Hamstring Tendon Grafts for Anterior Cruciate Ligament Reconstruction: The Effect of a 180° Twist Angle on Tensile Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Specimen Collection and Preparation
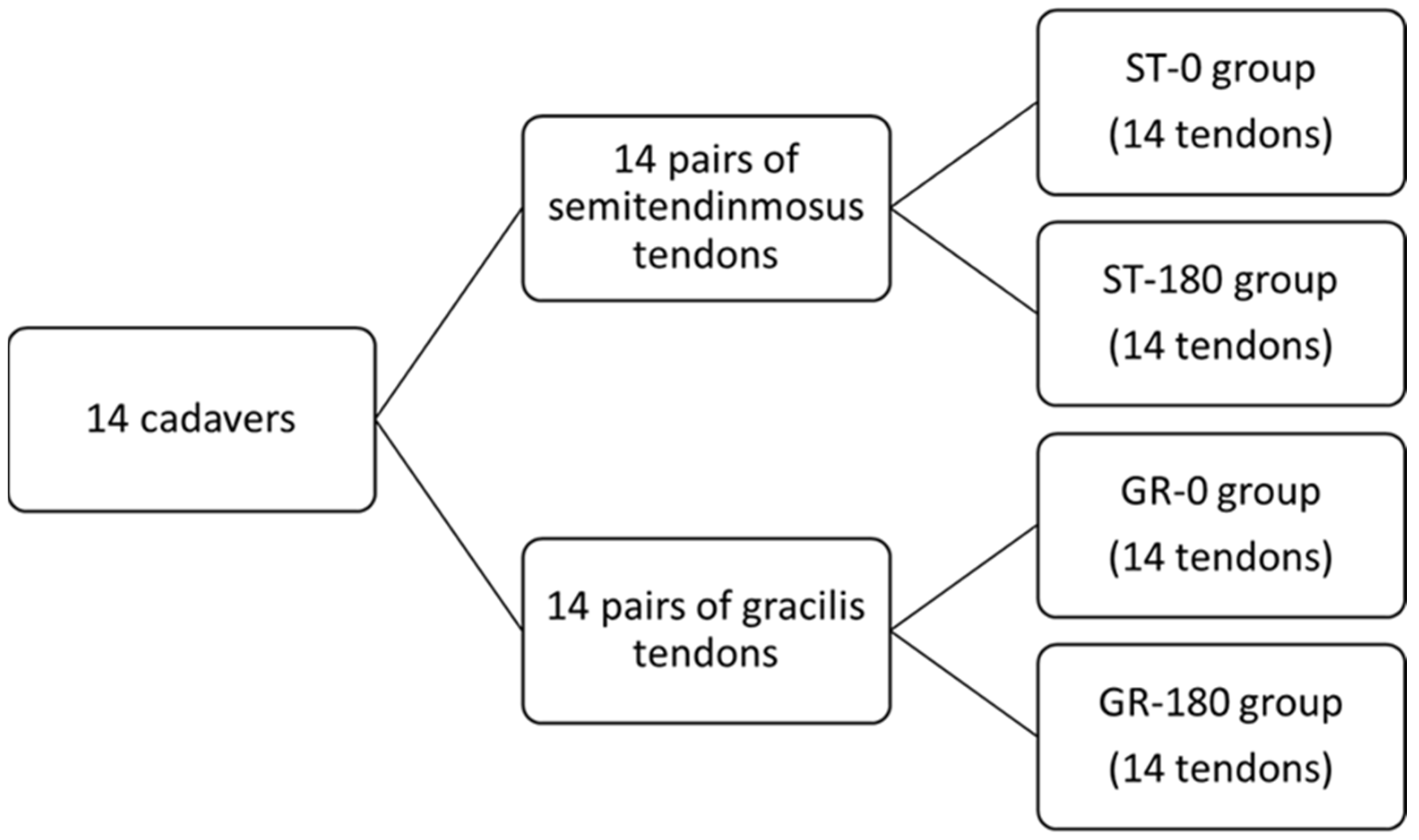
2.3. Graft Preparation and Grouping
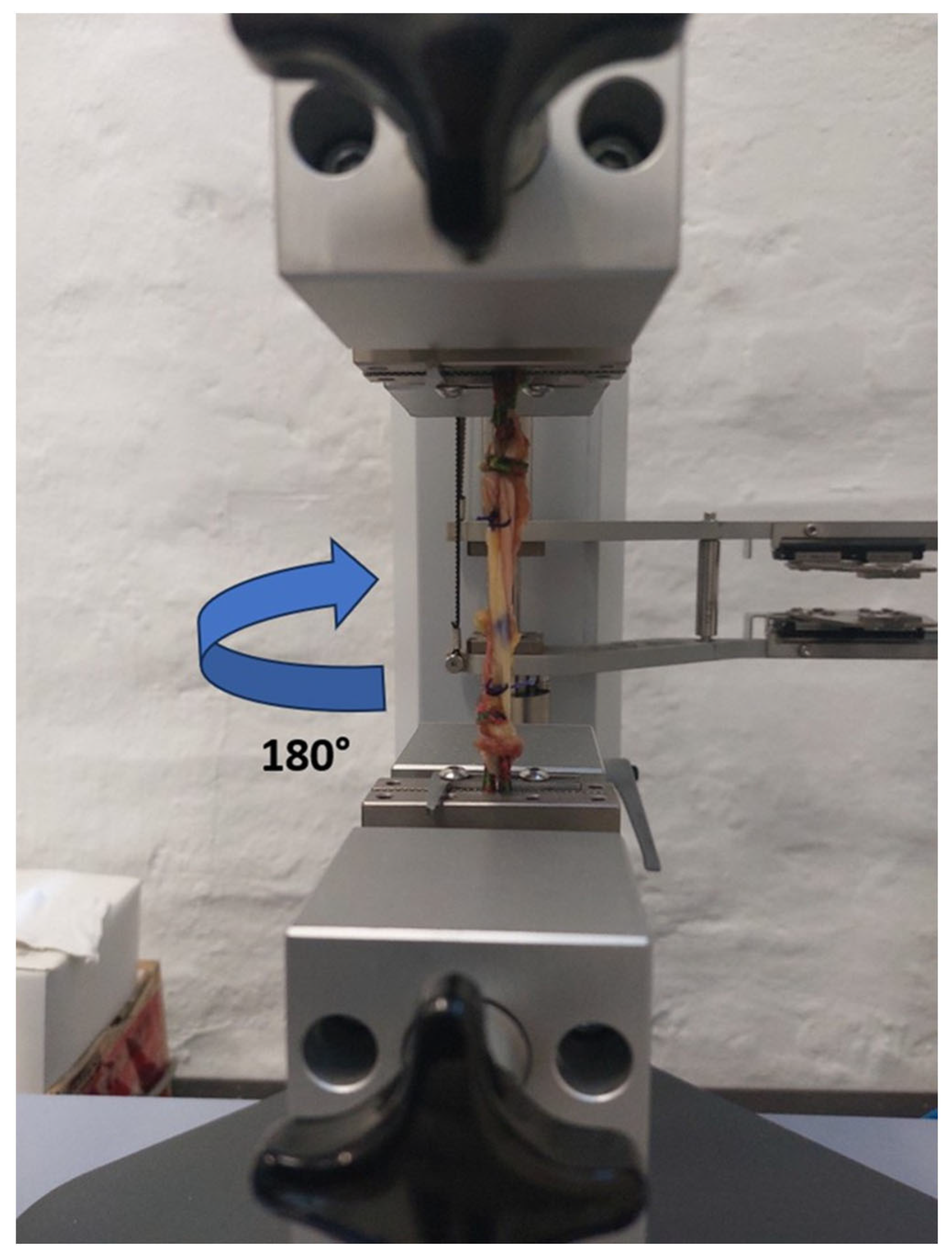
2.4. Biomechanical Testing
2.5. Statistical Analysis
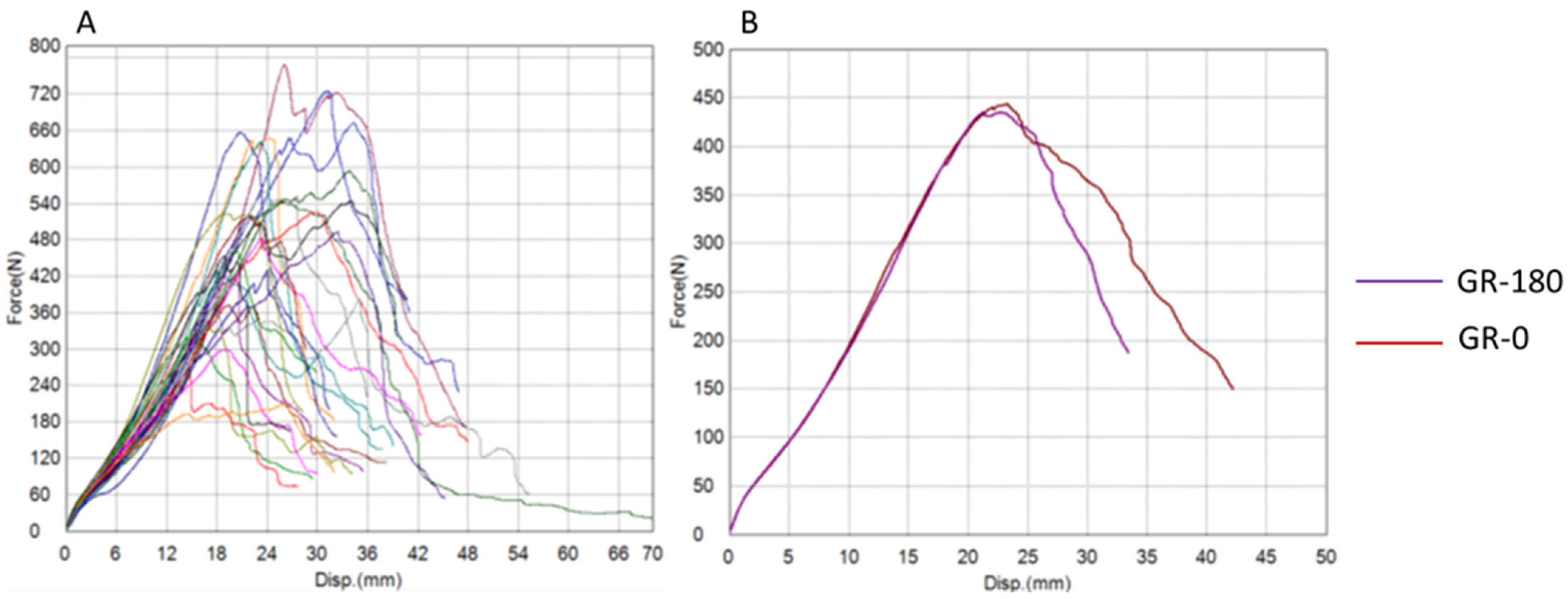
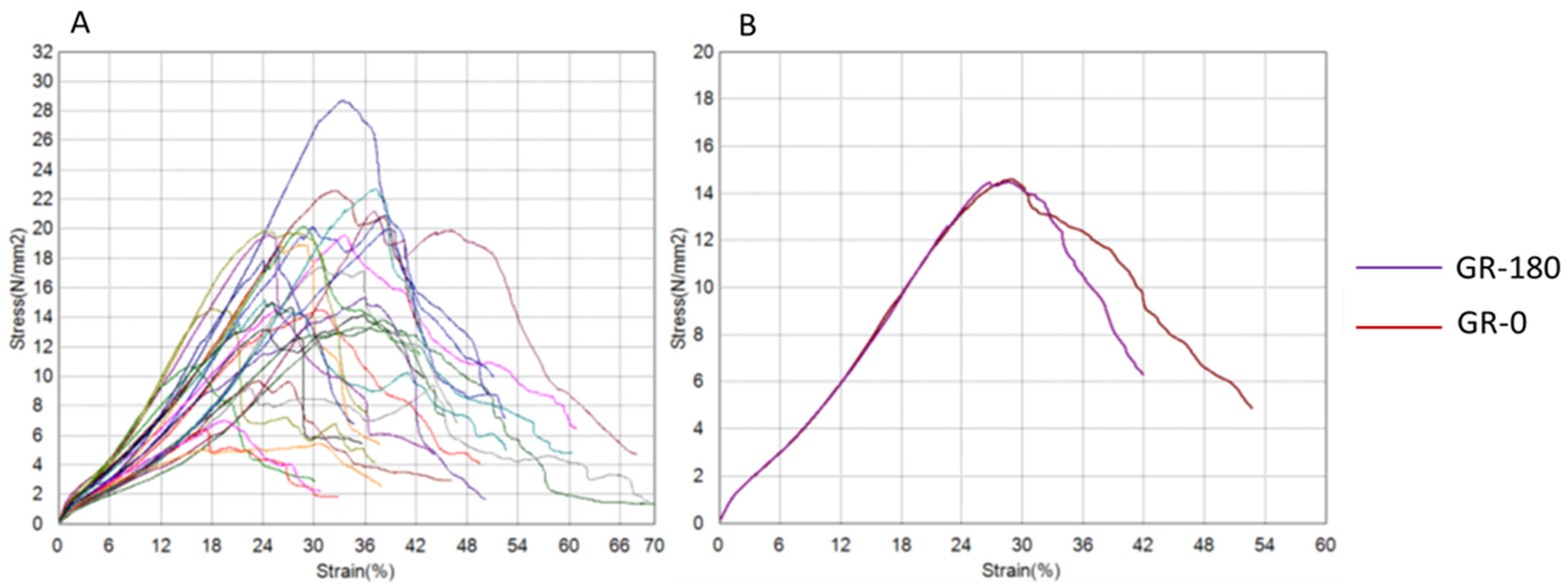
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicholls, M.; Aspelund, T.; Ingvarsson, T.; Briem, K. Nationwide study highlights a second peak in ACL tears for women in their early forties. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 648–654. [Google Scholar] [CrossRef]
- Nicholls, M.; Ingvarsson, T.; Filbay, S.; Lohmander, S.; Briem, K. Smoking and secondary ACL rupture are detrimental to knee health post ACL injury-a Bayesian analysis. J. Exp. Orthop. 2023, 10, 79. [Google Scholar] [CrossRef]
- Kaeding, C.C.; Léger-St-Jean, B.; Magnussen, R.A. Epidemiology and Diagnosis of Anterior Cruciate Ligament Injuries. Clin. Sports Med. 2017, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Paschos, N.K.; Howell, S.M. Anterior cruciate ligament reconstruction: Principles of treatment. EFORT Open Rev. 2017, 1, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Mall, N.A.; Chalmers, P.N.; Moric, M.; Tanaka, M.J.; Cole, B.J.; Bach, B.R., Jr.; Paletta, G.A., Jr. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am. J. Sports Med. 2014, 42, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Lohmander, L.S.; Englund, P.M.; Dahl, L.L.; Roos, E.M. The long-term consequence of anterior cruciate ligament and meniscus injuries: Osteoarthritis. Am. J. Sports Med. 2007, 35, 1756–1769. [Google Scholar] [CrossRef]
