Polycaprolactone for Hard Tissue Regeneration: Scaffold Design and In Vivo Implications
Abstract
1. Introduction to Tissue Engineering
2. Biomaterials: Basic Definitions and Classification
Why Poly (ε-Caprolactone)? Main Strengths and Weaknesses
| Advantages | Disadvantages | Ref | |
|---|---|---|---|
| Polycaprolactone (PCL) |
|
| [45,46,47] |
| Poly(lactic acid) (PLA) |
|
| [48,49,50] |
| Poly(lactic-co-glycolic acid) (PLGA) |
|
| [51,52,53] |
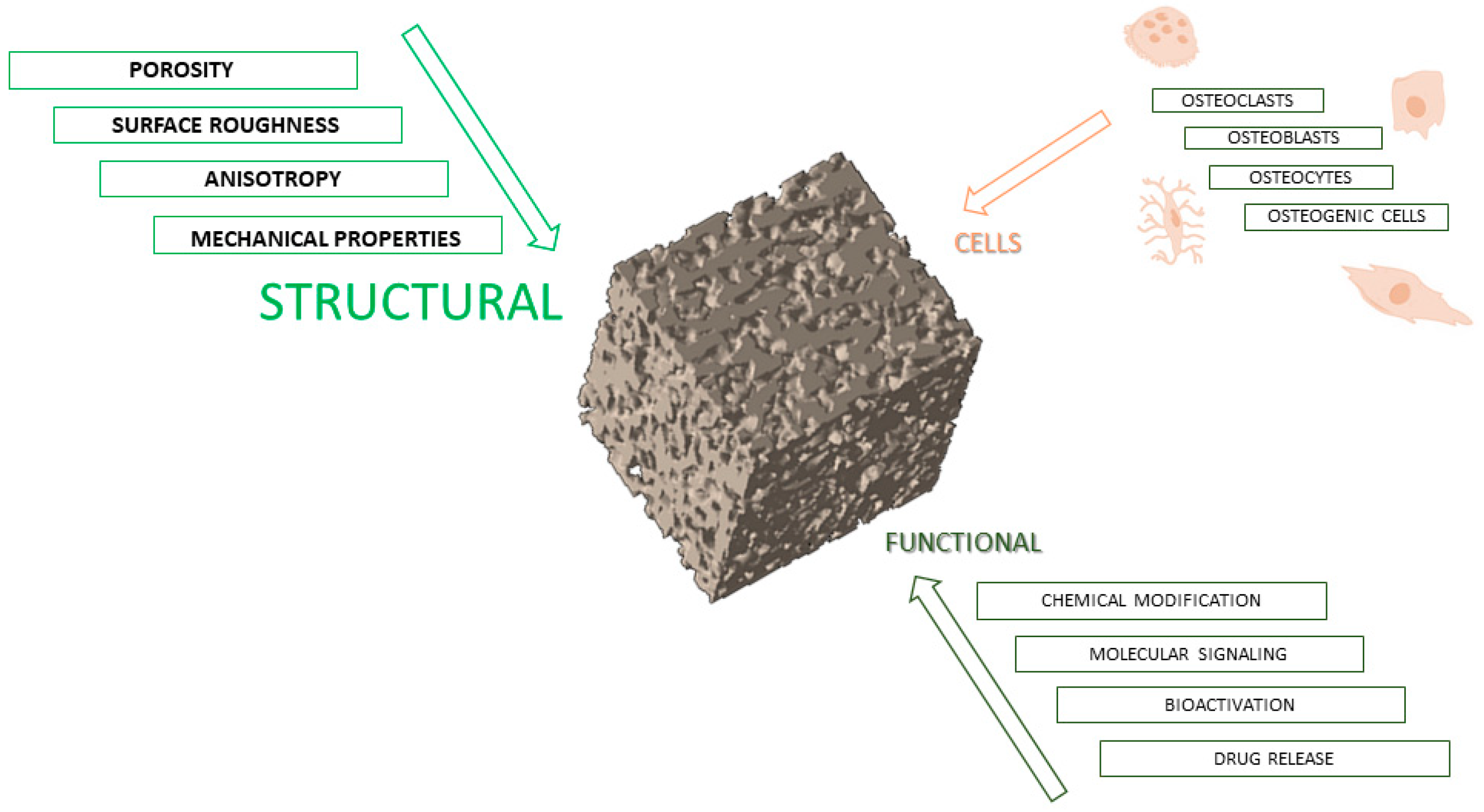
3. Structural Requirements for Scaffold Design
3.1. Porosity
3.2. Pore Size
3.3. Pore Interconnectivity
3.4. Mechanical Properties
3.5. Surface Roughness
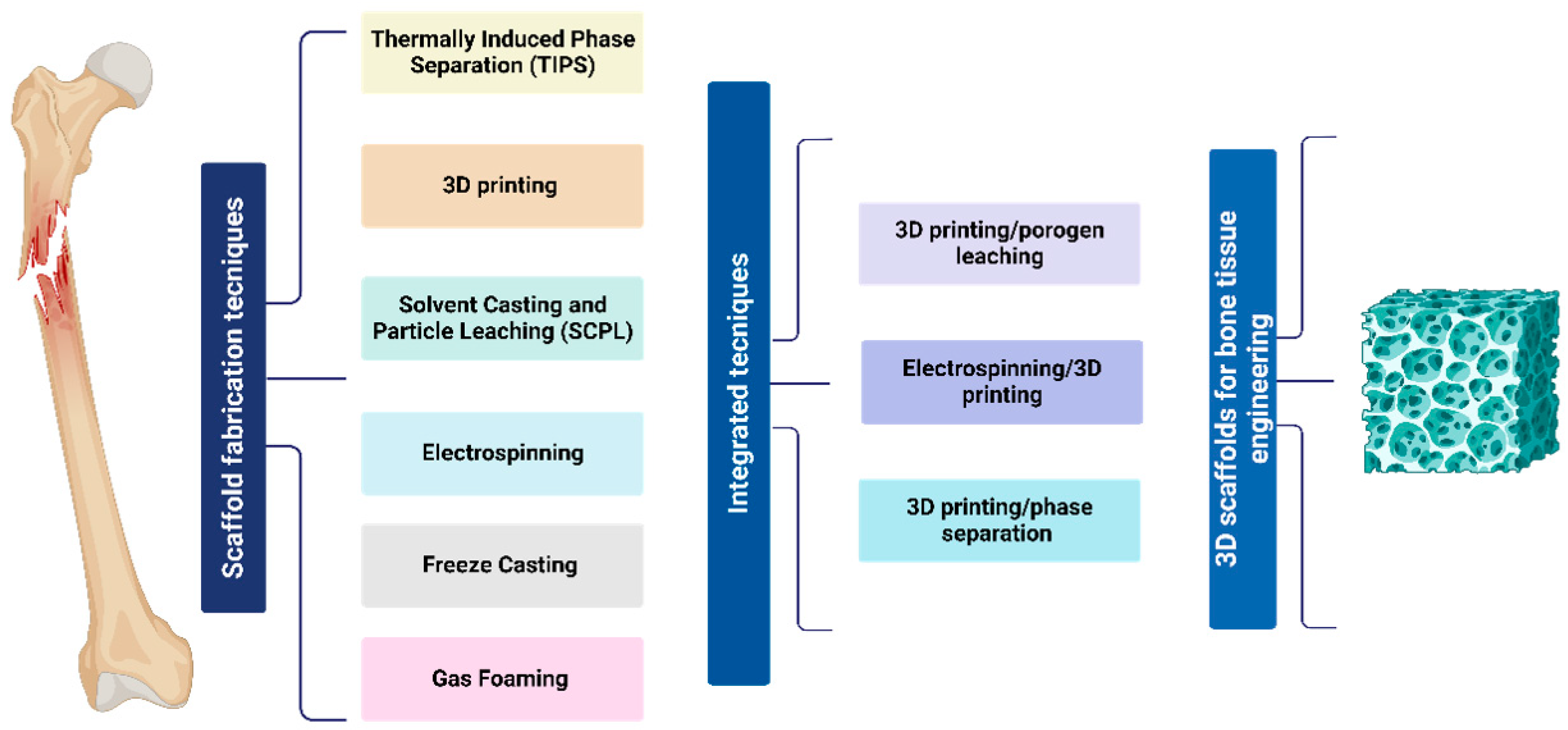
4. Fabrication Methods for Bone Tissue Regeneration and In Vivo Implications
4.1. Freeze Casting
4.2. Gas Foaming
4.3. Solvent Casting and Particle Leaching (SCPL)
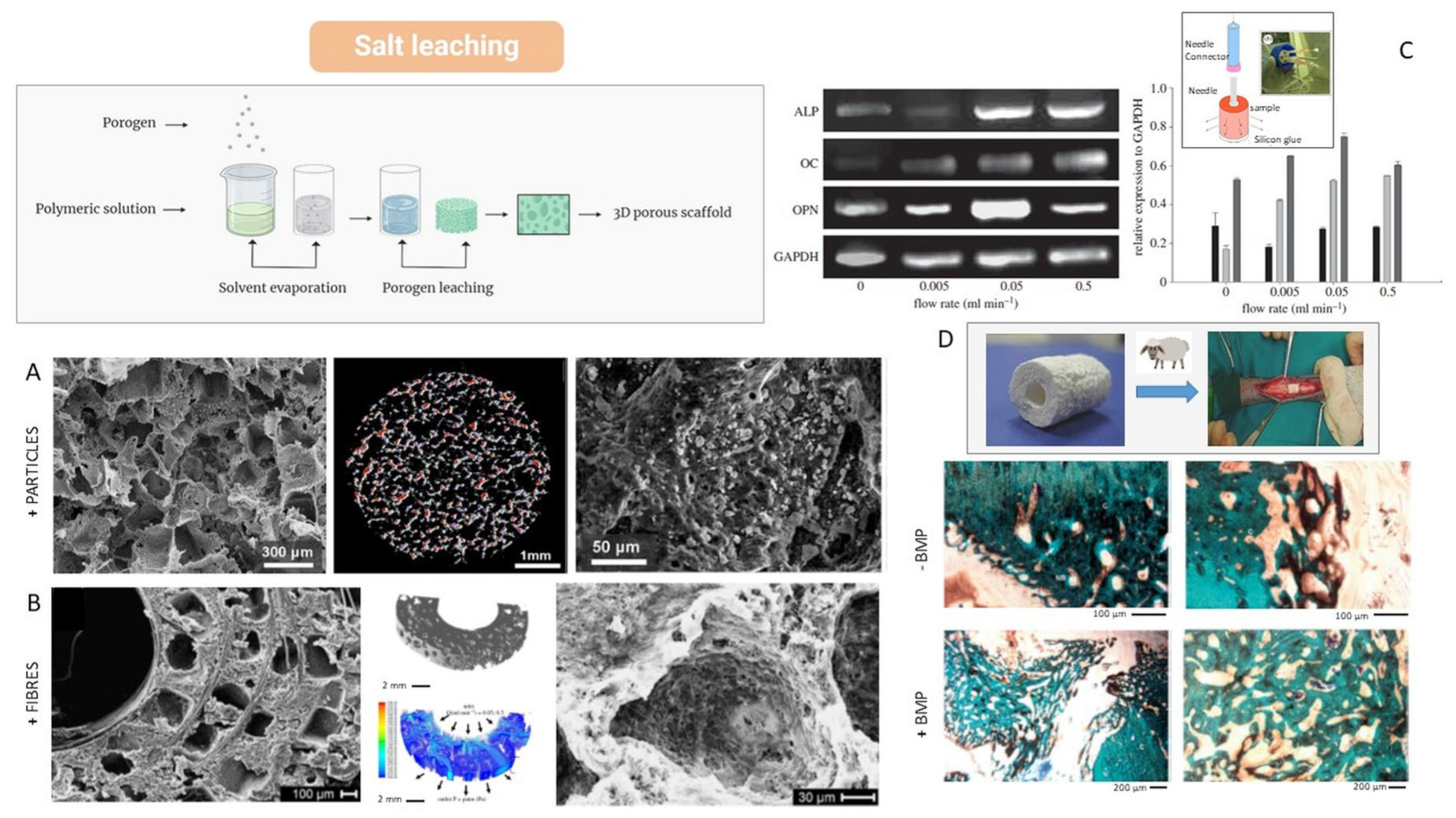
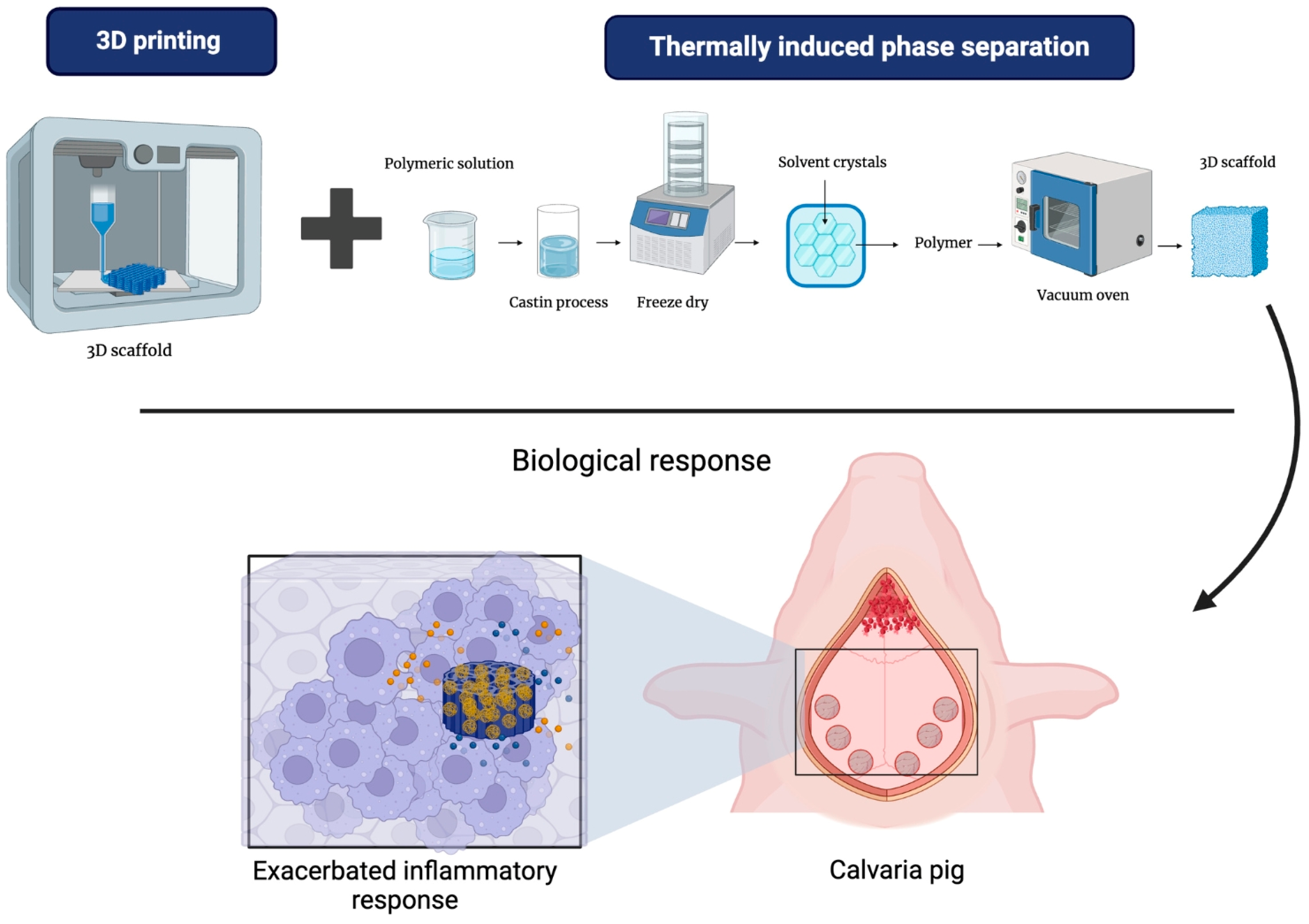
4.4. Thermally Induced Phase Separation (TIPS)
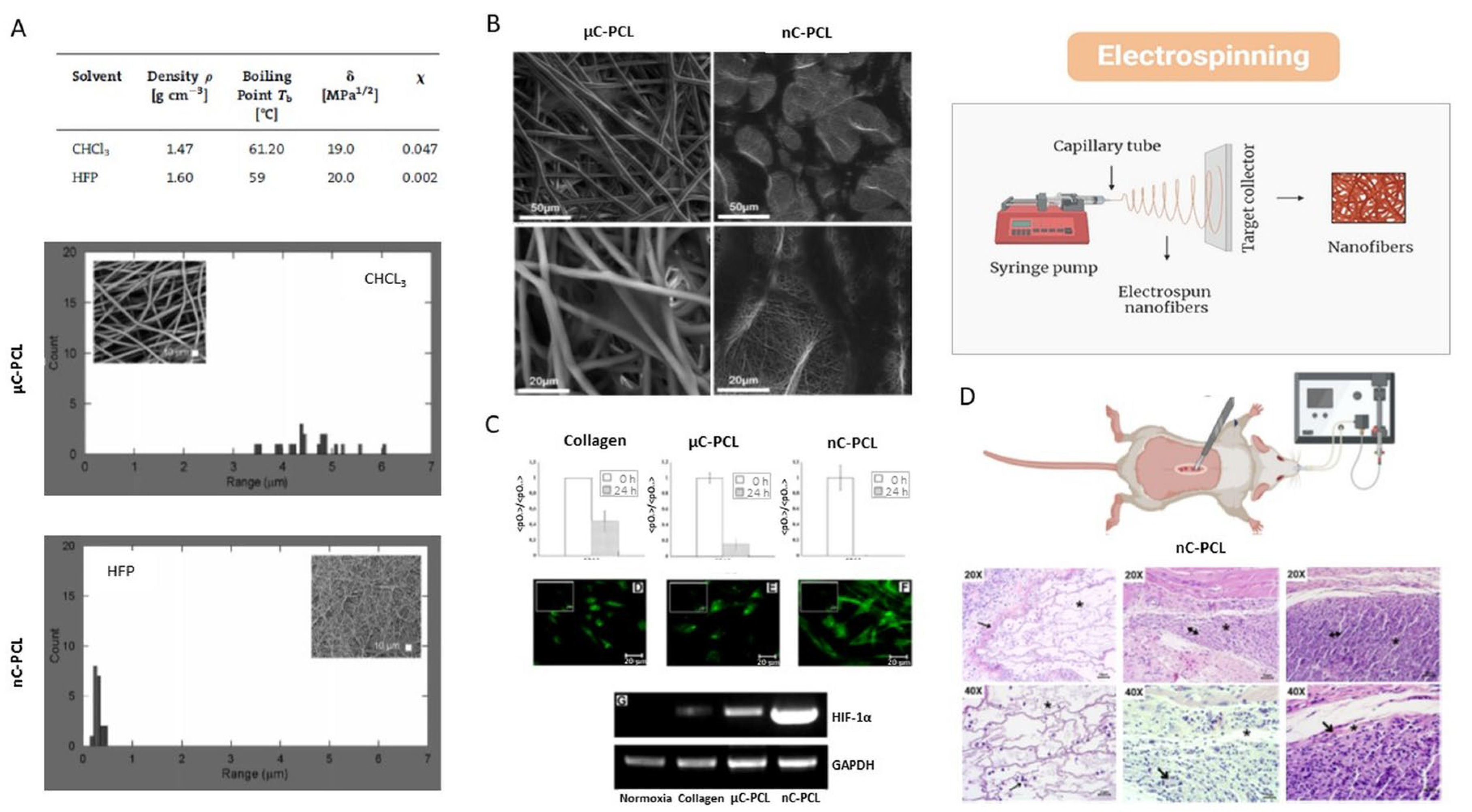
4.5. Electrospinning
4.6. 3D Printing
5. Integrated Techniques
5.1. 3D Printing/Porogen Leaching
5.2. 3D Printing/Phase Separation
5.3. 3D Printing/Electrospinning
6. Conclusions and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Dang, M.; Saunders, L.; Niu, X.; Fan, Y.; Ma, P.X. Biomimetic delivery of signals for bone tissue engineering. Bone Res. 2018, 6, 25. [Google Scholar] [CrossRef]
- Wei, H.; Cui, J.; Lin, K.; Xie, J.; Wang, X. Recent advances in smart stimuli-responsive biomaterials for bone therapeutics and regeneration. Bone Res. 2022, 10, 17. [Google Scholar] [CrossRef]
- Rosales Ibáñez, R.; Alvarado Estrada, K.N.; Ojeda Gutiérrez, F. Ingeniería Tisular en Odontología. Rev. Adm. 2012, LXIX, 164–167. [Google Scholar]
- Murphy, C.M.; O’brien, F.J.; Little, D.G.; Schindeler, A. Cell-scaffold interactions in the bone tissue engineering triad. Eur. Cell Mater. 2013, 26, 120–132. [Google Scholar] [CrossRef]
- Dolcimascolo, A.; Calabrese, G.; Conoci, S.; Parenti, R. Innovative Biomaterials for Tissue Engineering. In Biomaterial-Supported Tissue Reconstruction or Regeneration; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Kesharwani, R.K.; Keservani, R.K.; Sharma, A.K. Tissue Engineering; Apple Academic Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef]
- Puertas-Bartolomé, M.; Mora-Boza, A.; García-Fernández, L. Emerging Biofabrication Techniques: A Review on Natural Poly-mers for Biomedical Applications. Polymers 2021, 13, 1209. [Google Scholar] [CrossRef]
- Han, F.; Wang, J.; Ding, L.; Hu, Y.; Li, W.; Yuan, Z.; Guo, Q.; Zhu, C.; Yu, L.; Wang, H.; et al. Tissue Engineering and Regenerative Medicine: Achievements, Future, and Sustainability in Asia. Front. Bioeng. Biotechnol. 2020, 8, 83. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, R.; Deng, N.; Zhao, X.; Li, X.; Guo, C. Natural polymer-based scaffolds for soft tissue repair. Front. Bioeng. Biotechnol. 2022, 10, 954699. [Google Scholar] [CrossRef] [PubMed]
- Phutane, P.; Telange, D.; Agrawal, S.; Gunde, M.; Kotkar, K.; Pethe, A. Biofunctionalization and Applications of Polymeric Nanofibers in Tissue Engineering and Regenerative Medicine. Polymers 2023, 15, 1202. [Google Scholar] [CrossRef]
- Williams, D.F. Challenges With the Development of Biomaterials for Sustainable Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 127. [Google Scholar] [CrossRef]
- On, S.-W.; Cho, S.-W.; Byun, S.-H.; Yang, B.-E. Bioabsorbable Osteofixation Materials for Maxillofacial Bone Surgery: A Review on Polymers and Magnesium-Based Materials. Biomedicines 2020, 8, 300. [Google Scholar] [CrossRef]
