Integrating Modern Technologies into Traditional Anterior Cruciate Ligament Tissue Engineering
Abstract
1. Introduction
2. The Traditional ACL Field as We Know It
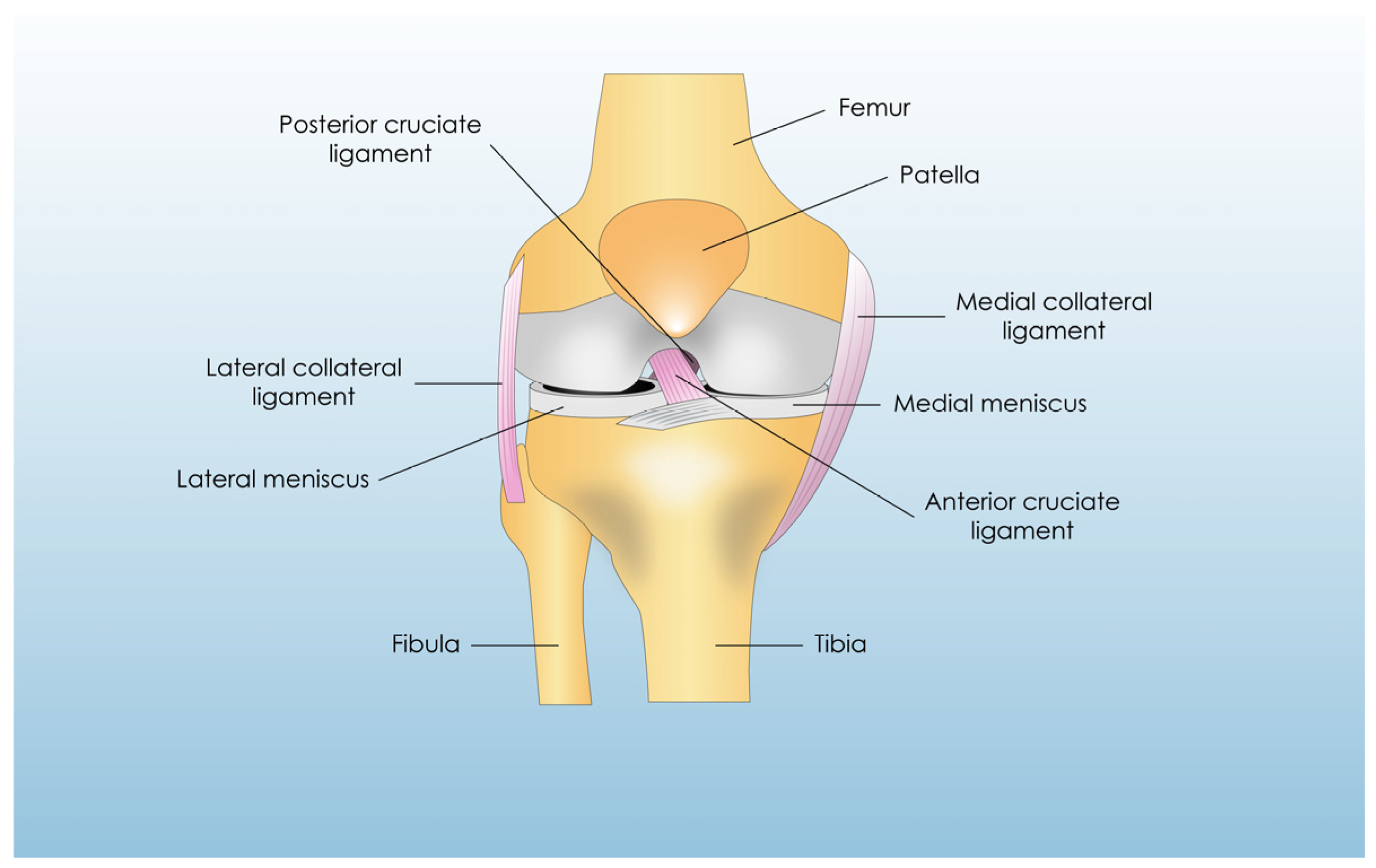
2.1. Anatomy, Biomechanics, and Physiology of the ACL
2.2. Injuries and Healing of ACL
2.3. Current Techniques Utilized for Treating the Ruptured ACL
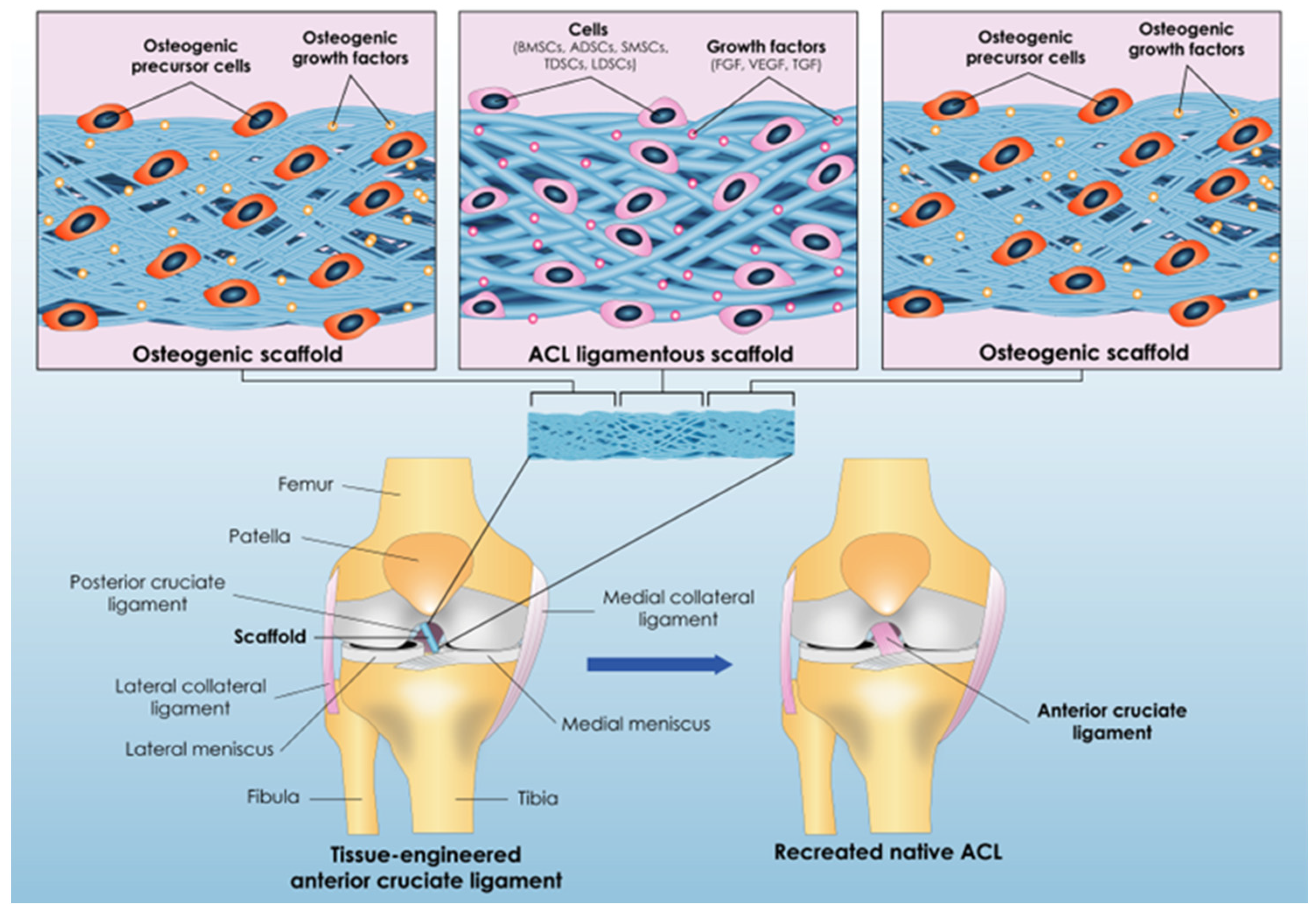
3. Tissue Engineering: Panacea for ACL’s Reconstruction Issues?
3.1. Issue No1: The Appropriate Scaffold Construction
3.1.1. The Process of Creating the Scaffold’s Structure
3.1.2. Constructing the Scaffold’s “Anchors”
3.1.3. Biomaterial Selection
- i.
- Natural biomaterials
- ii.
- Synthetic biomaterials
- iii.
- Composite biomaterials
3.2. Issue No2: The Suitable Cell Type of ACL Tissue Engineering
3.3. Issue No3: Facilitating the Right Combination of Growth Factors (GFs) and Mechanical Stimuli
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Evans, S.; Shaginaw, J.; Bartolozzi, A. Acl reconstruction—It’s all about timing. Int. J. Sports Phys. Ther. 2014, 9, 268–273. [Google Scholar] [PubMed]
- Csintalan, R.P.; Inacio, M.C.S.; Funahashi, T.T. Incidence rate of anterior cruciate ligament reconstructions. Perm. J. 2008, 12, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Ingham, S.J.M.; Maeyama, A.; Lertwanich, P.; Wang, J.H.; Mifune, Y.; Kramer, S.; Smolinski, P.; Fu, F.H. Biomechanics of the human triple-bundle anterior cruciate ligament. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Rahardja, R.; Zhu, M.; Love, H.; Clatworthy, M.G.; Monk, A.P.; Young, S.W. Factors associated with revision following anterior cruciate ligament reconstruction: A systematic review of registry data. Knee 2020, 27, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Borque, K.A.; Laughlin, M.S.; Pinheiro, V.H.; Jones, M.; Williams, A. Rebranding the ‘anatomic’ ACL reconstruction: Current concepts. J. ISAKOS 2023, 8, 23–28. [Google Scholar] [CrossRef]
- Browning, W.M., III; Kluczynski, M.A.; Curatolo, C.; Marzo, J.M. Suspensory Versus Aperture Fixation of a Quadrupled Hamstring Tendon Autograft in Anterior Cruciate Ligament Reconstruction: A Meta-analysis. Am. J. Sports Med. 2017, 45, 2418–2427. [Google Scholar] [CrossRef]
- Noyes, F.R.; Barber-Westin, S.D. Revision anterior cruciate surgery with use of bone-patellar tendon-bone autogenous grafts. The Journal of bone and joint surgery. Am. Vol. 2001, 83, 1131–1143. [Google Scholar] [CrossRef]
- Lai, C.C.H.; Ardern, C.L.; Feller, J.A.; Webster, K.E. Eighty-three per cent of elite athletes return to preinjury sport after anterior cruciate ligament reconstruction: A systematic review with meta-analysis of return to sport rates, graft rupture rates and performance outcomes. Br. J. Sports Med. 2018, 52, 128–138. [Google Scholar] [CrossRef]
- Kartus, J.; Movin, T.; Karlsson, J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy 2001, 17, 971–980. [Google Scholar] [CrossRef]
- Siebold, R.; Schuhmacher, P.; Fernandez, F.; Śmigielski, R.; Fink, C.; Brehmer, A.; Kirsch, J. Flat midsubstance of the anterior cruciate ligament with tibial “C”-shaped insertion site. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2015, 23, 3136–3142. [Google Scholar] [CrossRef]
- Van Zyl, R.; Van Schoor, A.-N.; Du Toit, P.J.; Suleman, F.E.; Velleman, M.D.; Glatt, V.; Tetsworth, K.; Hohmann, E. The Association Between Anterior Cruciate Ligament Length and Femoral Epicondylar Width Measured on Preoperative Magnetic Resonance Imaging or Radiograph. Arthrosc. Sports Med. Rehabil. 2020, 2, e23–e31. [Google Scholar] [CrossRef] [PubMed]
- Markatos, K.; Kaseta, M.K.; Lallos, S.N.; Korres, D.S.; Efstathopoulos, N. The anatomy of the ACL and its importance in ACL reconstruction. Eur. J. Orthop. Surg. Traumatol. 2013, 23, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Duthon, V.B.; Barea, C.; Abrassart, S.; Fasel, J.H.; Fritschy, D.; Ménétrey, J. Anatomy of the anterior cruciate ligament. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Hassebrock, J.D.; Gulbrandsen, M.T.; Asprey, W.L.; Makovicka, J.L.; Chhabra, A. Knee Ligament Anatomy and Biomechanics. Sports Med. Arthrosc. Rev. 2020, 28, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Kraeutler, M.J.; Wolsky, R.M.; Vidal, A.F.; Bravman, J.T. Anatomy and biomechanics of the native and reconstructed anterior cruciate ligament: Surgical implications. J. Bone Jt. Surg. Am. Vol. 2017, 99, 438–445. [Google Scholar] [CrossRef]
- Ferretti, M.; Ekdahl, M.; Shen, W.; Fu, F.H. Osseous landmarks of the femoral attachment of the anterior cruciate ligament: An anatomic study. J. Arthrosc. Relat. Surg. 2007, 23, 1218–1225. [Google Scholar] [CrossRef]
