PARKA AI: A Sensor-Integrated Mobile Application for Parkinson’s Disease Monitoring and Self-Management
Abstract
1. Introduction
- We design and implement an integrated patient–provider communication framework specifically for PD management.
- We develop accessibility-centered interfaces that accommodate motor and cognitive difficulties characteristic of PD.
- We implement an LLM-powered clinical reporting system that enhances workflow efficiency while maintaining medical accuracy.
- We demonstrate measurable improvements in patient engagement and clinical decision making through comprehensive use case walk-throughs.
2. Related Work
2.1. Digital Biomarkers for Parkinson’s Disease
2.2. Generative AI in Healthcare
2.3. Patient-Provider Communication Technologies
2.4. Data Visualizations for Health
2.5. Comparison with Existing PD Tools
3. Our Approach: PARKA AI
3.1. Design Requirements
- Presenting a holistic view of patient progress that consolidates motor (tremors and mobility) and non-motor (mood and sleep) data to streamline EMR navigation;
- Categorizing assessments by health domains to organize sensor-derived biomarkers and self-reported data for intuitive access;
- Providing periodic progress reports, particularly weekly summaries to foster meaningful patient–HCP discussions;
- Using simple, lay language to ensure comprehension for patients with cognitive impairments;
- Using accessible visualizations, leveraging intuitive charts and skeuomorphic elements to enhance usability across diverse users.
3.2. Prototype Implementation
4. Demonstrative Use Cases
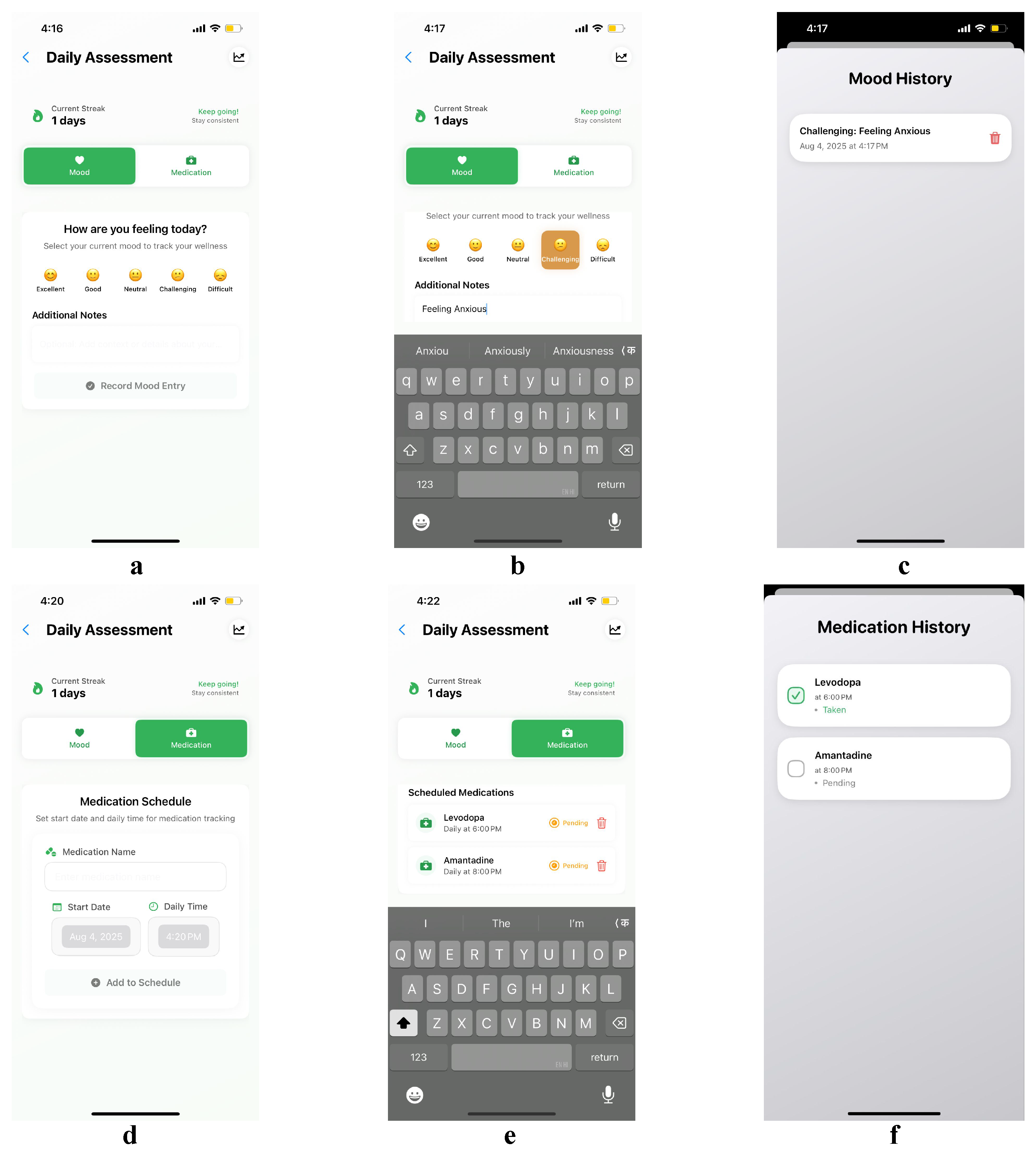
4.1. Use Case 1: Empowering Self-Management Through Symptom Tracking
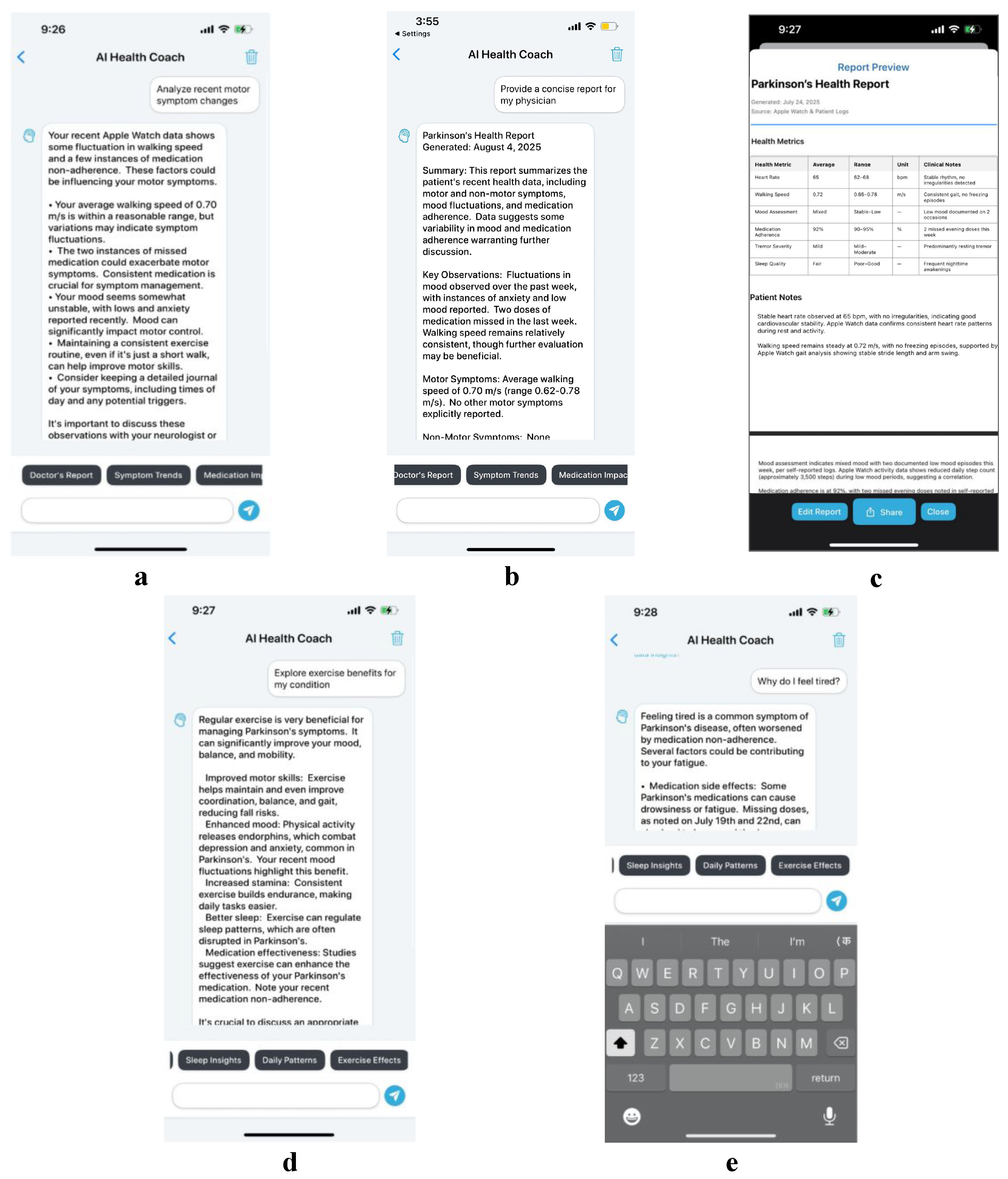
4.2. Use Case 2: Enhancing Patient Education via AI-Driven Insights
4.3. Use Case 3: Supporting Adherence Through Mood and Medication Tracking
5. Discussion and Future Work
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Ray Chaudhuri, K.; Weintraub, D. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Prim. 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.A. Effective physician-patient communication and health outcomes: A review. CMAJ Can. Med. Assoc. J. 1995, 152, 1423–1433. [Google Scholar]
- Effective Communication for Health Care Providers. (n.d.-d) Available online: https://www.cds.udel.edu/wp-content/uploads/2017/02/effective-communication.pdf (accessed on 18 August 2025).