- Mather, R.C., 3rd; Koenig, L.; Kocher, M.S.; Dall, T.M.; Gallo, P.; Scott, D.J.; Bach, B.R., Jr.; Spindler, K.P.; the MOON Knee Group. Societal and economic impact of anterior cruciate ligament tears. J. Bone Jt. Surg. 2013, 95, 1751–1759. [Google Scholar] [CrossRef]
- Costa, G.G.; Perelli, S.; Grassi, A.; Russo, A.; Zaffagnini, S.; Monllau, J.C. Minimizing the risk of graft failure after anterior cruciate ligament reconstruction in athletes. A narrative review of the current evidence. J. Exp. Orthop. 2022, 9, 26. [Google Scholar] [CrossRef]
- Chechik, O.; Amar, E.; Khashan, M.; Lador, R.; Eyal, G.; Gold, A. An international survey on anterior cruciate ligament reconstruction practices. Int. Orthop. 2013, 37, 201–206. [Google Scholar] [CrossRef]
- Mihelic, R.; Jurdana, H.; Jotanovic, Z.; Madjarevic, T.; Tudor, A. Long-term results of anterior cruciate ligament reconstruction: A comparison with non-operative treatment with a follow-up of 17–20 years. Int. Orthop. 2011, 35, 1093–1097. [Google Scholar] [CrossRef]
- De Marziani, L.; Orazi, S.; Boffa, A.; Andriolo, L.; Di Martino, A.; Zaffagnini, S.; Filardo, G. Knee temperature remains abnormal in patients successfully treated with anterior cruciate ligament reconstruction: An infrared thermography analysis. J. Exp. Orthop. 2024, 11, e70012. [Google Scholar] [CrossRef]
- Noyes, F.R.; Butler, D.L.; Grood, E.S.; Zernicke, R.F.; Hefzy, M.S. Biomechanical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. J. Bone Jt. Surg. 1984, 66, 344–352. [Google Scholar] [CrossRef]
- Wilson, T.W.; Zafuta, M.P.; Zobitz, M. A biomechanical analysis of matched bone-patellar tendon-bone and quadrupled hamstring grafts. Am. J. Sports Med. 1999, 27, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Lippe, J.; Armstrong, A.; Fulkerson, J.P. Autograft and allograft anterior cruciate ligament reconstruction: A review. Curr. Opin. Orthop. 2006, 17, 362–367. [Google Scholar]