- van Eck, C.F.; Morse, K.R.; Lesniak, B.P.; Kropf, E.J.; Tranovich, M.J.; van Dijk, C.N.; Fu, F.H. Does the lateral intercondylar ridge disappear in ACL deficient patients? Knee Surg Sports Traumatol Arthrosc. 2010, 18, 1184–1188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Śmigielski, R.; Zdanowicz, U.; Drwięga, M.; Ciszek, B.; Williams, A. The anatomy of the anterior cruciate ligament and its relevance to the technique of reconstruction. Bone Jt. J. 2016, 98B, 1020–1026. [Google Scholar] [CrossRef]
- Kopf, S.; Musahl, V.; Tashman, S.; Szczodry, M.; Shen, W.; Fu, F.H. A systematic review of the femoral origin and tibial insertion morphology of the ACL. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2009, 17, 213–219. [Google Scholar] [CrossRef]
- Yonetani, Y.; Kusano, M.; Tsujii, A.; Kinugasa, K.; Hamada, M.; Shino, K. Tibial insertion of the anterior cruciate ligament and anterior horn of the lateral meniscus share the lateral slope of the medial intercondylar ridge: A computed tomography study in a young, healthy population. Knee 2019, 26, 612–618. [Google Scholar] [CrossRef]
- Tensho, K.; Shimodaira, H.; Aoki, T.; Narita, N.; Kato, H.; Kakegawa, A.; Fukushima, N.; Moriizumi, T.; Fujii, M.; Fujinaga, Y.; et al. Bony Landmarks of the Anterior Cruciate Ligament Tibial Footprint: A Detailed Analysis Comparing 3-Dimensional Computed Tomography Images to Visual and Histological Evaluations. Am. J. Sports Med. 2014, 42, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Petersen, W.; Zantop, T. Anatomy of the anterior cruciate ligament with regard to its two bundles. Clin. Orthop. Relat. Res. 2007, 454, 35–47. [Google Scholar] [CrossRef]
- Marieswaran, M.; Jain, I.; Garg, B.; Sharma, V.; Kalyanasundaram, D. A review on biomechanics of anterior cruciate ligament and materials for reconstruction. Appl. Bionics Biomech. 2018, 2018, 4657824. [Google Scholar] [CrossRef]
- Woo, S.L.; Hollis, J.M.; Adams, D.J.; Lyon, R.M.; Takai, S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. Eff. Specim. Age Orientation. Am. J. Sports Med. 1990, 19, 217–225. [Google Scholar] [CrossRef]
- Laurent, C.P.; Ganghoffer, J.F.; Babin, J.; Six, J.L.; Wang, X.; Rahouadj, R. Morphological characterization of a novel scaffold for anterior cruciate ligament tissue engineering. J. Biomech. Eng. 2011, 133, 065001. [Google Scholar] [CrossRef]
- Lim, W.L.; Liau, L.L.; Ng, M.H.; Chowdhury, S.R.; Law, J.X. Current Progress in Tendon and Ligament Tissue Engineering. Tissue Eng. Regen. Med. 2019, 16, 549–571. [Google Scholar] [CrossRef]
- Wang, L.J.; Zeng, N.; Yan, Z.P.; Li, J.T.; Ni, G.X. Post-traumatic osteoarthritis following ACL injury. Arthritis Res. Ther. 2020, 22, 57. [Google Scholar] [CrossRef]
- Rodriguez, K.; Soni, M.; Joshi, P.K.; Patel, S.C.; Shreya, D.; Zamora, D.I.; Patel, G.S.; Grossmann, I.; Sange, I. Anterior Cruciate Ligament Injury: Conservative Versus Surgical Treatment. Cureus 2021, 13, e20206. [Google Scholar] [CrossRef]
- Takahashi, S.; Nagano, Y.; Ito, W.; Kido, Y.; Okuwaki, T. A retrospective study of mechanisms of anterior cruciate ligament injuries in high school basketball, handball, judo, soccer, and volleyball. Medicine 2019, 98, e16030. [Google Scholar] [CrossRef]
- Yu, B.; Garrett, W.E. Mechanisms of non-contact ACL injuries. Br. J. Sports Med. 2007, 41, i47–i51. [Google Scholar] [CrossRef]
- Murray, M.M.; Martin, S.D.; Martin, T.L.; Spector, M. Histological changes in the human anterior cruciate ligament after rupture. J. Bone Jt. Surg. Am. 2000, 82, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.M.; Fleming, B.C. Biology of anterior cruciate ligament injury and repair: Kappa delta ann doner vaughn award paper 2013. J. Orthop. Res. 2013, 31, 1501–1506. [Google Scholar] [CrossRef]
- El-Amin, S. Anterior Cruciate Ligament Tissue Engineering: A Review of Current Investigations. J. Nanotechnol. Mater. Sci. 2016, 3, 1–7. [Google Scholar] [CrossRef][Green Version]
- Uchida, R.; Horibe, S.; Nakamura, N. Stem cell-based therapy in anterior cruciate ligament repair. Ann. Jt. 2017, 2, 76. [Google Scholar] [CrossRef]
- Bray, R.C.; Leonard, C.A.; Salo, P.T. Vascular physiology and long-term healing of partial ligament tears. J. Orthop. Res. 2002, 20, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Delincé, P.; Ghafil, D. Anterior cruciate ligament tears: Conservative or surgical treatment? A critical review of the literature. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 48–61. [Google Scholar] [CrossRef]
- Ding, D.Y.; Tucker, L.Y.; Rugg, C.M. Comparison of Anterior Cruciate Ligament Tears Treated Nonoperatively Versus with Reconstruction: Risk of Subsequent Surgery. Am. J. Sports Med. 2022, 50, 652–661. [Google Scholar] [CrossRef]