- Al-Shalawi, F.D.; Ariff, A.H.M.; Jung, D.-W.; Ariffin, M.K.A.M.; Kim, C.L.S.; Brabazon, D.; Al-Osaimi, M.O. Biomaterials as Implants in the Orthopedic Field for Regenerative Medicine: Metal versus Synthetic Polymers. Polymers 2023, 15, 2601. [Google Scholar] [CrossRef]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef]
- Baino, F. Bioceramics and Composites for Orbital Implants: Current Trends and Clinical Performance. In Handbook of Bioceramics and Biocomposites; Springer International Publishing: Cham, Switzerland, 2016; pp. 1249–1274. [Google Scholar]
- Pina, S.; Reis, R.L.; Oliveira, J.M. Ceramic biomaterials for tissue engineering. In Fundamental Biomaterials: Ceramics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 95–116. [Google Scholar]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef]
- Pawan, K.; Brijnandan, S.D.; Anil, S. Bioceramics for Hard Tissue Engineering Applications: A Review. Int.-Natl. J. Appl. Eng. Res. 2018, 13, 2744–2752. [Google Scholar]
- Tanvir, A.H.; Khaleque, A.; Kim, G.-H.; Yoo, W.-Y.; Kim, Y.-Y. The Role of Bioceramics for Bone Regeneration: History, Mechanisms, and Future Perspectives. Biomimetics 2024, 9, 230. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, B.; Liu, G.; Tang, Y.; Lu, E.; Xie, K.; Lan, C.; Liu, J.; Qin, Z.; Wang, L. Metal Material, Properties and Design Methods of Porous Biomedical Scaffolds for Additive Manufacturing: A Review. Front. Bioeng. Biotechnol. 2021, 9, 641130. [Google Scholar] [CrossRef]
- Chowdhury, S.K.; Nagarjuna, V.; Bhaskar, B. Metallic Biomaterials in Tissue Engineering: Retrospect and Prospects. In Bio-Materials in Tissue Engineering and Regenerative Medicine; Springer: Singapore, 2021; pp. 19–60. [Google Scholar]
- Qi, J.; Yu, T.; Hu, B.; Wu, H.; Ouyang, H. Current Biomaterial-Based Bone Tissue Engineering and Translational Medicine. Int. J. Mol. Sci. 2021, 22, 10233. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, W.; Li, Z.; Cheng, L.; Zhang, Q.; Yue, D.; Shi, Z.; Wang, B.; Sun, W.; Zhang, N. Porous tantalum rods for treating osteonecrosis of the femoral head. Genet. Mol. Res. 2014, 13, 8342–8352. [Google Scholar] [CrossRef]
- Pham, M.H.; Mehta, V.A.; Tuchman, A.; Hsieh, P.C. Material Science in Cervical Total Disc Replacement. BioMed Res. Int. 2015, 2015, 719123. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Fernández-Montequín, J.; Valdés-Pérez, C.; Savigne-Gutiérrez, W.; Mendoza-Marí, Y.; García-Ojalvo, A.; Falcón-Cama, V.; del Barco-Herrera, D.G.; Fernández-Mayola, M.; Pérez-Saad, H.; et al. Mechanical Charac-terisation and Biomechanical and Biological Behaviours of Ti-Zr Binary-Alloy Dental Implants. BioMed Res. Int. 2017, 2017, 2923759. [Google Scholar]
- Sultana, N.; Hassan, M.I.; Lim, M.M. Composite Synthetic Scaffolds for Tissue Engineering and Regenerative Medicine; Springer Nature: Cham, Switzerland, 2015. [Google Scholar]
- Elena, S.; Claudio, M. Composite materials for biomedical applications: A review. J. Appl. Biomater. Biomech. 2003, 1, 3–18. [Google Scholar]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Gupta, D.; Dogra, V.; Verma, D.; Chaudhary, A.K.; Tewari, M. PCL-based composites and their utilizations in the medical sector. In Bioresorbable Polymers and Their Composites; Elsevier: Amsterdam, The Netherlands, 2024; pp. 63–83. [Google Scholar]
- Sowmya, B.; Hemavathi, A.B.; Panda, P.K. Poly (ε-caprolactone)-based electrospun nano-featured substrate for tissue engineering applications: A review. Prog. Biomater. 2021, 10, 91–117. [Google Scholar] [CrossRef]
- Mondal, D.; Griffith, M.; Venkatraman, S.S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 255–265. [Google Scholar] [CrossRef]
- Ouhadi, T.; Stevens, C.; Teyssié, P. Study of poly-ε-caprolactone bulk degradation. J. Appl. Polym. Sci. 1976, 20, 2963–2970. [Google Scholar] [CrossRef]
