The Recombinant Lactobacillus Strains with the Surface-Displayed Expression of Amuc_1100 Ameliorate Obesity in High-Fat Diet-Fed Adult Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Strains
2.2. Experimental Animals
2.3. The Construction of Recombinant Expression Vectors
2.4. The Detection of Fusion Protein with Fluorescence Microscopy
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Western Blotting
2.7. Hematoxylin and Eosin (H&E) Staining
2.8. Oral Glucose Tolerance Test (OGTT)
2.9. The Measure of LPS Concentration
2.10. Animals and Treatment
2.11. Statistical Analysis
3. Results
3.1. Construction, Expression, and Detection of Recombinant Fusion Proteins
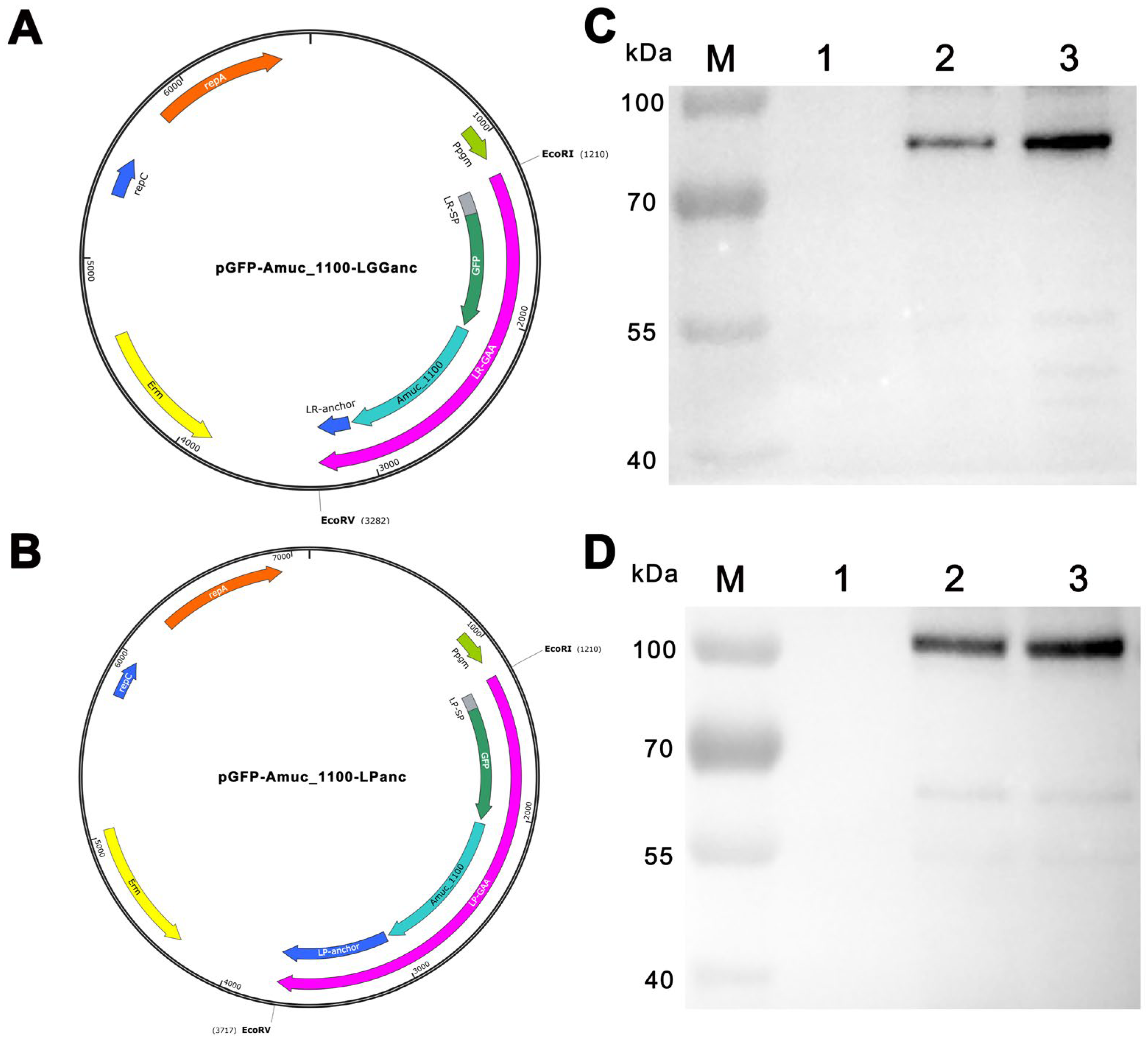
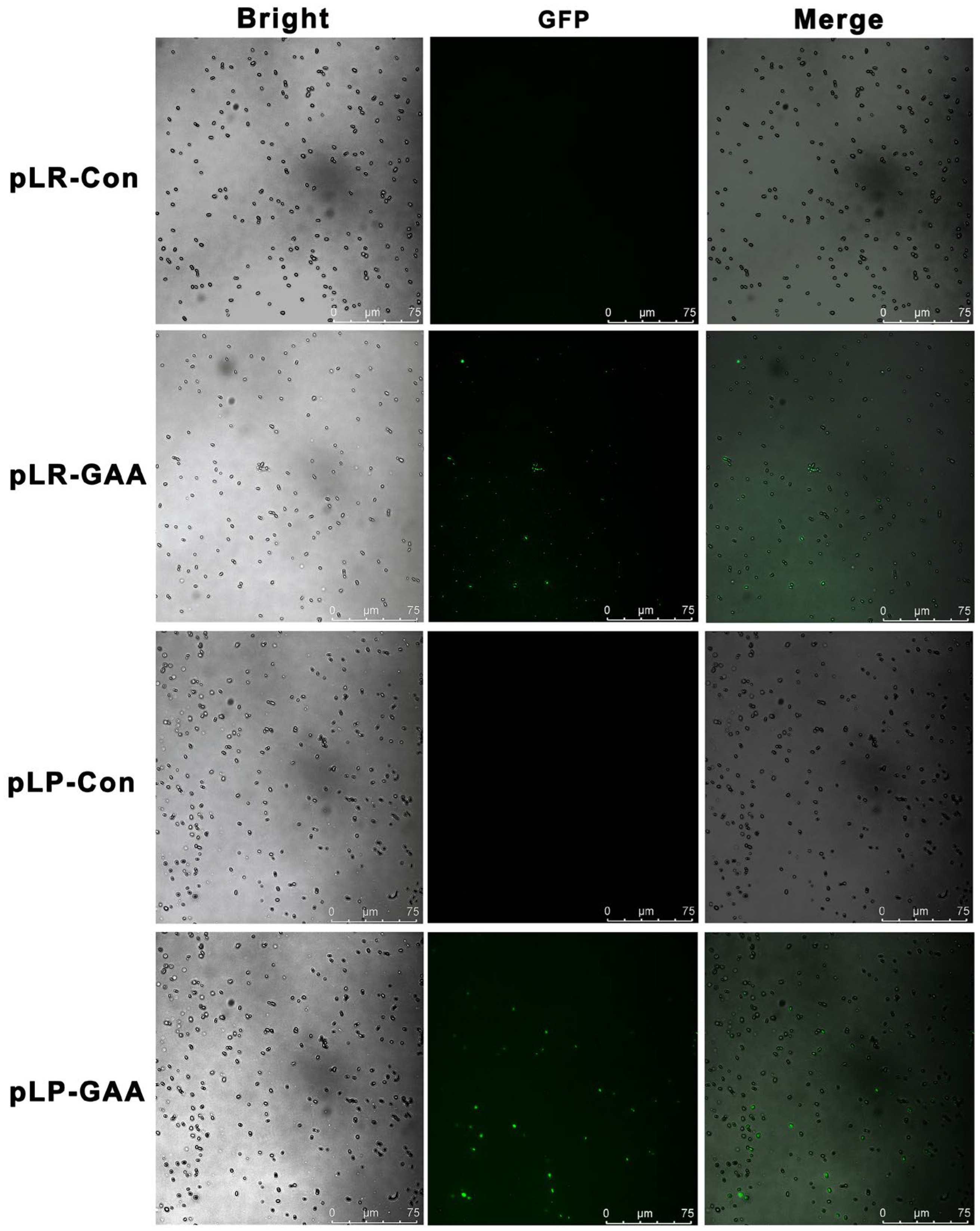
3.2. Recombinant Lactobacillus Strains Showed Beneficial Effects on the HFD-Induced Hyperglycemia
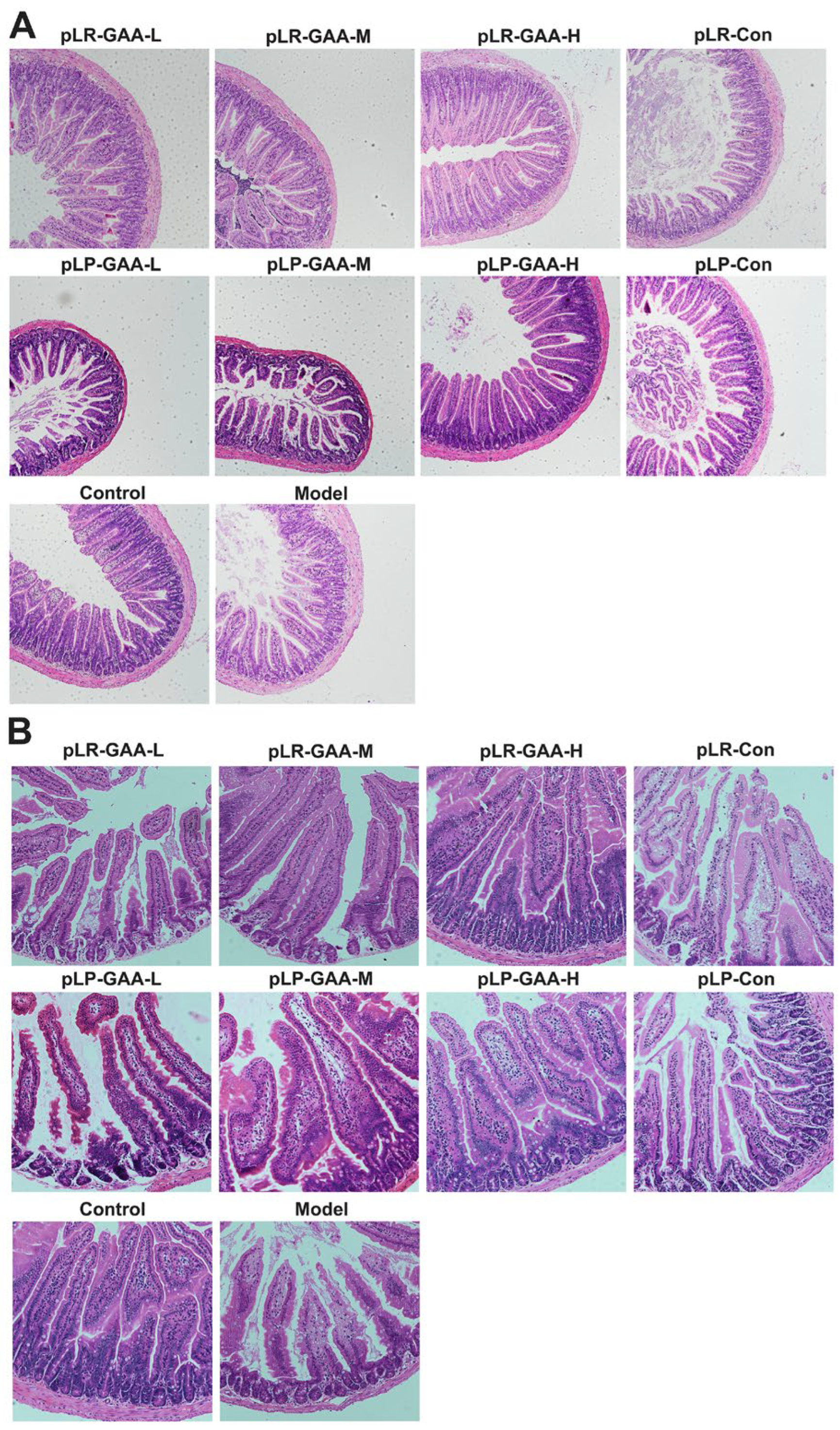
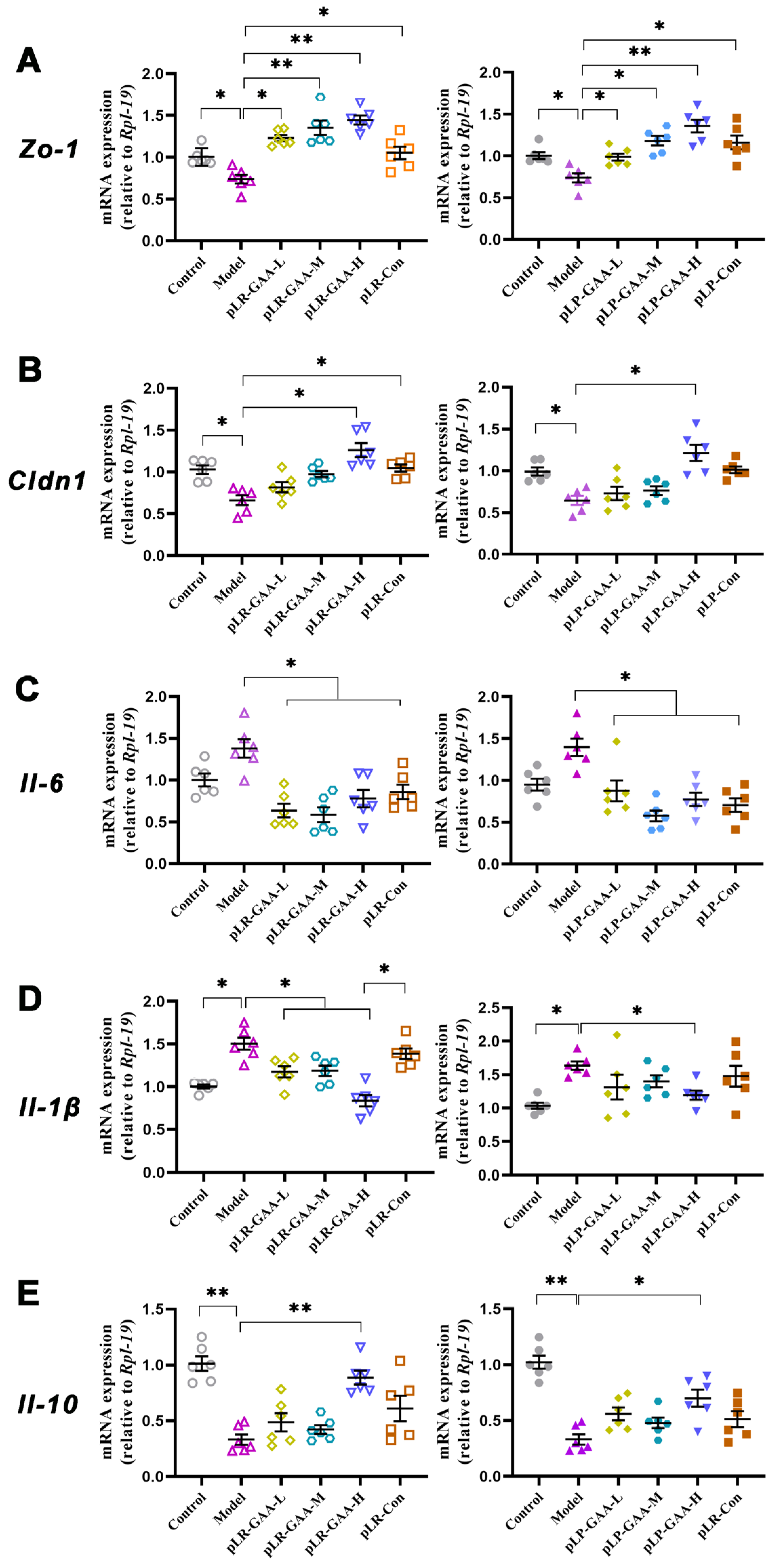
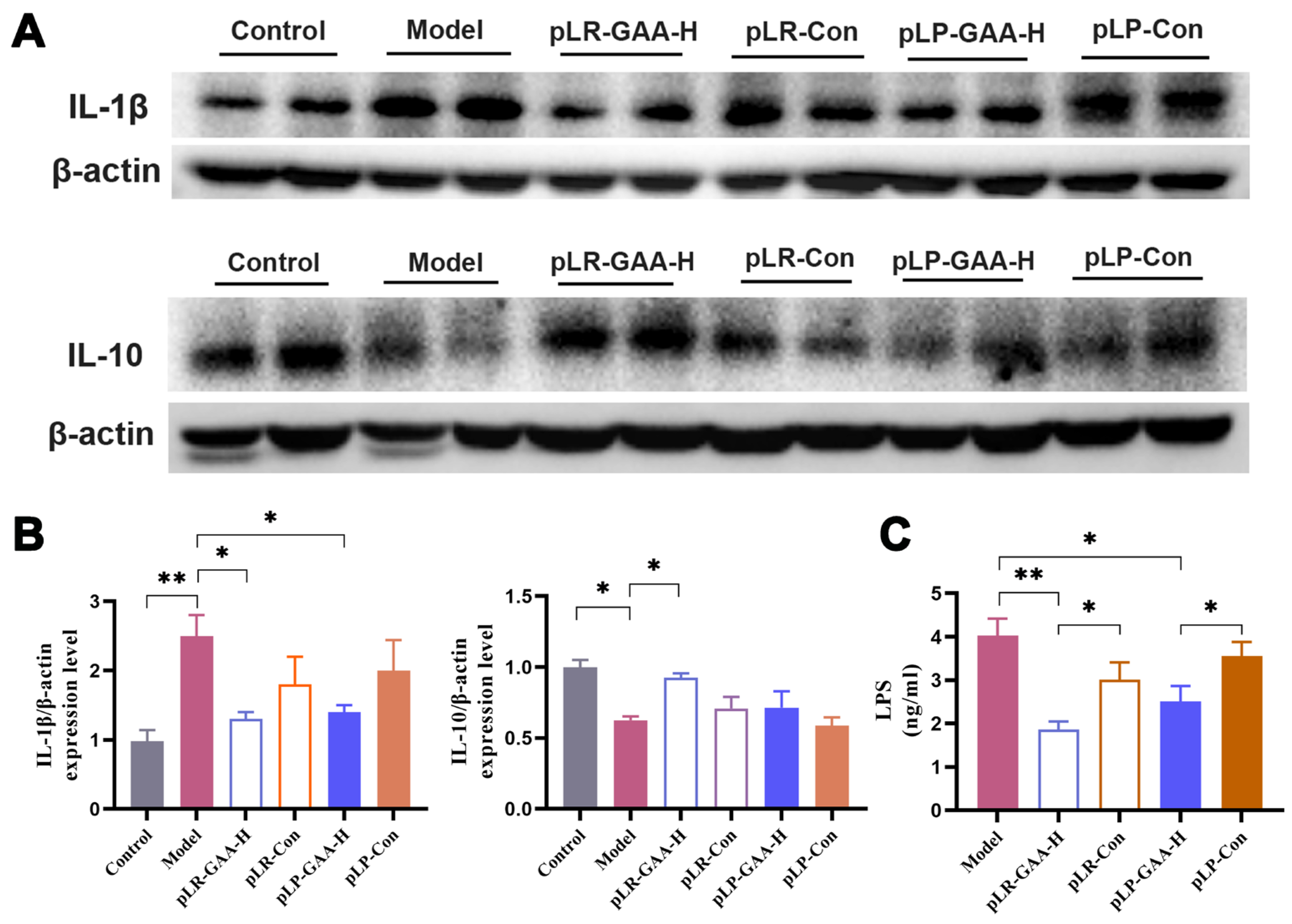
