Assessing the Impact of PRESERFLO MicroShunt on Intraocular Pressure in Porcine Eyes Ex Vivo Using Infusion Pump System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
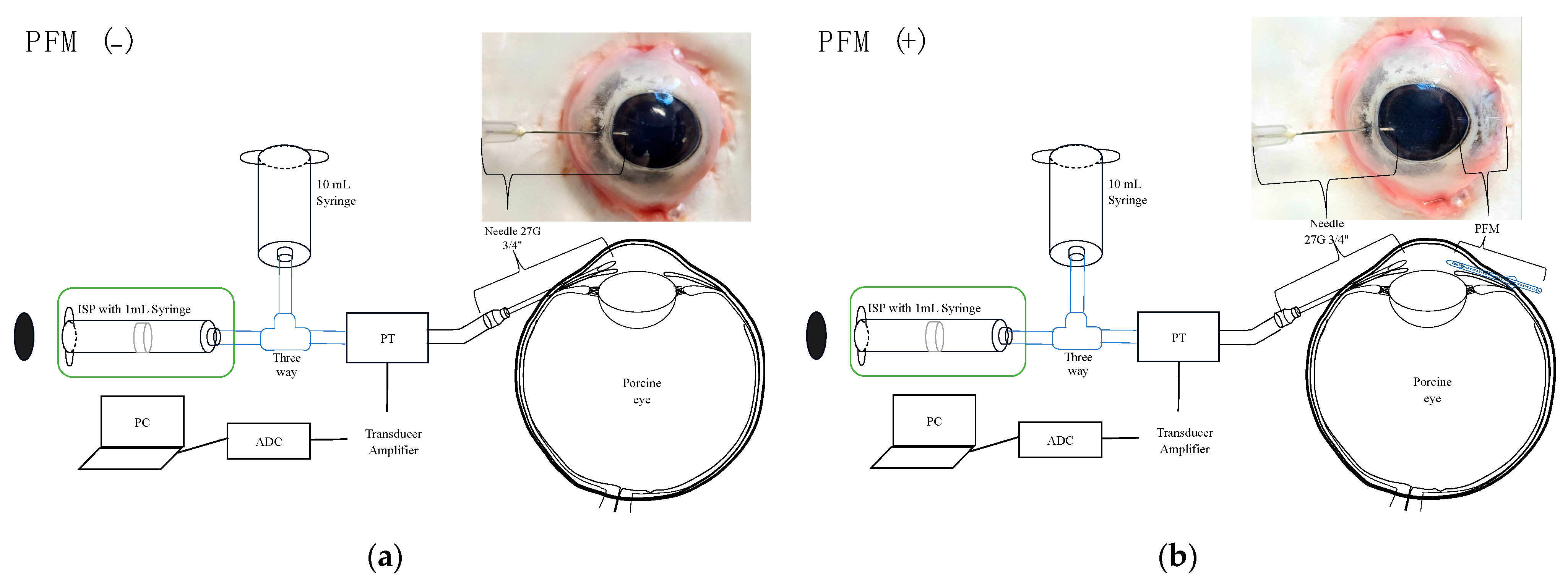
2.2. Experimental Setup
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armstrong, J.J.; De Francesco, T.; Ma, J.; Schlenker, M.B.; Ahmed, I.I.K. Ab Externo SIBS Microshunt with Mitomycin C for Open-Angle Glaucoma: Three-Year Results as a Primary Surgical Intervention. Ophthalmol. Glaucoma 2023, 6, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Schlenker, M.B.; Armstrong, J.J.; De Francesco, T.; Ahmed, I.I.K. All Consecutive Ab Externo SIBS Microshunt Implantations with Mitomycin C: One-Year Outcomes and Risk Factors for Failure. Am. J. Ophthalmol. 2023, 255, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, L.; Riss, I.; Batlle, J.F.; Kato, Y.P.; Martin, J.B.; Arrieta, E.; Palmberg, P.; Parrish, R.K.; Weber, B.A.; Kwon, Y.; et al. The use of poly(styrene- block -isobutylene- block -styrene) as a microshunt to treat glaucoma. Regen. Biomater. 2016, 3, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Sadruddin, O.; Pinchuk, L.; Angeles, R.; Palmberg, P. Ab externo implantation of the MicroShunt, a poly (styrene-block-isobutylene-block-styrene) surgical device for the treatment of primary open-angle glaucoma: A review. Eye Vis. 2019, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Nakashima, K.I.; Watanabe-Kitamura, F.; Watanabe, T.; Nakamura, K.; Maki, K.; Shimazaki, A.; Kato, M.; Tanihara, H.; Inoue, T. Intraocular Pressure-Lowering Effects of Trabeculectomy Versus MicroShunt Insertion in Rabbit Eyes. Transl. Vis. Sci. Technol. 2021, 10, 9. [Google Scholar] [CrossRef]