- Liu, G.; Wei, X.; Zhai, Y.; Zhang, J.; Li, J.; Zhao, Z.; Guan, T.; Zhao, D. 3D printed osteochondral scaffolds: Design strategies, present applications and future perspectives. Front. Bioeng. Biotechnol. 2024, 12, 1339916. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Deshpande, M.V.; Girase, A.; King, M.W. Degradation of Poly(ε-caprolactone) Resorbable Multifilament Yarn under Physiological Conditions. Polymers 2023, 15, 3819. [Google Scholar] [CrossRef]
- Javkhlan, Z.; Hsu, S.-H.; Chen, R.-S.; Chen, M.-H. 3D-printed polycaprolactone scaffolds coated with beta tricalcium phosphate for bone regeneration. J. Formos. Med. Assoc. 2024, 123, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Janmohammadi, M.; Nourbakhsh, M.S.; Bahraminasab, M.; Tayebi, L. Effect of Pore Characteristics and Alkali Treatment on the Physicochemical and Biological Properties of a 3D-Printed Polycaprolactone Bone Scaffold. ACS Omega 2023, 8, 7378–7394. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Nyberg, E.; Rindone, A.; Dorafshar, A.; Grayson, W.L. Comparison of 3D-Printed Poly-ε-Caprolactone Scaffolds Functionalized with Tricalcium Phosphate, Hydroxyapatite, Bio-Oss, or Decellularized Bone Matrix. Tissue Eng. Part A 2017, 23, 503–514. [Google Scholar] [CrossRef]
- Liang, H.-Y.; Lee, W.-K.; Hsu, J.-T.; Shih, J.-Y.; Ma, T.-L.; Vo, T.T.T.; Lee, C.-W.; Cheng, M.-T.; Lee, I.-T. Polycaprolactone in Bone Tissue Engineering: A Comprehensive Review of Innovations in Scaffold Fabrication and Surface Modifications. J. Funct. Biomater. 2024, 15, 243. [Google Scholar] [CrossRef]
- Park, S.H.; Park, S.A.; Kang, Y.G.; Shin, J.W.; Park, Y.S.; Gu, S.R.; Wu, Y.R.; Wei, J.; Shin, J.-W. PCL/β-TCP Composite Scaffolds Exhibit Positive Osteogenic Differentiation with Mechanical Stimulation. Tissue Eng. Regen. Med. 2017, 14, 349–358. [Google Scholar] [CrossRef]
- Re, F.; Borsani, E.; Rezzani, R.; Sartore, L.; Russo, D. Bone Regeneration Using Mesenchymal Stromal Cells and Biocompatible Scaffolds: A Concise Review of the Current Clinical Trials. Gels 2023, 9, 389. [Google Scholar] [CrossRef]
- Asaduzzaman, F.; Salmon, S. Controllable Water-Triggered Degradation of PCL Solution-Blown Nanofibrous Webs Made Possible by Lipase Enzyme Entrapment. Fibers 2023, 11, 49. [Google Scholar] [CrossRef]
- MacCraith, E.; O’brien, F.; Davis, N. Biodegradable materials for surgical management of stress urinary incontinence: A narrative review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 259, 153–160. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Zuhri, M.Y.M.; Norrrahim, M.N.F.; Misenan, M.S.M.; Jenol, M.A.; Samsudin, S.A.; Nurazzi, N.M.; Asyraf, M.R.M.; Supian, A.B.M.; Bangar, S.P.; et al. Natural Fiber-Reinforced Polycaprolactone Green and Hybrid Biocomposites for Various Advanced Applications. Polymers 2022, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.S.; Jardini, A.; Filho, R.M. Poly (Lactic Acid) Production for Tissue Engineering Applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef]
- Englert, C.; Brendel, J.C.; Majdanski, T.C.; Yildirim, T.; Schubert, S.; Gottschaldt, M.; Windhab, N.; Schubert, U.S. Pharmapolymers in the 21st century: Syn-thetic polymers in drug delivery applications. Prog. Polym. Sci. 2018, 87, 107–164. [Google Scholar] [CrossRef]
- Łysik, D.; Deptuła, P.; Chmielewska, S.; Bucki, R.; Mystkowska, J. Degradation of Polylactide and Polycaprolactone as a Result of Biofilm Formation Assessed under Experimental Conditions Simulating the Oral Cavity Environment. Materials 2022, 15, 7061. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Guzmán-Soria, A.; Moreno-Serna, V.; Canales, D.A.; García-Herrera, C.; Zapata, P.A.; Orihuela, P.A. Effect of Electrospun PLGA/Collagen Scaffolds on Cell Adhesion, Viability, and Collagen Release: Potential Applications in Tissue Engineering. Polymers 2023, 15, 1079. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Sugawara, Y.; Kamioka, H.; Honjo, T.; Tezuka, K.-I.; Takanoyamamoto, T. Three-dimensional reconstruction of chick calvarial osteocytes and their cell processes using confocal microscopy. Bone 2005, 36, 877–883. [Google Scholar] [CrossRef]
