.; Thomas, J.; Santis, A.; Acevedo, P.; Cabeza, I. Biogas production by pilot-acale anaerobic co-digestion and life cycle assessment using a real scale scenario: Independent parameters and co-substrates influence. Processes 2021, 9, 1875. [Google Scholar] [CrossRef]
- Shin, S.G.; Park, S.H.; Hwang, S. Substrate characteristics fluctuations in full-scale anaerobic digesters treating food waste at marginal organic loading rates: A case study. Energies 2022, 15, 3471. [Google Scholar] [CrossRef]
- Sasidhar, K.B.; Somasundaram, M.; Ekambaram, P.; Arumugam, S.K.; Nataraj, G.; Murugan, M.A. A critical review on the effects of pneumatic mixing in anaerobic digestion process. J. Clean. Prod. 2022, 378, 134513. [Google Scholar] [CrossRef]
- Filer, J.; Ding, H.H.; Chang, S. Biochemical methane potential (BMP) assay method for anaerobic digestion research. Water 2019, 11, 921. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Kulupa, T.; Kubiak, A.; Wolna-Maruwka, A.; Pilarski, K.; Niewiadomska, A. Anaerobic digestion of food waste—A short review. Energies 2023, 16, 5742. [Google Scholar] [CrossRef]
- Lima, D.; Li, L.; Appleby, G. Biogas production modelling based on a semi-continuous feeding operation in a municipal wastewater treatment plant. Energies 2025, 18, 1065. [Google Scholar] [CrossRef]
- Bhatia, P.; Fujiwara, M.; Salangsang, M.C.D.; Qian, J.; Liu, X.; Ban, S.; Myojin, M.; Toda, T. Effect of semi-continuous anaerobic digestion on the substrate solubilisation of lignin-rich steam-exploded ludwigia grandiflora. Appl. Sci. 2021, 11, 4452. [Google Scholar] [CrossRef]
- Manser, N.D.; Mihelcic, J.R.; Ergas, S.J. Semi-continuous mesophilic anaerobic digester performance under variations in solids retention time and feeding frequency. Bioresour. Technol. 2015, 190, 359–366. [Google Scholar] [CrossRef]
- Maaz, M.; Yasin, M.; Aslam, M.; Kumar, G.; Atabani, A.E.; Idrees, M.; Anjum, F.; Jamil, F.; Ahmad, R.; Khan, A.L.; et al. Anaerobic membrane bioreactors for wastewater treatment: Novel configurations, fouling control and energy considerations. Bioresour. Technol. 2019, 283, 358–372. [Google Scholar] [CrossRef]
- Muñoz, R.; Meier, L.; Diaz, I.; Jeison, D. A review on the state-of-the-art of physical/chemical and biological technologies for biogas upgrading. Rev. Environ. Sci. Biotechnol. 2015, 14, 727–759. [Google Scholar] [CrossRef]
- Mulbry, W.; Selmer, K.; Lansing, S. Effect of liquid surface area on hydrogen sulfide oxidation during micro-aeration in dairy manure digesters. PLoS ONE 2017, 12, e0185738. [Google Scholar] [CrossRef]
- Nguyen, D.; Gadhamshetty, V.; Nitayavardhana, S.; Khanal, S.K. Automatic process control in anaerobic digestion technology: A critical review. Bioresour. Technol. 2015, 193, 513–522. [Google Scholar] [CrossRef]
- Banerjee, S.; Prasad, N.; Selvaraju, S. Reactor design for biogas production—A short review. J. Energy Power Technol. 2021, 4, 004. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Manassa, P.; Dawson, M.; Fitzgerald, S.K. Oxidation reduction potential as a parameter to regulate micro-oxygen injection into anaerobic digester for reducing hydrogen sulphide concentration in biogas. Bioresour. Technol. 2014, 173, 443–447. [Google Scholar] [CrossRef]
