Color Change in Commercial Resin Composites with Different Photoinitiators
Abstract
1. Introduction
2. Materials and Methods
2.1. Resin Composites
2.2. Light Curing
2.3. Aging
2.4. Color Measurements
2.5. Color Change
2.6. Statistics
3. Results
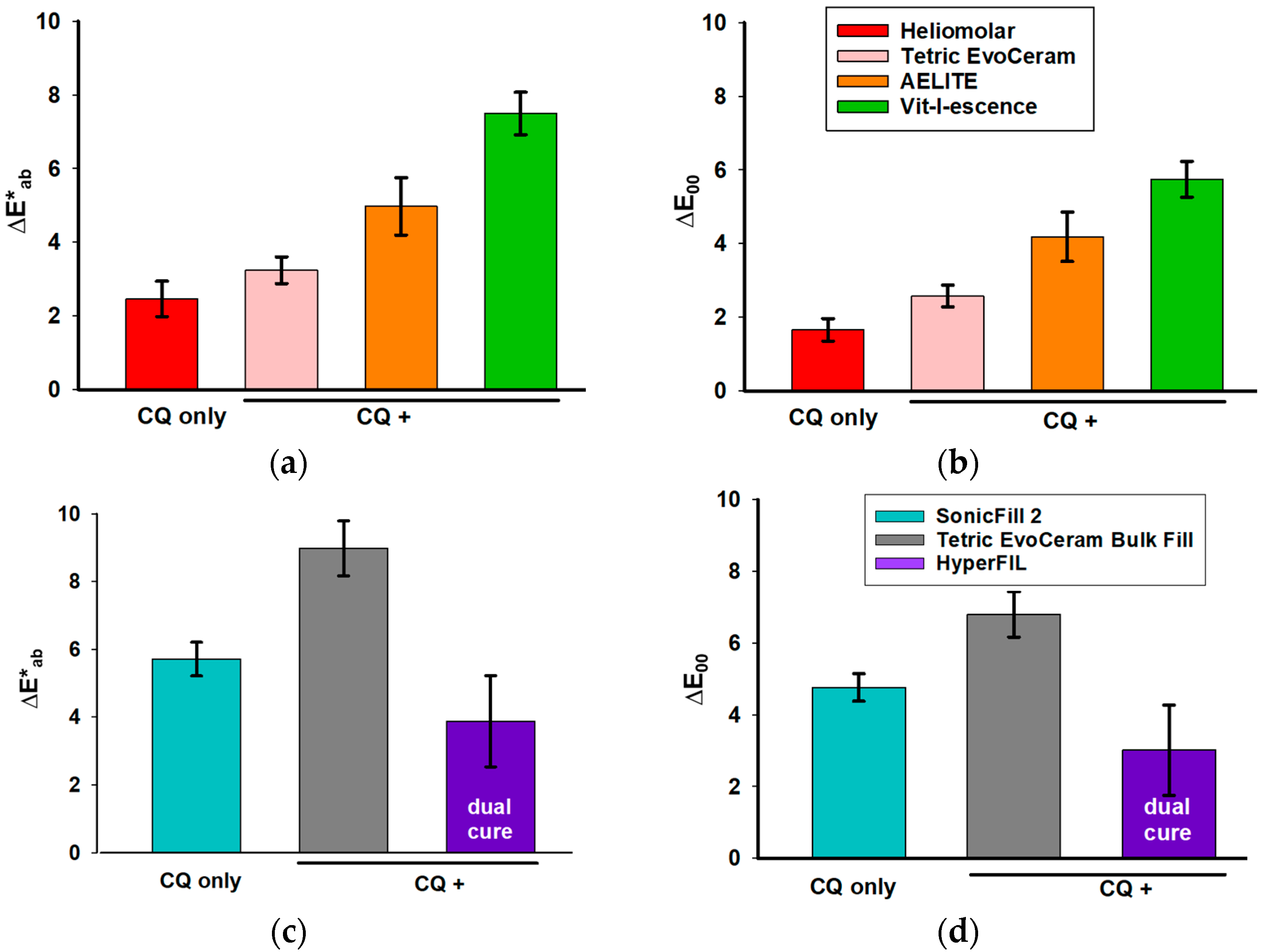
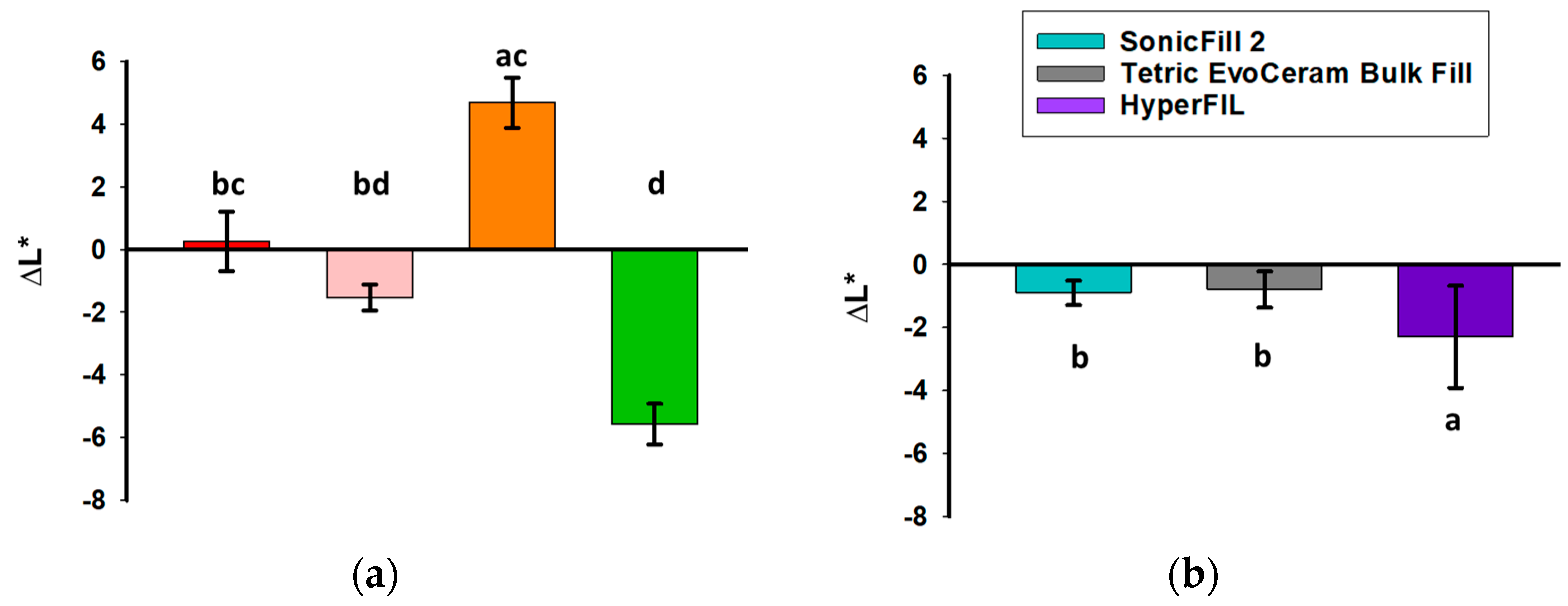
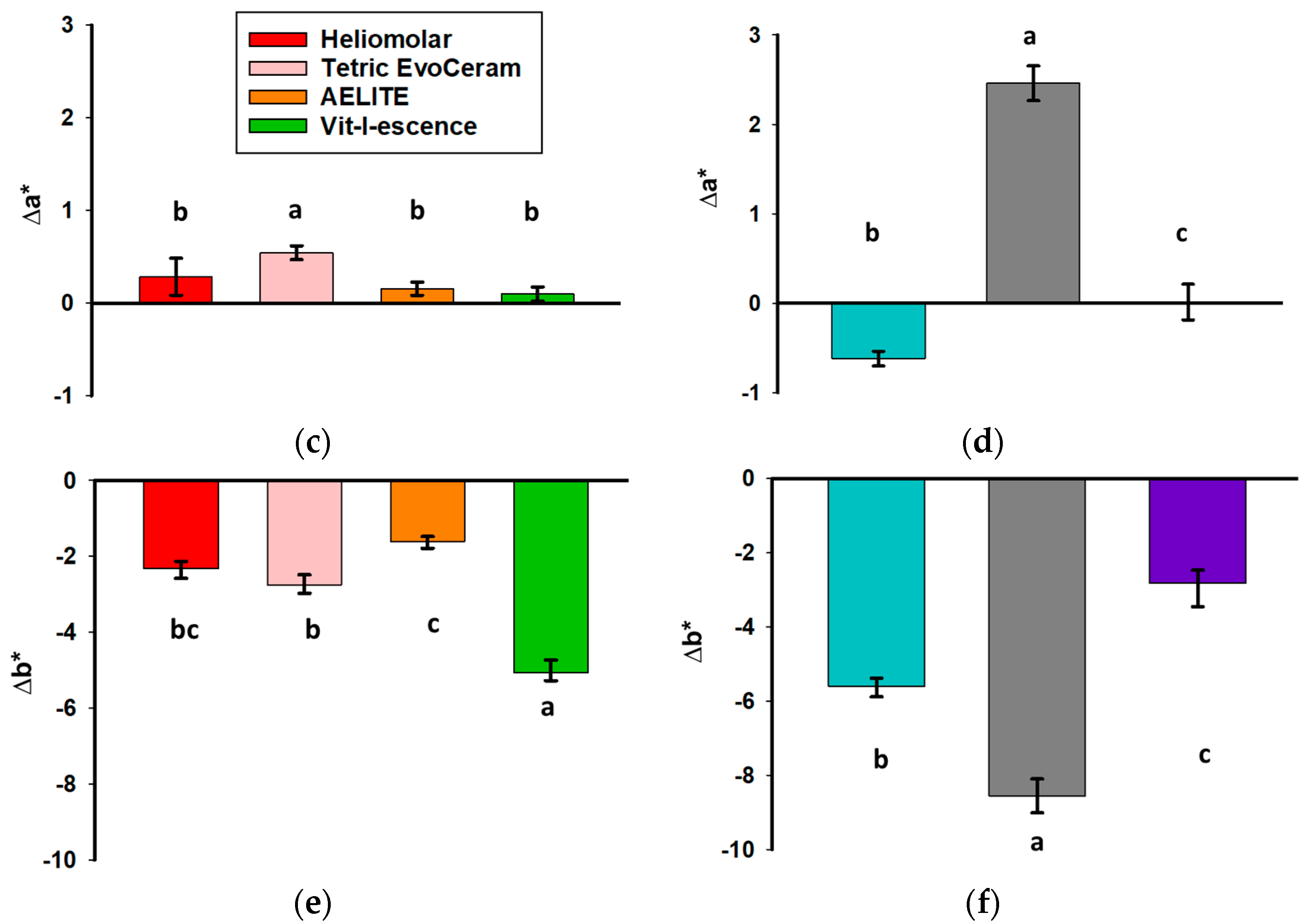
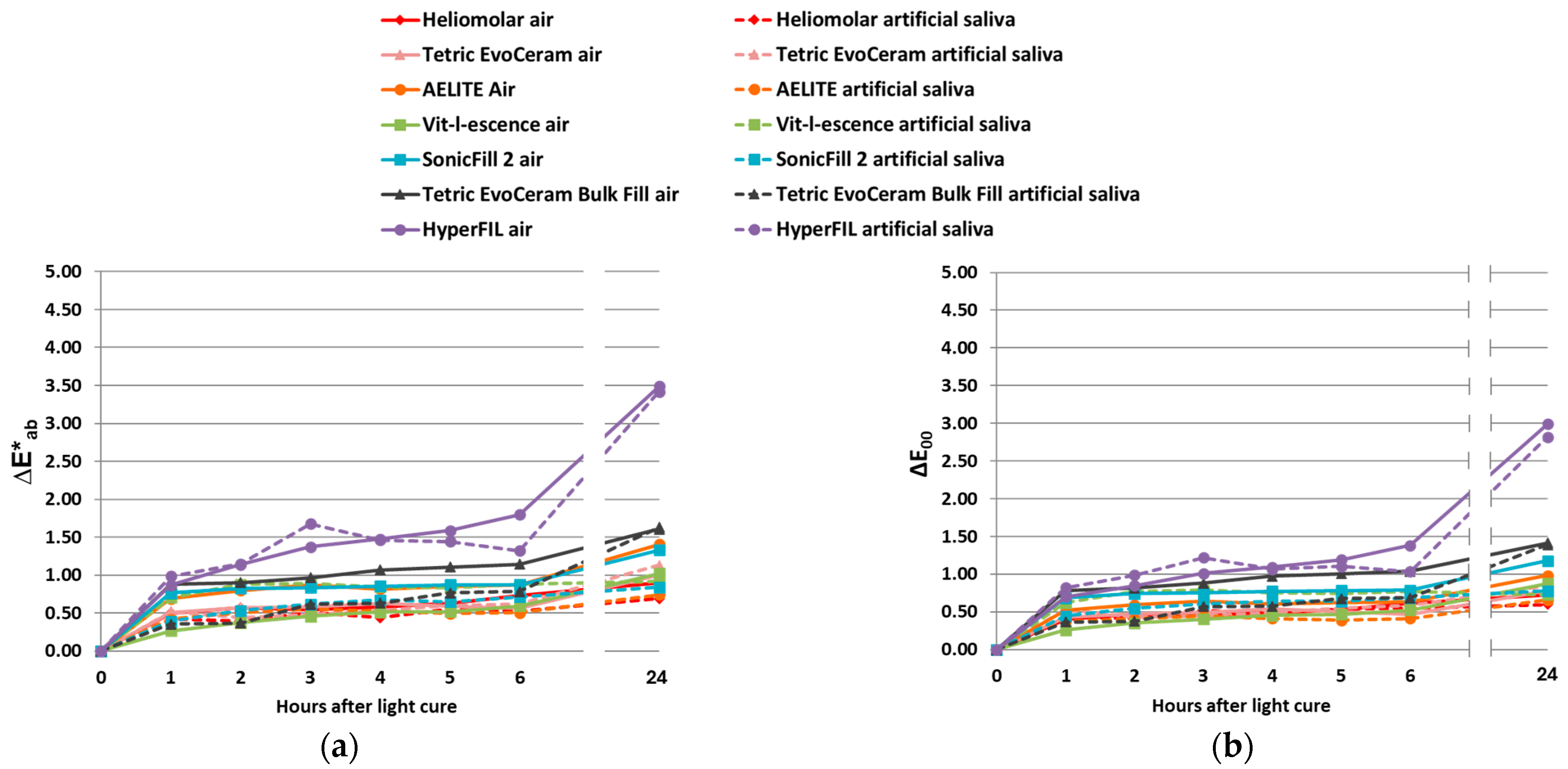
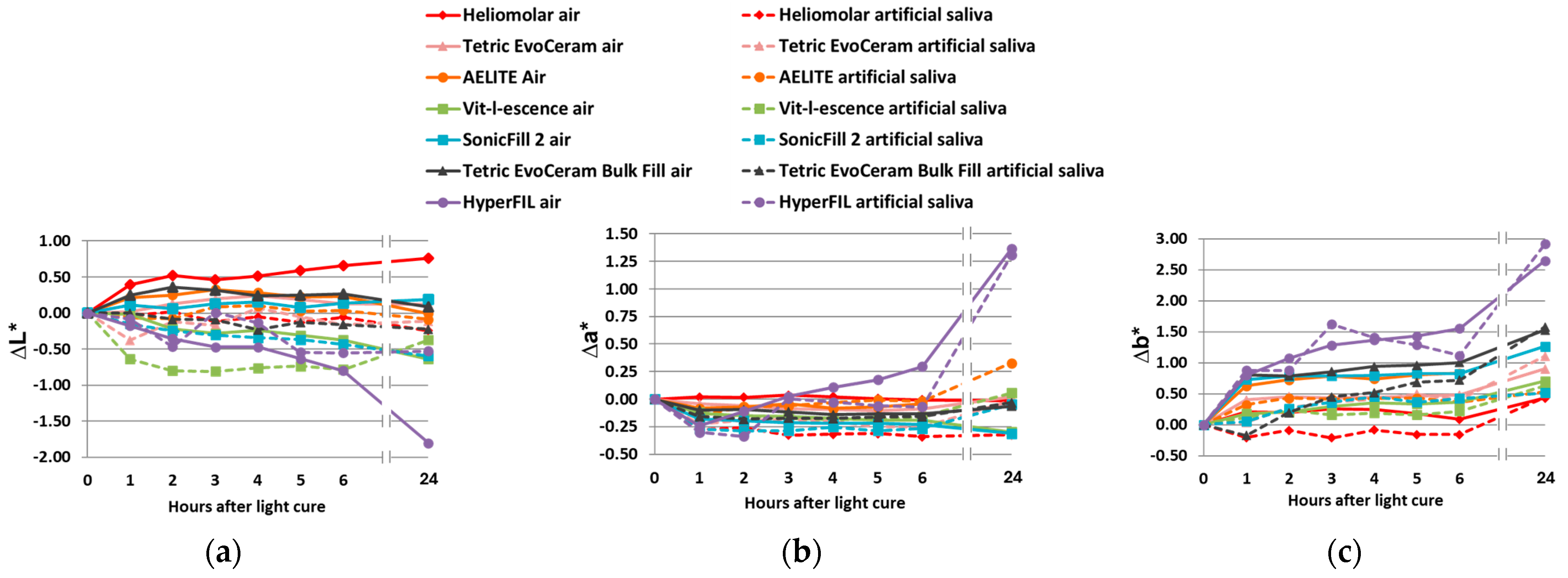
3.1. Color Change upon Polymerization
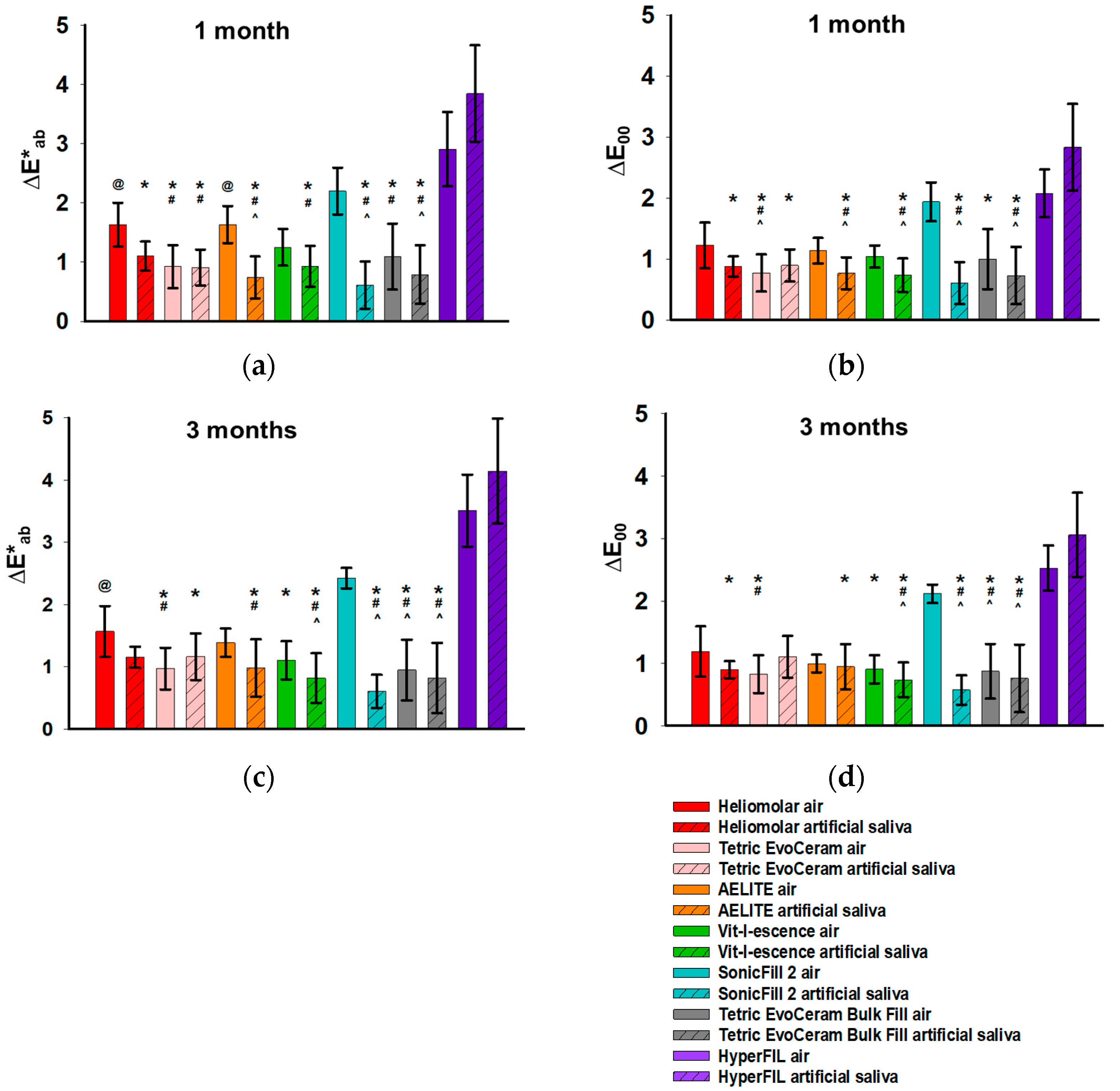
3.2. Color Change upon Aging
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CQ | Camphorquinone |
| CQ+ | CQ and additional photoinitiator(s) |
| TPO | Trimethylbenzoyl diphenylphosphine oxide |
| RCs | Resin composites |
| ΔE | Color change |
References
- Ferracane, J.L. A Historical Perspective on Dental Composite Restorative Materials. J. Funct. Biomater. 2024, 15, 173. [Google Scholar] [CrossRef]
- Bittencourt, B.F.; Dominguez, J.A.; Farago, P.V.; Pinheiro, L.A.; Gomes, O.M. Alternative coinitiators applicable to photocurable resin composites. Oral Health Dent. Manag. 2014, 13, 568–572. [Google Scholar]
- Kowalska, A.; Sokolowski, J.; Gozdek, T.; Krasowski, M.; Kopacz, K.; Bociong, K. The Influence of Various Photoinitiators on the Properties of Commercial Dental Composites. Polymers 2021, 13, 3972. [Google Scholar] [CrossRef]
- Santini, A.; Miletic, V.; Swift, M.D.; Bradley, M. Degree of conversion and microhardness of TPO-containing resin-based composites cured by polywave and monowave LED units. J. Dent. 2012, 40, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Palin, W.M.; Senyilmaz, D.P.; Marquis, P.M.; Shortall, A.C. Cure width potential for MOD resin composite molar restorations. Dent. Mater. 2008, 24, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Rueggeberg, F.A.; Giannini, M.; Arrais, C.A.G.; Price, R.B.T. Light curing in dentistry and clinical implications: A literature review. Braz. Oral Res. 2017, 31, e61. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Krifka, S.; Hiller, K.A.; Bolay, C.; Waha, C.; Van Meerbeek, B.; Schmalz, G.; Schweikl, H. Evaluation of cell responses toward adhesives with different photoinitiating systems. Dent. Mater. 2015, 31, 916–927. [Google Scholar] [CrossRef]
