Can Artificial Intelligence Improve the Appropriate Use and Decrease the Misuse of REBOA?
Abstract
1. Introduction
2. Materials and Methods
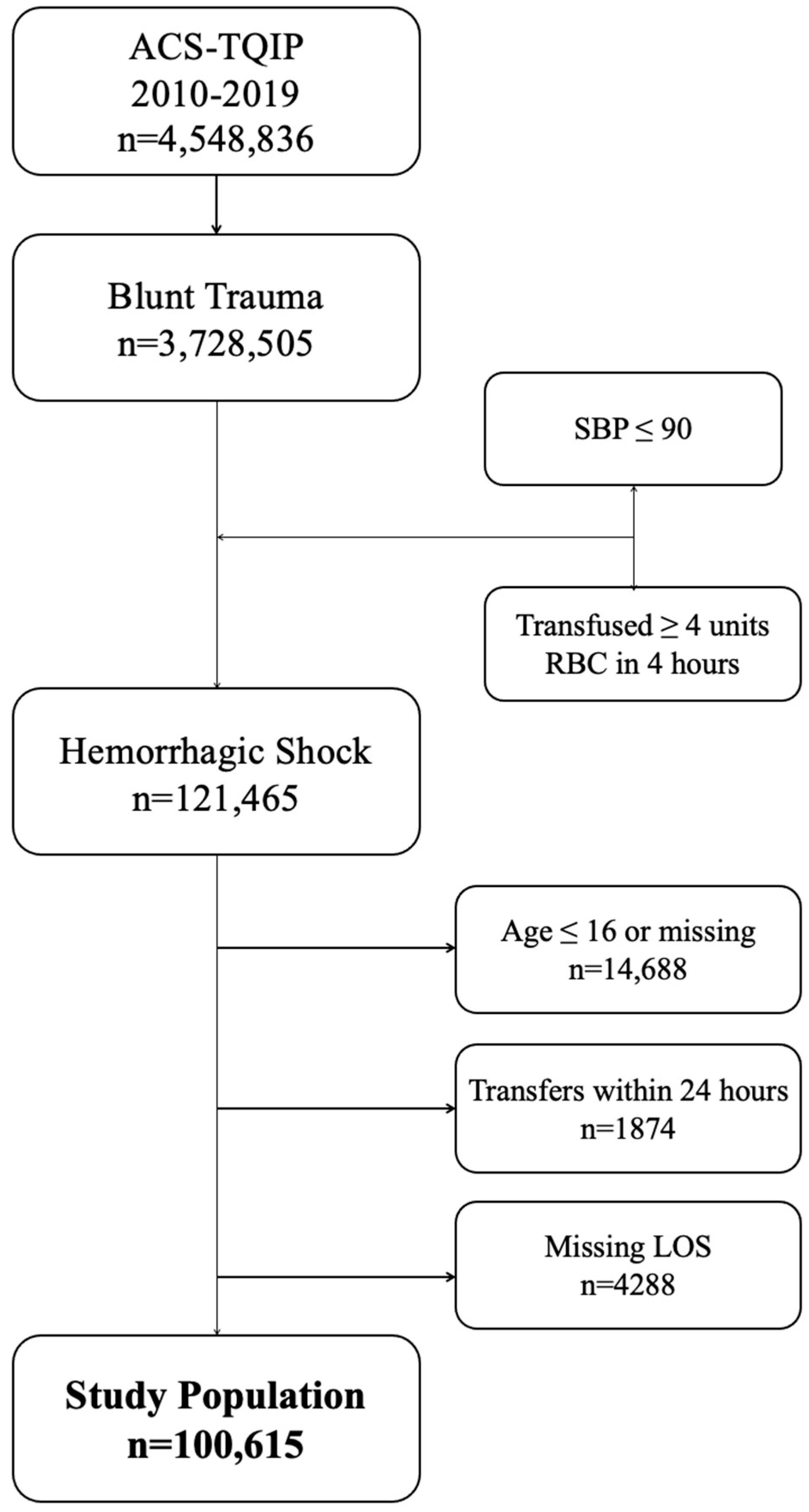
2.1. Patient Population
2.2. Data Points
2.3. Outcome Definitions
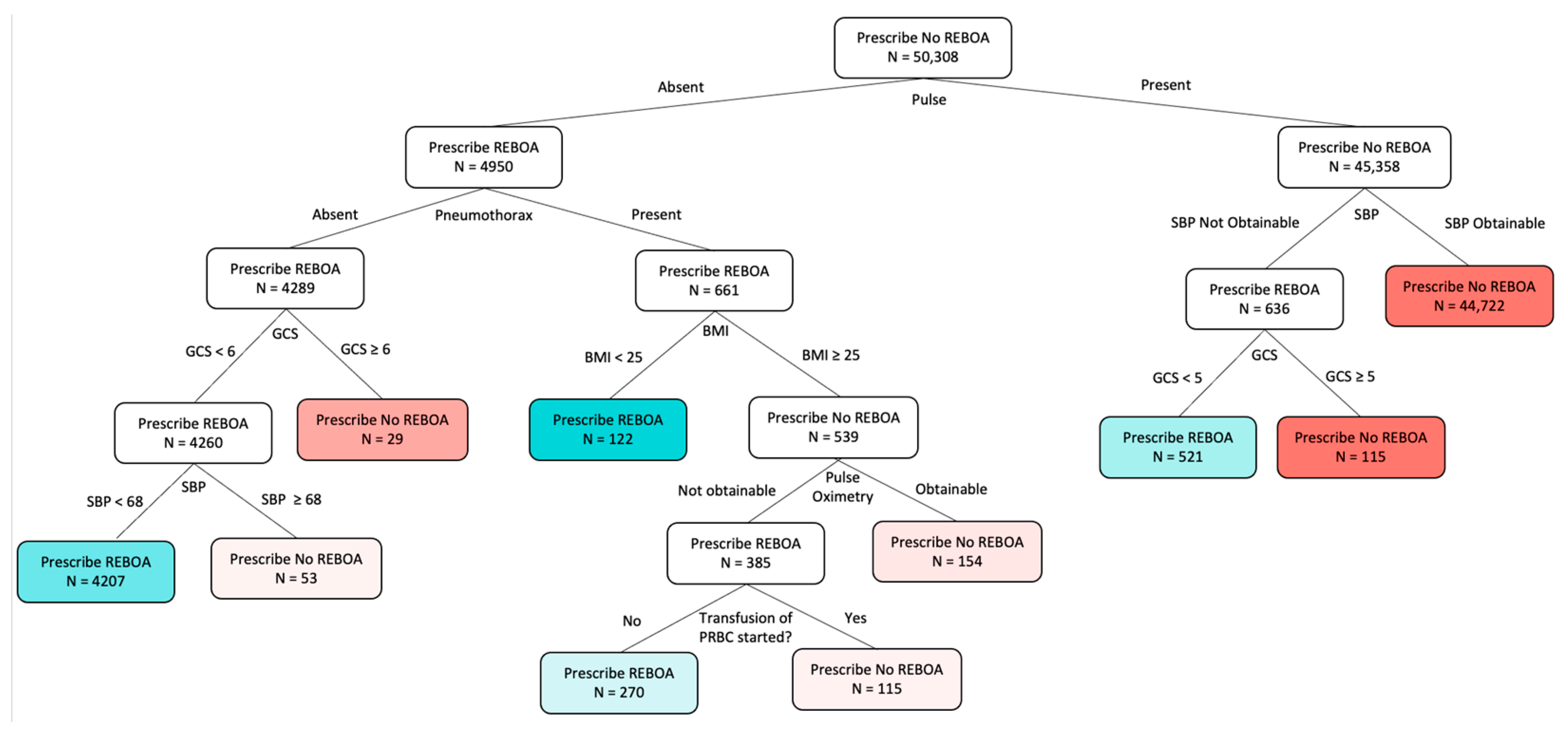
2.4. Optimal Policy Trees
2.5. Measurement of Model Performance
2.5.1. Policy Evaluation
2.5.2. Other Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Outcomes
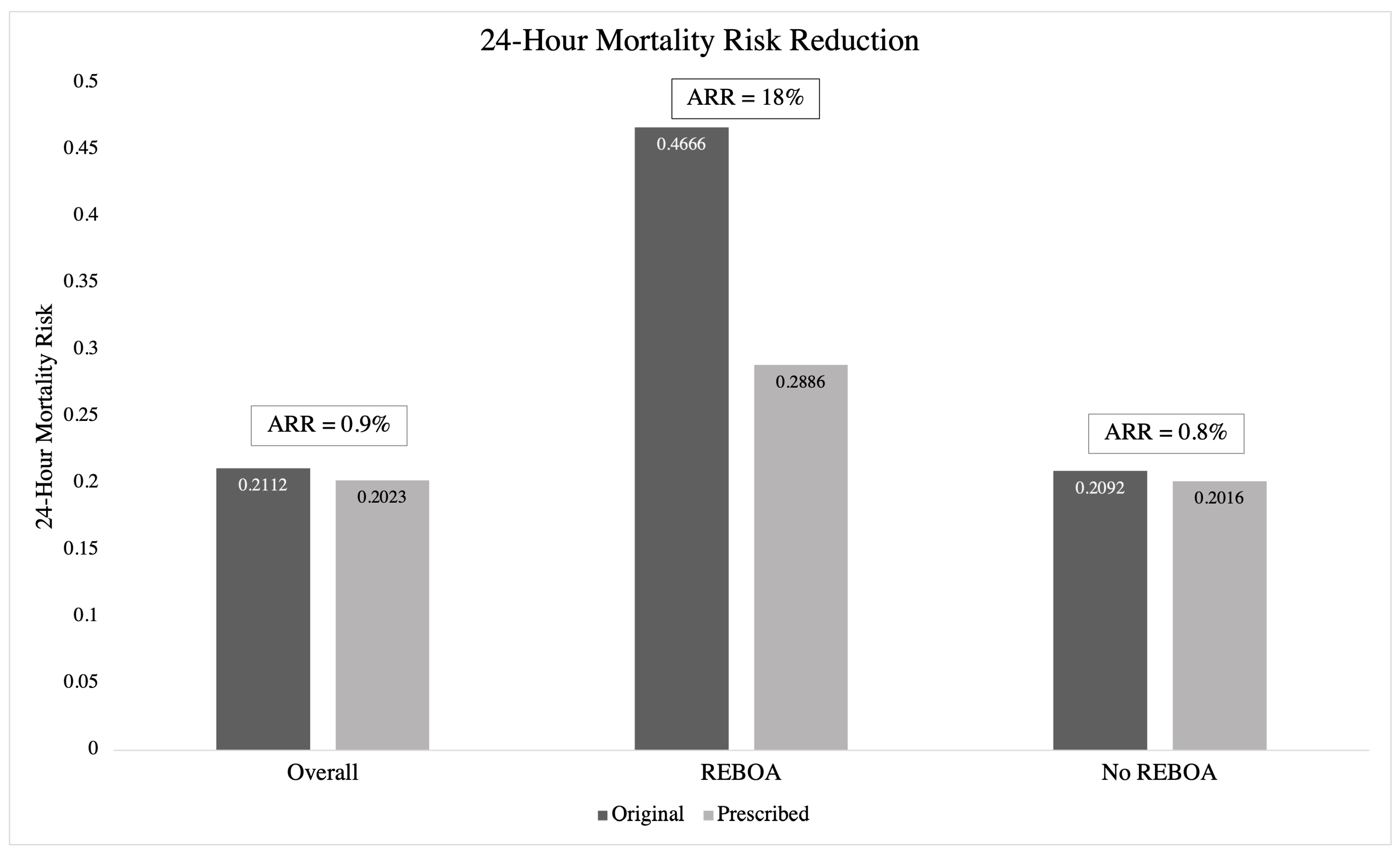
3.3. OPT and 24 h Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inoue, J.; Shiraishi, A.; Yoshiyuki, A.; Haruta, K.; Matsui, H.; Otomo, Y. Resuscitative endovascular balloon occlusion of the aorta might be dangerous in patients with severe torso trauma: A propensity score analysis. J. Trauma Acute Care Surg. 2016, 80, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Zeeshan, M.; Sakran, J.V.; Hamidi, M.; Kulvatunyou, N.; Khan, M.; O’kEeffe, T.; Rhee, P. Nationwide Analysis of Resuscitative Endovascular Balloon Occlusion of the Aorta in Civilian Trauma. JAMA Surg. 2019, 154, 500–508. [Google Scholar] [CrossRef]
- Yamamoto, R.; Cestero, R.F.; Suzuki, M.; Funabiki, T.; Sasaki, J. Resuscitative endovascular balloon occlusion of the aorta (REBOA) is associated with improved survival in severely injured patients: A propensity score matching analysis. Am. J. Surg. 2019, 218, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Abe, T.; Hagiwara, S.; Saitoh, D.; Oshima, K. Resuscitative endovascular balloon occlusion of the aorta may contribute to improved survival. Scand. J. Trauma Resusc. Emerg. Med. 2020, 28, 62. [Google Scholar] [CrossRef]