- Chen, J.; Chen, T.B.; Gao, D.; Lei, M.; Zheng, G.D.; Liu, H.T.; Guo, S.L.; Cai, L. Reducing H2S production by O2 feedback control during large-scale sewage sludge composting. Waste Manag. 2011, 31, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Lanko, I.; Flores, L.; Garfí, M.; Todt, V.; Posada, J.A.; Jenicek, P.; Ferrer, I. Life cycle assessment of the mesophilic, thermophilic, and temperature-phased anaerobic digestion of sewage sludge. Water. 2020, 12, 3140. [Google Scholar] [CrossRef]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Conventional mesophilic vs. thermophilic anaerobic digestion: A trade-off between performance and stability? Water Res. 2014, 53, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Shen, C. Thermophilic-mesophilic temperature phase anaerobic co-digestion compared with single phase co-digestion of sewage sludge and food waste. Sci. Rep. 2024, 14, 11967. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Peng, J.; Wang, L.; Yang, J.; Kou, X.; Chai, B.; Gao, L.; Han, X. A comparative study on mesophilic and thermophilic anaerobic digestion of different total solid content sludges produced in a long sludge-retention-time system. Results Eng. 2023, 19, 101228. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, W.; Qi, D.; Ding, Y.; Zhao, Z. Review on microaeration-based anaerobic digestion: State of the art, challenges, and prospectives. Sci. Total Environ. 2020, 710, 136388. [Google Scholar] [CrossRef]
- Costa, L.; Duarte, M.S.; Magalhaes, C.P.; Pereira, M.A.; Cavaleiro, A.J. Micro-aeration for improving anaerobic treatment and biogas production from organic pollutants. Appl. Microbiol. Biotechnol. 2025, 109, 131. [Google Scholar] [CrossRef]
- Wentzel, M.C.; Ekama, G.A.; Loewenthal, R.E. Fundamentals of biological behaviour and wastewater strength tests. In Handbook of Water and Wastewater Microbiology; Academic Press: Cambridge, MA, USA, 2003; pp. 145–173. [Google Scholar] [CrossRef]
- Leitsch, D.; Williams, C.F.; Hrdy, I. Redox pathways as drug targets in microaerophilic parasites. Trends Parasitol. 2018, 34, 576–589. [Google Scholar] [CrossRef]
- Jenicek, P.; Horejs, J.; Pokorna-Krayzelova, L.; Bindzar, J.; Bartacek, J. Simple biogas desulfurization by microaeration -Full scale experience. Anaerobe 2017, 46, 41–45. [Google Scholar] [CrossRef]
- Diaz, I.; Perez, S.I.; Ferrero, E.M.; Fdz-Polanco, M. Effect of oxygen dosing point and mixing on the microaerobic removal of hydrogen sulphide in sludge digesters. Bioresour. Technol. 2011, 102, 3768–3775. [Google Scholar] [CrossRef]
- Luca, A.-V.; Simon-Várhelyi, M.; Mihály, N.-B.; Cristea, V.-M. Fault type diagnosis of the WWTP dissolved oxygen sensor based on fisher siscriminant analysis and assessment of associated environmental and economic impact. Appl. Sci. 2023, 13, 2554. [Google Scholar] [CrossRef]
- De Vleeschauwer, F.; Caluwé, M.; Dobbeleers, T.; Stes, H.; Dockx, L.; Kiekens, F.; Copot, C.; Dries, J. A dynamic control system for aerobic granular sludge reactors treating high COD/P wastewater, using pH and DO sensors. J. Water Process Eng. 2020, 33, 101065. [Google Scholar] [CrossRef]
- Karnieli, O. Bioreactors and downstream processing for stem cell manufacturing. In Stem Cell Manufacturing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 141–160. [Google Scholar]
- Di Costanzo, N.; Di Capua, F.; Cesaro, A.; Carraturo, F.; Salamone, M.; Guida, M.; Esposito, G.; Giordano, A. Headspace micro-oxygenation as a strategy for efficient biogas desulfurization and biomethane generation in a centralized sewage sludge digestion plant. Biomass Bioenerg. 2024, 183, 107151. [Google Scholar] [CrossRef]