- Magnussen, R.A.; Lawrence, J.T.; West, R.L.; Toth, A.P.; Taylor, D.C.; Garrett, W.E. Graft size and patient age are predictors of early revision after ACL reconstruction with hamstring autograft. Arthroscopy 2012, 28, 526–531. [Google Scholar] [CrossRef]
- Kaeding, C.C.; Aros, B.; Pedroza, A.; Pifel, E.; Amendola, A.; Andrish, J.T.; Dunn, W.R.; Marx, R.G.; McCarty, E.C.; Parker, R.D.; et al. Allograft versus autograft anterior cruciate ligament reconstruction: Predictors of failure from a MOON prospective longitudinal cohort. Sports Health 2011, 3, 73–81. [Google Scholar] [CrossRef]
- Wiggins, A.J.; Grandhi, R.K.; Schneider, D.K.; Stanfield, D.; Webster, K.E.; Myer, G.D. Risk of secondary injury in younger athletes after ACL reconstruction: A systematic review and meta-analysis. Am. J. Sports Med. 2016, 44, 1861–1876. [Google Scholar] [CrossRef]
- Mariscalco, M.W.; Flanigan, D.C.; Mitchell, J.; Pedroza, A.D.; Jones, M.H.; Andrish, J.T.; Parker, R.D.; Kaeding, C.C.; Magnussen, R.A. The influence of hamstring autograft size on patient-reported outcomes and risk of revision after ACL reconstruction. Arthroscopy 2013, 29, 1948–1953. [Google Scholar] [CrossRef]
- Conte, E.J.; Hyatt, A.E.; Gatt, C.J., Jr.; Dhawan, A. Hamstring autograft size can be predicted and is a potential risk factor for anterior cruciate ligament reconstruction failure. Arthroscopy 2014, 30, 882–890. [Google Scholar] [CrossRef]
- Spragg, L.; Chen, J.; Mirzayan, R.; Love, R.; Maletis, G.B. The effect of autologous hamstring graft diameter on the likelihood for revision of anterior cruciate ligament reconstruction. Am. J. Sports Med. 2016, 44, 1475–1481. [Google Scholar] [CrossRef]