- Kowalska, A.; Sokolowski, J.; Bociong, K. The Photoinitiators Used in Resin Based Dental Composite—A Review and Future Perspectives. Polymers 2021, 13, 470. [Google Scholar] [CrossRef]
- Kowalska, A.; Sokolowski, J.; Szynkowska-Jozwik, M.I.; Gozdek, T.; Kopacz, K.; Bociong, K. Can TPO as Photoinitiator Replace “Golden Mean” Camphorquinone and Tertiary Amines in Dental Composites? Testing Experimental Composites Containing Different Concentration of Diphenyl(2,4,6-trimethylbenzoyl)phosphine Oxide. Int. J. Mol. Sci. 2022, 23, 11594. [Google Scholar] [CrossRef]
- Porto, I.C.; Soares, L.E.; Martin, A.A.; Cavalli, V.; Liporoni, P.C. Influence of the photoinitiator system and light photoactivation units on the degree of conversion of dental composites. Braz. Oral Res. 2010, 24, 475–481. [Google Scholar] [CrossRef]
- Sim, J.S.; Seol, H.J.; Park, J.K.; Garcia-Godoy, F.; Kim, H.I.; Kwon, Y.H. Interaction of LED light with coinitiator-containing composite resins: Effect of dual peaks. J. Dent. 2012, 40, 836–842. [Google Scholar] [CrossRef]
- de Oliveira, D.; Rocha, M.G.; Correa, I.C.; Correr, A.B.; Ferracane, J.L.; Sinhoreti, M.A.C. The effect of combining photoinitiator systems on the color and curing profile of resin-based composites. Dent. Mater. 2016, 32, 1209–1217. [Google Scholar] [CrossRef]
- Guiraldo, R.D.; Consani, S.; Consani, R.L.; Berger, S.B.; Mendes, W.B.; Sinhoreti, M.A.; Correr-Sobrinho, L. Comparison of silorane and methacrylate-based composite resins on the curing light transmission. Braz. Dent. J. 2010, 21, 538–542. [Google Scholar] [CrossRef]
- Price, R.B.; Felix, C.A. Effect of delivering light in specific narrow bandwidths from 394 to 515nm on the micro-hardness of resin composites. Dent. Mater. 2009, 25, 899–908. [Google Scholar] [CrossRef]