- Wang, C.; Xu, D.; Lin, L.; Li, S.; Hou, W.; He, Y.; Sheng, L.; Yi, C.; Zhang, X.; Li, H.; et al. Large-pore-size Ti6Al4V scaffolds with different pore structures for vascularized bone regeneration. Mater. Sci. Eng. C 2021, 131, 112499. [Google Scholar] [CrossRef]
- Iviglia, G.; Kargozar, S.; Baino, F. Biomaterials, Current Strategies, and Novel Nano-Technological Approaches for Periodontal Regeneration. J. Funct. Biomater. 2019, 10, 3. [Google Scholar] [CrossRef]
- Mohammadi, H.; Sepantafar, M.; Muhamad, N.; Bakar Sulong, A. How Does Scaffold Porosity Conduct Bone Tissue Regeneration? Adv. Eng. Mater. 2021, 23, 2100463. [Google Scholar] [CrossRef]
- Chang, H.-I.; Wang, Y. Cell Responses to Surface and Architecture of Tissue Engineering Scaffolds. In Regenerative Medicine and Tissue Engineering—Cells and Biomaterials; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Cheng, M.-Q.; Wahafu, T.; Jiang, G.-F.; Liu, W.; Qiao, Y.-Q.; Peng, X.-C.; Cheng, T.; Zhang, X.-L.; He, G.; Liu, X.-Y. A novel open-porous magnesium scaffold with controllable microstructures and properties for bone regeneration. Sci. Rep. 2016, 6, 24134. [Google Scholar] [CrossRef]
- Lim, T.C.; Chian, K.S.; Leong, K.F. Cryogenic prototyping of chitosan scaffolds with controlled micro and macro architecture and their effect on in vivo neo-vascularization and cellular infiltration. J. Biomed. Mater. Res. Part A 2010, 94A, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Morejón, L.; Delgado, J.A.; Ribeiro, A.A.; de Oliveira, M.V.; Mendizábal, E.; García, I.; Alfonso, A.; Poh, P.; van Griensven, M.; Balmayor, E.R. Development, Characteri-zation and In Vitro Biological Properties of Scaffolds Fabricated From Calcium Phosphate Nanoparticles. Int. J. Mol. Sci. 2019, 20, 1790. [Google Scholar] [CrossRef]
- Guarino, V.; Scaglione, S.; Sandri, M.; Alvarez-Perez, M.A.; Tampieri, A.; Quarto, R.; Ambrosio, L. MgCHA particles dispersion in porous PCL scaffolds: In vitro mineralization and in vivo bone formation. J. Tissue Eng. Regen. Med. 2014, 8, 291–303. [Google Scholar] [CrossRef]
- Mukherjee, S.; Darzi, S.; Paul, K.; Werkmeister, J.A.; Gargett, C.E. Mesenchymal stem cell-based bioengineered constructs: Foreign body response, cross-talk with macrophages and impact of biomaterial design strategies for pelvic floor disorders. Interface Focus 2019, 9, 20180089. [Google Scholar] [CrossRef] [PubMed]
- Reis, R.L.; Roma, S. Biodegradable Systems in Tissue Engineering and Regenerative Medicine; CRC Press: Boca Raton, FL, USA, 2005; 568p. [Google Scholar]
- Sauerova, P.; Suchy, T.; Supova, M.; Bartos, M.; Klima, J.; Juhasova, J.; Juhas, S.; Kubikova, T.; Tonar, Z.; Sedlacek, R.; et al. Positive impact of dynamic seeding of mesenchymal stem cells on bone-like biodegradable scaffolds with increased content of calcium phosphate nanoparticles. Mol. Biol. Rep. 2019, 46, 4483–4500. [Google Scholar] [CrossRef]
- Murphy, C.M.; O’Brien, F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adh. Migr. 2010, 4, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Nune, K.C.; Murr, L.E.; Misra, R.D.K. Biocompatibility and mechanical behaviour of three-dimensional scaffolds for biomedical devices: Process–structure–property paradigm. Int. Mater. Rev. 2016, 61, 20–45. [Google Scholar] [CrossRef]