- Masdipa, A.; Kaidzu, S.; Tanito, M. Exploring the Pressure Characteristics of the PRESERFLO MicroShunt in In Vitro Studies and Effects of Sclera on Device Performance. J. Clin. Med. 2023, 12, 7266. [Google Scholar] [CrossRef] [PubMed]
- Middleton, S. Porcine ophthalmology. Vet. Clin. North. Am. Food Anim. Pract. 2010, 26, 557–572. [Google Scholar] [CrossRef]
- McMenamin, P.G.; Steptoe, R.J. Normal anatomy of the aqueous humour outflow system in the domestic pig eye. J. Anat. 1991, 178, 65–77. [Google Scholar] [PubMed]
- Masdipa, A.; Kaidzu, S.; Tanito, M. Flow Pressure Characteristics of the Ahmed Glaucoma Valve and Possible Effect of Entrapped Air in the Tube. Transl. Vis. Sci. Technol. 2023, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- McLaren, J.W. Measurement of aqueous humor flow. Exp. Eye Res. 2009, 88, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, D.K.; Sapra, A. Physiology, Aqueous Humor Circulation. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Brubaker, R.F. Goldmann’s equation and clinical measures of aqueous dynamics. Exp. Eye Res. 2004, 78, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Hejkal, J.J.; Camras, C.B.; Toris, C.B. Aqueous humor dynamics during the day and night in juvenile and adult rabbits. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3145–3151. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, L.; Xu, J.; Chen, X.; Gu, Y.; Ren, Y.; Wang, K. Comparability of three intraocular pressure measurement: iCare pro rebound, non-contact and Goldmann applanation tonometry in different IOP group. BMC Ophthalmol. 2019, 19, 225. [Google Scholar] [CrossRef] [PubMed]