3.3. The Analysis of Histomorphology and the Expression of Inflammatory Factors in Gut
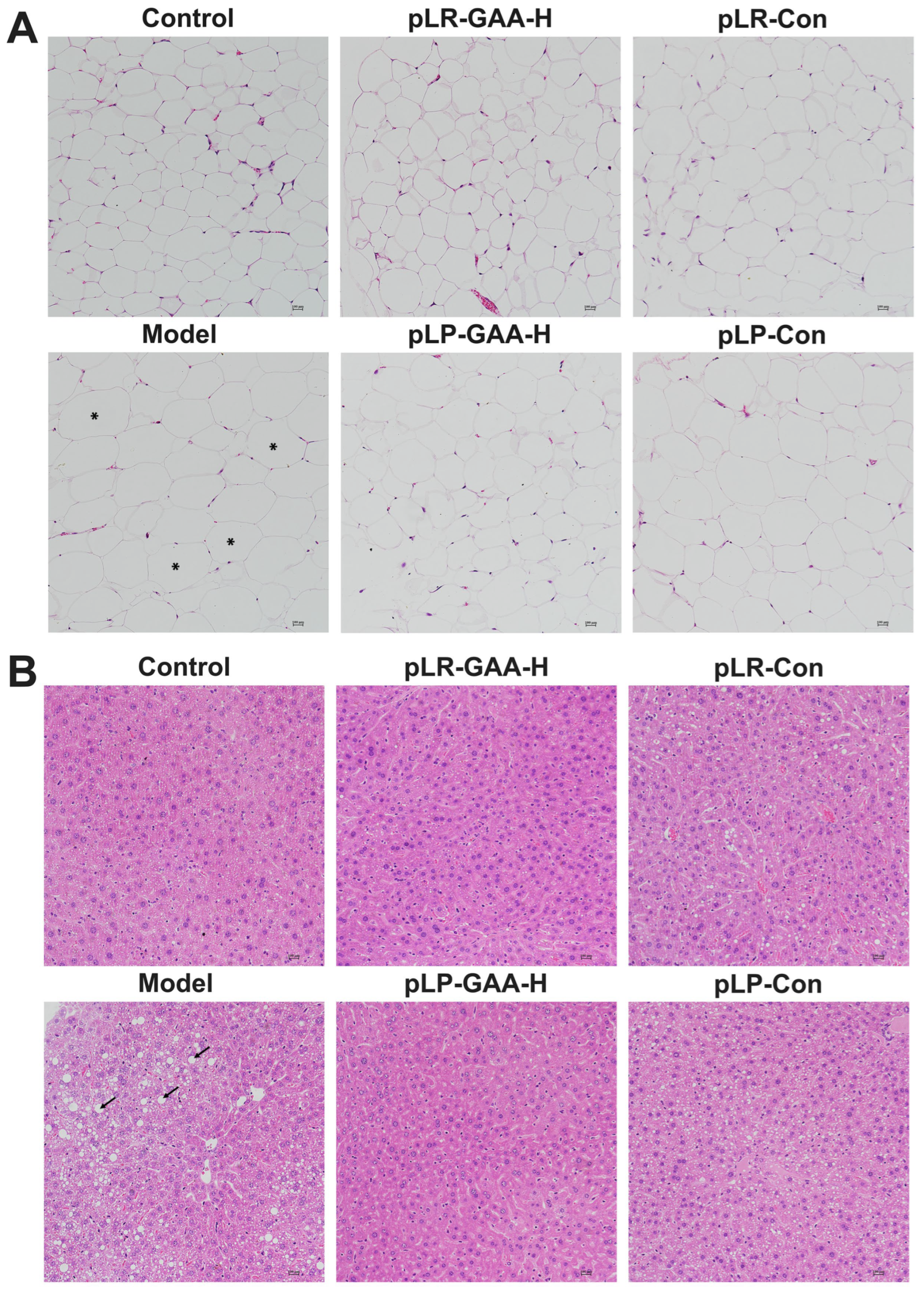
3.4. Effects of Recombinant Lactobacillus Strains on the Histomorphology of Liver and Adipose Tissues
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kivimäki, M.; Kuosma, E.; Ferrie, J.E.; Luukkonen, R.; Nyberg, S.T.; Alfredsson, L.; Batty, G.D.; Brunner, E.J.; Fransson, E.; Goldberg, M.; et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: Pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health 2017, 2, e277–e285. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rodriguez, E.; Guillen-Grima, F.; Martí, A.; Brugos-Larumbe, A. Comorbidity associated with obesity in a large population: The APNA study. Obes. Res. Clin. Pract. 2015, 9, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Schoof, B.; Stangenberg, M.; Mende, K.C.; Thiesen, D.M.; Ntalos, D.; Dreimann, M. Obesity in spontaneous spondylodiscitis: A relevant risk factor for severe disease courses. Sci. Rep. 2020, 10, 21919. [Google Scholar] [CrossRef]
- Leung, M.Y.; Pollack, L.M.; Colditz, G.A.; Chang, S.H. Life years lost and lifetime health care expenditures associated with diabetes in the U.S., National Health Interview Survey, 1997–2000. Diabetes Care 2015, 38, 460–468. [Google Scholar] [CrossRef]
- World Obesity Federation. World Obesity Atlas. 2023. Available online: https://data.worldobesity.org/publications/?cat=19 (accessed on 19 January 2024).