- García, A.F.; Manzano-Nunez, R.; Orlas, C.P.; Ruiz-Yucuma, J.; Londoño, A.; Salazar, C.; Melendez, J.; Sánchez, Á.I.; Puyana, J.C.; Ordoñez, C.A. Association of resuscitative endovascular balloon occlusion of the aorta (REBOA) and mortality in penetrating trauma patients. Eur. J. Trauma Emerg. Surg. 2021, 47, 1779–1785. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Teeter, W.; Hoehn, M.; Pasley, J.; Hu, P.; Yang, S.; Romagnoli, A.; Diaz, J.; Stein, D.; Scalea, T. Use of Resuscitative Endovascular Balloon Occlusion of the Aorta for Proximal Aortic Control in Patients with Severe Hemorrhage and Arrest. JAMA Surg. 2018, 153, 130–135. [Google Scholar] [CrossRef]
- DuBose, J.J.; Scalea, T.M.; Brenner, M.; Skiada, D.; Inaba, K.; Cannon, J.; Moore, L.; Holcomb, J.; Turay, D.; Arbabi, C.N.; et al. The AAST prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) registry: Data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J. Trauma Acute Care Surg. 2016, 81, 409–419. [Google Scholar] [CrossRef]
- Brenner, M.; Zakhary, B.; Coimbra, R.; Morrison, J.; Scalea, T.; Moore, L.J.; Podbielski, J.; Holcomb, J.B.; Inaba, K.; Cannon, J.W.; et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA) may be superior to resuscitative thoracotomy (RT) in patients with traumatic brain injury (TBI). Trauma Surg. Acute Care Open 2022, 7, e000715. [Google Scholar] [CrossRef]
- Hughes, C.W. Use of an intra-aortic balloon catheter tamponade for controlling intra-abdominal hemorrhage in man. Surgery 1954, 36, 65–68. [Google Scholar]
- Brenner, M.; Bulger, E.M.; Perina, D.G.; Henry, S.; Kang, C.S.; Rotondo, M.F.; Chang, M.C.; Weireter, L.J.; Coburn, M.; Winchell, R.J.; et al. Joint statement from the American College of Surgeons Committee on Trauma (ACS COT) and the American College of Emergency Physicians (ACEP) regarding the clinical use of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA). Trauma Surg. Acute Care Open 2018, 3, e000154. [Google Scholar] [CrossRef] [PubMed]