- Rocha, M.G.; de Oliveira, D.; Correa, I.C.; Correr-Sobrinho, L.; Sinhoreti, M.; Ferracane, J.L.; Correr, A.B. Light-emitting Diode Beam Profile and Spectral Output Influence on the Degree of Conversion of Bulk Fill Composites. Oper. Dent. 2017, 42, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, J.; Vani, K.; Alagarsamy, V.; J, P.; Sabarathinam, J. Analyzing the Bioactivity of a Novel Bone Cement: An In Vitro Study. Cureus 2024, 16, e68422. [Google Scholar] [CrossRef]
- Pecho, O.E.; Ghinea, R.; Alessandretti, R.; Perez, M.M.; Della Bona, A. Visual and instrumental shade matching using CIELAB and CIEDE2000 color difference formulas. Dent. Mater. 2016, 32, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Durand, L.B.; Ruiz-Lopez, J.; Perez, B.G.; Ionescu, A.M.; Carrillo-Perez, F.; Ghinea, R.; Perez, M.M. Color, lightness, chroma, hue, and translucency adjustment potential of resin composites using CIEDE2000 color difference formula. J. Esthet. Restor. Dent. 2021, 33, 836–843. [Google Scholar] [CrossRef]
- Paravina, R.D.; Ghinea, R.; Herrera, L.J.; Bona, A.D.; Igiel, C.; Linninger, M.; Sakai, M.; Takahashi, H.; Tashkandi, E.; Perez Mdel, M. Color difference thresholds in dentistry. J. Esthet. Restor. Dent. 2015, 27 (Suppl. S1), S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Miletic, V.; Santini, A. Micro-Raman spectroscopic analysis of the degree of conversion of composite resins containing different initiators cured by polywave or monowave LED units. J. Dent. 2012, 40, 106–113. [Google Scholar] [CrossRef]
- Ilie, N.; Hickel, R. Can CQ be completely replaced by alternative initiators in dental adhesives? Dent. Mater. J. 2008, 27, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Randolph, L.D.; Palin, W.M.; Bebelman, S.; Devaux, J.; Gallez, B.; Leloup, G.; Leprince, J.G. Ultra-fast light-curing resin composite with increased conversion and reduced monomer elution. Dent. Mater. 2014, 30, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.F.; Pfeifer, C.S.; Consani, S.; Prahl, S.A.; Ferracane, J.L. Influence of photoinitiator type on the rate of polymerization, degree of conversion, hardness and yellowing of dental resin composites. Dent. Mater. 2008, 24, 1169–1177. [Google Scholar] [CrossRef]