- Andreides, M.; Pokorná-Krayzelová, L.; Bartáček, J. Importance of digester’s headspace geometry for the efficient H2S removal through microaeration; experimental and simulation study. Fuel 2024, 362, 130900. [Google Scholar] [CrossRef]
- Li, X.; Yan, Y.-J.; Lu, C.-s.; Jiang, H.; Ma, H.; Hu, Y. Micro-aeration based anaerobic digestion for food waste treatment: A review. J. Water Process Eng. 2024, 58, 104814. [Google Scholar] [CrossRef]
- Bayat, F.; Maddiboina, D.; Didar, T.F.; Hosseinidoust, Z. Regenerating heavily biofouled dissolved oxygen sensors using bacterial viruses. RSC Adv. 2021, 11, 8346–8355. [Google Scholar] [CrossRef]
- Ruano, M.V.; Ribes, J.; Ruiz-Martinez, A.; Seco, A.; Robles, A. An advanced control system for nitrogen removal and energy consumption optimization in full-scale wastewater treatment plants. J. Water Process Eng. 2024, 57, 104705. [Google Scholar] [CrossRef]
- Andrade Cruz, I.; Chuenchart, W.; Long, F.; Surendra, K.C.; Renata Santos Andrade, L.; Bilal, M.; Liu, H.; Tavares Figueiredo, R.; Khanal, S.K.; Fernando Romanholo Ferreira, L. Application of machine learning in anaerobic digestion: Perspectives and challenges. Bioresour. Technol. 2022, 345, 126433. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Xia, Y.; Wang, X.; Liu, H.; Liu, H.; Xun, L. H2S biotreatment with sulfide-oxidizing heterotrophic bacteria. Biodegradation 2018, 29, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Krayzelova, L.; Bartacek, J.; Díaz, I.; Jeison, D.; Volcke, E.I.P.; Jenicek, P. Microaeration for hydrogen sulfide removal during anaerobic treatment: A review. Rev. Environ. Sci. Biotechnol. 2015, 14, 703–725. [Google Scholar] [CrossRef]
- Gomez-Silvan, C.; Molina-Munoz, M.; Poyatos, J.M.; Ramos, A.; Hontoria, E.; Rodelas, B.; Gonzalez-Lopez, J. Structure of archaeal communities in membrane-bioreactor and submerged-biofilter wastewater treatment plants. Bioresour. Technol. 2010, 101, 2096–2105. [Google Scholar] [CrossRef]
- Magalhaes, C.P.; Duarte, M.S.; Pereira, M.A.; Stams, A.J.M.; Cavaleiro, A.J. Facultative anaerobic bacteria enable syntrophic fatty acids degradation under micro-aerobic conditions. Bioresour. Technol. 2025, 417, 131829. [Google Scholar] [CrossRef]
- Elsayed, A.; Laqa Kakar, F.; Mustafa Abdelrahman, A.; Ahmed, N.; AlSayed, A.; Sherif Zagloul, M.; Muller, C.; Bell, K.Y.; Santoro, D.; Norton, J.; et al. Enhancing anaerobic digestion Efficiency: A comprehensive review on innovative intensification technologies. Energy Convers. Manag. 2024, 320, 118979. [Google Scholar] [CrossRef]
- Song, Y.; Mahdy, A.; Hou, Z.; Lin, M.; Stinner, W.; Qiao, W.; Dong, R. Air supplement as a stimulation approach for the in situ desulfurization and methanization enhancement of anaerobic digestion of chicken manure. Energy Fuels 2020, 34, 12606–12615. [Google Scholar] [CrossRef]
- Calderon, K.; Gonzalez-Martinez, A.; Gomez-Silvan, C.; Osorio, F.; Rodelas, B.; Gonzalez-Lopez, J. Archaeal diversity in biofilm technologies applied to treat urban and industrial wastewater: Recent advances and future prospects. Int. J. Mol. Sci. 2013, 14, 18572–18598. [Google Scholar] [CrossRef]
- Liu, T.; Li, X.; Yekta, S.S.; Bjorn, A.; Mu, B.Z.; Masuda, L.S.M.; Schnurer, A.; Enrich-Prast, A. Absence of oxygen effect on microbial structure and methane production during drying and rewetting events. Sci. Rep. 2022, 12, 16570. [Google Scholar] [CrossRef]
- Wormald, R.M.; Rout, S.P.; Mayes, W.; Gomes, H.; Humphreys, P.N. Hydrogenotrophic methanogenesis under alkaline conditions. Front. Microbiol. 2020, 11, 614227. [Google Scholar] [CrossRef]