- Prodromos, C.C.; Joyce, B.T.; Shi, K.; Keller, B.L. A meta-analysis of stability after anterior cruciate ligament reconstruction as a function of hamstring versus patellar tendon graft and fixation method. Arthroscopy 2005, 21, 1202–1208. [Google Scholar] [CrossRef]
- Carey, J.L.; Dunn, W.R.; Dahm, D.L.; Zeger, S.L.; Spindler, K.P. A systematic review of anterior cruciate ligament reconstruction with autograft compared with allograft. J. Bone Jt. Surg. 2009, 91, 2242–2250. [Google Scholar] [CrossRef]
- Claes, S.; Verdonk, P.; Forsyth, R.; Bellemans, J. The “ligamentization” process in anterior cruciate ligament reconstruction: What happens to the human graft? A systematic review of the literature. Am. J. Sports Med. 2011, 39, 2476–2483. [Google Scholar] [CrossRef] [PubMed]
- Zaffagnini, S.; Marcacci, M.; Lo Presti, M.; Giordano, G.; Iacono, F.; Neri, M.P. Prospective and randomized evaluation of ACL reconstruction with three techniques: A clinical and radiographic evaluation at 5 years follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Cerulli, G.; Placella, G.; Sebastiani, E.; Tei, M.M.; Speziali, A.; Manfreda, F. ACL Reconstruction: Choosing the Graft. Joints 2013, 1, 18–24. [Google Scholar] [PubMed]
- Baawa-Ameyaw, J.; Plastow, R.; Begum, F.A.; Kayani, B.; Jeddy, H.; Haddad, F. Current concepts in graft selection for anterior cruciate ligament reconstruction. EFORT Open Rev. 2021, 6, 808–815. [Google Scholar] [CrossRef]
- Runer, A.; Keeling, L.; Wagala, N.; Nugraha, H.; Özbek, E.A.; Hughes, J.D.; Musahl, V. Current trends in graft choice for anterior cruciate ligament reconstruction—part I: Anatomy, biomechanics, graft incorporation and fixation. J. Exp. Orthop. 2023, 10, 37. [Google Scholar] [CrossRef]
- Frank, R.M.; Hamamoto, J.T.; Bernardoni, E.; Cvetanovich, G.; Bach, B.R., Jr.; Verma, N.N.; Bush-Joseph, C.A. ACL Reconstruction Basics: Quadruple (4-Strand) Hamstring Autograft Harvest. Arthrosc. Tech. 2017, 6, e1309–e1313. [Google Scholar] [CrossRef]