- Schroeder, W.F.; Cook, W.D.; Vallo, C.I. Photopolymerization of N,N-dimethylaminobenzyl alcohol as amine co-initiator for light-cured dental resins. Dent. Mater. 2008, 24, 686–693. [Google Scholar] [CrossRef]
- Leprince, J.G.; Palin, W.M.; Vanacker, J.; Sabbagh, J.; Devaux, J.; Leloup, G. Physico-mechanical characteristics of commercially available bulk-fill composites. J. Dent. 2014, 42, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Popal, M.; Volk, J.; Leyhausen, G.; Geurtsen, W. Cytotoxic and genotoxic potential of the type I photoinitiators BAPO and TPO on human oral keratinocytes and V79 fibroblasts. Dent. Mater. 2018, 34, 1783–1796. [Google Scholar] [CrossRef]
- Lu, H.; Stansbury, J.W.; Bowman, C.N. Impact of curing protocol on conversion and shrinkage stress. J. Dent. Res. 2005, 84, 822–826. [Google Scholar] [CrossRef]
- Pfeifer, C.S.; Shelton, Z.R.; Braga, R.R.; Windmoller, D.; Machado, J.C.; Stansbury, J.W. Characterization of dimethacrylate polymeric networks: A study of the crosslinked structure formed by monomers used in dental composites. Eur. Polym. J. 2011, 47, 162–170. [Google Scholar] [CrossRef]
- Stansbury, J.W. Curing dental resins and composites by photopolymerization. J. Esthet. Dent. 2000, 12, 300–308. [Google Scholar] [CrossRef]
- Ogunyinka, A.; Palin, W.M.; Shortall, A.C.; Marquis, P.M. Photoinitiation chemistry affects light transmission and degree of conversion of curing experimental dental resin composites. Dent. Mater. 2007, 23, 807–813. [Google Scholar] [CrossRef]
- Schneider, L.F.; Cavalcante, L.M.; Consani, S.; Ferracane, J.L. Effect of co-initiator ratio on the polymer properties of experimental resin composites formulated with camphorquinone and phenyl-propanedione. Dent. Mater. 2009, 25, 369–375. [Google Scholar] [CrossRef]
