The Self-Expandable Impella CP (ECP) as a Mechanical Resuscitation Device
Abstract
1. Introduction
2. Materials and Methods
2.1. Impella ECP pLVAD
2.2. Preparation
2.3. Cardiac Arrest and Resuscitation
2.4. Follow-Up
2.5. Measurements
2.6. Statistical Analysis
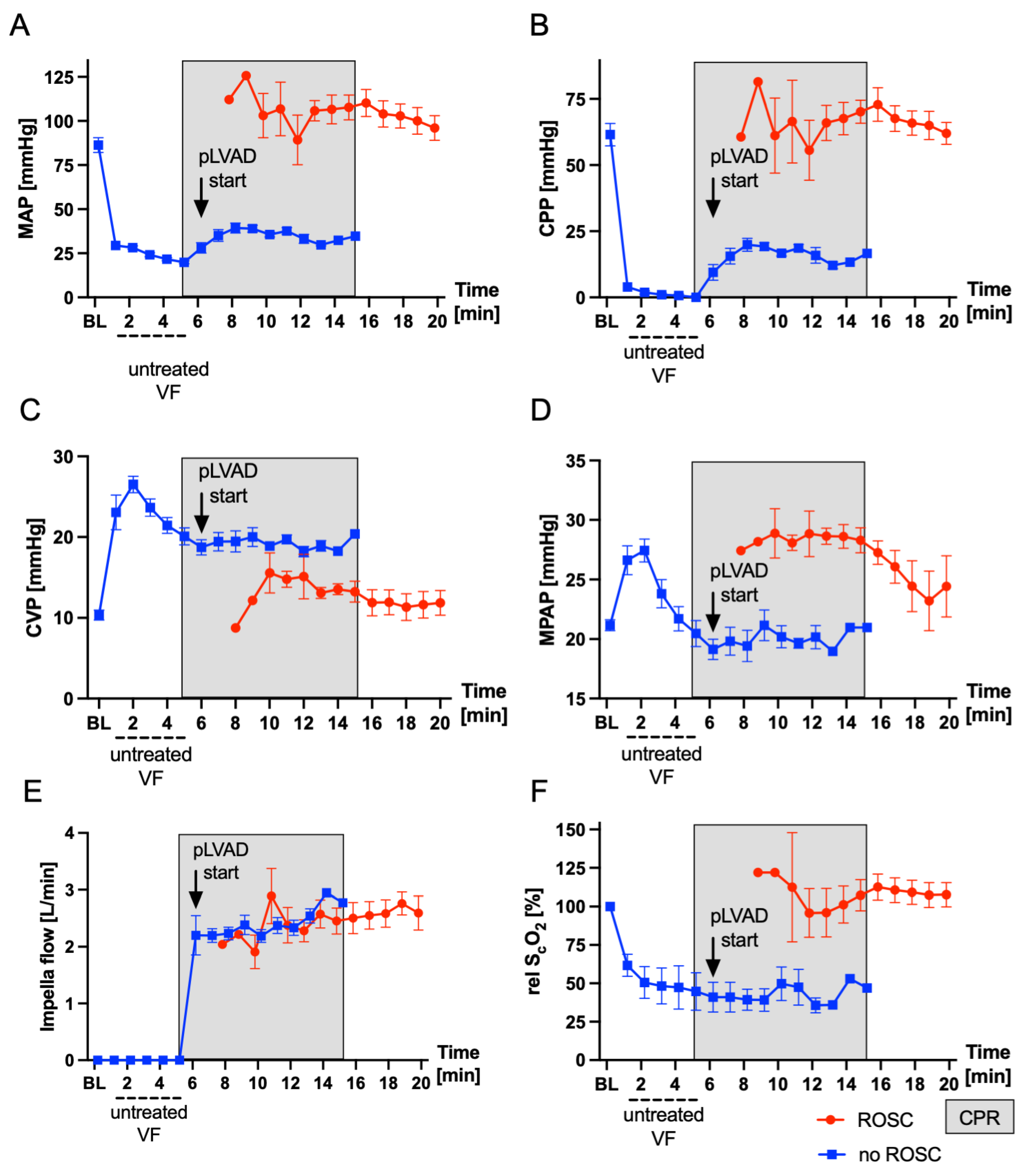
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grasner, J.T.; Lefering, R.; Koster, R.W.; Masterson, S.; Bottiger, B.W.; Herlitz, J.; Wnent, J.; Tjelmeland, I.B.; Ortiz, F.R.; Maurer, H.; et al. Corrigendum to “EuReCa One-27 Nations, One Europe, One Registry a Prospective One Month Analysis of out-of-Hospital Cardiac Arrest Outcomes in 27 Countries in Europe” [Resuscitation 105 (2016) 188–195]. Resuscitation 2016, 109, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Stecker, E.C.; Reinier, K.; Marijon, E.; Narayanan, K.; Teodorescu, C.; Uy-Evanado, A.; Gunson, K.; Jui, J.; Chugh, S.S. Burden of Sudden Cardiac Death in the United. Circ. Arrhythmia Electrophysiol. 2014, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- Waalewijn, R.A.; Tijssen, J.G.; Koster, R.W. Bystander Initiated Actions in out-of-Hospital Cardiopulmonary Resuscitation: Results from the Amsterdam Resuscitation Study (Arresust). Resuscitation 2001, 50, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; Travers, A.H.; Berg, R.A.; Castren, M.; Considine, J.; Escalante, R.; Gazmuri, R.J.; Koster, R.W.; Lim, S.H.; Nation, K.J.; et al. Part 3: Adult Basic Life Support and Automated External Defibrillation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Resuscitation 2015, 95, e43–e69. [Google Scholar] [CrossRef] [PubMed]
- Talikowska, M.; Tohira, H.; Finn, J. Cardiopulmonary Resuscitation Quality and Patient Survival Outcome in Cardiac Arrest: A Systematic Review and Meta-Analysis. Resuscitation 2015, 96, 66–77. [Google Scholar] [CrossRef]
- Wallace, S.K.; Abella, B.S.; Becker, L.B. Quantifying the Effect of Cardiopulmonary Resuscitation Quality on Cardiac Arrest Outcome: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Derwall, M.; Brucken, A.; Bleilevens, C.; Ebeling, A.; Fohr, P.; Rossaint, R.; Kern, K.B.; Nix, C.; Fries, M. Doubling Survival and Improving Clinical Outcomes Using a Left Ventricular Assist Device Instead of Chest Compressions for Resuscitation after Prolonged Cardiac Arrest: A Large Animal Study. Crit. Care 2015, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Lotun, K.; Truong, H.T.; Cha, K.C.; Alsakka, H.; Gianotto-Oliveira, R.; Smith, N.; Rao, P.; Bien, T.; Chatelain, S.; Kern, M.C.; et al. Cardiac Arrest in the Cardiac Catheterization Laboratory: Combining Mechanical Chest Compressions and Percutaneous LV Assistance. JACC Cardiovasc. Interv. 2019, 12, 1840–1849. [Google Scholar] [CrossRef]
