Toward Supportive Decision-Making for Ureteral Stent Removal: Development of a Morphology-Based X-Ray Analysis
Abstract
1. Introduction
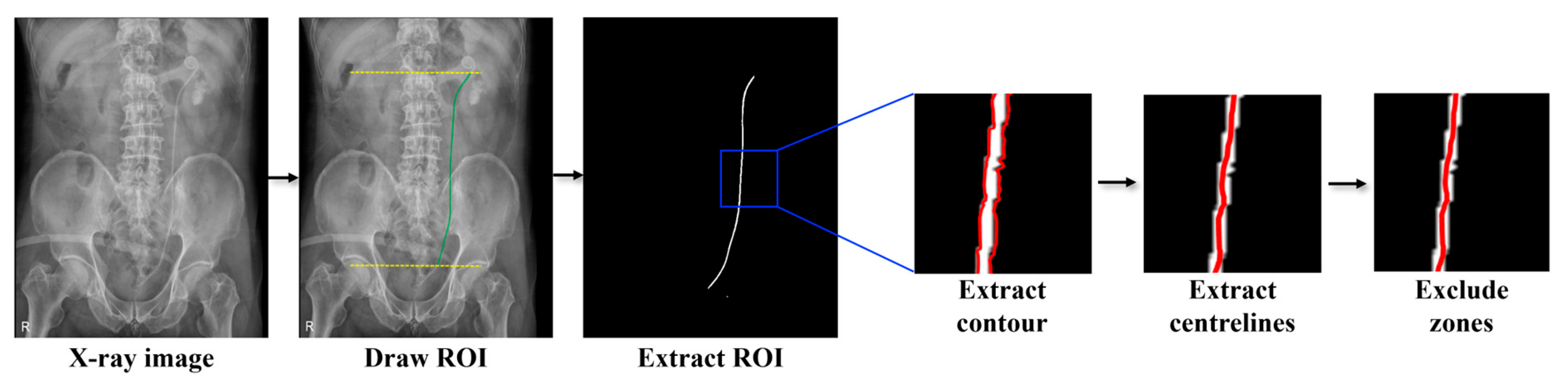
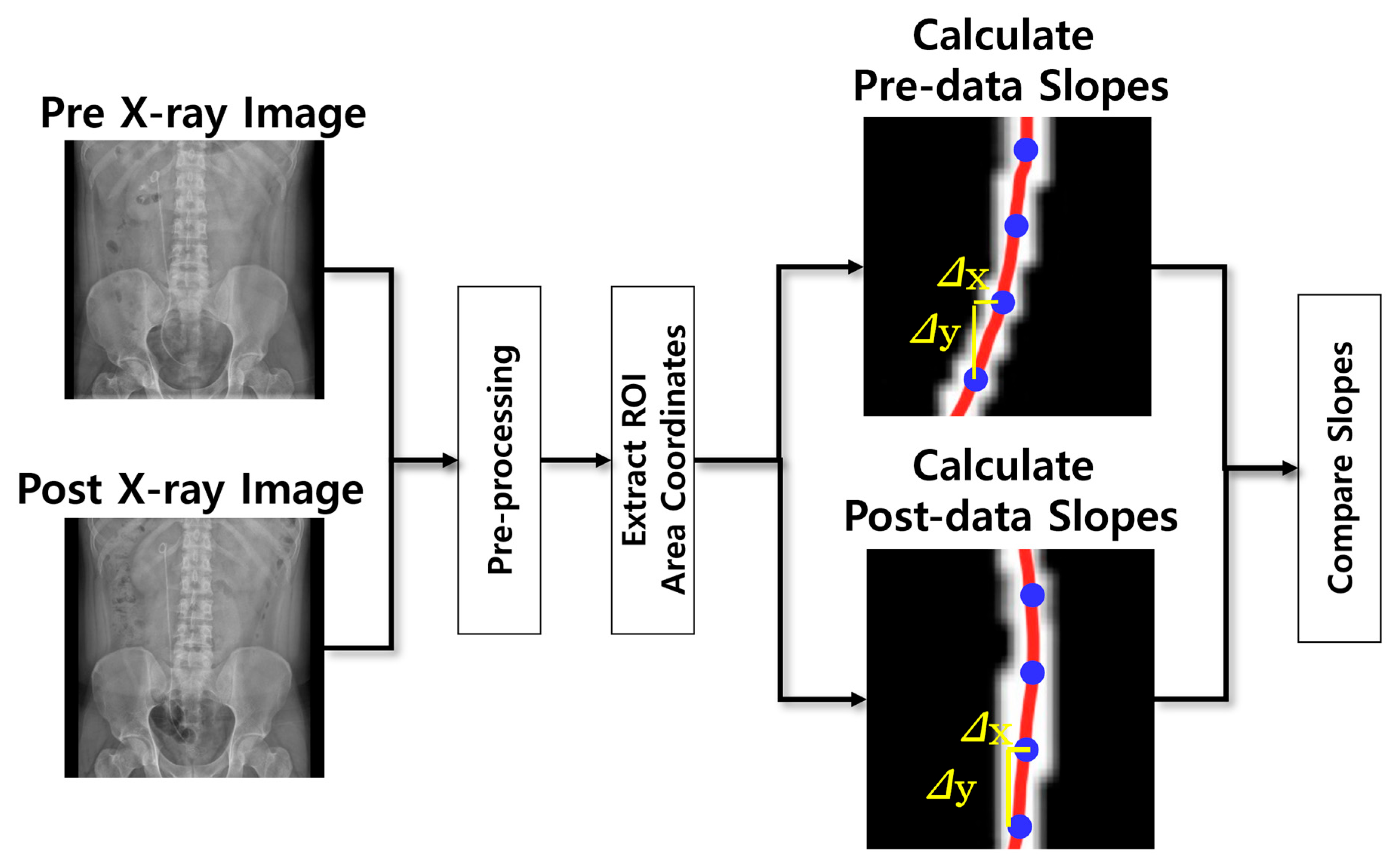
2. Materials and Methods
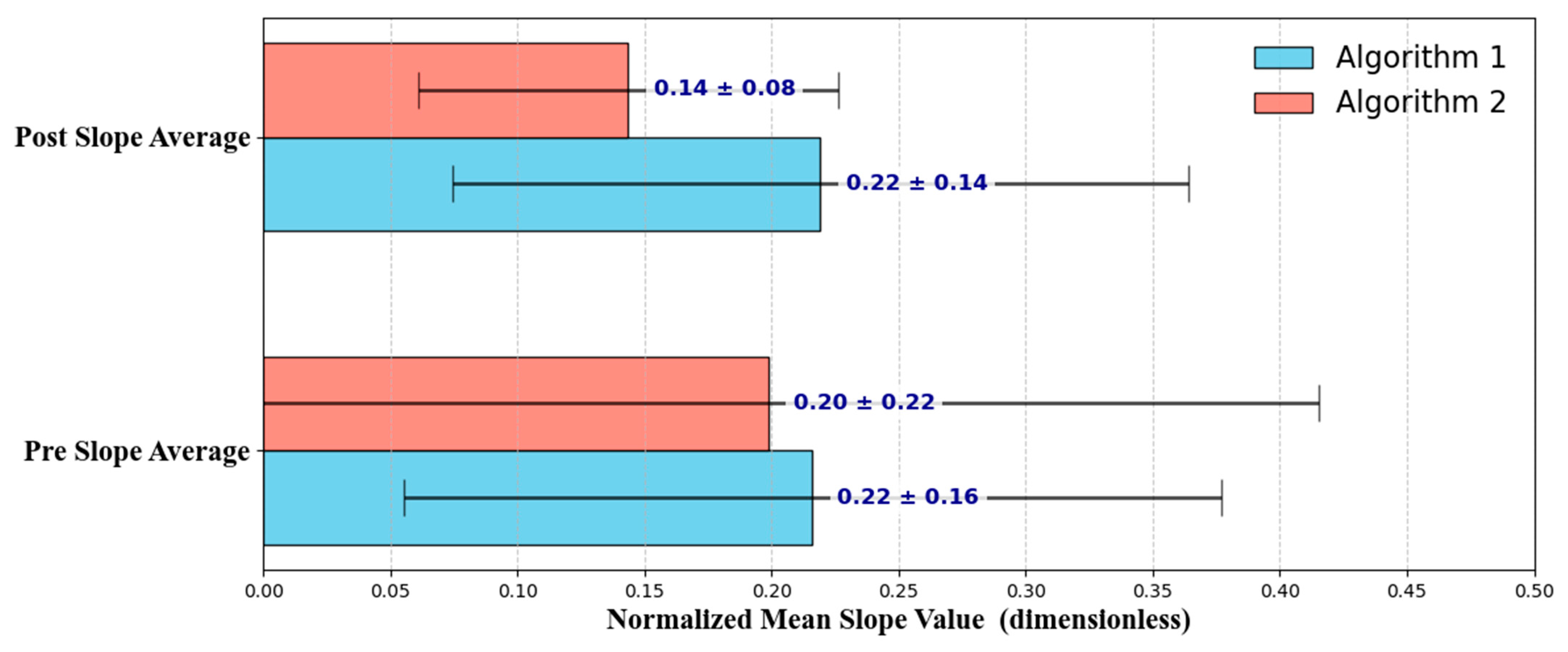
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bohnenpoll, T.; Kispert, A. Ureter growth and differentiation. Semin. Cell Dev. Biol. 2014, 36, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, V.P.; Johnson, E.U.; Wong, K.; Iskander, M.; Javed, S.; Gupta, N.; McCabe, J.E.; Kavoussi, L. Contemporary management of ureteral strictures. J. Clin. Urol. 2019, 12, 20–31. [Google Scholar] [CrossRef]
- Goldfischer, E.R.; Gerber, G.S. Endoscopic management of ureteral strictures. J. Urol. 1997, 157, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, I.; Mamoulakis, C.; Miyazawa, K.; Rodgers, A.; Talati, J.; Lotan, Y. Epidemiology of stone disease across the world. World J. Urol. 2017, 35, 1301–1320. [Google Scholar] [CrossRef] [PubMed]
- Edvardsson, V.O.; Indridason, O.S.; Haraldsson, G.; Kjartansson, O.; Palsson, R. Temporal trends in the incidence of kidney stone disease. Kidney Int. 2013, 83, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Skolarikos, A.; Somani, B.; Neisius, A.; Jung, H.; Petřík, A.; Tailly, T.; Davis, N.; Tzelves, L.; Geraghty, R.; Lombardo, R.; et al. Metabolic evaluation and recurrence prevention for urinary stone patients: An EAU guidelines update. Eur. Urol. 2024, 86, 343–363. [Google Scholar] [CrossRef] [PubMed]