- Smith, T.O.; Postle, K.; Penny, F.; McNamara, I.; Mann, C.J. Is reconstruction the best management strategy for anterior cruciate ligament rupture? A systematic review and meta-analysis comparing anterior cruciate ligament reconstruction versus non-operative treatment. Knee 2014, 21, 462–470. [Google Scholar] [CrossRef]
- Filbay, S.R.; Grindem, H. Evidence-based recommendations for the management of anterior cruciate ligament (ACL) rupture. Best. Pract. Research. Clin. Rheumatol. 2019, 33, 33–47. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, Y.; Zhang, A.; Zhou, L.; Zhang, Q.; Ji, Z.; Xu, W. Global research status of anterior cruciate ligament reconstruction: A bibliometric analysis. EFORT Open Rev. 2022, 7, 808–816. [Google Scholar] [CrossRef]
- Di Benedetto, P.; Di Benedetto, E.; Fiocchi, A.; Beltrame, A.; Causero, A. Causes of Failure of Anterior Cruciate Ligament Reconstruction and Revision Surgical Strategies. Knee Surg. Relat. Res. 2016, 28, 319–324. [Google Scholar] [CrossRef]
- Samitier, G.; Marcano, A.I.; Alentorn-Geli, E.; Cugat, R.; Farmer, K.W.; Moser, M.W. Failure of Anterior Cruciate Ligament Reconstruction. Arch. Bone Jt. Surg. 2015, 3, 220–240. [Google Scholar] [PubMed]
- Yang, Y.T.; Cai, Z.J.; He, M.; Liu, D.; Xie, W.Q.; Li, Y.S.; Xiao, W.-F. All-Inside Anterior Cruciate Ligament Reconstruction: A Review of Advance and Trends. Front. Biosci. Landmark 2022, 27, 91. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.H.; Bennett, C.H.; Lattermann, C.; Ma, C.B. Current trends in anterior cruciate ligament reconstruction. Part 1: Biology and biomechanics of reconstruction. Am. J. Sports Med. 1999, 27, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.-Y.; Wu, C.; Dede, O.; Vercillo, F.; Noorani, S. Biomechanics and anterior cruciate ligament reconstruction. J. Orthop. Surg. Res. 2006, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Zhang, K.; Li, J.; Fu, W. Supplementary Lateral Extra-articular Tenodesis for Residual Anterolateral Rotatory Instability in Patients Undergoing Single-Bundle Anterior Cruciate Ligament Reconstruction: A Meta-analysis of Randomized Controlled Trials. Orthop. J. Sports Med. 2021, 9, 23259671211002282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagaraj, R.; Kumar, M.N. Revision anterior cruciate ligament reconstruction in the nonathlete population. Indian J. Orthop. 2019, 53, 154–159. [Google Scholar] [CrossRef]
- Wilde, J.; Bedi, A.; Altchek, D.W. Revision anterior cruciate ligament reconstruction. Sports Health 2014, 6, 504–518. [Google Scholar] [CrossRef]
- George, M.S.; Dunn, W.R.; Spindler, K.P. Current concepts review: Revision anterior cruciate ligament reconstruction. Am. J. Sports Med. 2006, 34, 2026–2037. [Google Scholar] [CrossRef]
- Nau, T.; Teuschl, A. Regeneration of the anterior cruciate ligament: Current strategies in tissue engineering. World J. Orthop. 2015, 6, 127–136. [Google Scholar] [CrossRef]
- Paschos, N.K.; Howell, S.M. Anterior cruciate ligament reconstruction: Principles of treatment. EFORT Open Rev. 2016, 1, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Guglielmetti, L.G.B.; Salas, V.E.R.; Jorge, P.B.; Severino, F.R.; Duarte, A.; de Oliveira, V.M.; Cury, R.d.P.L. Prospective and Randomized Clinical Evaluation of Hamstring Versus Patellar Tendon Autograft for Anterior Cruciate Ligament Reconstruction in Soccer Players. Orthop. J. Sports Med. 2021, 9, 23259671211028168. [Google Scholar] [CrossRef] [PubMed]
- Laboute, E.; James-Belin, E.; Puig, P.L.; Trouve, P.; Verhaeghe, E. Graft failure is more frequent after hamstring than patellar tendon autograft. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3537–3546. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, M.; Deng, M.; Xing, J.; He, L.; Wang, C. Outcome of bone-patellar tendon-bone vs hamstring tendon autograft for anterior cruciate ligament reconstruction: A meta-analysis of randomized controlled trials with a 5-year minimum follow-up. Medicine 2020, 99, e23476. [Google Scholar] [CrossRef]
- Lin, K.M.; Boyle, C.; Marom, N.; Marx, R.G. Graft Selection in Anterior Cruciate Ligament Reconstruction. Sports Med. Arthrosc. Rev. 2020, 28, 41–48. [Google Scholar] [CrossRef]
- Mouarbes, D.; Menetrey, J.; Marot, V.; Courtot, L.; Berard, E.; Cavaignac, E. Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis of Outcomes for Quadriceps Tendon Autograft Versus Bone–Patellar Tendon–Bone and Hamstring-Tendon Autografts. Am. J. Sports Med. 2019, 47, 3531–3540. [Google Scholar] [CrossRef]
- Baawa-Ameyaw, J.; Plastow, R.; Begum, F.A.; Kayani, B.; Jeddy, H.; Haddad, F. Current concepts in graft selection for anterior cruciate ligament reconstruction. EFORT Open Rev. 2021, 6, 808–815. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheung, E.C.; DiLallo, M.; Feeley, B.T.; Lansdown, D.A. Osteoarthritis and ACL Reconstruction-Myths and Risks. Curr. Rev. Musculoskelet. Med. 2020, 13, 115–122. [Google Scholar] [CrossRef]