- Piaggio, A.L.; Sasidhar, K.B.; Khande, P.; Balakrishnan, M.; van Lier, J.B.; de Kreuk, M.K.; Lindeboom, R.E.F. Effects of low oxygen dosages on an anaerobic membrane bioreactor, simulating the oxygen load in an anaerobic digester-dissolved air flotation (AD-DAF) system. ACS ES T Water. 2023, 3, 4133–4142. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, C.; Nges, I.A.; Liu, J. The effects of pre-aeration and inoculation on solid-state anaerobic digestion of rice straw. Bioresour. Technol. 2017, 224, 78–86. [Google Scholar] [CrossRef]
- Calabro, P.S.; Paone, E.; Komilis, D. Strategies for the sustainable management of orange peel waste through anaerobic digestion. J. Environ. Manag. 2018, 212, 462–468. [Google Scholar] [CrossRef]
- Ding, K.; Wu, B.; Wang, Y.; Xu, L.; Liu, M.; Xiang, J.; Chen, Y.; Gu, L.; Li, J.; Li, L.; et al. Study on synergistic effect of carrier combined with micro-aeration on anaerobic digestion of food waste. Chem. Eng. J. 2024, 498, 155731. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, D.-H.; Zheng, Y.; Zou, H.; Fu, S.-F. Understanding the mechanisms behind micro-aeration to enhance anaerobic digestion of corn straw. Fuel 2022, 318, 123604. [Google Scholar] [CrossRef]
- Zhu, R.; Zhang, Y.-f.; Zou, H.; Guo, R.-B.; Fu, S.-F. The effects of micro-aeration on semi-continued anaerobic digestion of corn straw with increasing organic loading rates. Renew. Energy 2022, 195, 1194–1201. [Google Scholar] [CrossRef]
- Mahdy, A.; Song, Y.; Salama, A.; Qiao, W.; Dong, R. Simultaneous H2S mitigation and methanization enhancement of chicken manure through the introduction of the micro-aeration approach. Chemosphere 2020, 253, 126687. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Deng, L.; Wu, J.; Wang, W.; Zheng, D.; Wang, Z.; Liu, Y. Intermittent air mixing system for anaerobic digestion of animal wastewater: Operating conditions and full-scale validation. Bioresour. Technol. 2021, 335, 125304. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Wu, Z.; Shrestha, S.; Lee, P.H.; Raskin, L.; Khanal, S.K. Intermittent micro-aeration: New strategy to control volatile fatty acid accumulation in high organic loading anaerobic digestion. Water Res. 2019, 166, 115080. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Zhu, J.; Xiao, Y.; Schrader, L.C.; Xiao Wu, S.; Aka Robinson, N., Jr.; Wang, Z. Employing micro-aeration in anaerobic digestion of poultry litter and wheat straw: Batch kinetics and continuous performance. Bioresour. Technol. 2023, 368, 128351. [Google Scholar] [CrossRef]
- González-González, A.; Cuadros, F. Effect of aerobic pretreatment on anaerobic digestion of olive mill wastewater (OMWW): An ecoefficient treatment. Food Bioprod. Process. 2015, 95, 339–345. [Google Scholar] [CrossRef]
- Ni, Z.; Liu, J.; Zhang, M. Short-term pre-aeration applied to the dry anaerobic digestion of MSW, with a focus on the spectroscopic characteristics of dissolved organic matter. Chem. Eng. J. 2017, 313, 1222–1232. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.G.; Vasileiou, S.A.; Lyberatos, G.; Angelidaki, I. Effect of micro-aeration and inoculum type on the biodegradation of lignocellulosic substrate. Bioresour. Technol. 2017, 225, 246–253. [Google Scholar] [CrossRef]