- Eshraghi, S.; Das, S. Mechanical and microstructural properties of polycaprolactone scaffolds with one-dimensional, two-dimensional, and three-dimensional orthogonally oriented porous architectures produced by selective laser sintering. Acta Biomater. 2010, 6, 2467–2476. [Google Scholar] [CrossRef]
- Vincenzo, G.; Filippo, C.; Luigi, A. Porosity and Mechanical Properties Relationship in PCL Porous Scaffolds. Appl. Biomater. Biomech. 2007, 5, 149–157. [Google Scholar]
- Guarino, V.; Gloria, A.; Raucci, M.G.; De Santis, R.; Ambrosio, L. Bio-inspired composite and cell instructive platforms for bone regeneration. Int. Mater. Rev. 2012, 57, 256–275. [Google Scholar] [CrossRef]
- Yaszemski, M.J.; Payne, R.G.; Hayes, W.C.; Langer, R.; Mikos, A.G. Evolution of bone transplantation: Molecular, cellular and tissue strategies to engineer human bone. Biomaterials 1996, 17, 175–185. [Google Scholar] [CrossRef]
- Velasco, M.A.; Narváez-Tovar, C.A.; Garzón-Alvarado, D.A. Design, Materials, and Mechanobiology of Biodegradable Scaffolds for Bone Tissue Engineering. BioMed Res. Int. 2015, 2015, 729076. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, X.; Zhang, M.; Damanik, F.; Baker, M.B.; Leferink, A.; Yuan, H.; Truckenmüller, R.; van Blitterswijk, C.; Moroni, L. Tailoring surface nanoroughness of electrospun scaffolds for skeletal tissue engineering. Acta Biomater. 2017, 59, 82–93. [Google Scholar] [CrossRef]
- Calore, A.R.; Srinivas, V.; Groenendijk, L.; Serafim, A.; Stancu, I.C.; Wilbers, A.; Leoné, N.; Sanchez, A.A.; Auhl, D.; Mota, C.; et al. Manufacturing of scaffolds with interconnected internal open porosity and surface roughness. Acta Biomater. 2023, 156, 158–176. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Prasad, B.L.V. Surface Modification of Polymers for Tissue Engineering Applications: Arginine Acts as a Sticky Protein Equivalent for Viable Cell Accommodation. ACS Omega 2018, 3, 4242–4251. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Liu, X.; Xu, A.; Wang, L.; Luo, Z.; Zheng, Y.; Deng, F.; Wei, J.; Tang, Z.; Wei, S. Effect of surface roughness on osteogenesis in vitro and osseointegration in vivo of carbon fiber-reinforced polyetheretherketone-nanohydroxyapatite composite. Int. J. Nanomed. 2015, 10, 1425–1447. [Google Scholar]
- Han, J.; Li, Z.; Sun, Y.; Cheng, F.; Zhu, L.; Zhang, Y.; Zhang, Z.; Wu, J.; Wang, J. Surface Roughness and Biocompatibility of Polycaprolactone Bone Scaffolds: An Energy-Density-Guided Parameter Optimization for Selective Laser Sintering. Front. Bioeng. Biotechnol. 2022, 10, 888267. [Google Scholar] [CrossRef]
- Ghorbani, F.; Zamanian, A.; Sahranavard, M. Mussel-inspired polydopamine-mediated surface modification of freeze-cast poly (ε-caprolactone) scaffolds for bone tissue engineering applications. Biomed. Eng. Biomed. Technol. 2020, 65, 273–287. [Google Scholar] [CrossRef]
- Lor Huai Chong Zarith, N.Z.; Sultana, N. Poly(Caprolactone)/chitosan-based scaffold using freeze drying technique for bone tissue engineering application. In Proceedings of the 2015 10th Asian Control Conference (ASCC), Kota Kinabalu, Malaysia, 31 May–3 June 2015; IEEE: New York, NY, USA, 2015; pp. 1–4. [Google Scholar]
- Wang, S.; Yang, Y.; Koons, G.L.; Mikos, A.G.; Qiu, Z.; Song, T.; Cui, F.; Wang, X. Tuning pore features of mineralized collagen/PCL scaffolds for cranial bone regeneration in a rat model. Mater. Sci. Eng. C 2020, 106, 110186. [Google Scholar] [CrossRef] [PubMed]
- Manoukian, O.S.; Sardashti, N.; Stedman, T.; Gailiunas, K.; Ojha, A.; Penalosa, A.; Mancuso, C.; Hobert, M.; Kumbar, S.G. Biomaterials for Tissue Engineering and Regenerative Medicine. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Duarte, R.M.; Correia-Pinto, J.; Reis, R.L.; Duarte, A.R.C. Subcritical carbon dioxide foaming of polycaprolactone for bone tissue regeneration. J. Supercrit. Fluids 2018, 140, 1–10. [Google Scholar] [CrossRef]