- Bodkin, S.G.; Werner, B.C.; Slater, L.V.; Hart, J.M. Post-traumatic osteoarthritis diagnosed within 5 years following ACL reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 790–796. [Google Scholar] [CrossRef]
- Santos, M.L.; Rodrigues, M.T.; Domingues, R.M.A.; Reis, R.L.; Gomes, M.E. Biomaterials as Tendon and Ligament Substitutes: Current Developments. Stud. Mechanobiol. Tissue Eng. Biomater. 2017, 21, 349–371. [Google Scholar] [CrossRef]
- Laurent, C.; Liu, X.; De Isla, N.; Wang, X.; Rahouadj, R. Defining a scaffold for ligament tissue engineering: What has been done, and what still needs to be done. J. Cell. Immunother. 2018, 4, 4–9. [Google Scholar] [CrossRef]
- Cooper, J.A.; Lu, H.H.; Ko, F.K.; Freeman, J.W.; Laurencin, C.T. Fiber-based tissue-engineered scaffold for ligament replacement: Design considerations and in vitro evaluation. Biomaterials 2005, 26, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Surrao, D.C.; Waldman, S.D.; Amsden, B.G. Biomimetic poly(lactide) based fibrous scaffolds for ligament tissue engineering. Acta Biomater. 2012, 8, 3997–4006. [Google Scholar] [CrossRef] [PubMed]
- Tovar, N.; Sanjeeva Murthy, N.; Kohn, J.; Gatt, C.; Dunn, M. ACL reconstruction using a novel hybrid scaffold composed of polyarylate fibers and collagen fibers. J. Biomed. Mater. Res. A 2012, 100A, 2913–2920. [Google Scholar] [CrossRef]
- Tang, Y.; Tian, J.; Li, L.; Huang, L.; Shen, Q.; Guo, S.; Jiang, Y. Biomimetic Biphasic Electrospun Scaffold for Anterior Cruciate Ligament Tissue Engineering. Tissue Eng. Regen. Med. 2021, 18, 819–830. [Google Scholar] [CrossRef]
- Leong, N.L.; Petrigliano, F.A.; McAllister, D.R. Current tissue engineering strategies in anterior cruciate ligament reconstruction. J. Biomed. Mater. Res. A 2014, 102, 1614–1624. [Google Scholar] [CrossRef]
- Chi, J.; Wang, M.; Chen, J.; Hu, L.; Chen, Z.; Backman, L.J.; Zhang, W. Topographic Orientation of Scaffolds for Tissue Regeneration: Recent Advances in Biomaterial Design and Applications. Biomimetics 2022, 7, 131. [Google Scholar] [CrossRef]
- Aminatun Huriah, R.; Hikmawati, D.; Hadi, S.; Amrillah, T.; Abdullah, C.A.C. Nanofiber Scaffold Based on Polylactic Acid-Polycaprolactone for Anterior Cruciate Ligament Injury. Polymers 2022, 14, 2983. [Google Scholar] [CrossRef]
- Sahoo, S.; Cho-Hong, J.G.; Siew-Lok, T. Development of hybrid polymer scaffolds for potential applications in ligament and tendon tissue engineering. Biomed. Mater. 2007, 2, 169–173. [Google Scholar] [CrossRef]
- Wu, Y.; Wong, Y.S.; Fuh, J.Y.H. Degradation behaviors of geometric cues and mechanical properties in a 3D scaffold for tendon repair. J. Biomed. Mater. Res. A 2017, 105, 1138–1149. [Google Scholar] [CrossRef]
- Avossa, J.; Herwig, G.; Toncelli, C.; Itel, F.; Rossi, R.M. Electrospinning based on benign solvents: Current definitions, implications and strategies. Green. Chem. 2022, 24, 2347–2375. [Google Scholar] [CrossRef]
- Emonts, C.; Wienen, D.; Bauer, B.; Idrissi, A.; Gries, T. 3D-Braided Poly-ε-Caprolactone-Based Scaffolds for Ligament Tissue Engineering. J. Funct. Biomater. 2022, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.W. Tissue engineered devices for ligament repair, replacement and regeneration. Afr. J. Biotechnol. 2009, 8, 7182–7189. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Jones, A.C.; Arns, C.H.; Hutmacher, D.W.; Milthorpe, B.K.; Sheppard, A.P.; Knackstedt, M.A. The correlation of pore morphology, interconnectivity and physical properties of 3D ceramic scaffolds with bone ingrowth. Biomaterials 2009, 30, 1440–1451. [Google Scholar] [CrossRef]
- Noyes, F.R.; Grood, E.S. The strength of the anterior cruciate ligament in humans and Rhesus monkeys. J. Bone Jt. Surg. Am. 1976, 58, 1074–1082. [Google Scholar] [CrossRef]
- Roldán, E.; Reeves, N.D.; Cooper, G.; Andrews, K. In vivo mechanical behaviour of the anterior cruciate ligament: A study of six daily and high impact activities. Gait Posture 2017, 58, 201–207. [Google Scholar] [CrossRef]
- Vieira, A.C.; Guedes, R.M.; Tita, V. Damage-induced hydrolyses modelling of biodegradable polymers for tendons and ligaments repair. J. Biomech. 2015, 48, 3478–3485. [Google Scholar] [CrossRef]
- Vieira, A.C.; Guedes, R.M.; Tita, V. Constitutive modeling of biodegradable polymers: Hydrolytic degradation and time-dependent behavior. Int. J. Solids Struct. 2014, 51, 1164–1174. [Google Scholar] [CrossRef]
- Lu, H.H.; Spalazzi, J.P. Biomimetic stratified scaffold design for ligament-to-bone interface tissue engineering. Comb. Chem. High. Throughput Screen. 2009, 12, 589–597. [Google Scholar] [CrossRef]