- Kaeding, C.C.; Pedroza, A.D.; Reinke, E.K.; Huston, L.J.; Consortium, M.; Spindler, K.P. Risk Factors and Predictors of Subsequent ACL Injury in Either Knee After ACL Reconstruction: Prospective Analysis of 2488 Primary ACL Reconstructions from the MOON Cohort. Am. J. Sports Med. 2015, 43, 1583–1590. [Google Scholar] [CrossRef]
- Rahardja, R.; Zhu, M.; Love, H.; Clatworthy, M.G.; Monk, A.P.; Young, S.W. Factors associated with revision following anterior cruciate ligament reconstruction: A systematic review of registry data. Knee 2020, 27, 287–299. [Google Scholar] [CrossRef]
- Samuelsen, B.T.; Webster, K.E.; Johnson, N.R.; Hewett, T.E.; Krych, A.J. Hamstring autograft versus patellar tendon autograft for ACL reconstruction: Is there a difference in graft failure rate? A meta-analysis of 47,613 patients. Clin. Orthop. Relat. Res. 2017, 475, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Josipović, M.; Vlaić, J.; Serdar, J.; Šimunović, M.; Nizić, D.; Schauperl, Z.; Bojanić, I.; Jelić, M. Plantaris tendon: A novel graft for anterolateral ligament reconstruction and additional reinforcement for anterior cruciate ligament autografts in combined reconstructive procedures. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 2604–2608. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Shim, J.C.; Yang, S.J.; Cho, S.I.; Kim, J.G. Functional Effects of Single Semitendinosus Tendon Harvesting in Anatomic Anterior Cruciate Ligament Reconstruction: Comparison of Single versus Dual Hamstring Harvesting. Clin. Orthop. Surg. 2019, 11, 60–72. [Google Scholar] [CrossRef]
- Zamarra, G.; Fisher, M.B.; Woo, S.L.; Cerulli, G. Biomechanical evaluation of using one hamstrings tendon for ACL reconstruction: A human cadaveric study. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 11–19. [Google Scholar] [CrossRef]