- Crawley, L.; Zamir, S.M.; Cordeiro, M.F.; Guo, L. Clinical options for the reduction of elevated intraocular pressure. Ophthalmol. Eye Dis. 2012, 4, 43–64. [Google Scholar] [CrossRef] [PubMed]
- Majoulet, A.; Scemla, B.; Hamard, P.; Brasnu, E.; Hage, A.; Baudouin, C.; Labbé, A. Safety and Efficacy of the Preserflo® Microshunt in Refractory Glaucoma: A One-Year Study. J. Clin. Med. 2022, 11, 7086. [Google Scholar] [CrossRef] [PubMed]
- Pawiroredjo, S.S.M.; Bramer, W.M.; Pawiroredjo, N.D.; Pals, J.; Poelman, H.J.; de Vries, V.A.; Wolfs, R.C.W.; Ramdas, W.D. Efficacy of the PRESERFLO MicroShunt and a Meta-Analysis of the Literature. J. Clin. Med. 2022, 11, 7149. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Sugihara, K.; Tsutsui, A.; Hara, K.; Manabe, K.; Matsuoka, Y. Effects of Preoperative Intraocular Pressure Level on Surgical Results of Microhook Ab Interno Trabeculotomy. J. Clin. Med. 2021, 10, 3327. [Google Scholar] [CrossRef]
- Sakamoto, T.; Nisiwaki, H. Factors associated with 1-year outcomes and transient intraocular pressure elevation in minimally invasive glaucoma surgery using Kahook Dual Blades. Sci. Rep. 2023, 13, 15206. [Google Scholar] [CrossRef]
- Ishida, A.; Miki, T.; Naito, T.; Ichioka, S.; Takayanagi, Y.; Tanito, M. Surgical Results of Trabeculectomy among Groups Stratified by Prostaglandin-Associated Periorbitopathy Severity. Ophthalmology 2023, 130, 297–303. [Google Scholar] [CrossRef]

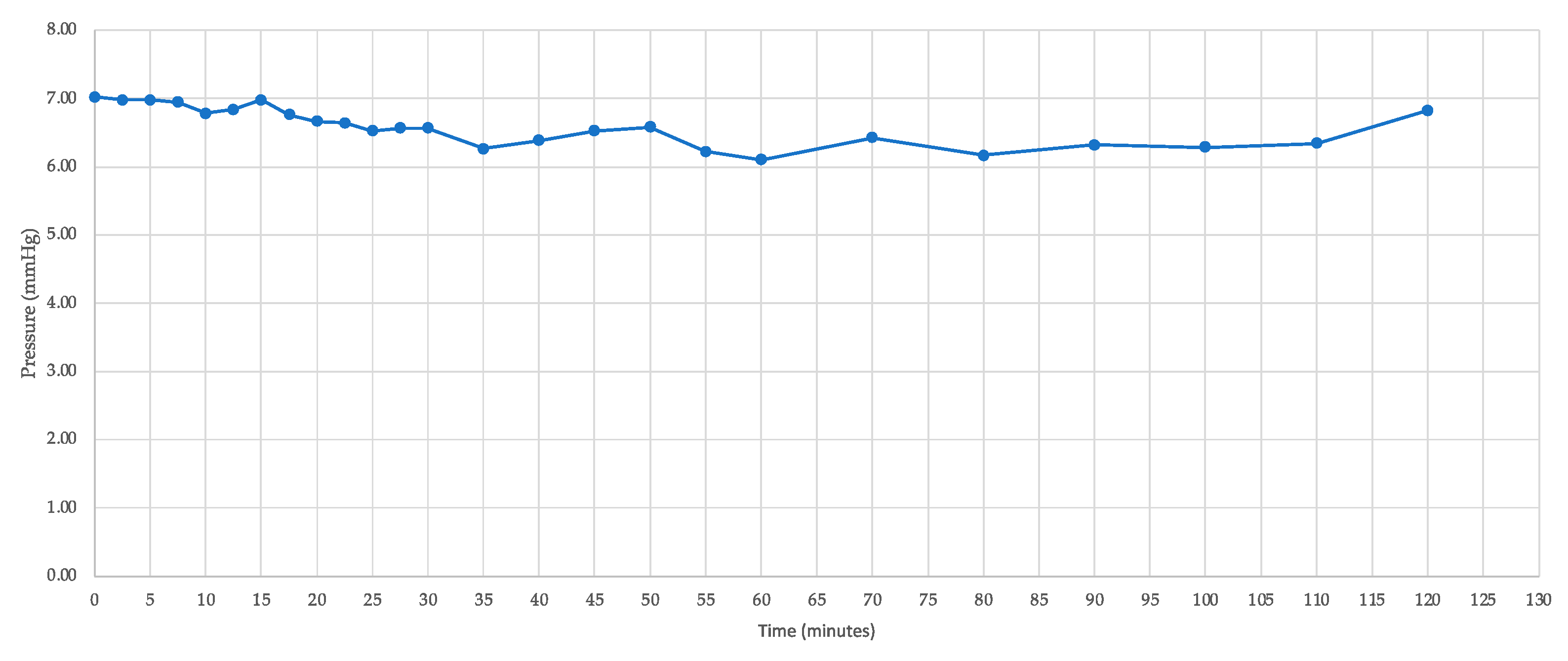

| Time | IOP (mmHg) |
|---|---|
| 0 | 6.9 |
| 10 | 6.8 |
| 20 | 6.7 |
| 30 | 6.6 |
| 40 | 6.5 |
| 50 | 6.7 |
| 60 | 6.1 |
| 70 | 6.4 |
| 80 | 6.2 |
| 90 | 6.3 |
| 100 | 6.3 |
| 110 | 6.3 |
| 120 | 6.8 |
| Mean ± SD | 6.5 ± 0.3 |
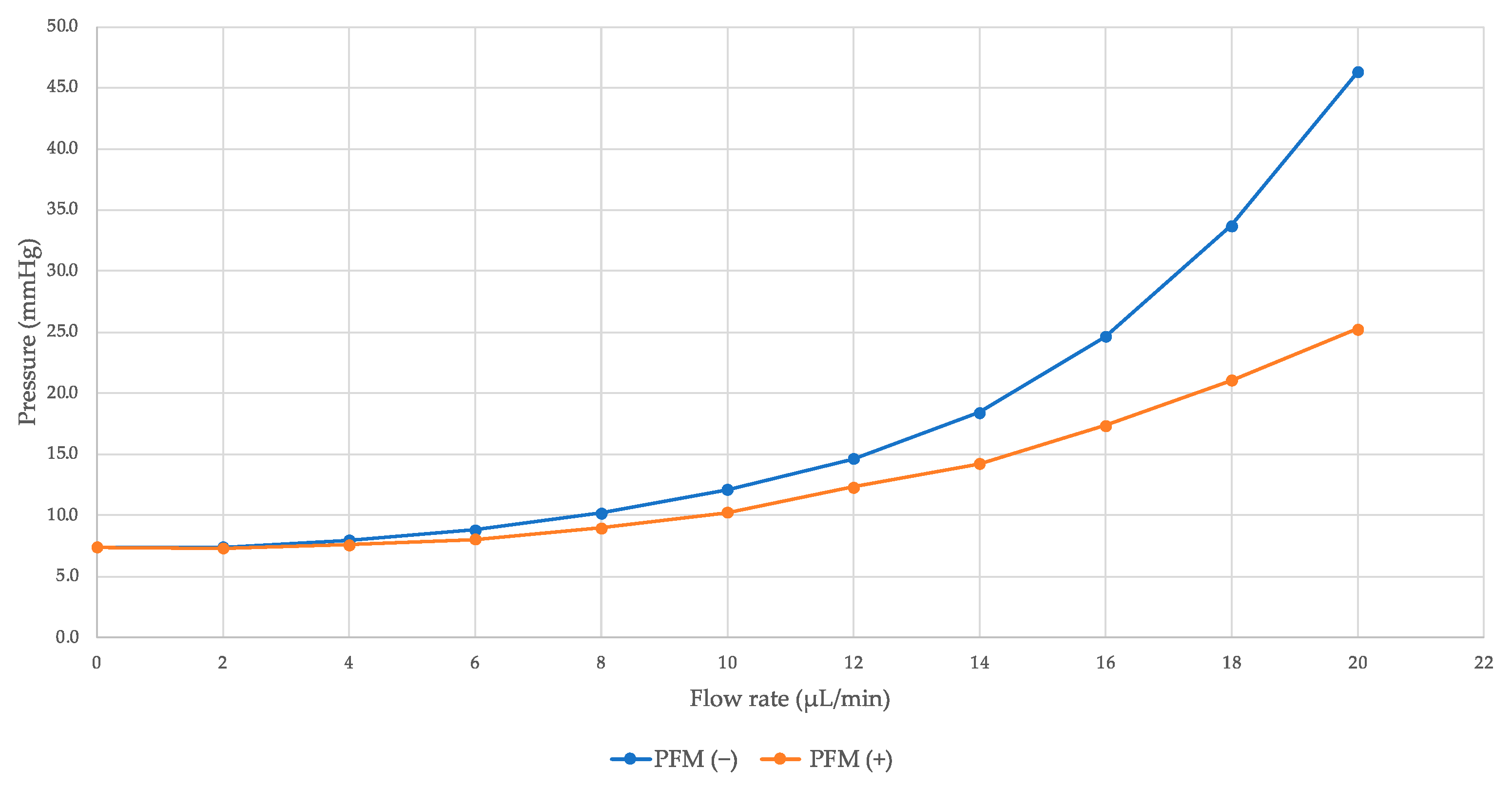