- Qu, D.; Subramony, S.D.; Boskey, A.L.; Pleshko, N.; Doty, S.B.; Lu, H.H. Compositional mapping of the mature anterior cruciate ligament-to-bone insertion. J. Orthop. Res. 2017, 35, 2513–2523. [Google Scholar] [CrossRef]
- Boys, A.J.; McCorry, M.C.; Rodeo, S.; Bonassar, L.J.; Estroff, L.A. Next generation tissue engineering of orthopedic soft tissue-to-bone interfaces. MRS Commun. 2017, 7, 289–308. [Google Scholar] [CrossRef]
- Patel, S.; Caldwell, J.-M.; Doty, S.B.; Levine, W.N.; Rodeo, S.; Soslowsky, L.J.; Thomopoulos, S.; Lu, H.H. Integrating soft and hard tissues via interface tissue engineering. J. Orthop. Res. 2018, 36, 1069–1077. [Google Scholar] [CrossRef]
- Gögele, C.; Hahn, J.; Schulze-Tanzil, G. Anatomical Tissue Engineering of the Anterior Cruciate Ligament Entheses. Int. J. Mol. Sci. 2023, 24, 9745. [Google Scholar] [CrossRef]
- Dunn, M.G.; Liesch, J.B.; Tiku, M.L.; Zawadsky, J.P. Development of fibroblast-seeded ligament analogs for ACL reconstruction. J. Biomed. Mater. Res. 1995, 29, 1363–1371. [Google Scholar] [CrossRef]
- Bakirci, E.; Guenat, O.T.; Ahmad, S.S.; Gantenbein, B. Tissue Engineering Approaches For The Repair And Regeneration Of The Anterior Cruciate Ligament: Towards 3D Bioprinted Acl-On-Chip. Eur. Cell Mater. 2022, 44, 21–42. [Google Scholar] [CrossRef]
- Ge, Z.; Yang, F.; Goh, J.C.H.; Ramakrishna, S.; Lee, E.H. Biomaterials and scaffolds for ligament tissue engineering. J. Biomed. Mater. Res. A 2006, 77, 639–652. [Google Scholar] [CrossRef]
- Walters, V.I.; Kwansa, A.L.; Freeman, J.W. Design and analysis of braid-twist collagen scaffolds. Connect. Tissue Res. 2012, 53, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Ferreira, F.N.; Alves, N.M.; Paiva, M.C. Biodegradable polymer nanocomposites for ligament/tendon tissue engineering. J. Nanobiotechnology 2020, 18, 23. [Google Scholar] [CrossRef]
- Bi, F.; Shi, Z.; Liu, A.; Guo, P.; Yan, S. Anterior cruciate ligament reconstruction in a rabbit model using silk-collagen scaffold and comparison with autograft. PLoS ONE 2015, 10, e0125900. [Google Scholar] [CrossRef]
- Bi, F.; Chen, Y.; Liu, J.; Hu, W.; Tian, K. Bone Mesenchymal Stem Cells Contribute to Ligament Regeneration and Graft-Bone Healing after Anterior Cruciate Ligament Reconstruction with Silk-Collagen Scaffold. Stem Cells Int. 2021, 2021, 6697969. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Ouyang, H.W.; Goh, J.C.H.; Mo, X.M.; Teoh, S.H.; Lee, E.H. Characterization of anterior cruciate ligament cells and bone marrow stromal cells on various biodegradable polymeric films. Mater. Sci. Eng. C 2002, 20, 63–69. [Google Scholar] [CrossRef]
- Lu, H.H.; Cooper, J.A.; Manuel, S.; Freeman, J.W.; Attawia, M.A.; Ko, F.K.; Laurencin, C.T. Anterior cruciate ligament regeneration using braided biodegradable scaffolds: In vitro optimization studies. Biomaterials 2005, 26, 4805–4816. [Google Scholar] [CrossRef]
- Petrigliano, F.A.; Arom, G.A.; Nazemi, A.N.; Yeranosian, M.G.; Wu, B.M.; McAllister, D.R. In vivo evaluation of electrospun polycaprolactone graft for anterior cruciate ligament engineering. Tissue Eng. Part A 2015, 21, 1228–1236. [Google Scholar] [CrossRef]
- Sahoo, S.; Toh, S.L.; Goh, J.C.H. A bFGF-releasing silk/PLGA-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells. Biomaterials 2010, 31, 2990–2998. [Google Scholar] [CrossRef]
- Hevesi, M.; LaPrade, M.; Saris, D.B.F.; Krych, A.J. Stem Cell Treatment for Ligament Repair and Reconstruction. Curr. Rev. Musculoskelet. Med. 2019, 12, 446–450. [Google Scholar] [CrossRef]
- Wang, C.; Hu, Y.; Zhang, S.; Ruan, D.; Huang, Z.; He, P.; Cai, H.; Heng, B.C.; Chen, X.; Shen, W. Application of Stem Cell Therapy for ACL Graft Regeneration. Stem Cells Int. 2021, 2021, 6641818. [Google Scholar] [CrossRef]
- Ogata, Y.; Mabuchi, Y.; Shinoda, K.; Horiike, Y.; Mizuno, M.; Otabe, K.; Suto, E.G.; Suzuki, N.; Sekiya, I.; Akazawa, C. Anterior cruciate ligament-derived mesenchymal stromal cells have a propensity to differentiate into the ligament lineage. Regen. Ther. 2018, 8, 20–28. [Google Scholar] [CrossRef]
- Ge, Z.; Goh, J.C.H.; Lee, E.H. Selection of cell source for ligament tissue engineering. Cell Transplant. 2005, 14, 573–583. [Google Scholar] [CrossRef]
- Liu, H.; Fan, H.; Toh, S.L.; Goh, J.C.H. A comparison of rabbit mesenchymal stem cells and anterior cruciate ligament fibroblasts responses on combined silk scaffolds. Biomaterials 2008, 29, 1443–1453. [Google Scholar] [CrossRef]
- Cooper, J.A.; Bailey, L.A.O.; Carter, J.N.; Castiglioni, C.E.; Kofron, M.D.; Ko, F.K.; Laurencin, C.T. Evaluation of the anterior cruciate ligament, medial collateral ligament, achilles tendon and patellar tendon as cell sources for tissue-engineered ligament. Biomaterials 2006, 27, 2747–2754. [Google Scholar] [CrossRef]