- Chen, K.; Shen, Z.; Gu, W.; Lyu, Z.; Qi, X.; Mu, Y.; Ning, Y.; Meinian Investigator Group. Prevalence of obesity and associated complications in China: A cross-sectional, real-world study in 15.8 million adults. Diabetes Obes. Metab. 2023, 25, 3390–3399. [Google Scholar] [CrossRef] [PubMed]
- Tremmel, M.; Gerdtham, U.G.; Nilsson, P.M.; Saha, S. Economic Burden of Obesity: A Systematic Literature Review. Int. J. Environ. Res. Public Health 2017, 14, 435. [Google Scholar] [CrossRef]
- Geng, J.; Ni, Q.; Sun, W.; Li, L.; Feng, X. The links between gut microbiota and obesity and obesity related diseases. Biomed. Pharmacother. 2022, 147, 112678. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Stanislawski, M.A.; Dabelea, D.; Lange, L.A.; Wagner, B.D.; Lozupone, C.A. Gut microbiota phenotypes of obesity. NPJ Biofilms Microbiomes 2019, 5, 18. [Google Scholar] [CrossRef]
- Velloso, L.A.; Folli, F.; Saad, M.J. TLR4 at the Crossroads of Nutrients, Gut Microbiota, and Metabolic Inflammation. Endocr. Rev. 2015, 36, 245–271. [Google Scholar] [CrossRef]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lan, C.; Li, H.; Ouyang, Q.; Kong, F.; Wu, A.; Ren, Z.; Tian, G.; Cai, J.; Yu, B.; et al. Rational consideration of Akkermansia muciniphila targeting intestinal health: Advantages and challenges. npj Biofilms Microbiomes 2022, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Van Hul, M.; Everard, A.; Delzenne, N.M.; De Vos, W.M.; Cani, P.D. Pasteurized Akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice. Gut Microbes 2020, 11, 1231–1245. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, W.; Wang, R.; Cheng, R.; Tang, Z.; Zhang, M. The outer membrane protein Amuc_1100 of Akkermansia muciniphila promotes intestinal 5-HT biosynthesis and extracellular availability through TLR2 signalling. Food Funct. 2021, 12, 3597–3610. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ye, C.; Yan, B.; He, X.; Xing, K. Assessing the Influence of Dietary History on Gut Microbiota. Curr. Microbiol. 2019, 76, 237–247. [Google Scholar] [CrossRef]
- Shen, Y.L.; Zhang, L.Q.; Yang, Y.; Yin, B.C.; Ye, B.C.; Zhou, Y. Advances in role and mechanism of lactic acid bacteria in treating obesity. Food Bioeng. 2022, 1, 101–115. [Google Scholar] [CrossRef]
- Ahn, H.Y.; Kim, M.; Ahn, Y.T.; Sim, J.H.; Choi, I.D.; Lee, S.H.; Lee, J.H. The triglyceride-lowering effect of supplementation with dual probiotic strains, Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032: Reduction of fasting plasma lysophosphatidylcholines in nondiabetic and hypertriglyceridemic subjects. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 724–733. [Google Scholar] [CrossRef]
- Chung, H.J.; Yu, J.G.; Lee, I.A.; Liu, M.J.; Shen, Y.F.; Sharma, S.P.; Jamal, M.A.; Yoo, J.H.; Kim, H.J.; Hong, S.T. Intestinal removal of free fatty acids from hosts by Lactobacilli for the treatment of obesity. FEBS Open Bio 2016, 6, 64–76. [Google Scholar] [CrossRef]
- Mobini, R.; Tremaroli, V.; Ståhlman, M.; Karlsson, F.; Levin, M.; Ljungberg, M.; Sohlin, M.; Bertéus Forslund, H.; Perkins, R.; Bäckhed, F.; et al. Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: A randomized controlled trial. Diabetes Obes. Metab. 2017, 19, 579–589. [Google Scholar] [CrossRef]
- Nagata, S.; Chiba, Y.; Wang, C.; Yamashiro, Y. The effects of the Lactobacillus casei strain on obesity in children: A pilot study. Benef. Microbes 2017, 8, 535–543. [Google Scholar] [CrossRef]
- Gomes, A.C.; de Sousa, R.G.; Botelho, P.B.; Gomes, T.L.; Prada, P.O.; Mota, J.F. The additional effects of a probiotic mix on abdominal adiposity and antioxidant Status: A double-blind, randomized trial. Obesity 2017, 25, 30–38. [Google Scholar] [CrossRef]