- Shehab, M.; El Helali, A.; Abdelkhalek, M.; Abdelshafy, M.; Mourad, M.; El Helaly, H.; Zikry, M. Role of ureteric stents in relieving obstruction in patients with obstructive uropathy. Urol. Ann. 2013, 5, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, V.; Tozzi, M.; Pietropaolo, A.; De Coninck, V.; Somani, B.K.; Tailly, T.; Bres-Niewada, E.; Mykoniatis, I.; Gregori, A.; Talso, M. Comprehensive overview of ureteral stents based on clinical aspects, material and design. Cent. Eur. J. Urol. 2023, 76, 49–56. [Google Scholar] [CrossRef]
- Nabi, G.; Cook, J.; N’dow, J.; McClinton, S. Outcomes of stenting after uncomplicated ureteroscopy: Systematic review and meta-analysis. BMJ 2007, 334, 572. [Google Scholar] [CrossRef] [PubMed]
- Ringel, A.; Richter, S.; Shalev, M.; Nissenkorn, I. Late complications of ureteral stents. Eur. Urol. 2000, 38, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.J.; Brooks, N.A.; Ghareeb, G.M.; Tracy, C.R. Pilot study to determine optimal stent duration following ureteroscopy: Three versus seven days. Curr. Urol. 2018, 11, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Porto, B.C.; Hobaica, N.C.; Passerotti, C.C.; Sardenberg, R.A.; Otoch, J.P.; da Cruz, J.A.S. Optimal stenting duration following ureteroscopy and nephrolithotomy: Systematic review and meta-analysis. BMC Urol. 2025, 25, 76. [Google Scholar] [CrossRef] [PubMed]
- Heidenberg, D.J.; Nauheim, J.; Grant, C.; Mi, L.; Van Der Walt, C.; Mufarrij, P. Timing of ureteral stent removal after ureteroscopy on stent-related symptoms: A validated questionnaire comparison of 3 and 7 days stent duration. J. Endourol. 