- Van Eijk, F.; Saris, D.B.F.; Riesle, J.; Willems, W.J.; Van Blitterswijk, C.A.; Verbout, A.J.; Dhert, W. Tissue engineering of ligaments: A comparison of bone marrow stromal cells, anterior cruciate ligament, and skin fibroblasts as cell source. Tissue Eng. 2004, 10, 893–903. [Google Scholar] [CrossRef]
- Kobayashi, K.; Healey, R.M.; Sah, R.L.; Clark, J.J.; Tu, B.P.; Goomer, R.S.; Akeson, W.H.; Moriya, H.; Amiel, D. Novel method for the quantitative assessment of cell migration: A study on the motility of rabbit anterior cruciate (ACL) and medial collateral ligament (MCL) cells. Tissue Eng. 2000, 6, 29–38. [Google Scholar] [CrossRef]
- Liu, H.; Wei, X.; Ding, X.; Li, X.; Zhou, G.; Li, P.; Fan, Y. Comparison of cellular responses of mesenchymal stem cells derived from bone marrow and synovium on combined silk scaffolds. J. Biomed. Mater. Res. A 2015, 103, 115–125. [Google Scholar] [CrossRef]
- Eagan, M.J.; Zuk, P.A.; Zhao, K.W.; Bluth, B.E.; Brinkmann, E.J.; Wu, B.M.; McAllister, D.R. The suitability of human adipose-derived stem cells for the engineering of ligament tissue. J. Tissue Eng. Regen. Med. 2012, 6, 702–709. [Google Scholar] [CrossRef]
- Tan, Q.; Lui, P.P.Y.; Rui, Y.F.; Wong, Y.M. Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Eng. Part A 2012, 18, 840–851. [Google Scholar] [CrossRef]
- Petrigliano, F.A.; McAllister, D.R.; Wu, B.M. Tissue Engineering for Anterior Cruciate Ligament Reconstruction: A Review of Current Strategies. Arthrosc. J. Arthrosc. Relat. Surg. 2006, 22, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Pauly, H.M.; Sathy, B.N.; Olvera, D.; McCarthy, H.O.; Kelly, D.J.; Popat, K.C.; Dunne, N.J.; Donahue, T.L.H. Hierarchically structured electrospun scaffolds with chemically conjugated growth factor for ligament tissue engineering. Tissue Eng. Part A 2017, 23, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.-Y.; Debski, R.E.; Withrow, J.D.; Janaushek, M.A. Biomechanics of Knee Ligaments. Am. J. Sports Med. 1999, 27, 533–543. [Google Scholar] [CrossRef]
- Li, B.; Li, F.; Puskar, K.M.; Wang, J.H.-C. Spatial patterning of cell proliferation and differentiation depends on mechanical stress magnitude. J. Biomech. 2009, 42, 1622–1627. [Google Scholar] [CrossRef]
- Altman, G.H.; Horan, R.L.; Martin, I.; Farhadi, J.; Stark, P.R.H.; Volloch, V.; Richmond, J.C.; Vunjak-Novakovic, G.; Kaplan, D.L. Cell differentiation by mechanical stress. FASEB J. 2002, 16, 1–13. [Google Scholar] [CrossRef]
- Sun, L.; Qu, L.; Zhu, R.; Li, H.; Xue, Y.; Liu, X.; Fan, J.; Fan, H. Effects of Mechanical Stretch on Cell Proliferation and Matrix Formation of Mesenchymal Stem Cell and Anterior Cruciate Ligament Fibroblast. Stem Cells Int. 2016, 2016, 9842075. [Google Scholar] [CrossRef]
- Gögele, C.; Hoffmann, C.; Konrad, J.; Merkel, R.; Schwarz, S.; Tohidnezhad, M.; Hoffmann, B.; Schulze-Tanzil, G.G. Cyclically stretched ACL fibroblasts emigrating from spheroids adapt their cytoskeleton and ligament-related expression profile. Cell Tissue Res. 2021, 384, 675–690. [Google Scholar] [CrossRef]
- Troop, L.D.; Puetzer, J.L. Intermittent cyclic stretch of engineered ligaments drives hierarchical collagen fiber maturation in a dose- and organizational-dependent manner. Acta Biomater. 2024, 185, 296–311. [Google Scholar] [CrossRef]
- Hohlrieder, M.; Teuschl, A.H.; Cicha, K.; van Griensven, M.; Redl, H.; Stampfl, J. Bioreactor and scaffold design for the mechanical stimulation of anterior cruciate ligament grafts. Biomed. Mater. Eng. 2013, 23, 225–237. [Google Scholar] [CrossRef]
- Subramony, S.D.; Su, A.; Yeager, K.; Lu, H.H. Combined effects of chemical priming and mechanical stimulation on mesenchymal stem cell differentiation on nanofiber scaffolds. J. Biomech. 2014, 47, 2189–2196. [Google Scholar] [CrossRef]
- Wang, Y.; Shimmin, A.; Ghosh, P.; Marks, P.; Linklater, J.; Connell, D.; Hall, S.; Skerrett, D.; Itescu, S.; Cicuttini, F.M. Safety, tolerability, clinical, and joint structural outcomes of a single intra-articular injection of allogeneic mesenchymal precursor cells in patients following anterior cruciate ligament reconstruction: A controlled double-blind randomised trial. Arthritis Res. Ther. 2017, 19, 180. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.S.; Proffen, B.L.; Kiapour, A.M.; Sieker, J.T.; Fleming, B.C.; Murray, M.M. Bench-to-bedside: Bridge-enhanced anterior cruciate ligament repair. J. Orthop. Res. 2017, 35, 2606–2612. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Zhou, J.; Hwang, J.; Xu, X. Effects of Platelet-Rich Plasma on Clinical Outcomes After Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis. Orthop. J. Sports Med. 2022, 10, 232596712110615. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Huang, B.; Zheng, Z.; Fu, L.; Chen, L. Clinical Use of Platelet-Rich Plasma to Promote Tendon–Bone Healing and Graft Maturation in Anterior Cruciate Ligament Reconstruction—A Randomized Controlled Study. Indian. J. Orthop. 2022, 56, 805–811. [Google Scholar] [CrossRef]