- Satpayeva, A.; Rojas, A.; Tyrka, M.; Ksepko, E.; Galotto, M.J.; Zizovic, I. Supercritical Foaming and Impregnation of Polycaprolactone and Polycaprolactone-Hydroxyapatite Composites with Carvacrol. Processes 2022, 10, 482. [Google Scholar] [CrossRef]
- Luo, K.; Wang, L.; Chen, X.; Zeng, X.; Zhou, S.; Zhang, P.; Li, J. Biocompatible Poly(ε-caprolactone)-based Shape-memory Polyu-rethane Composite Scaffold with Bone-induced Activity. J. Bionic. Eng. 2022, 19, 167–178. [Google Scholar] [CrossRef]
- Cho, Y.S.; Hong, M.W.; Quan, M.; Kim, S.; Lee, S.; Lee, S.; Kim, Y.Y. Assessments for bone regeneration using the polycaprolactone SLUP (salt-leaching using powder) scaffold. J. Biomed. Mater. Res. Part A 2017, 105, 3432–3444. [Google Scholar] [CrossRef]
- Sempertegui, N.D.; Narkhede, A.A.; Thomas, V.; Rao, S.S. A combined compression molding, heating, and leaching process for fabrication of micro-porous poly(ε-caprolactone) scaffolds. J. Biomater. Sci. Polym. Ed. 2018, 29, 1978–1993. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Fan, F.-Y.; Shen, Y.-K.; Wang, C.-H.; Huang, Y.-T.; Chern, M.-J.; Wang, Y.-H.; Wang, L. 3D poly-ε-caprolactone/graphene porous scaffolds for bone tissue engineering. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125393. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef]
- Ruiz-Aguilar, C.; Olivares-Pinto, U.; Drew, R.A.L.; Aguilar-Reyes, E.A.; Alfonso, I. Porogen Effect on Structural and Physical Prop-erties of β-TCP Scaffolds for Bone Tissue Regeneration. IRBM 2020, 42, 302–312. [Google Scholar] [CrossRef]
- Guarino, V.; Causa, F.; Taddei, P.; di Foggia, M.; Ciapetti, G.; Martini, D.; Fagnano, C.; Baldini, N.; Ambrosio, L. Polylactic acid fibre-reinforced polycaprolactone scaffolds for bone tissue engineering. Biomaterials 2008, 29, 3662–3670. [Google Scholar] [CrossRef]
- Guarino, V.; Urciuolo, F.; Alvarez-Perez, M.A.; Mele, B.; Netti, P.A.; Ambrosio, L. Osteogenic differentiation and mineralization in fibre-reinforced tubular scaffolds: Theoretical study and experimental evidences. J. R. Soc. Interface 2012, 9, 2201–2212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guarino, V.; Ambrosio, L. The synergic effect of polylactide fiber and calcium phosphate particle reinforcement in poly ε-caprolactone-based composite scaffolds. Acta Biomater. 2008, 4, 1778–1787. [Google Scholar] [CrossRef]
- Ronca, A.; Guarino, V.; Raucci, M.G.; Salamanna, F.; Martini, L.; Zeppetelli, S.; Fini, M.; Kon, E.; Filardo, G.; Marcacci, M.; et al. Large defect-tailored composite scaffolds for in vivo bone regeneration. J. Biomater. Appl. 2014, 29, 715–727. [Google Scholar] [CrossRef]
- Guarino, V.; Ambrosio, L. Temperature-driven processing techniques for manufacturing fully interconnected porous scaffolds in bone tissue engineering. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 1389–1400. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Yousefi, A. Effects of processing parameters in thermally induced phase separation technique on porous ar-chitecture of scaffolds for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Guaccio, A.; Guarnieri, D.; Netti, P.A.; Ambrosio, L. Binary system thermodynamics to control pore architecture of PCL scaffold via temperature-driven phase separation process. J. Biomater. Appl. 2012, 27, 241–254. [Google Scholar] [CrossRef]
- Milián, L.; Oliver-Ferrándiz, M.; Peregrín, I.; Sancho-Tello, M.; Martín-De-Llano, J.J.; Martínez-Ramos, C.; Carda, C.; Mata, M. Alginate Improves the Chondrogenic Capacity of 3D PCL Scaffolds In Vitro: A Histological Approach. Curr. Issues Mol. Biol. 2024, 46, 3563–3578. [Google Scholar] [CrossRef]