- Bulger, E.M.; Perina, D.G.; Qasim, Z.; Beldowicz, B.; Brenner, M.; Guyette, F.; Rowe, D.; Kang, C.S.; Gurney, J.; DuBose, J.; et al. Clinical use of resuscitative endovascular balloon occlusion of the aorta (REBOA) in civilian trauma systems in the USA, 2019: A joint statement from the American College of Surgeons Committee on Trauma, the American College of Emergency Physicians, the National Association of Emergency Medical Services Physicians and the National Association of Emergency Medical Technicians. Trauma Surg. Acute Care Open 2019, 4, e000376. [Google Scholar] [CrossRef]
- Inaba, K.; Alam, H.B.; Brasel, K.J.; Brenner, M.; Brown, C.V.R.; Ciesla, D.J.; de Moya, M.A.; DuBose, J.J.; Moore, E.E.; Moore, L.J.; et al. A Western Trauma Association critical decisions algorithm: Resuscitative endovascular balloon occlusion of the aorta. J. Trauma Acute Care Surg. 2022, 92, 748–753. [Google Scholar] [CrossRef]
- Bertsimas, D.; Dunn, J.; Velmahos, G.C.; Kaafarani, H.M.A. Surgical Risk Is Not Linear: Derivation and Validation of a Novel, User-friendly, and Machine-learning-based Predictive OpTimal Trees in Emergency Surgery Risk (POTTER) Calculator. Ann. Surg. 2018, 268, 574–583. [Google Scholar] [CrossRef]
- Maurer, L.R.; Bertsimas, D.; Bouardi, H.T.; Hechi, M.E.; Moheb, M.E.; Giannoutsou, K.; Zhuo, D.; Dunn, J.; Velmahos, G.C.; Kaafarani, H.M.A. Trauma outcome predictor: An artificial intelligence interactive smartphone tool to predict outcomes in trauma patients. J. Trauma Acute Care Surg. 2021, 91, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Bertsimas, D.; Dunn, J.; Mundru, N. Optimal prescriptive trees. Inf. J. Optim. 2019, 1, 164–183. [Google Scholar] [CrossRef]