- Ruan, D.; Zhou, Z.; Pang, H.; Yao, J.; Chen, G.; Qiu, Z. Enhancing methane production of anaerobic sludge digestion by microaeration: Enzyme activity stimulation, semi-continuous reactor validation and microbial community analysis. Bioresour. Technol. 2019, 289, 121643. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, Y.J.; Wu, H.M.; Ibrahim Gadow, S.; Jiang, H.; Kong, Z.; Hu, Y. Enhancing mesophilic methanogenesis in oleate-rich environments through optimized micro-aeration pretreatment. Waste Manag. 2025, 193, 171–179. [Google Scholar] [CrossRef]
- Yu, N.; Guo, B.; Zhang, Y.; Zhang, L.; Zhou, Y.; Liu, Y. Different micro-aeration rates facilitate production of different end-products from source-diverted blackwater. Water Res. 2020, 177, 115783. [Google Scholar] [CrossRef]
- Chuenchart, W.; Sawaya, C.; Surendra, K.C.; Smith, A.L.; Khanal, S.K. In-situ intermittent micro-aeration in food waste and sewage sludge anaerobic co-digestion: Performance, stability, and microbial dynamics. Bioresour. Technol. 2025, 427, 132398. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Nguyen, D.; Shrestha, S.; Raskin, L.; Khanal, S.K.; Lee, P.H. Evaluation of nanaerobic digestion as a mechanism to explain surplus methane production in animal rumina and engineered digesters. Environ. Sci. Technol. 2023, 57, 12302–12314. [Google Scholar] [CrossRef] [PubMed]
- Biogasclean. 2016. Available online: https://biogasclean.com/wp-content/uploads/2021/03/9-21.01.16.-Biogasclean-safety.TD_.pdf (accessed on 5 October 2025).
- Rafieenia, R.; Girotto, F.; Peng, W.; Cossu, R.; Pivato, A.; Raga, R.; Lavagnolo, M.C. Effect of aerobic pre-treatment on hydrogen and methane production in a two-stage anaerobic digestion process using food waste with different compositions. Waste Manag. 2017, 59, 194–199. [Google Scholar] [CrossRef]
- Krayzelova, L.; Bartacek, J.; Kolesarova, N.; Jenicek, P. Microaeration for hydrogen sulfide removal in UASB reactor. Bioresour. Technol. 2014, 172, 297–302. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, R.C.; Xu, X.J.; Fang, N.; Wang, A.J.; Ren, N.Q.; Lee, D.J. Enhanced performance of denitrifying sulfide removal process at high carbon to nitrogen ratios under micro-aerobic condition. Bioresour. Technol. 2017, 232, 417–422. [Google Scholar] [CrossRef]
- Kraakman, N.J.R.; Diaz, I.; Fdz-Polanco, M.; Muñoz, R. Large-scale micro-aerobic digestion studies at municipal water resource recovery facilities for process-integrated biogas desulfurization. J. Water Process Eng. 2023, 53, 103643. [Google Scholar] [CrossRef]
- Khadir, A.; Nakhla, G.; Karki, R.; Raskin, L.; Muller, C.; Guevarra, K.; Summers, A.; Pierce, L.; Shahbaz, P.; Bell, K.; et al. Micro-aeration for hydrogen sulfide reduction in full-scale anaerobic digesters with limited headspace: Performance and sulfide reduction kinetics. Process Saf. Environ. Prot. 2025, 196, 106911. [Google Scholar] [CrossRef]
- Pokorna-Krayzelova, L.; Bartacek, J.; Theuri, S.N.; Segura Gonzalez, C.A.; Prochazka, J.; Volcke, E.I.P.; Jenicek, P. Microaeration through a biomembrane for biogas desulfurization: Lab-scale and pilot-scale experiences. Environ. Sci. Water Res. Technol. 2018, 4, 1190–1200. [Google Scholar] [CrossRef]
- Zhang, R.C.; Xu, X.J.; Chen, C.; Shao, B.; Zhou, X.; Yuan, Y.; Lee, D.J.; Ren, N.Q. Bioreactor performance and microbial community analysis of autotrophic denitrification under micro-aerobic condition. Sci. Total Environ. 2019, 647, 914–922. [Google Scholar] [CrossRef]
- Ramos, I.; Fdz-Polanco, M. The potential of oxygen to improve the stability of anaerobic reactors during unbalanced conditions: Results from a pilot-scale digester treating sewage sludge. Bioresour. Technol. 2013, 140, 80–85. [Google Scholar] [CrossRef]