- Haupt, E.; Okeefe, K.J.; Clay, T.B.; Kenney, N.; Farmer, K.W. Biomechanical Properties of Small-Size Hamstring Autografts. Cureus 2020, 12, e8728. [Google Scholar] [CrossRef]
- Fabbri, M.; Monaco, E.; Lanzetti, R.M.; Perugia, D.; Guzzini, M.; Labianca, L.; Ferretti, A. Single harvesting in the all-inside graft-link technique: Is the graft length crucial for success? A biomechanical study. J. Orthop. Traumatol. 2017, 18, 17–22. [Google Scholar] [CrossRef]
- Ferretti, A.; Conteduca, F.; Morelli, F.; Monteleone, L.; Nanni, F.; Valente, M. Biomechanics of anterior cruciate ligament reconstruction using twisted doubled hamstring tendons. Int. Orthop. 2003, 27, 22–25. [Google Scholar] [CrossRef]
- Kim, D.H.; Wilson, D.R.; Hecker, A.T.; Jung, T.M.; Brown, C.H., Jr. Twisting and braiding reduces the tensile strength and stiffness of human hamstring tendon grafts used for anterior cruciate ligament reconstruction. Am. J. Sports Med. 2003, 31, 861–867. [Google Scholar] [CrossRef]
- Sidwell, A.; Wilson, D.; Wild, P. Effect of twisting hamstring tendon grafts on strength and joint laxity through the range of knee movement. Proc. Inst. Mech. Eng. Part H 2004, 218, 69–77. [Google Scholar] [CrossRef]
- Lawhorn, K.W.; Howell, S.M. Principles for using hamstring tendons for anterior cruciate ligament reconstruction. Clin. Sports Med. 2007, 26, 567–585. [Google Scholar] [CrossRef]
- Pearsall, A.W., 4th; Hollis, J.M.; Russell, G.V., Jr.; Scheer, Z. A biomechanical comparison of three lower extremity tendons for ligamentous reconstruction about the knee. Arthroscopy 2003, 19, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Pailhé, R.; Cavaignac, E.; Murgier, J.; Laffosse, J.M.; Swider, P. Biomechanical study of ACL reconstruction grafts. J. Orthop. Res. 2015, 33, 1188–1196. [Google Scholar] [CrossRef]