- Kim, E.H.; Jung, K.H.; Son, S.A.; Hur, B.; Kwon, Y.H.; Park, J.K. Effect of resin thickness on the microhardness and optical properties of bulk-fill resin composites. Restor. Dent. Endod. 2015, 40, 128–135. [Google Scholar] [CrossRef]
- Kim, I.J.; Lee, Y.K. Changes in color and color parameters of dental resin composites after polymerization. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 541–546. [Google Scholar] [CrossRef]
- Seghi, R.R.; Gritz, M.D.; Kim, J. Colorimetric changes in composites resulting from visible-light-initiated polymerization. Dent. Mater. 1990, 6, 133–137. [Google Scholar] [CrossRef]
- Lee, Y.K.; Yu, B.; Lim, H.N.; Lim, J.I. Difference in the color stability of direct and indirect resin composites. J. Appl. Oral Sci. 2011, 19, 154–160. [Google Scholar] [CrossRef]
- Silami, F.D.; Mundim, F.M.; Garcia Lda, F.; Sinhoreti, M.A.; Pires-de-Souza Fde, C. Color stability of experimental composites containing different photoinitiators. J. Dent. 2013, 41 (Suppl. S3), e62–e66. [Google Scholar] [CrossRef]
- de Oliveira, D.C.; Rocha, M.G.; Gatti, A.; Correr, A.B.; Ferracane, J.L.; Sinhoret, M.A. Effect of different photoinitiators and reducing agents on cure efficiency and color stability of resin-based composites using different LED wavelengths. J. Dent. 2015, 43, 1565–1572. [Google Scholar] [CrossRef]
- Albuquerque, P.P.; Moreira, A.D.; Moraes, R.R.; Cavalcante, L.M.; Schneider, L.F. Color stability, conversion, water sorption and solubility of dental composites formulated with different photoinitiator systems. J. Dent. 2013, 41 (Suppl S3), e67–e72. [Google Scholar] [CrossRef] [PubMed]
- Paravina, R.D.; Perez, M.M.; Ghinea, R. Acceptability and perceptibility thresholds in dentistry: A comprehensive review of clinical and research applications. J. Esthet. Restor. Dent. 2019, 31, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Milagres, V.; Teixeira, M.L.; Miranda, M.E.; Osorio Silva, C.H.; Ribeiro Pinto, J.R. Effect of gender, experience, and value on color perception. Oper. Dent. 2012, 37, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Kurt, M.; Nemli, S.K.; Gungor, M.B.; Bal, B.T.; Ozturk, E. Perceptibility and acceptability thresholds for color differences of light and dark maxillofacial skin replications. Vis. Res. 2024, 223, 108474. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Polo, C.; Portillo Munoz, M.; Lorenzo Luengo, M.C.; Vicente, P.; Galindo, P.; Martin Casado, A.M. Comparison of the CIELab and CIEDE2000 color difference formulas. J. Prosthet. Dent. 2016, 115, 65–70. [Google Scholar] [CrossRef]
- Ragain, J.C., Jr.; Johnston, W.M. Minimum color differences for discriminating mismatch between composite and tooth color. J. Esthet. Restor. Dent. 2001, 13, 41–48. [Google Scholar] [CrossRef]
- Da Silva, J.D.; Park, S.E.; Weber, H.P.; Ishikawa-Nagai, S. Clinical performance of a newly developed spectrophotometric system on tooth color reproduction. J. Prosthet. Dent. 2008, 99, 361–368. [Google Scholar] [CrossRef]
- Celik, E.U.; Aladag, A.; Turkun, L.S.; Yilmaz, G. Color changes of dental resin composites before and after polymerization and storage in water. J. Esthet. Restor. Dent. 2011, 23, 179–188. [Google Scholar] [CrossRef]
- Salgado, V.E.; Rego, G.F.; Schneider, L.F.; Moraes, R.R.; Cavalcante, L.M. Does translucency influence cure efficiency and color stability of resin-based composites? Dent. Mater. 2018, 34, 957–966. [Google Scholar] [CrossRef]
- Barutcigil, C.; Barutcigil, K.; Ozarslan, M.M.; Dundar, A.; Yilmaz, B. Color of bulk-fill composite resin restorative materials. J. Esthet. Restor. Dent. 2018, 30, E3–E8. [Google Scholar] [CrossRef]
- Paolone, G.; Pavan, F.; Guglielmi, P.C.; Scotti, N.; Cantatore, G.; Vichi, A. In vitro procedures for color stability evaluation of dental resin-based composites exposed to smoke: A scoping review. Dent. Mater. J. 2022, 41, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Alghazzawi, T.F. The effects of monomer type, filler size, and filler content of three resin cements on the color stability of laminate veneers exposed to accelerated aging. Dent. Mater. 2024, 40, e23–e30. [Google Scholar] [CrossRef]