- Adem, M.K.; Morris, I.C.; Shin, C.; Tilmans, S.H.; Mitch, W.A.; Criddle, C.S. Efficient sulfide and methane removal in anaerobic secondary effluent using a pilot-scale membrane-aerated biofilm reactor. Chem. Eng. J. 2024, 486, 150066. [Google Scholar] [CrossRef]
- Giordano, A.; Di Capua, F.; Esposito, G.; Pirozzi, F. Long-term biogas desulfurization under different microaerobic conditions in full-scale thermophilic digesters co-digesting high-solid sewage sludge. Int. Biodeterior. Biodegrad. 2019, 142, 131–136. [Google Scholar] [CrossRef]
- Diaz, I.; Lopes, A.C.; Perez, S.I.; Fdz-Polanco, M. Performance evaluation of oxygen, air and nitrate for the microaerobic removal of hydrogen sulphide in biogas from sludge digestion. Bioresour. Technol. 2010, 101, 7724–7730. [Google Scholar] [CrossRef]
- Yang, H.; Deng, L. Using air instead of biogas for mixing and its effect on anaerobic digestion of animal wastewater with high suspended solids. Bioresour. Technol. 2020, 318, 124047. [Google Scholar] [CrossRef]
- Kobayashi, T.; Li, Y.Y.; Kubota, K.; Harada, H.; Maeda, T.; Yu, H.Q. Characterization of sulfide-oxidizing microbial mats developed inside a full-scale anaerobic digester employing biological desulfurization. Appl. Microbiol. Biotechnol. 2012, 93, 847–857. [Google Scholar] [CrossRef]
- Lim, J.W.; Chiam, J.A.; Wang, J.Y. Microbial community structure reveals how microaeration improves fermentation during anaerobic co-digestion of brown water and food waste. Bioresour. Technol. 2014, 171, 132–138. [Google Scholar] [CrossRef]
- Zhen, X.; Zhang, X.; Li, S.; Li, M.; Kang, J. Effect of micro-oxygen pretreatment on gas production characteristics of anaerobic digestion of kitchen waste. J. Mater. Cycles Waste Manag. 2020, 22, 1852–1858. [Google Scholar] [CrossRef]
- Sarkar, O.; Venkata Mohan, S. Pre-aeration of food waste to augment acidogenic process at higher organic load: Valorizing biohydrogen, volatile fatty acids and biohythane. Bioresour. Technol. 2017, 242, 68–76. [Google Scholar] [CrossRef]
- Yin, J.; Yu, X.; Zhang, Y.; Shen, D.; Wang, M.; Long, Y.; Chen, T. Enhancement of acidogenic fermentation for volatile fatty acid production from food waste: Effect of redox potential and inoculum. Bioresour. Technol. 2016, 216, 996–1003. [Google Scholar] [CrossRef]
- Canul Bacab, F.; España Gamboa, E.; Ruiz Espinoza, J.E.; Leal-Bautista, R.M.; Tapia Tussell, R.; Domínguez Maldonado, J.; Canto Canché, B.; Alzate-Gaviria, L. Two phase anaerobic digestion system of municipal solid waste by utilizing microaeration and granular activated carbon. Energies 2020, 13, 933. [Google Scholar] [CrossRef]
- Wen, Y.; Jiang, H.; Qian, R.; Liu, S.; Tang, X.; Huang, W.; Chen, H. Micro-aerated fermentation enhances acetate production from high-rate activated sludge to supply carbon source for heterotrophic denitratation. Chem. Eng. J. 2022, 446, 136894. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Wang, P.; Guo, S. Improving hydrolysis acidification by limited aeration in the pretreatment of petrochemical wastewater. Bioresour. Technol. 2015, 194, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.; Zhou, Z.; Shen, X.; Qiao, W.; Jiang, L.M.; Pan, W.; Zhou, J. Effects of dissolved oxygen on performance and microbial community structure in a micro-aerobic hydrolysis sludge in situ reduction process. Water Res. 2016, 90, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Kythreotou, N.; Florides, G.; Tassou, S.A. A review of simple to scientific models for anaerobic digestion. Renew. Energy 2014, 71, 701–714. [Google Scholar] [CrossRef]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.M.; Siegrist, H.; Vavilin, V.A. The IWA Anaerobic Digestion Model No 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef]