- Laurent, C.P.; Durville, D.; Mainard, D.; Ganghoffer, J.F.; Rahouadj, R. A multilayer braided scaffold for Anterior Cruciate Ligament: Mechanical modeling at the fiber scale. J. Mech. Behav. Biomed. Mater. 2012, 12, 184–196. [Google Scholar] [CrossRef]
- Martin, R.K.; Wastvedt, S.; Pareek, A.; Persson, A.; Visnes, H.; Fenstad, A.M.; Moatshe, G.; Wolfson, J.; Lind, M.; Engebretsen, L. Machine learning algorithm to predict anterior cruciate ligament revision demonstrates external validity. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 368–375. [Google Scholar] [CrossRef]
- Andriollo, L.; Picchi, A.; Sangaletti, R.; Perticarini, L.; Rossi, S.M.P.; Logroscino, G.; Benazzo, F. The Role of Artificial Intelligence in Anterior Cruciate Ligament Injuries: Current Concepts and Future Perspectives. Healthcare 2024, 12, 300. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, T.; Wu, C.; Qiao, Y.; Su, W.; Chen, J.; Xie, G.; Dong, S.; Xu, J.; Zhao, J. Predicting the Objective and Subjective Clinical Outcomes of Anterior Cruciate Ligament Reconstruction: A Machine Learning Analysis of 432 Patients. Am. J. Sports Med. 2022, 50, 3786–3795. [Google Scholar] [CrossRef]
- Rafieyan, S.; Vasheghani-Farahani, E.; Baheiraei, N.; Keshavarz, H. MLATE: Machine learning for predicting cell behavior on cardiac tissue engineering scaffolds. Comput. Biol. Med. 2023, 158, 106804. [Google Scholar] [CrossRef]
- Conev, A.; Litsa, E.E.; Perez, M.R.; Diba, M.; Mikos, A.G.; Kavraki, L.E. Machine learning-guided three-dimensional printing of tissue engineering scaffolds. Tissue Eng. Part A 2020, 26, 1359–1368. [Google Scholar] [CrossRef]
- Barrera, M.D.B.; Franco-Martínez, F.; Lantada, A.D. Artificial intelligence aided design of tissue engineering scaffolds employing virtual tomography and 3d convolutional neural networks. Materials 2021, 14, 5278. [Google Scholar] [CrossRef] [PubMed]
- Entekhabi, E.; Haghbin Nazarpak, M.; Sedighi, M.; Kazemzadeh, A. Predicting degradation rate of genipin cross-linked gelatin scaffolds with machine learning. Mater. Sci. Eng. C 2020, 107, 110362. [Google Scholar] [CrossRef] [PubMed]
- Al-Kharusi, G.; Dunne, N.J.; Little, S.; Levingstone, T.J. The Role of Machine Learning and Design of Experiments in the Advancement of Biomaterial and Tissue Engineering Research. Bioengineering 2022, 9, 561. [Google Scholar] [CrossRef] [PubMed]
| Hamstrings | Bone–Pattelar-Tendon–Bone | Quadriceps Tendon | Allograft | Synthetic | |
|---|---|---|---|---|---|
| Complications | Higher rupture rate than patellar tendon [53], Saphenous neuromas [57], Decreased flexion strength [57], Unpredictable graft size [57], Increased tunnel widening [57], and knee laxity [53,54] | Anterior/kneeling knee pain [57], Patellar fractures [57], Decreased extension strength [54], Graft-tunnel mismatch [55] | Technically demanding [56], Post-operative hematoma [56], Donor-site pain [56], Decreased extension strength [56] | Slow revascularization [57], Poor incorporation [57], Residual instability [55], Higher failure rate than autografts [55], Risk of disease transmission [57], High cost [57], Sterilization further increases graft failure [55] | Foreign body inflammation [57], Material degradation- wear debris [57], Higher graft rupture rate than autograft [57] |
| Study Group | Technology Utilized | Purpose | Function of the Program | Results |
|---|---|---|---|---|
| Rafieyan et al. [129] | A total of 28 ML algorithms were applied to determine the most suitable one. | Prediction of cell differentiation and behavior in different cardiac tissue-engineered scaffolds. | A cardiac tissue-engineered scaffold dataset was created, and then the cardiac tissue-engineered scaffolds were rated based on cell behavior. Finally, 28 ML algorithms were utilized to conclude which one was the most successful in predicting cell behavior on cardiac tissue-engineered scaffolds. | The highest-performing individual algorithm was XGBoost, achieving an accuracy of 87%. The highest-performing algorithms for ensemble learning were Adaboost Classifier and VotingClassifier, achieving an accuracy of 93%. The results show that ML can accurately predict cell behavior on cardiac tissue-engineered scaffolds. |