- Kim, J.; Yun, J.M.; Kim, M.K.; Kwon, O.; Cho, B. Lactobacillus gasseri BNR17 Supplementation Reduces the Visceral Fat Accumulation and Waist Circumference in Obese Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Med. Food 2018, 21, 454–461. [Google Scholar] [CrossRef]
- Castañeda-Márquez, A.C.; Díaz-Benítez, C.E.; Bahena-Roman, M.; Campuzano-Benítez, G.E.; Galván-Portillo, M.; Campuzano-Rincón, J.C.; Lagunas-Martínez, A.; Bermudez-Morales, V.H.; Orbe-Orihuela, Y.C.; Peralta-Romero, J.; et al. Lactobacillus paracasei as a protective factor of obesity induced by an unhealthy diet in children. Obes. Res. Clin. Pract. 2020, 14, 271–278. [Google Scholar] [CrossRef]
- Oraphruek, P.; Chusak, C.; Ngamukote, S.; Sawaswong, V.; Chanchaem, P.; Payungporn, S.; Suantawee, T.; Adisakwattana, S. Effect of a Multispecies Synbiotic Supplementation on Body Composition, Antioxidant Status, and Gut Microbiomes in Overweight and Obese Subjects: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2023, 15, 1863. [Google Scholar] [CrossRef]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]
- Fredriksen, L.; Mathiesen, G.; Sioud, M.; Eijsink, V.G. Cell wall anchoring of the 37-kilodalton oncofetal antigen by Lactobacillus plantarum for mucosal cancer vaccine delivery. Appl. Environ. Microbiol. 2010, 76, 7359–7362. [Google Scholar] [CrossRef]
- von Ossowski, I.; Satokari, R.; Reunanen, J.; Lebeer, S.; De Keersmaecker, S.C.; Vanderleyden, J.; de Vos, W.M.; Palva, A. Functional characterization of a mucus-specific LPXTG surface adhesin from probiotic Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 2011, 77, 4465–4472. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, W.; Li, B.; Qian, S.; Wei, B.; Gong, S.; Wang, J.; Liu, M.; Wei, M. Nuciferine modulates the gut microbiota and prevents obesity in high-fat diet-fed rats. Exp. Mol. Med. 2020, 52, 1959–1975. [Google Scholar] [CrossRef]
- Xiong, J.; Deng, I.; Kelliny, S.; Lin, L.; Bobrovskaya, L.; Zhou, X.F. Long term high fat diet induces metabolic disorders and aggravates behavioral disorders and cognitive deficits in MAPT P301L transgenic mice. Metab. Brain Dis. 2022, 37, 1941–1957. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xiao, Y.; Li, D.; Zhang, S.; Wu, Y.; Zhang, Q.; Bai, W. New Insights Intothe Mechanisms of High-Fat Diet Mediated GutMicrobiota in Chronic Diseases. iMeta 2023, 2, e69. [Google Scholar] [CrossRef]
- Duan, Y.; Zeng, L.; Zheng, C.; Song, B.; Li, F.; Kong, X.; Xu, K. Inflammatory Links between High Fat Diets and Diseases. Front. Immunol. 2018, 9, 2649. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Reunanen, J.; Meijerink, M.; Pietilä, T.E.; Kainulainen, V.; Klievink, J.; Huuskonen, L.; Aalvink, S.; Skurnik, M.; Boeren, S.; et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE 2017, 12, e0173004. [Google Scholar] [CrossRef] [PubMed]
- Mulhall, H.; DiChiara, J.M.; Deragon, M.; Iyer, R.; Huck, O.; Amar, S. Akkermansia muciniphila and Its Pili-Like Protein Amuc_1100 Modulate Macrophage Polarization in Experimental Periodontitis. Infect. Immun. 2020, 89, e00500-20. [Google Scholar] [CrossRef]