- Alkhudhairy, F.; Vohra, F.; Naseem, M.; Owais, M.M.; Amer, A.H.B.; Almutairi, K.B. Color stability and degree of conversion of a novel dibenzoyl germanium derivative containing photo-polymerized resin luting cement. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800020917326. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K. Comparison of CIELAB ΔE* and CIEDE2000 color-differences after polymerization and thermocycling of resin composites. Dent. Mater. 2005, 21, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Acar, O.; Yilmaz, B.; Altintas, S.H.; Chandrasekaran, I.; Johnston, W.M. Color stainability of CAD/CAM and nanocomposite resin materials. J. Prosthet. Dent. 2016, 115, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Ghinea, R.; Perez, M.M.; Herrera, L.J.; Rivas, M.J.; Yebra, A.; Paravina, R.D. Color difference thresholds in dental ceramics. J. Dent. 2010, 38 (Suppl. S2), e57–e64. [Google Scholar] [CrossRef] [PubMed]

| Resin Composites | Manufacturer/Shade | Photoinitiator | Reference |
|---|---|---|---|
| Heliomolar | Ivoclar a/A2 | Traditional CQ | [13] |
| Tetric EvoCeram | Ivoclar a/A2 | Traditional CQ + TPO | [5] |
| AELITE | Bisco b/A2 | Traditional CQ + TPO | [14] |
| Vit-l-escence | Ultradent c/A2 | Traditional CQ + TPO | [4] |
| SonicFill 2 | Kerr d/A2 | Bulk-fill CQ | [15] |
| Tetric EvoCeram Bulk Fill | Ivoclar a/(IVA/Universal A) | Bulk-fill CQ + Ivocerin + TPO | [15] |
| HyperFIL | Parkell e/Universal (A2/B2) | Bulk-fill dual-cure CQ + unknown | Manufacturer |
| Resin Composites/Shade | Group | Aging Method | Before Light (n = 8) | |||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||
| Mean | SD | Mean | SD | Mean | SD | |||
| Heliomolar/A2 | Traditional CQ | Artificial Saliva | 61.97 | 1.05 | −1.43 | 0.25 | 14.29 | 0.70 |
| Air | 62.26 | 1.29 | −1.50 | 0.42 | 14.44 | 0.65 | ||
| Tetric EvoCeram/A2 | Traditional CQ+ | Artificial Saliva | 56.64 | 0.70 | −0.50 | 0.05 | 10.23 | 0.24 |