- Amram, M.; Dunn, J.; Zhuo, Y.D. Optimal policy trees. Mach. Learn. 2022, 111, 2741–2768. [Google Scholar] [CrossRef]
- Funk, M.J.; Westreich, D.; Wiesen, C.; Stürmer, T.; Brookhart, M.A.; Davidian, M. Doubly robust estimation of causal effects. Am. J. Epidemiol. 2011, 173, 761–767. [Google Scholar] [CrossRef]
- Wager, S.; Athey, S. Estimation and inference of heterogeneous treatment effects using random forests. J. Am. Stat. Assoc. 2018, 113, 1228–1242. [Google Scholar] [CrossRef]
- Hang, C.N.; Yu, P.-D.; Chen, S.; Tan, C.W.; Chen, G. MEGA: Machine Learning-Enhanced Graph Analytics for Infodemic Risk Management. IEEE J. Biomed. Health Inform. 2023, 27, 6100–6111. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Ma, T.; Xiao, C.; Sun, J. Pre-training of Graph Augmented Transformers for Medication Recommendation. In Proceedings of the 28th International Joint Conference on Artificial Intelligence (IJCAI), Macau, China, 10–16 August 2019; pp. 5953–5959. [Google Scholar]
- Choi, E.; Xu, Z.; Li, Y.; Dusenberry, M.; Flores, G.; Xue, E.; Dai, A.M. Learning the Graphical Structure of Electronic Health Records with Graph Convolutional Transformer. Proc. AAAI Conf. Artif. Intell. 2020, 34, 606–613. [Google Scholar] [CrossRef]
- Bertsimas, D.; Pawlowski, C.; Zhuo, Y.D. From predictive methods to missing data imputation: An optimization approach. J. Mach. Learn. Res. 2018, 18, 1–39. [Google Scholar]
- Moore, L.J.; Fox, E.E.; Meyer, D.E.; Wade, C.E.; Podbielski, J.M.; Xu, X.; Morrison, J.J.M.; Scalea, T.; Fox, C.J.; Moore, E.E.; et al. Prospective Observational Evaluation of the ER-REBOA Catheter at 6 U.S. Trauma Centers. Ann. Surg. 2022, 275, e520–e526. [Google Scholar] [CrossRef]