- Michon, C.; Langella, P.; Eijsink, V.G.; Mathiesen, G.; Chatel, J.M. Display of recombinant proteins at the surface of lactic acid bacteria: Strategies and applications. Microb. Cell Factories 2016, 15, 70. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, G.; Øverland, L.; Kuczkowska, K.; Eijsink, V.G.H. Anchoring of heterologous proteins in multiple Lactobacillus species using anchors derived from Lactobacillus plantarum. Sci. Rep. 2020, 10, 9640. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Pham, M.L.; Stelzer, E.M.; Plattner, E.; Grabherr, R.; Mathiesen, G.; Peterbauer, C.K.; Haltrich, D.; Nguyen, T.H. Constitutive expression and cell-surface display of a bacterial β-mannanase in Lactobacillus plantarum. Microb. Cell Factories 2019, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yang, Y.; Zhang, Z.; Yao, Y.; Xia, R.; Gao, C.; Du, D.; Hu, J.; Ran, C.; Liu, Z.; et al. Surface-Displayed Amuc_1100 From Akkermansia muciniphila on Lactococcus lactis ZHY1 Improves Hepatic Steatosis and Intestinal Health in High-Fat-Fed Zebrafish. Front. Nutr. 2021, 8, 726108. [Google Scholar] [CrossRef]
- Guo, M.; Liu, H.; Yu, Y.; Zhu, X.; Xie, H.; Wei, C.; Mei, C.; Shi, Y.; Zhou, N.; Qin, K.; et al. Lactobacillus rhamnosus GG ameliorates osteoporosis in ovariectomized rats by regulating the Th17/Treg balance and gut microbiota structure. Gut Microbes 2023, 15, 2190304. [Google Scholar] [CrossRef]
- Sun, M.; Wu, T.; Zhang, G.; Liu, R.; Sui, W.; Zhang, M.; Geng, J.; Yin, J.; Zhang, M. Lactobacillus rhamnosus LRa05 improves lipid accumulation in mice fed with a high fat diet via regulating the intestinal microbiota, reducing glucose content and promoting liver carbohydrate metabolism. Food Funct. 2020, 11, 9514–9525. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Günzel, D.; Yu, A.S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef]
- Moreira, A.P.; Texeira, T.F.; Ferreira, A.B.; Peluzio Mdo, C.; Alfenas Rde, C. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.; Glazier, J.; Mimee, M. Genetic Engineering of Resident Bacteria in the Gut Microbiome. J. Bacteriol. 2023, 205, e0012723. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jin, W.; Hoover, A.; Chao, Y.; Ma, Y. Decoding the microbiome: Advances in genetic manipulation for gut bacteria. Trends Microbiol. 2023, 31, 1143–1161. [Google Scholar] [CrossRef]
- Braat, H.; Rottiers, P.; Hommes, D.W.; Huyghebaert, N.; Remaut, E.; Remon, J.P.; van Deventer, S.J.; Neirynck, S.; Peppelenbosch, M.P.; Steidler, L. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin. Gastroenterol. Hepatol. 2006, 4, 754–759. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Jiang, L.; Xie, C.; Mo, Y.; Zhang, Z.; Xu, S.; Guo, X.; Xing, K.; Wang, Y.; Su, Z. The Recombinant Lactobacillus Strains with the Surface-Displayed Expression of Amuc_1100 Ameliorate Obesity in High-Fat Diet-Fed Adult Mice. Bioengineering 2024, 11, 574. https://doi.org/10.3390/bioengineering11060574
Zhang X, Jiang L, Xie C, Mo Y, Zhang Z, Xu S, Guo X, Xing K, Wang Y, Su Z. The Recombinant Lactobacillus Strains with the Surface-Displayed Expression of Amuc_1100 Ameliorate Obesity in High-Fat Diet-Fed Adult Mice. Bioengineering. 2024; 11(6):574. https://doi.org/10.3390/bioengineering11060574
Chicago/Turabian StyleZhang, Xueni, Lei Jiang, Cankun Xie, Yidi Mo, Zihao Zhang, Shengxia Xu, Xiaoping Guo, Ke Xing, Yina Wang, and Zhijian Su. 2024. "The Recombinant Lactobacillus Strains with the Surface-Displayed Expression of Amuc_1100 Ameliorate Obesity in High-Fat Diet-Fed Adult Mice" Bioengineering 11, no. 6: 574. https://doi.org/10.3390/bioengineering11060574
APA StyleZhang, X., Jiang, L., Xie, C., Mo, Y., Zhang, Z., Xu, S., Guo, X., Xing, K., Wang, Y., & Su, Z. (2024). The Recombinant Lactobacillus Strains with the Surface-Displayed Expression of Amuc_1100 Ameliorate Obesity in High-Fat Diet-Fed Adult Mice. Bioengineering, 11(6), 574. https://doi.org/10.3390/bioengineering11060574