- Geethan, I.; Santhosh Sahanand, K.; Ashwin Vijay, P.R.; Rajan, D.V. Mechanical assessment of tripled hamstring tendon graft when using suspensory fixation for cruciate ligament reconstruction. J. Exp. Orthop. 2018, 5, 48. [Google Scholar] [CrossRef]
- Raoulis, V.A.; Zibis, A.; Chiotelli, M.D.; Kermanidis, A.T.; Banios, K.; Schuster, P.; Hantes, M.E. Biomechanical evaluation of three patellar fixation techniques for MPFL reconstruction: Load to failure did not differ but interference screw stabilization was stiffer than suture anchor and suture-knot fixation. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 3697–3705. [Google Scholar] [CrossRef]


| Semitendinosus Group | ST-0 | ST-180 | p-Value |
|---|---|---|---|
| Tendon mass (g) | 5.03 ± 1.6 | 5.06 ± 1.9 | 0.965 |
| Tendon length (cm) | 30.113 ± 3.261 | 29.831 ± 2.814 | 0.288 |
| Graft length (cm) | 6.711 ± 1.23 | 6.558 ± 1.121 | 0.479 |
| Graft diameter (mm) | 8.15 ± 0.82 | 8.11 ± 0.88 | 0.9251 |
| Gracilis Group | GR-0 | GR 180-0 | p-Value |
|---|---|---|---|
| Tendon mass (g) | 2.345 ± 0.92 | 2.44 ± 0.9 | 0.8 |
| Tendon length (cm) | 25.808 ± 2.612 | 25.663 ± 2.438 | 0.611 |
| Graft length (cm) | 5.566 ± 1.003 | 5.355 ± 0.787 | 0.5031 |
| Graft diameter (mm) | 6.240 ± 0.793 | 6.340 ± 0.828 | 0.4854 |
| Semitendinosus Group (ST) | ST-0 | ST-180 | t-Test (p) | Wilcoxon (p) |
|---|---|---|---|---|
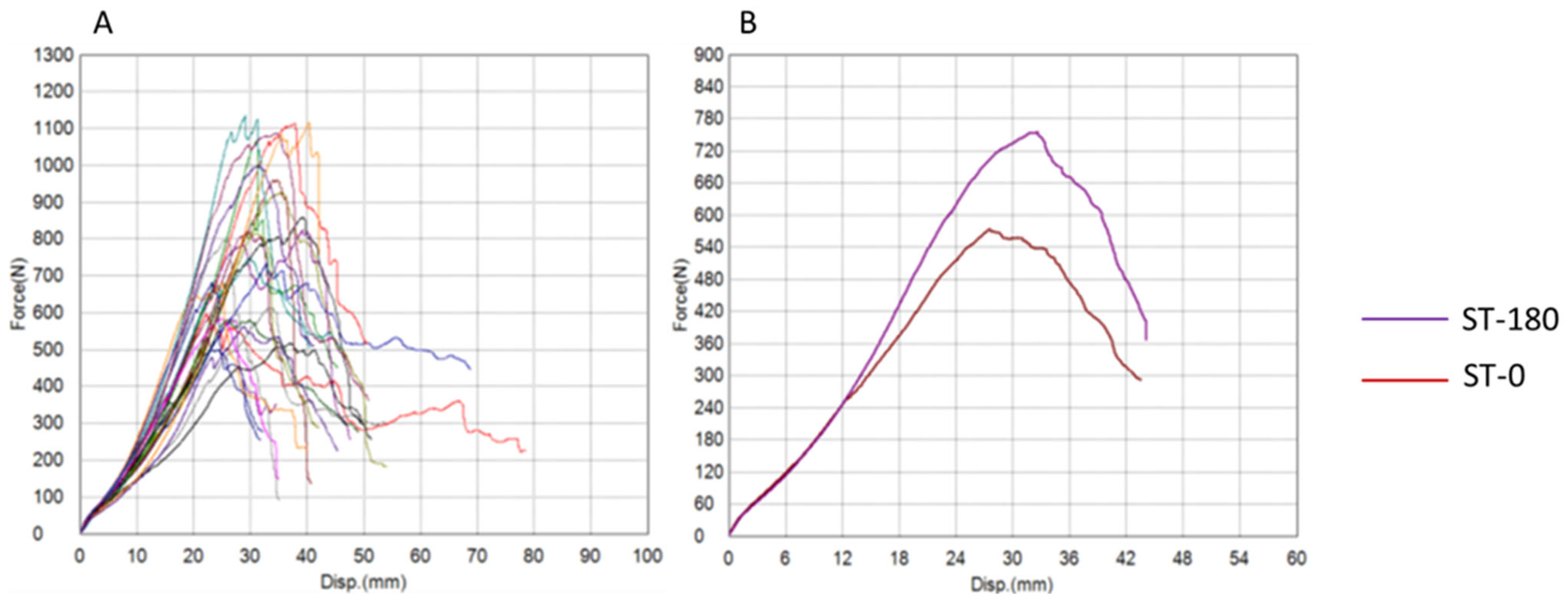
| Maximum Force (N) | 648.72 ± 287.71 | 853.11 ± 189.14 | 0.037 | 0.062 |
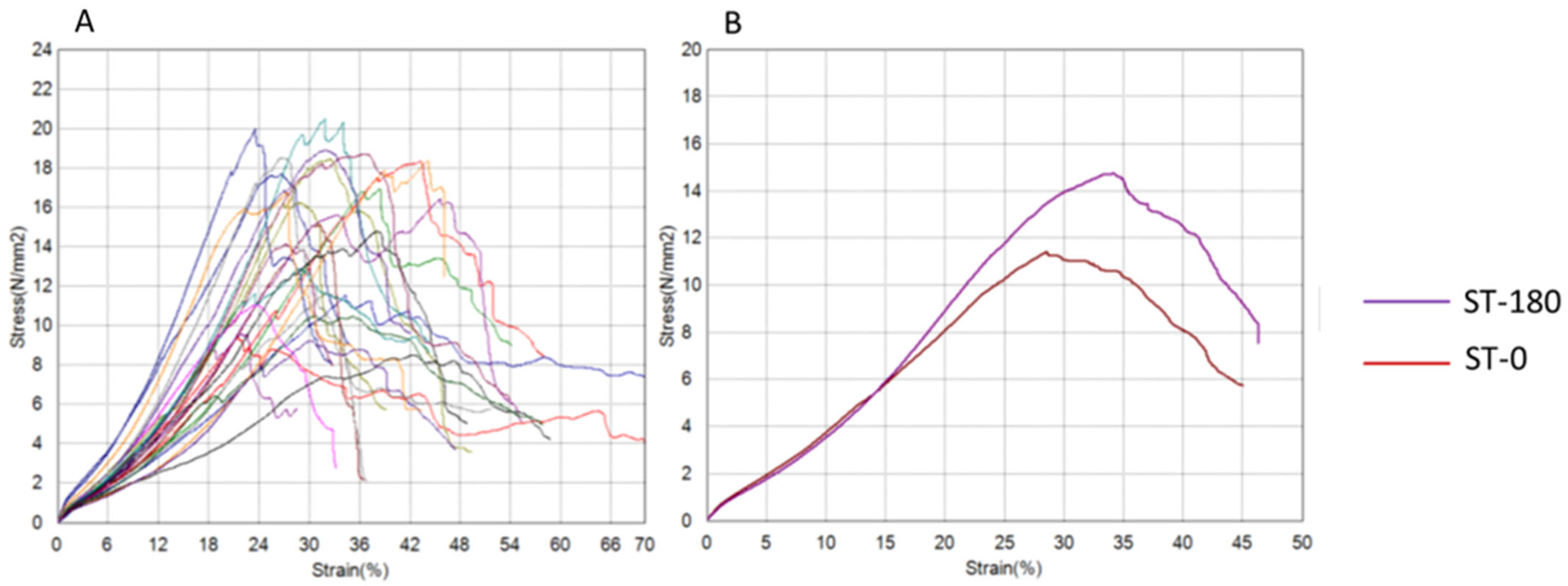
| Tensile Strength (N/mm2) | 12.74 ± 5.71 | 16.87 ± 6.23 | 0.081 | 0.194 |
| Energy (J) | 9.21 ± 4.47 | 13.48 ± 4.95 | 0.024 | 0.062 |
| Tensile Modulus (N/mm2) | 52.11 ± 21.84 | 56.68 ± 16.02 | 0.534 | 0.067 |
| Stiffness (N/mm) | 26.57 ± 10.23 | 30.30 ± 6.96 | 0.261 | 0.352 |