- Romagnoli, A.; Teeter, W.; Pasley, J.; Hu, P.; Hoehn, M.; Stein, D.; Scalea, T.; Brenner, M. Time to aortic occlusion: It’s all about access. J. Trauma Acute Care Surg. 2017, 83, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- El Hechi, M.; Ward, T.M.; An, G.C.; Maurer, L.; Moheb, M.E.; Tsoulfas, G.; Kaafarani, H.M. Artificial Intelligence, Machine Learning, and Surgical Science: Reality Versus Hype. J. Surg. Res. 2021, 264, A1–A9. [Google Scholar] [CrossRef] [PubMed]
- Maurer, L.R.; Bertsimas, D.; Kaafarani, H.M.A. Machine Learning Reimagined: The Promise of Interpretability to Combat Bias. Ann. Surg. 2022, 275, e738–e739. [Google Scholar] [CrossRef]
| No REBOA | REBOA | p-Value | |
|---|---|---|---|
| N (%) | 99,812 (99.2%) | 803 (0.8%) | |
| Age, median (IQR) | 52 (33, 66) | 48 (29, 61) | <0.001 |
| Sex, n (%) | 0.008 | ||
| Male | 64,471 (64.6%) | 555 (69.1%) | |
| Female | 35,317 (35.4%) | 248 (30.9%) | |
| Admission physiology, median (IQR) | |||
| SBP | 82 (70, 90) | 86 (69, 118) | <0.001 |
| Pulse | 90 (70, 112) | 108 (80, 130) | <0.001 |
| Temperature | 36.4 (36, 36.7) | 36.1 (35.6, 36.5) | <0.001 |
| GCS | 14 (3, 15) | 3 (3, 14) | <0.001 |
| Respiratory rate | 18 (16, 22) | 20 (16, 25) | <0.001 |
| Pulse oximetry | 97 (93, 100) | 97 (91, 100) | 0.016 |
| Supplemental oxygen, n (%) | 49,406 (55.4%) | 547 (79.3%) | <0.001 |
| Intubated in ED, n (%) | 25,577 (25.6%) | 322 (40.1%) | <0.001 |
| Height, median (IQR) | 172.72 (165, 180) | 175 (165.1, 180.3) | 0.034 |
| Weight, median (IQR) | 80.29 (68, 97) | 85 (72, 100) | <0.001 |
| BMI, median (IQR) | 26.9 (23.5, 31.7) | 28.7 (24.7, 33.4) | <0.001 |
| Signs of life in ED, n (%) | 0.57 | ||
| No signs of life | 8628 (8.6%) | 74 (9.2%) | |
| Signs of life | 91,184 (91.4%) | 729 (90.8%) | |
| Teaching status, n (%) | <0.001 | ||
| Community | 34,933 (35.1%) | 187 (23.4%) | |
| Nonteaching | 11,753 (11.8%) | 52 (6.5%) | |
| University | 52,735 (53.0%) | 559 (70.1%) | |