- Adachi, T.; Tsunekawa, Y.; Tanimura, D. Association between cognitive impairment and medication adherence score, including psychological aspects in older patients with cardiovascular disease. Geriatr. Nurs. 2025, 62 Pt B, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Reyes, D.M.; Hahn, J.; Shen, L.; Yan, P. Language Modeling Screens Parkinson’s Disease with Self-reported Questionnaires. medRxiv 2024. [Google Scholar] [CrossRef]
- Rodríguez-Martín, D.; Pérez-López, C. Commercial symptom monitoring devices in Parkinson’s disease: Benefits, limitations, and trends. Front. Neurol. 2024, 15, 1470928. [Google Scholar] [CrossRef]
- Sun, Y.M.; Wang, Z.Y.; Liang, Y.Y.; Hao, C.W.; Shi, C.H. Digital biomarkers for precision diagnosis and monitoring in Parkinson’s disease. NPJ Digit. Med. 2024, 7, 218. [Google Scholar] [CrossRef]
- Mahadevan, N.; Demanuele, C.; Zhang, H.; Volfson, D.; Ho, B.; Erb, M.K.; Patel, S. Development of digital biomarkers for resting tremor and Bradykinesia using a wrist-worn wearable device. Npj Digit. Med. 2020, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Advancing Medical Ai with Med-Gemini. Google Research. (n.d.) Available online: https://research.google/blog/advancing-medical-ai-with-med-gemini/ (accessed on 18 August 2025).
- Interaction Design Foundation—IxDF. (2021, June 14). What Is Human-Centered Design (HCD)? Available online: https://www.interaction-design.org/literature/topics/human-centered-design (accessed on 18 August 2025).
- A Task by Data Type Taxonomy for Information Visualizations. (n.d.-a) Available online: https://www.cs.umd.edu/~ben/papers/Shneiderman1996eyes.pdf (accessed on 18 August 2025).
- Nobbs, D.; Piwko, W.; Bull, C.; Cormack, F.; Ahmaniemi, T.; Holst, S.C.; Chatterjee, M.; Maetzler, W.; Avey, S.; Ng, W.F. Regulatory qualification of a cross-disease digital measure: Benefits and challenges from the perspective of IMI consortium idea-fast. Digit. Biomarkers 2023, 7, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Moreau, C.; Rouaud, T.; Grabli, D.; Benatru, I.; Remy, P.; Marques, A.R.; Drapier, S.; Mariani, L.L.; Roze, E.; Devos, D.; et al. Overview on wearable sensors for the management of Parkinson’s disease. NPJ Park. Dis. 2023, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Rashik, M.; Sweth, S.; Agrawal, N.; Kochar, S.; Smith, K.M.; Rajabiyazdi, F.; Setlur, V.; Mahyar, N.; Sarvghad, A. Ai-enabled conversational journaling for advancing parkinson’s disease symptom tracking. In Proceedings of the 2025 CHI Conference on Human Factors in Computing Systems, Yokohama, Japan, 26 April–1 May 2025; pp. 1–23. [Google Scholar] [CrossRef]
- Talk2Care: Facilitating Asynchronous Patient-Provider Communication with Large-Language-Model. (n.d.) Available online: https://www.researchgate.net/publication/385667900_Talk2Care_Facilitating_Asynchronous_Patient-Provider_Communication_with_Large-Language-Model (accessed on 18 August 2025).