- Fedorovich, S.V.K.V.V. Mathematical modeling of competition between sulohate reduction and methanogenesis in anaeronic reactors. Bioresour. Technol. 1998, 65, 227–242. [Google Scholar] [CrossRef]
- Kalyuzhnyi, S.; Fedorovich, V.; Lens, P.; Hulshoff Pol, L.; Lettinga, G. Mathematical modelling as a tool to study population dynamics between sulfate reducing and methanogenic bacteria. Biodegradation 1998, 9, 187–199. [Google Scholar] [CrossRef]
- Botheju, D.; Bakke, R. Oxygen effects in anaerobic digestion—A review. Open Waste Manag. J. 2011, 4, 1–19. [Google Scholar] [CrossRef]
- Botheju, D.; Lie, B.; Bakke, R. Oxygen effects in anaerobic digestion—II. Model. Identif. Control. A Nor. Res. Bull. 2010, 31, 55–65. [Google Scholar] [CrossRef]
- Pokorna-Krayzelova, L.; Mampaey, K.E.; Vannecke, T.P.W.; Bartacek, J.; Jenicek, P.; Volcke, E.I.P. Model-based optimization of microaeration for biogas desulfurization in UASB reactors. Biochem. Eng. J. 2017, 125, 171–179. [Google Scholar] [CrossRef]
- Batstone, D.J. Mathematical modelling of anaerobic reactors treating domestic wastewater: Rational criteria for model use. Rev. Environ. Sci. Biotechnol. 2006, 5, 57–71. [Google Scholar] [CrossRef]
- Fedorovich, V.; Lens, P.; Kalyuzhnyi, S. Extension of anaerobic digestion model no. 1 with processes of sulfate reduction. Appl. Biochem. Biotechnol. 2003, 109, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Tawai, A.; Sriariyanun, M.; Gentili, P.L. Nonlinear optimization-based robust control approach for a two-stage anaerobic digestion process. J. Chem. 2022, 2022, 1–18. [Google Scholar] [CrossRef]
- Ganeshan, P.; Rajendran, K. Dynamic simulation and optimization of anaerobic digestion processes using MATLAB. Bioresour. Technol. 2022, 351, 126970. [Google Scholar] [CrossRef]
- Anthony, N.; Knobel, A.E.L. A mathematical model of a high sulphate wastewater anaerobic treatment system. Water Res. 2002, 36, 257–265. [Google Scholar]
- Mainardis, M.; Buttazzoni, M.; Goi, D. Up-flow anaerobic sludge blanket (UASB) technology for energy recovery: A review on state-of-the-art and recent technological advances. Bioengineering 2020, 7, 43. [Google Scholar] [CrossRef] [PubMed]
- Valdes, F.; Camiloti, P.R.; Bartacek, J.; Torres-Aravena, A.; Toledo-Alarcon, J.; Zaiat, M.; Jeison, D. Micro-oxygenation in upflow anaerobic sludge bed (UASB) reactors using a silicon membrane for sulfide oxidation. Polymers 2020, 12, 1990. [Google Scholar] [CrossRef]
- Abubakar, U.A.; Lemar, G.S.; Bello, A.D.; Ishaq, A.; Dandajeh, A.A.; Jagun, Z.T.; Houmsi, M.R. Evaluation of traditional and machine learning approaches for modeling volatile fatty acid concentrations in anaerobic digestion of sludge: Potential and challenges. Environ. Sci. Pollut. Res. Int. 2024. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Zhang, L.; Hou, J.; Zhang, Z.; Liu, H.; You, S.; Sik Ok, Y.; Li, W. Review of explainable machine learning for anaerobic digestion. Bioresour. Technol. 2023, 369, 128468. [Google Scholar] [CrossRef]
- Long, F.; Wang, L.; Cai, W.; Lesnik, K.; Liu, H. Predicting the performance of anaerobic digestion using machine learning algorithms and genomic data. Water Res. 2021, 199, 117182. [Google Scholar] [CrossRef]