| ACS verification level, n (%) | <0.001 | ||
| 1 | 49,277 (58.8%) | 589 (86.7%) | |
| 2 | 21,719 (25.9%) | 80 (11.8%) | |
| 3 | 12,848 (15.3%) | 10 (1.5%) | |
| ED Procedures, n (%) | |||
| Chest tube placement | 942 (0.9%) | 24 (3.0%) | <0.001 |
| Transfusion of RBCs | 19,865 (19.9%) | 519 (64.6%) | <0.001 |
| Transfusion of whole blood | 1643 (1.6%) | 77 (9.6%) | <0.001 |
| ED Diagnoses, n (%) | |||
| Pelvic fx | 9105 (9.1%) | 287 (35.7%) | <0.001 |
| Femur fx | 2077 (2.1%) | 33 (4.1%) | <0.001 |
| Hemothorax | 6851 (6.9%) | 160 (19.9%) | <0.001 |
| Pneumothorax | 20,938 (21.0%) | 376 (46.8%) | <0.001 |
| Thoracic aorta injury | 2419 (2.4%) | 51 (6.4%) | <0.001 |
| No REBOA | REBOA | p-Value | |
|---|---|---|---|
| N (%) | 99,812 (99.2%) | 803 (0.8%) | |
| Transfusion volume (units) in 4 h, median (IQR) | |||
| RBC | 5 (4, 9) | 12 (7, 21) | <0.001 |
| FFP | 4 (2, 7) | 8 (4, 15) | <0.001 |
| PLT | 5 (0, 8) | 7 (4, 15) | <0.001 |
| Cryo | 0 (0, 0) | 0 (0, 2) | <0.001 |
| AIS Body Region, median (IQR) | |||
| Head | 1 (0, 3) | 2 (0, 3) | <0.001 |
| Face | 0 (0, 1) | 0 (0, 1) | <0.001 |
| Thorax | 2 (0, 3) | 3 (2, 4) | <0.001 |
| Abdomen | 1 (0, 2) | 3 (2, 4) | <0.001 |
| Extremity | 2 (0, 3) | 3 (2, 4) | <0.001 |
| ISS, median (IQR) | 21 (10, 33) | 34 (26, 45) | <0.001 |
| Hemorrhage Control Procedures in 4 h, n (%) | |||
| Laparotomy | 12,045 (12.1%) | 373 (46.5%) | <0.001 |
| Thoracotomy/Sternotomy | 2256 (2.3%) | 90 (11.2%) | <0.001 |
| Extremity vascular | 2900 (2.9%) | 34 (4.2%) | 0.026 |
| Preperitoneal pelvic packing | 1880 (1.9%) | 99 (12.3%) | <0.001 |
| External fixation of pelvis | 147 (0.1%) | 19 (2.4%) | <0.001 |
| Angioembolization of pelvis | 1868 (1.9%) | 122 (15.2%) | <0.001 |
| Comorbidities, n (%) | |||
| Alcoholism | 8657 (8.8%) | 42 (5.4%) | <0.001 |