| Conev et al. [130] | ML-based software (Random Forests) that utilizes a direct classification-based approach and an indirect approach. | Prediction of printing quality given the printed conditions in 3D bioprinting of scaffolds used for bone tissue engineering. Proposal of the most suitable parameters affecting the quality of the print for dataset construction. | The ML software used polymeric biomaterials and different printing configurations as inputs to determine if the resulting product quality was high or low. They used two different metrics to assess the printing quality, material accuracy, and machine precision. | Both models were able to correctly label the majority of the tested configurations. The most important parameters affecting the quality of a print were material composition, printing speed, and printing pressure. The results indicate the potential of ML for identifying suitable printing parameters and reducing experimentation. |
| Barrera et al. [131] | 3D convolutional neural networks. | Prediction of tissue-engineered scaffolds’ mechanical properties with a more efficient and time-saving method by utilizing trained 3D convolutional neural networks. | The software is trained by inputting 2D-layered images of digital tomographies from the computer-aided design models and values from computer-aided design measurements and Finite Element Method simulations. They were utilized to predict the properties of a new set of scaffolds. Their performance has been assessed by the already trained 3D convolutional neural networks. | This methodology is a promising future aspect as a cost-effective tool for predicting the mechanical properties of 3D scaffolds and has the capability of being constantly updated by an expanding dataset. |
| Martin et al. [126] | NKLR (Norwegian Knee Ligament Register) ML-based tool. | External validation of the NKLR ML predictive models for ACL revision risk by evaluating their performance on different groups of patients from the Danish Knee Ligament Registry (DKLR). | 10,922 DKLR patients were used, which satisfied five variables. The predicted revision probabilities were calculated for all DKLR patients for ACL revision. Finally, the tool’s performance was evaluated using the same metrics as the NKLR study. | The tool produced similar results when applied to the DKLR population compared to the original NKLR database (DKLR: 0.68; NKLR: 0.68–0.69). This suggests that the algorithm can be applied outside of the initial patient population and represents the first predictive ML model that has been externally validated for ACL reconstruction revision. |
| Ye et al. [128] | Different ML-based software. | Identifying the best-performing machine learning models for predicting the objective and subjective clinical outcomes of ACL reconstruction and determining the most important predictors. | A total of 432 patients who underwent ACL reconstruction were included. A variety of ML models were used in order to conclude which software had the highest performance for each clinical outcome (graft failure, residual laxity, PROMs, and return to sports). | The results were determined based on the area under the curve and the accuracy. The most successful results were as follows: (1) Graft failure: 0.944 (excellent) and 98.6%. (2) Residual laxity: 0.920 (excellent) and 91.4%. (3) Failure to achieve the minimal clinically important difference of the Lysholm score: 0.930 (excellent) and 91.0%. (4) Failure to achieve the minimal clinically important difference of the International Knee Documentation Committee score: 0.942 (excellent) and 95.1%. The results provide reliable predictions for the outcome of the ACL reconstruction surgery. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sopilidis, A.; Stamatopoulos, V.; Giannatos, V.; Taraviras, G.; Panagopoulos, A.; Taraviras, S. Integrating Modern Technologies into Traditional Anterior Cruciate Ligament Tissue Engineering. Bioengineering 2025, 12, 39. https://doi.org/10.3390/bioengineering12010039
Sopilidis A, Stamatopoulos V, Giannatos V, Taraviras G, Panagopoulos A, Taraviras S. Integrating Modern Technologies into Traditional Anterior Cruciate Ligament Tissue Engineering. Bioengineering. 2025; 12(1):39. https://doi.org/10.3390/bioengineering12010039
Chicago/Turabian StyleSopilidis, Aris, Vasileios Stamatopoulos, Vasileios Giannatos, Georgios Taraviras, Andreas Panagopoulos, and Stavros Taraviras. 2025. "Integrating Modern Technologies into Traditional Anterior Cruciate Ligament Tissue Engineering" Bioengineering 12, no. 1: 39. https://doi.org/10.3390/bioengineering12010039
APA StyleSopilidis, A., Stamatopoulos, V., Giannatos, V., Taraviras, G., Panagopoulos, A., & Taraviras, S. (2025). Integrating Modern Technologies into Traditional Anterior Cruciate Ligament Tissue Engineering. Bioengineering, 12(1), 39. https://doi.org/10.3390/bioengineering12010039