- Guarino, V.; Causa, F.; Salerno, A.; Ambrosio, L.; Netti, P.A. Design and manufacture of microporous polymeric materials with hierarchal complex structure for biomedical application. Mater. Sci. Technol. 2008, 24, 1111–1117. [Google Scholar] [CrossRef]
- Ghalia, M.A.; Dahman, Y. Advanced nanobiomaterials in tissue engineering: Synthesis, properties, and applications. In Nanobiomaterials in Soft Tissue Engineering: Applications of Nanobiomaterials; Elsevier: Toronto, ON, Canada, 2016. [Google Scholar]
- Samadian, H.; Farzamfar, S.; Vaez, A.; Ehterami, A.; Bit, A.; Alam, M.; Goodarzi, A.; Darya, G.; Salehi, M. A tailored polylactic acid/polycaprolactone biodegradable and bioactive 3D porous scaffold containing gelatin nanofibers and Taurine for bone regeneration. Sci. Rep. 2020, 10, 13366. [Google Scholar] [CrossRef]
- Tayeed, M.H.; Tehranchi, M.; Ehterami, A.; Shanei, F.; Taleghani, F.; Semyari, H.; Mehrnia, N.; Bozorgzadeh, S.; Salehi, M. Bone Regeneration in Rat Using a PCL/gelatin/Nanoclay Nanocomposite Scaffold Containing Silybin. Res. Sq. 2021. preprint. [Google Scholar] [CrossRef]
- Zaiss, S.; Brown, T.D.; Reichert, J.C.; Berner, A. Poly(ε-caprolactone) Scaffolds Fabricated by Melt Electrospinning for Bone Tissue Engineering. Materials 2016, 9, 232. [Google Scholar] [CrossRef]
- Guarino, V.; Cirillo, V.; Taddei, P.; Alvarez-Perez, M.A.; Ambrosio, L. Tuning Size Scale and Crystallinity of PCL Electrospun Fibres via Solvent Permittivity to Address hMSC Response. Macromol. Biosci. 2011, 11, 1694–1705. [Google Scholar] [CrossRef] [PubMed]
- Guaccio, A.; Guarino, V.; Perez, M.A.A.; Cirillo, V.; Netti, P.A.; Ambrosio, L. Influence of electrospun fiber mesh size on hMSC oxygen metabolism in 3D collagen matrices: Experimental and theoretical evidences. Biotechnol. Bioeng. 2011, 108, 1965–1976. [Google Scholar] [CrossRef]
- Cruz-Maya, I.; Cirillo, V.; Serrano-Bello, J.; Serri, C.; Alvarez-Perez, M.A.; Guarino, V. Optimization of Diclofenac-Loaded Bicom-ponent Nanofibers: Effect of Gelatin on In Vitro and In Vivo Response. Pharmaceutics 2024, 16, 925. [Google Scholar] [CrossRef]
- Delaine-Smith, R.M.; Hann, A.J.; Green, N.H.; Reilly, G.C. Electrospun Fiber Alignment Guides Osteogenesis and Matrix Organi-zation Differentially in Two Different Osteogenic Cell Types. Front. Bioeng. Biotechnol. 2021, 9, 672959. [Google Scholar] [CrossRef]
- Madrid, A.P.M.; Vrech, S.M.; Sanchez, M.A.; Rodriguez, A.P. Advances in additive manufacturing for bone tissue engineering scaffolds. Mater. Sci. Eng. C 2019, 100, 631–644. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Liang, H.; Gao, C.; Peng, S.; Shen, L.; Shuai, C. Additive manufacturing of bone scaffolds. Int. J. Bioprint. 2018, 5, 148. [Google Scholar] [CrossRef]
- Wang, F.; Tankus, E.B.; Santarella, F.; Rohr, N.; Sharma, N.; Märtin, S.; Michalscheck, M.; Maintz, M.; Cao, S.; Thieringer, F.M. Fabrication and Characterization of PCL/HA Filament as a 3D Printing Material Using Thermal Extrusion Technology for Bone Tissue Engineering. Polymers 2022, 14, 669. [Google Scholar] [CrossRef]
- Daskalakis, E.; Huang, B.; Vyas, C.; Acar, A.A.; Fallah, A.; Cooper, G.; Weightman, A.; Koc, B.; Blunn, G.; Bartolo, P. Novel 3D Bioglass Scaffolds for Bone Tissue Regeneration. Polymers 2022, 14, 445. [Google Scholar] [CrossRef]
- Guarino, V.; Gloria, A.; De Santis, R.; Ambrosio, L. Manufacturing Multifunctional Scaffolds for Tissue Engineering. In Polymeric Biomaterials; Dumitriu, S., Popa, V., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 507–532. [Google Scholar]
- Rosales-Ibáñez, R.; Cubo-Mateo, N.; Rodríguez-Navarrete, A.; González-González, A.M.; Villamar-Duque, T.E.; Flores-Sánchez, L.O.; Rodríguez-Lorenzo, L.M. Assessment of a PCL-3D Printing-Dental Pulp Stem Cells Triplet for Bone Engineering: An In Vitro Study. Polymers 2021, 13, 1154. [Google Scholar] [CrossRef]