| Gracilis Group (GR) | GR-0 | GR-180 | t-Test (p) | Wilcoxon (p) |
|---|---|---|---|---|
| Maximum Force (N) | 506.81 ± 139.52 | 474.36 ± 148.14 | 0.411 | 0.545 |
| Tensile Strength (N/mm2) | 16.44 ± 5.33 | 15.71 ± 6.02 | 0.602 | 1 |
| Energy (J) | 6.52 ± 3.02 | 5.88 ± 2.77 | 0.412 | 0.448 |
| Tensile Modulus (N/mm2) | 66.25 ± 17.75 | 58.97 ± 17.58 | 0.371 | 0.418 |
| Stiffness (N/mm) | 25.17 ± 6.5 | 22.66 ± 4.45 | 0.264 | 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serdar, J.; Pilipović, A.; Filardo, G.; Martinović, S.; Mihić, A.G.; Plečko, M.; Basal, O.; Smoljanović, T. Hamstring Tendon Grafts for Anterior Cruciate Ligament Reconstruction: The Effect of a 180° Twist Angle on Tensile Properties. Bioengineering 2025, 12, 1105. https://doi.org/10.3390/bioengineering12101105
Serdar J, Pilipović A, Filardo G, Martinović S, Mihić AG, Plečko M, Basal O, Smoljanović T. Hamstring Tendon Grafts for Anterior Cruciate Ligament Reconstruction: The Effect of a 180° Twist Angle on Tensile Properties. Bioengineering. 2025; 12(10):1105. https://doi.org/10.3390/bioengineering12101105
Chicago/Turabian StyleSerdar, Jure, Ana Pilipović, Giuseppe Filardo, Slavica Martinović, Anita Galić Mihić, Mihovil Plečko, Ozgur Basal, and Tomislav Smoljanović. 2025. "Hamstring Tendon Grafts for Anterior Cruciate Ligament Reconstruction: The Effect of a 180° Twist Angle on Tensile Properties" Bioengineering 12, no. 10: 1105. https://doi.org/10.3390/bioengineering12101105
APA StyleSerdar, J., Pilipović, A., Filardo, G., Martinović, S., Mihić, A. G., Plečko, M., Basal, O., & Smoljanović, T. (2025). Hamstring Tendon Grafts for Anterior Cruciate Ligament Reconstruction: The Effect of a 180° Twist Angle on Tensile Properties. Bioengineering, 12(10), 1105. https://doi.org/10.3390/bioengineering12101105