- Panagides, V.; Vase, H.; Shah, S.P.; Basir, M.B.; Mancini, J.; Kamran, H.; Batra, S.; Laine, M.; Eiskjaer, H.; Christensen, S.; et al. Impella CP Implantation during Cardiopulmonary Resuscitation for Cardiac Arrest: A Multicenter Experience. J. Clin. Med. 2021, 10, 339. [Google Scholar] [CrossRef]
- Burzotta, F.; Russo, G.; Previ, L.; Bruno, P.; Aurigemma, C.; Trani, C. Impella: Pumps Overview and Access Site Management. Minerva Cardioangiol. 2018, 66, 606–611. [Google Scholar] [CrossRef]
- Glazier, J.J.; Kaki, A. The Impella Device: Historical Background, Clinical Applications and Future Directions. Int. J. Angiol. 2019, 28, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Philipson, D.J.; Cohen, D.J.; Fonarow, G.C.; Ziaeian, B. Analysis of Adverse Events Related to Impella Usage (from the Manufacturer and User Facility Device Experience and National Inpatient Sample Databases). Am. J. Cardiol. 2021, 140, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Vase, H.; Christensen, S.; Christiansen, A.; Therkelsen, C.J.; Christiansen, E.H.; Eiskjær, H.; Poulsen, S.H. The Impella CP Device for Acute Mechanical Circulatory Support in Refractory Cardiac Arrest. Resuscitation 2017, 112, 70–74. [Google Scholar] [CrossRef]
- Kamran, H.; Batra, S.; Venesy, D.M.; Patten, R.D.; Waxman, S.; Pyne, C.; Shah, S.P. Outcomes of Impella CP Insertion during Cardiac Arrest: A Single Center Experience. Resuscitation 2020, 147, 53–56. [Google Scholar] [CrossRef]
- Davidsen, C.; Packer, E.J.S.; Loland, K.H.; Rotevatn, S.; Nygreen, E.L.; Eriksen, E.; Oksnes, A.; Herstad, J.; Haaverstad, R.; Bleie, O.; et al. Impella Use in Acute Myocardial Infarction Complicated by Cardiogenic Shock and Cardiac Arrest: Analysis of 10 Years Registry Data. Resuscitation 2019, 140, 178–184. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The Arrive Guidelines 2.0: Updated Guidelines for Reporting Animal Research. Exp. Physiol. 2020, 105, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Billig, S.; Zayat, R.; Ebeling, A.; Steffen, H.; Nix, C.; Hatam, N.; Schnoring, H.; Derwall, M. Transesophageal Echocardiography in Swine: Evaluation of Left and Right Ventricular Structure, Function and Myocardial Work. Int. J. Cardiovasc. Imaging 2021, 37, 835–846. [Google Scholar] [CrossRef]
- Niemann, J.T.; Rosborough, J.P.; Ung, S.; Criley, J.M. Coronary Perfusion Pressure during Experimental Cardiopulmonary Resuscitation. Ann. Emerg. Med. 1982, 11, 127–131. [Google Scholar] [CrossRef]
- Tuseth, V. Percutaneous Assist Device for Cardiopulmonary Resuscitation. Interv. Cardiol. Clin. 2013, 2, 429–443. [Google Scholar] [CrossRef]