- [PDF] Lifelines: Visualizing Personal Histories | Semantic SCHOLAR. (n.d.-b) Available online: https://www.semanticscholar.org/paper/LifeLines:-visualizing-personal-histories-Plaisant-Milash/6c47a8d6a27c24ad2a5ad73e90eb2c6a54017be3 (accessed on 18 August 2025).
- Heer, J.; Bostock, M.; Ogievetsky, V. A Tour Through the Visualization Zoo. Commun. ACM 2010, 53, 59–67. [Google Scholar] [CrossRef]
- (PDF) ArmSleeve: A Patient Monitoring System to Support Occupational Therapists in Stroke Rehabilitation. Available online: https://www.researchgate.net/publication/303901748_ArmSleeve_A_Patient_Monitoring_System_to_Support_Occupational_Therapists_in_Stroke_Rehabilitation (accessed on 18 August 2025).
- Ramesh, S.H.; Ouskine, A.; Khorasani, E.; Ebrahimipour, M.; Finestone, H.; Chan, A.D.; Rajabiyazdi, F. A data visualization tool for patients and healthcare providers to communicate during inpatient stroke rehabilitation. In Proceedings of the 50th Graphics Interface Conference, Halifax, NS, Canada, 3–6 June 2024; pp. 1–14. [Google Scholar] [CrossRef]
- Adams, J.L.; Kangarloo, T.; Gong, Y.; Khachadourian, V.; Tracey, B.; Volfson, D.; Latzman, R.D.; Cosman, J.; Edgerton, J.; Anderson, D.; et al. Using a smartwatch and smartphone to assess early Parkinson’s disease in the WATCH-PD study over 12 months. NPJ Park. Dis. 2024, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- The Visual Display of Quantitative Information. (n.d.-f) Available online: https://kyl.neocities.org/books/%5BTEC%20TUF%5D%20the%20visual%20display%20of%20quantitative%20information.pdf (accessed on 18 August 2025).
- Stableford, S.; Mettger, W. Plain Language: A Strategic Response to the Health Literacy Challenge. J. Public Health Policy 2007, 28, 71–93. [Google Scholar] [CrossRef] [PubMed]
- MLC Team. Introduction to MLC LLM. Available online: https://llm.mlc.ai/docs/get_started/introduction (accessed on 18 August 2025).
- Center for Drug Evaluation and Research. (n.d.); Digital Health Technologies for Remote Data Acquisition in Clinical in. U.S. Food and Drug Administration. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/digital-health-technologies-remote-data-acquisition-clinical-investigations (accessed on 18 August 2025).
- Zhang, P.; Zeng, G.; Wang, T.; Lu, W. Tinyllama: An open-source small language model. arXiv 2024, arXiv:2401.02385. [Google Scholar]
- Dorsey, E.R.; Venkataraman, V.; Grana, M.J.; Bull, M.T.; George, B.P.; Boyd, C.M.; Beck, C.A.; Rajan, B.; Seidmann, A.; Biglan, K.M. Randomized controlled clinical trial of “virtual house calls” for Parkinson disease. JAMA Neurol. 2013, 70, 565–570. [Google Scholar] [CrossRef]
- Ebby, C.G.; Tse, G.; Bethel, J.; Zhao, Q.; Gerber, D.M.; Kelly, M.M. Large Language Models to Summarize Pediatric Admission Notes Into Plain Language. Pediatrics 2025, 155, e2024069515. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhalala, K.S.; Mansoor, H. PARKA AI: A Sensor-Integrated Mobile Application for Parkinson’s Disease Monitoring and Self-Management. Bioengineering 2025, 12, 1059. https://doi.org/10.3390/bioengineering12101059
Bhalala KS, Mansoor H. PARKA AI: A Sensor-Integrated Mobile Application for Parkinson’s Disease Monitoring and Self-Management. Bioengineering. 2025; 12(10):1059. https://doi.org/10.3390/bioengineering12101059
Chicago/Turabian StyleBhalala, Krisha Sanjay, and Hamid Mansoor. 2025. "PARKA AI: A Sensor-Integrated Mobile Application for Parkinson’s Disease Monitoring and Self-Management" Bioengineering 12, no. 10: 1059. https://doi.org/10.3390/bioengineering12101059
APA StyleBhalala, K. S., & Mansoor, H. (2025). PARKA AI: A Sensor-Integrated Mobile Application for Parkinson’s Disease Monitoring and Self-Management. Bioengineering, 12(10), 1059. https://doi.org/10.3390/bioengineering12101059