| Bleeding disorder | 3028 (3.1%) | 10 (1.3%) | 0.004 |
| CHF | 4121 (4.2%) | 7 (0.9%) | <0.001 |
| Smoker | 17,240 (17.5%) | 101 (13.1%) | 0.001 |
| CKD | 1751 (1.8%) | 5 (0.6%) | 0.017 |
| Diabetes | 11,619 (11.8%) | 54 (7.0%) | <0.001 |
| MI | 1138 (1.2%) | 4 (0.5%) | 0.097 |
| PAD | 709 (0.7%) | 3 (0.4%) | 0.28 |
| HTN | 26,725 (27.2%) | 106 (13.7%) | <0.001 |
| COPD | 6899 (7.0%) | 19 (2.5%) | <0.001 |
| Steroid use | 802 (0.8%) | 2 (0.3%) | 0.086 |
| Cirrhosis | 2043 (2.1%) | 14 (1.8%) | 0.61 |
| Substance abuse | 4522 (4.6%) | 41 (5.3%) | 0.35 |
| No REBOA | REBOA | p-Value | |
|---|---|---|---|
| N (%) | 99,812 (99.2%) | 803 (0.8%) | |
| Hospital complications, n (%) | |||
| CAUTI | 732 (0.7%) | 13 (1.6%) | 0.003 |
| CLABSI | 336 (0.3%) | 5 (0.6%) | 0.16 |
| Superficial SSI | 649 (0.7%) | 4 (0.5%) | 0.6 |
| Deep SSI | 779 (0.8%) | 18 (2.3%) | <0.001 |
| Organ space SSI | 561 (0.6%) | 4 (0.5%) | 0.81 |
| Sepsis | 1598 (1.6%) | 30 (3.8%) | <0.001 |
| Pressure ulcer | 2426 (2.4%) | 36 (4.5%) | <0.001 |
| DVT | 2126 (2.1%) | 42 (5.3%) | <0.001 |
| PE | 1387 (1.4%) | 20 (2.5%) | 0.008 |
| Compartment syndrome | 455 (0.5%) | 20 (2.5%) | <0.001 |
| Cardiac arrest | 7043 (7.1%) | 175 (21.8%) | <0.001 |
| MI | 581 (0.6%) | 3 (0.4%) | 0.44 |
| ARDS | 2796 (2.8%) | 28 (3.5%) | 0.24 |
| VAP | 4975 (5.0%) | 30 (3.8%) | 0.11 |
| AKI | 3211 (3.2%) | 80 (10.0%) | <0.001 |
| Unplanned intubation | 3131 (3.1%) | 24 (3.0%) | 0.82 |
| Unplanned return to OR | 2184 (2.2%) | 61 (7.6%) | <0.001 |
| Unplanned ICU admission | 2745 (2.8%) | 25 (3.1%) | 0.52 |
| Ventilator days, median (IQR) | 1 (0, 4) | 2 (1, 7) | <0.001 |
| ICU LOS, median (IQR) | 2 (0, 8) | 2 (0, 12) | 0.019 |
| 24 h mortality, n (%) | 20,828 (20.9%) | 377 (46.9%) | <0.001 |
| In-hospital mortality, n (%) | 30,706 (30.8%) | 496 (61.8%) | <0.001 |