- Diaz, I.; Ramos, I.; Fdz-Polanco, M. Economic analysis of microaerobic removal of H2S from biogas in full-scale sludge digesters. Bioresour. Technol. 2015, 192, 280–286. [Google Scholar] [CrossRef]
- Song, C.; Li, W.; Cai, F.; Liu, G.; Chen, C. Anaerobic and microaerobic pretreatment for improving methane production from paper waste in anaerobic digestion. Front. Microbiol. 2021, 12, 688290. [Google Scholar] [CrossRef]
- Jiang, C.; Qi, R.; Hao, L.; McIlroy, S.J.; Nielsen, P.H. Monitoring foaming potential in anaerobic digesters. Waste Manag. 2018, 75, 280–288. [Google Scholar] [CrossRef]
- Mutegoa, E.; Sahini, M.G. Approaches to mitigation of hydrogen sulfide during anaerobic digestion process—A review. Heliyon. 2023, 9, e19768. [Google Scholar] [CrossRef]
- Huilinir, C.; Pages-Diaz, J.; Vargas, G.; Vega, S.; Lauzurique, Y.; Palominos, N. Microaerobic condition as pretreatment for improving anaerobic digestion: A review. Bioresour. Technol. 2023, 384, 129249. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lu, Q.; Du, M.; Xu, Q.; Wang, D. Hormesis-Like Effects of Tetrabromobisphenol A on Anaerobic Digestion: Responses of Metabolic Activity and Microbial Community. Environ. Sci. Technol. 2022, 56, 11277–11287. [Google Scholar] [CrossRef] [PubMed]

| Reactor Type | Materials | Digestion Temperature | Working Volume | Aeration Rate | Effect | Reference |
|---|---|---|---|---|---|---|
| Batch | Corn straw | 55 °C | 200 mL | 2.5–20 mL air/day | 1.6–10.1% increase VS removal | [20] |
| Batch | Synthetic food waste | 30 ± 2 °C | 500 mL | 274 L air/kgTS/day | 36% increase VFA | [101] |
| Batch | Synthetic food waste | 35 ± 1 °C | 1 L | 5 L air/h for 24 h | 14.5–37.6% decrease VFA 45.8% increase energy generation | [85] |
| Batch | Municipal solid waste | 38 ± 1 °C | 1.5 L | 254 L air/kgTS/day | 200% increase decomposition 250% increase VFA | [102] |
| Batch | Food waste | 37 °C | 1 L | 5–20 mL O2 pretreatment | 28% decrease-23% increase COD removal | [99] |
| Batch | Sludge | 25 °C | 1 L | 4.6–15.4 mL air/L/min | 46–123% increase VFA | [103] |
| Semi-continuous | Food waste | 30 ± 2 °C | 20 L | 400 L pretreatment | 10% increase VFA 25% increase COD removal | [100] |
| Semi-continuous | Blackwater | 22 °C | 1.5 L | 5–150 mg O2/L | 39.9–48.7% increase hydrolysis | [81] |
| Continuous | Wastewater | --- | 2 L | 0.5 m3 air/h for 72 h | 86.9% increase COD | [104] |
| Continuous | Wastewater | --- | 50 L | 3–4 mg O2/L | 59.2% increase sludge reduction | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Froelich, E.B.; Akdeniz, N. Enhancing Anaerobic Digestion of Agricultural By-Products: Insights and Future Directions in Microaeration. Bioengineering 2025, 12, 1117. https://doi.org/10.3390/bioengineering12101117
Froelich EB, Akdeniz N. Enhancing Anaerobic Digestion of Agricultural By-Products: Insights and Future Directions in Microaeration. Bioengineering. 2025; 12(10):1117. https://doi.org/10.3390/bioengineering12101117
Chicago/Turabian StyleFroelich, Ellie B., and Neslihan Akdeniz. 2025. "Enhancing Anaerobic Digestion of Agricultural By-Products: Insights and Future Directions in Microaeration" Bioengineering 12, no. 10: 1117. https://doi.org/10.3390/bioengineering12101117
APA StyleFroelich, E. B., & Akdeniz, N. (2025). Enhancing Anaerobic Digestion of Agricultural By-Products: Insights and Future Directions in Microaeration. Bioengineering, 12(10), 1117. https://doi.org/10.3390/bioengineering12101117