2024, 38, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yan, M.; Liu, Z.; Qiu, J.; Dai, Y.; Tang, Y. A method for detecting ureteral stent encrustations in medical CT images based on Mask-RCNN and 3D morphological analysis. Front. Physiol. 2024, 15, 1432121. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Yan, M.; Wang, H.; Liu, Z.; Wang, G.; Wu, X.; Gao, Q.; Hu, H.; Chen, J.; Dai, Y. Identifying ureteral stent encrustation using machine learning based on CT radiomics features: A bicentric study. Front. Med. 2023, 10, 1202486. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhao, Z.; Liu, Y.; Nai, R.; Yuan, C.; Wu, P.; Li, J.; Zhang, X.; Wang, H. Measurement of ureteral length: Comparison of deep learning-based method and other estimation methods on CT and KUB. Comput. Biol. Med. 2025, 184, 109374. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Lu, W. Image edge detection based on OpenCV. Int. J. Electron. Electr. Eng. 2013, 1, 104–106. [Google Scholar] [CrossRef]
- York, D. Least-squares fitting of a straight line. Can. J. Phys. 1966, 44, 1079–1086. [Google Scholar] [CrossRef]
- Kim, T.K. T test as a parametric statistic. Korean J. Anesthesiol. 2015, 68, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Singh, U.; Pandey, C.M.; Mishra, P.; Pandey, G. Application of Student’s t-test, analysis of variance, and covariance. Ann. Card. Anaesth. 2019, 22, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.; Lachenbruch, P.A. Paired t test. In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]

| Variable | Category | n | Percent | Mean ± SD |
|---|---|---|---|---|
| Sex | M | 100 | 57.80% | - |
| F | 73 | 42.20% | - | |
| Age | ≤20 | 0 | 3.30% | 0.0 ± 0.0 |
| 21–39 | 23 | 13.30% | 33.8 ± 5.0 | |
| 40–59 | 64 | 37.00% | 50.1 ± 6.1 | |