- Park, S.A.; Lee, H.-J.; Kim, K.-S.; Lee, S.J.; Lee, J.-T.; Kim, S.-Y.; Chang, N.-H.; Park, S.-Y. In Vivo Evaluation of 3D-Printed Polycaprolactone Scaffold Implantation Combined with β-TCP Powder for Alveolar Bone Augmentation in a Beagle Defect Model. Materials 2018, 11, 238. [Google Scholar] [CrossRef]
- Dang, H.P.; Vaquette, C.; Shabab, T.; Pérez, R.A.; Yang, Y.; Dargaville, T.R.; Shafiee, A.; Tran, P.A. Porous 3D Printed Scaffolds For Guided Bone Re-generation In a Rat Calvarial Defect Model. Appl. Mater. Today 2020, 20, 100706. [Google Scholar] [CrossRef]
- Jensen, J.; Rölfing, J.H.D.; Le, D.Q.S.; Kristiansen, A.A.; Nygaard, J.V.; Hokland, L.B.; Bendtsen, M.; Kassem, M.; Lysdahl, H.; Bünger, C.E. Surface-modified functionalized poly-caprolactone scaffolds for bone repair: In vitro and in vivo experiments. J. Biomed. Mater. Res. A 2014, 102, 2993–3003. [Google Scholar] [CrossRef]
- Yang, D.; Faraz, F.; Wang, J.; Radacsi, N. Combination of 3D Printing and Electrospinning Techniques for Biofabrication (Adv. Mater. Technol. 7/2022). Adv. Mater. Technol. 2022, 7, 2101309. [Google Scholar] [CrossRef]
- Liu, J.; Zou, Q.; Wang, C.; Lin, M.; Li, Y.; Zhang, R.; Li, Y. Electrospinning and 3D printed hybrid bi-layer scaffold for guided bone regeneration. Mater. Des. 2021, 210, 110047. [Google Scholar] [CrossRef]
- Helaehil, J.V.; Lourenço, C.B.; Huang, B.; Helaehil, L.V.; de Camargo, I.X.; Chiarotto, G.B.; Santamaria, M., Jr.; Bártolo, P.; Caetano, G.F. In Vivo Investigation of Poly-mer-Ceramic PCL/HA and PCL/β-TCP 3D Composite Scaffolds and Electrical Stimulation for Bone Regeneration. Polymers 2021, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhang, C.; Xiong, W.; Song, Y.; Wang, Q.; Zhang, H.; Guo, S.; Yang, S.; Liu, H. Advances in electroactive biomaterials: Through the lens of electrical stimulation promoting bone regeneration strategy. J. Orthop. Transl. 2024, 47, 191–206. [Google Scholar] [CrossRef]
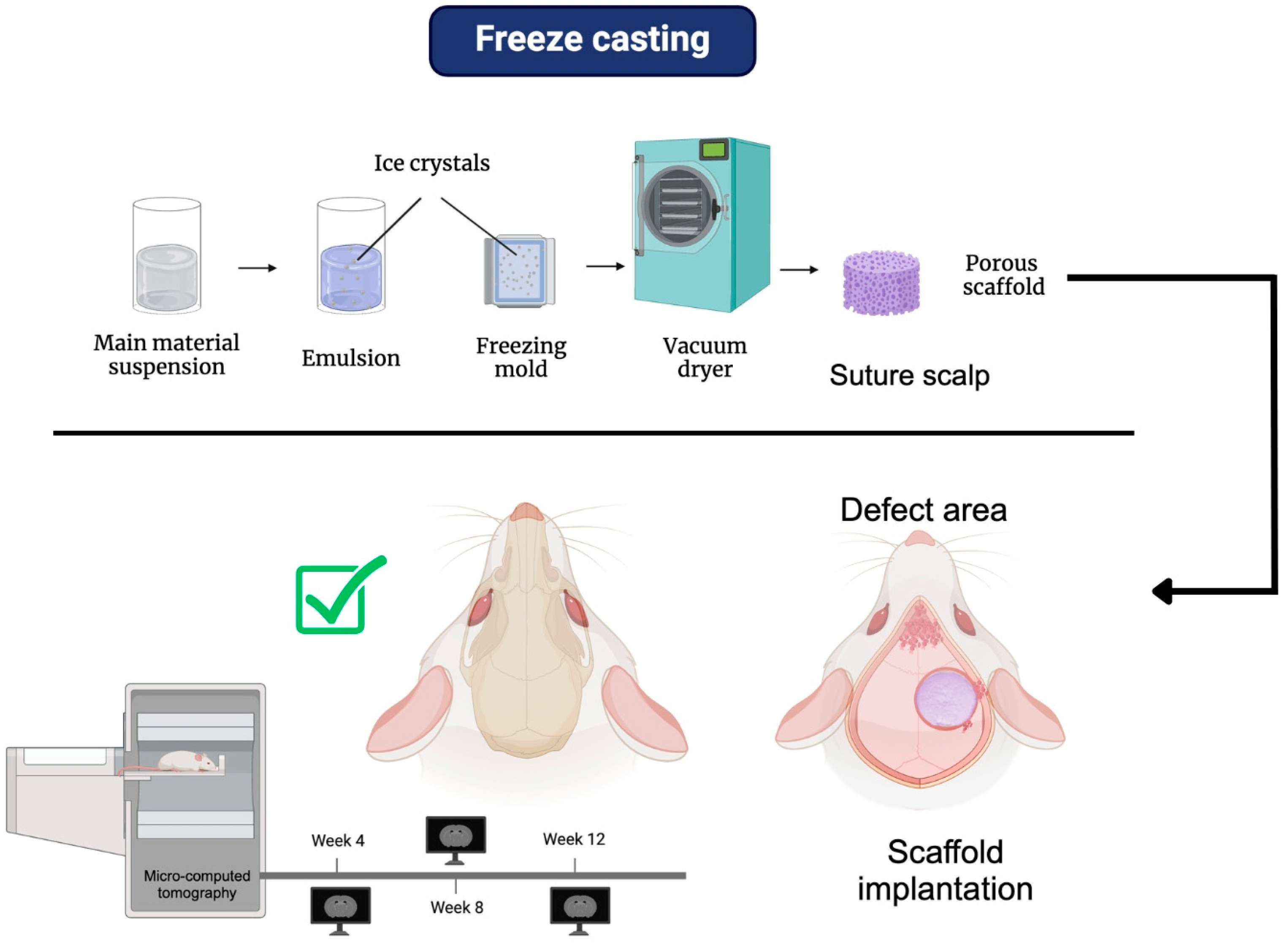

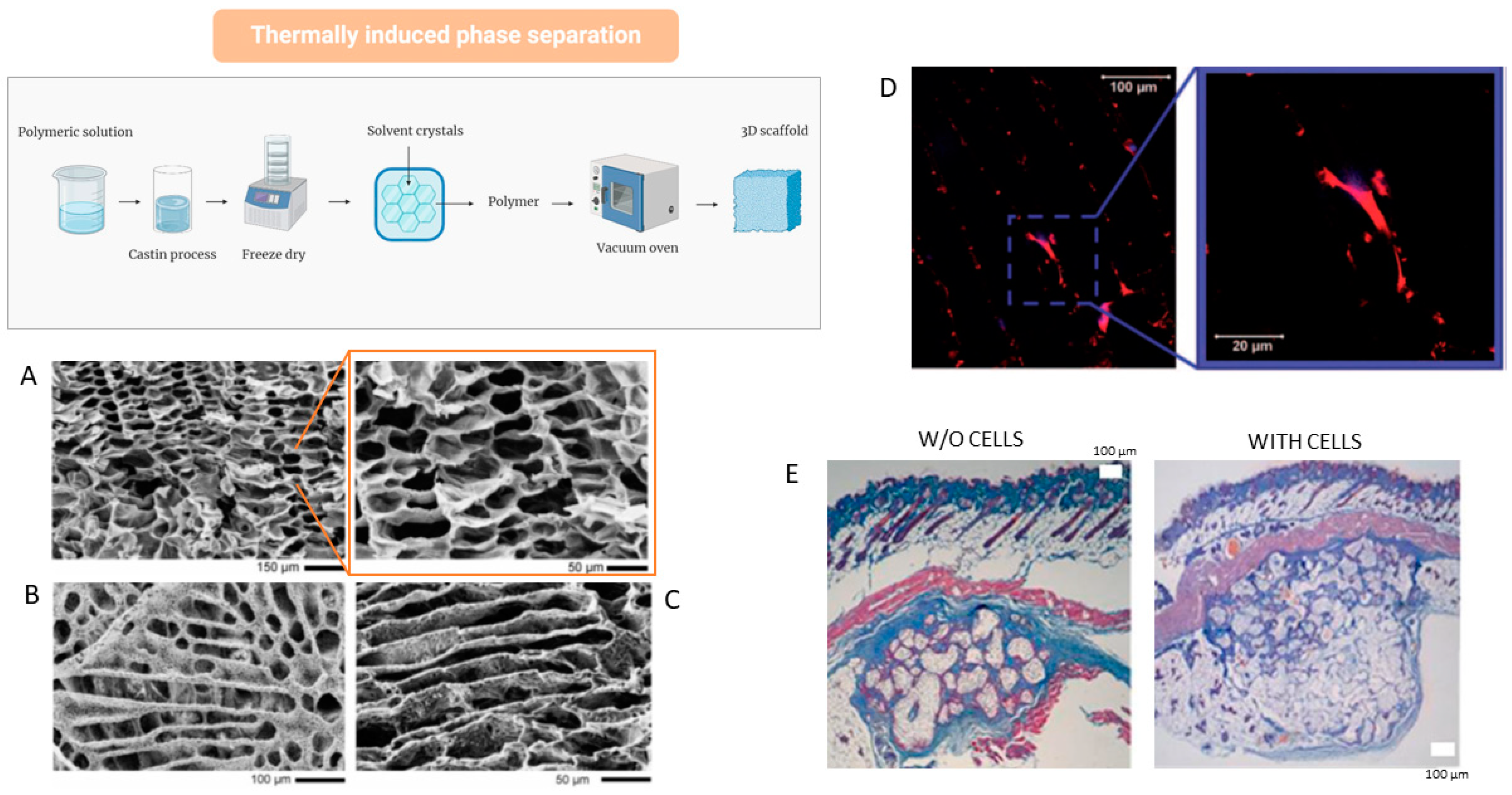
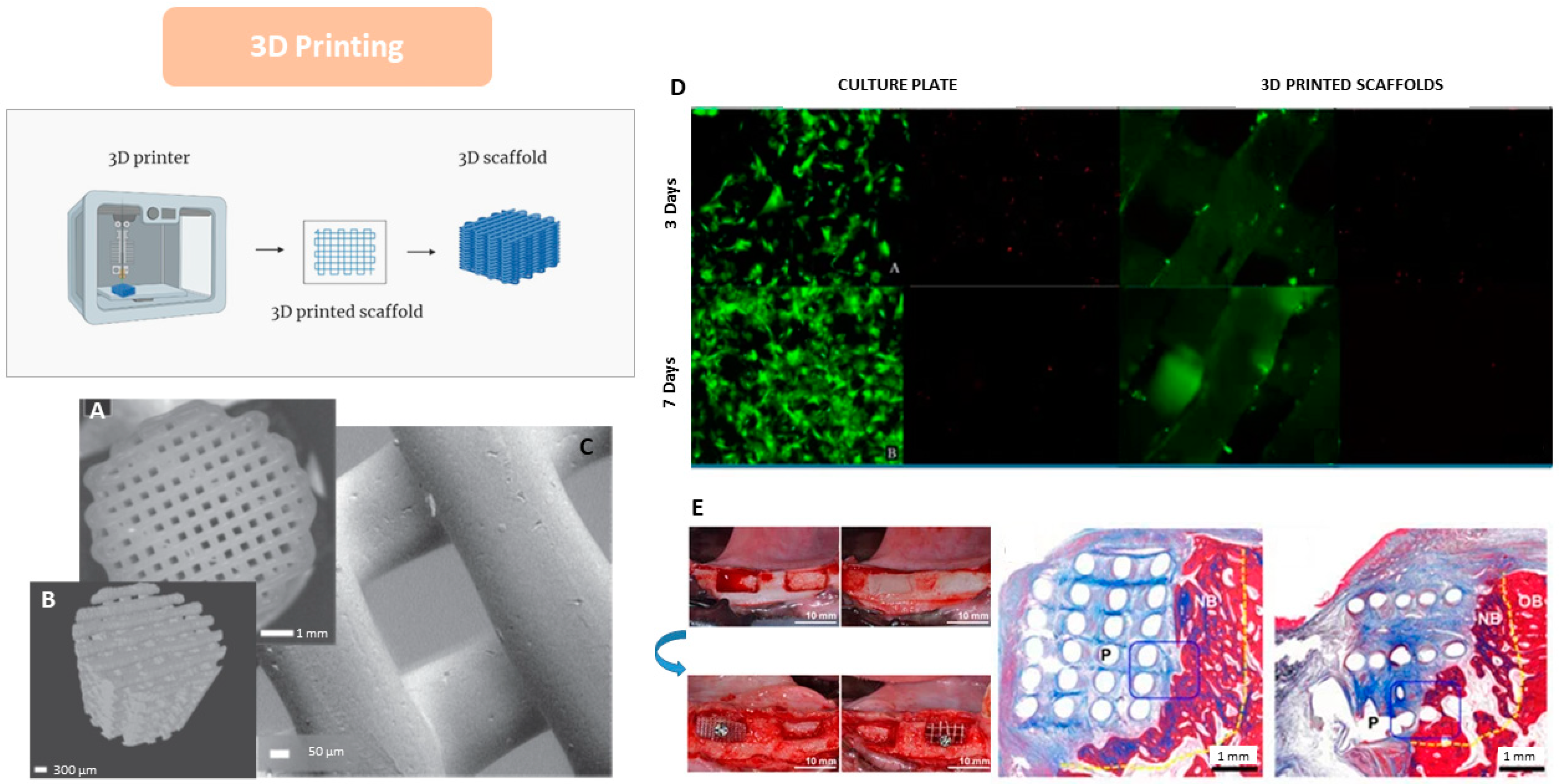
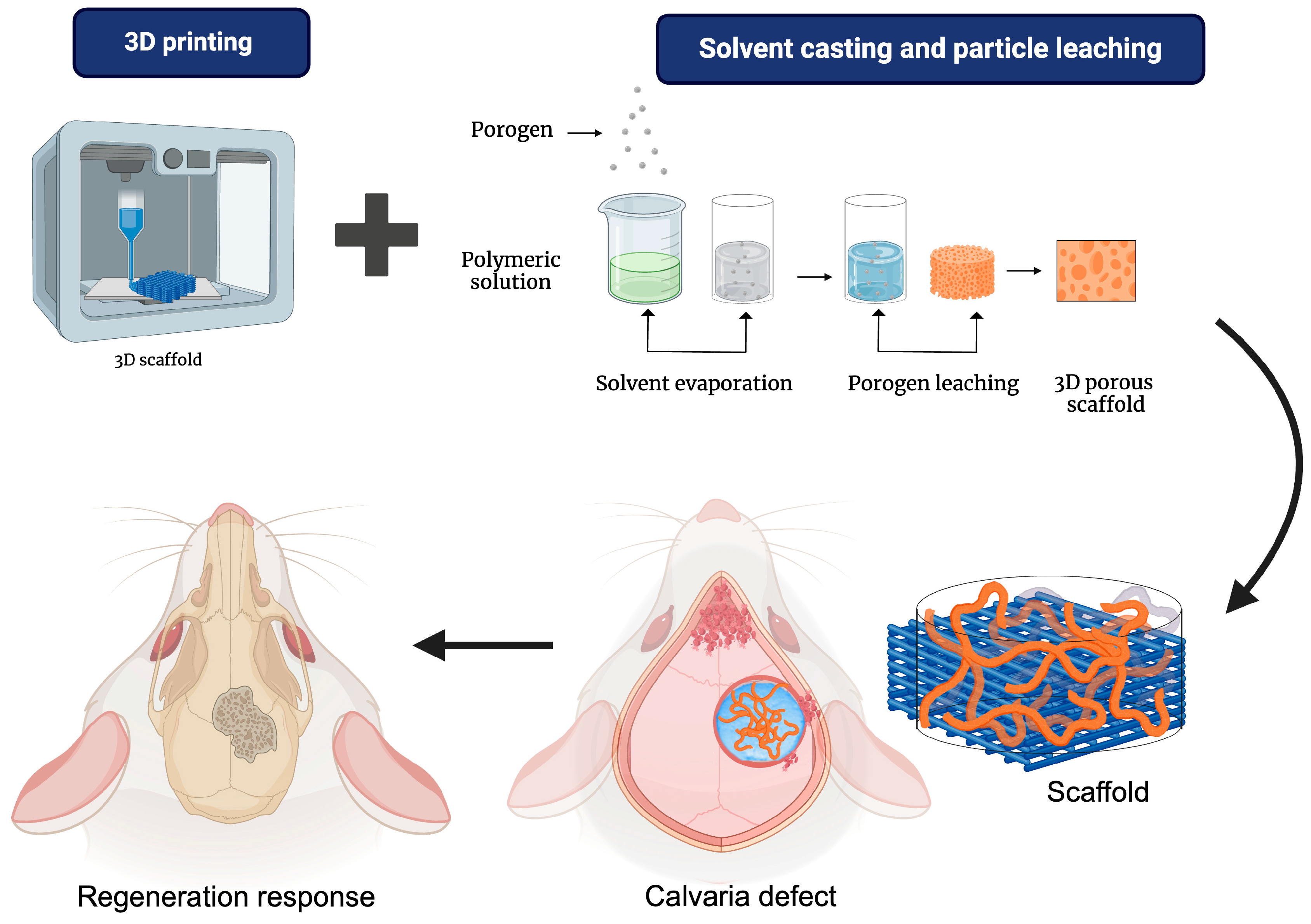

| Biomaterials | Advantages | Disadvantages | Examples | Product Name | Applications | Refs |
|---|---|---|---|---|---|---|
| Natural Polymers |
|
|
|
|
| [7,8,9,10,11] |
| Synthetic Polymers |
|
|
|
|
| [7,10,12,13,14,15,16,17] |
| Bioceramics |
|
|
|
|
| [7,16,17,18,19,20,21] |
| Metals |
|
|
|
|
| [7,22,23,24,25,26,27] |
| Composites |
|
|
|
|
| [7,11,28,29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Ruiz, F.; Núñez-Tapia, I.; Piña-Barba, M.C.; Alvarez-Pérez, M.A.; Guarino, V.; Serrano-Bello, J. Polycaprolactone for Hard Tissue Regeneration: Scaffold Design and In Vivo Implications. Bioengineering 2025, 12, 46. https://doi.org/10.3390/bioengineering12010046
Ramírez-Ruiz F, Núñez-Tapia I, Piña-Barba MC, Alvarez-Pérez MA, Guarino V, Serrano-Bello J. Polycaprolactone for Hard Tissue Regeneration: Scaffold Design and In Vivo Implications. Bioengineering. 2025; 12(1):46. https://doi.org/10.3390/bioengineering12010046
Chicago/Turabian StyleRamírez-Ruiz, Fernanda, Israel Núñez-Tapia, María Cristina Piña-Barba, Marco Antonio Alvarez-Pérez, Vincenzo Guarino, and Janeth Serrano-Bello. 2025. "Polycaprolactone for Hard Tissue Regeneration: Scaffold Design and In Vivo Implications" Bioengineering 12, no. 1: 46. https://doi.org/10.3390/bioengineering12010046
APA StyleRamírez-Ruiz, F., Núñez-Tapia, I., Piña-Barba, M. C., Alvarez-Pérez, M. A., Guarino, V., & Serrano-Bello, J. (2025). Polycaprolactone for Hard Tissue Regeneration: Scaffold Design and In Vivo Implications. Bioengineering, 12(1), 46. https://doi.org/10.3390/bioengineering12010046