| Flow (µL/Minute) | IOP (mmHg) | %ΔIOP | p Value | |
|---|---|---|---|---|
| PFM (−) | PFM (+) | |||
| 0 | 7.4 ± 0.4 | 7.4 ± 0.4 | 0.0% | >0.99 |
| 2 | 7.4 ± 0.4 | 7.3 ± 0.5 | 1.0% | 0.46 |
| 4 | 8.0 ± 0.7 | 7.6 ± 0.7 | 4.4% | 0.11 |
| 6 | 8.8 ± 0.9 | 8.1 ± 0.8 | 8.8% | 0.0011 |
| 8 | 10.2 ± 1.4 | 9.0 ± 1.0 | 12.0% | 0.0082 |
| 10 | 12.1 ± 1.9 | 10.2 ± 1.5 | 15.6% | 0.0099 |
| 12 | 14.6 ± 2.2 | 12.3 ± 2.4 | 15.8% | 0.0041 |
| 14 | 18.4 ± 3.4 | 14.3 ± 3.6 | 22.6% | 0.0033 |
| 16 | 24.7 ± 6.1 | 17.4 ± 5.1 | 29.5% | 0.0014 |
| 18 | 33.7 ± 9.5 | 21.1 ± 7.0 | 37.6% | 0.0009 |
| 20 | 46.3 ± 14.7 | 25.3 ± 8.9 | 45.4% | 0.0009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masdipa, A.; Kaidzu, S.; Tanito, M. Assessing the Impact of PRESERFLO MicroShunt on Intraocular Pressure in Porcine Eyes Ex Vivo Using Infusion Pump System. Bioengineering 2024, 11, 669. https://doi.org/10.3390/bioengineering11070669
Masdipa A, Kaidzu S, Tanito M. Assessing the Impact of PRESERFLO MicroShunt on Intraocular Pressure in Porcine Eyes Ex Vivo Using Infusion Pump System. Bioengineering. 2024; 11(7):669. https://doi.org/10.3390/bioengineering11070669
Chicago/Turabian StyleMasdipa, Andi, Sachiko Kaidzu, and Masaki Tanito. 2024. "Assessing the Impact of PRESERFLO MicroShunt on Intraocular Pressure in Porcine Eyes Ex Vivo Using Infusion Pump System" Bioengineering 11, no. 7: 669. https://doi.org/10.3390/bioengineering11070669
APA StyleMasdipa, A., Kaidzu, S., & Tanito, M. (2024). Assessing the Impact of PRESERFLO MicroShunt on Intraocular Pressure in Porcine Eyes Ex Vivo Using Infusion Pump System. Bioengineering, 11(7), 669. https://doi.org/10.3390/bioengineering11070669