| Air | 56.91 | 1.05 | −0.54 | 0.08 | 10.07 | 0.28 | ||
| AELITE/A2 | Traditional CQ+ | Artificial Saliva | 59.82 | 0.67 | 0.78 | 0.10 | 10.09 | 0.47 |
| Air | 59.60 | 0.62 | 0.79 | 0.11 | 10.71 | 0.20 | ||
| Vit-l-escence/A2 | Traditional CQ+ | Artificial Saliva | 66.98 | 0.99 | −0.10 | 0.13 | 13.31 | 0.39 |
| Air | 66.21 | 0.93 | −0.06 | 0.11 | 13.51 | 0.16 | ||
| SonicFill Bulk/A2 | Bulk CQ | Artificial Saliva | 58.46 | 0.43 | −0.96 | 0.06 | 8.65 | 0.24 |
| Air | 57.84 | 0.92 | −0.91 | 0.09 | 7.89 | 0.36 | ||
| Tetric EvoCeram Bulk/IVA/Universal A | Bulk CQ+ | Artificial Saliva | 53.19 | 0.42 | −4.14 | 0.09 | 11.64 | 0.37 |
| Air | 53.89 | 0.84 | −4.41 | 0.17 | 11.90 | 0.54 | ||
| HyperFIL Bulk/Universal (A2/B2) | Bulk CQ+ | Artificial Saliva | 60.26 | 1.37 | 0.30 | 0.51 | 11.41 | 1.17 |
| Air | 58.74 | 0.58 | 0.48 | 0.17 | 11.57 | 0.48 | ||
| Source of Variation/p | ΔE*ab | ΔE00 | ||||
|---|---|---|---|---|---|---|
| 1 d | 1 m | 3 m | 1 d | 1 m | 3 m | |
| photoinitiator (CQ only or CQ+) | # <0.001 | 0.566 | 0.519 | # <0.001 | 0.801 | 0.625 |
| aging method (air or artificial saliva) | 0.186 | # 0.004 | # 0.016 | 0.217 | # 0.004 | # 0.012 |
| photoinitiator × aging | 0.56 | # 0.021 | # 0.015 | 0.646 | # 0.005 | # 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, F.; Berzins, D.W. Color Change in Commercial Resin Composites with Different Photoinitiators. Bioengineering 2025, 12, 1047. https://doi.org/10.3390/bioengineering12101047
Gao F, Berzins DW. Color Change in Commercial Resin Composites with Different Photoinitiators. Bioengineering. 2025; 12(10):1047. https://doi.org/10.3390/bioengineering12101047
Chicago/Turabian StyleGao, Feng, and David W. Berzins. 2025. "Color Change in Commercial Resin Composites with Different Photoinitiators" Bioengineering 12, no. 10: 1047. https://doi.org/10.3390/bioengineering12101047
APA StyleGao, F., & Berzins, D. W. (2025). Color Change in Commercial Resin Composites with Different Photoinitiators. Bioengineering, 12(10), 1047. https://doi.org/10.3390/bioengineering12101047