| Leaf # | Original | Prescribed | ARR | ARR (%) |
|---|---|---|---|---|
| 5 | 0.959 | 0.879 | 0.080 | 7.984 |
| 6 | 0.719 | 0.718 | 0.001 | 0.080 |
| 7 | 0.406 | 0.406 | 0.000 | 0.000 |
| 9 | 0.848 | 0.834 | 0.015 | 1.477 |
| 12 | 0.919 | 0.888 | 0.031 | 3.059 |
| 13 | 0.888 | 0.888 | 0.000 | 0.005 |
| 14 | 0.820 | 0.819 | 0.001 | 0.087 |
| 17 | 0.740 | 0.685 | 0.054 | 5.438 |
| 18 | 0.190 | 0.182 | 0.008 | 0.831 |
| 19 | 0.123 | 0.121 | 0.002 | 0.153 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokenkamp, M.; Ma, Y.; Dorken-Gallastegi, A.; Proaño-Zamudio, J.A.; Gebran, A.; Velmahos, G.C.; Bertsimas, D.; Kaafarani, H.M.A. Can Artificial Intelligence Improve the Appropriate Use and Decrease the Misuse of REBOA? Bioengineering 2025, 12, 1025. https://doi.org/10.3390/bioengineering12101025
Bokenkamp M, Ma Y, Dorken-Gallastegi A, Proaño-Zamudio JA, Gebran A, Velmahos GC, Bertsimas D, Kaafarani HMA. Can Artificial Intelligence Improve the Appropriate Use and Decrease the Misuse of REBOA? Bioengineering. 2025; 12(10):1025. https://doi.org/10.3390/bioengineering12101025
Chicago/Turabian StyleBokenkamp, Mary, Yu Ma, Ander Dorken-Gallastegi, Jefferson A. Proaño-Zamudio, Anthony Gebran, George C. Velmahos, Dimitris Bertsimas, and Haytham M. A. Kaafarani. 2025. "Can Artificial Intelligence Improve the Appropriate Use and Decrease the Misuse of REBOA?" Bioengineering 12, no. 10: 1025. https://doi.org/10.3390/bioengineering12101025
APA StyleBokenkamp, M., Ma, Y., Dorken-Gallastegi, A., Proaño-Zamudio, J. A., Gebran, A., Velmahos, G. C., Bertsimas, D., & Kaafarani, H. M. A. (2025). Can Artificial Intelligence Improve the Appropriate Use and Decrease the Misuse of REBOA? Bioengineering, 12(10), 1025. https://doi.org/10.3390/bioengineering12101025