| 60–79 | 78 | 45.10% | 67.4 ± 5.1 | |
| ≥80 | 8 | 4.60% | 85.6 ± 3.5 | |
| BMI | Underweight (<18.5) | 5 | 2.89% | 18.02 ± 0.36 |
| Normal (18.5–22.9) | 36 | 20.81% | 21.27 ± 0.92 | |
| Overweight (23–24.9) | 55 | 31.79% | 24.16 ± 0.54 | |
| Obese (≥25) | 77 | 44.51% | 28.24 ± 3.03 | |
| Stent Location | Left | 83 | 47.98% | - |
| Right | 71 | 41.04% | - | |
| Both | 19 | 10.98% | - | |
| Stent Duration | ≤30 days | 80 | 46.24% | 22.45 ± 4.82 |
| 31–60 days | 78 | 45.09% | 37.04 ± 5.13 | |
| 61–120 days | 9 | 5.20% | 77.22 ± 13.53 | |
| ≥121 days | 6 | 3.47% | 290.83 ± 178.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Kim, Y.J.; Park, T.Y.; Kim, K.G. Toward Supportive Decision-Making for Ureteral Stent Removal: Development of a Morphology-Based X-Ray Analysis. Bioengineering 2025, 12, 1084. https://doi.org/10.3390/bioengineering12101084
Lee SH, Kim YJ, Park TY, Kim KG. Toward Supportive Decision-Making for Ureteral Stent Removal: Development of a Morphology-Based X-Ray Analysis. Bioengineering. 2025; 12(10):1084. https://doi.org/10.3390/bioengineering12101084
Chicago/Turabian StyleLee, So Hyeon, Young Jae Kim, Tae Young Park, and Kwang Gi Kim. 2025. "Toward Supportive Decision-Making for Ureteral Stent Removal: Development of a Morphology-Based X-Ray Analysis" Bioengineering 12, no. 10: 1084. https://doi.org/10.3390/bioengineering12101084
APA StyleLee, S. H., Kim, Y. J., Park, T. Y., & Kim, K. G. (2025). Toward Supportive Decision-Making for Ureteral Stent Removal: Development of a Morphology-Based X-Ray Analysis. Bioengineering, 12(10), 1084. https://doi.org/10.3390/bioengineering12101084