- Derwall, M.; Ebeling, A.; Nolte, K.W.; Weis, J.; Rossaint, R.; Ichinose, F.; Nix, C.; Fries, M.; Brucken, A. Inhaled Nitric Oxide Improves Transpulmonary Blood Flow and Clinical Outcomes after Prolonged Cardiac Arrest: A Large Animal Study. Crit. Care 2015, 19, 328. [Google Scholar] [CrossRef]
- Crowley, J.; Cronin, B.; Essandoh, M.; D’Alessandro, D.; Shelton, K.; Dalia, A.A. Transesophageal Echocardiography for Impella Placement and Management. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2663–2668. [Google Scholar] [CrossRef] [PubMed]
- Pieri, M.; Pappalardo, F. Bedside Insertion of Impella Percutaneous Ventricular Assist Device in Patients with Cardiogenic Shock. Int. J. Cardiol. 2020, 316, 26–30. [Google Scholar] [CrossRef]
- Vetrovec, G.W.; Anderson, M.; Schreiber, T.; Popma, J.; Lombardi, W.; Maini, B.; Moller, J.E.; Schafer, A.; Dixon, S.R.; Hall, S.; et al. The cVAD Registry for Percutaneous Temporary Hemodynamic Support: A Prospective Registry of Impella Mechanical Circulatory Support Use in High-Risk PCI, Cardiogenic Shock, and Decompensated Heart Failure. Am. Heart J. 2018, 199, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Lemor, A.; Dabbagh, M.F.; Cohen, D.; Villablanca, P.; Tehrani, B.; Alaswad, K.; Alqarqaz, M.; Lasorda, D.; Kaki, A.; Genereux, P.; et al. Rates and Impact of Vascular Complications in Mechanical Circulatory Support. Catheter. Cardiovasc. Interv. 2022, 99, 1702–1711. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, L.; Mahabadi, A.A.; Totzeck, M.; Krueger, A.; Janosi, R.A.; Rassaf, T.; Al-Rashid, F. Access Site Complications following Impella-Supported High-Risk Percutaneous Coronary Interventions. Sci. Rep. 2019, 9, 17844. [Google Scholar] [CrossRef] [PubMed]
- Abaunza, M.; Kabbani, L.S.; Nypaver, T.; Greenbaum, A.; Balraj, P.; Qureshi, S.; Alqarqaz, M.A.; Shepard, A.D. Incidence and Prognosis of Vascular Complications after Percutaneous Placement of Left Ventricular Assist Device. J. Vasc. Surg. 2015, 62, 417–423. [Google Scholar] [CrossRef]
- Maier, G.W.; Tyson, G.S., Jr.; Olsen, C.O.; Kernstein, K.H.; Davis, J.W.; Conn, E.H.; Rankin, J.S. The Physiology of External Cardiac Massage: High-Impulse Cardiopulmonary Resuscitation. Circulation 1984, 70, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Brucken, A.; Derwall, M.; Bleilevens, C.; Stoppe, C.; Gotzenich, A.; Gaisa, N.T.; Weis, J.; Nolte, K.W.; Rossaint, R.; Ichinose, F.; et al. Brief Inhalation of Nitric Oxide Increases Resuscitation Success and Improves 7-Day-Survival after Cardiac Arrest in Rats: A Randomized Controlled Animal Study. Crit. Care 2015, 19, 408. [Google Scholar] [CrossRef]
- Bartsch, B.; Haase, K.K.; Voelker, W.; Schobel, W.A.; Karsch, K.R. Risk of Invasive Diagnosis with Retrograde Catheterization of the Left Ventricle in Patients with Acquired Aortic Valve Stenosis. Z. Kardiol. 1999, 88, 255–260. [Google Scholar] [CrossRef]

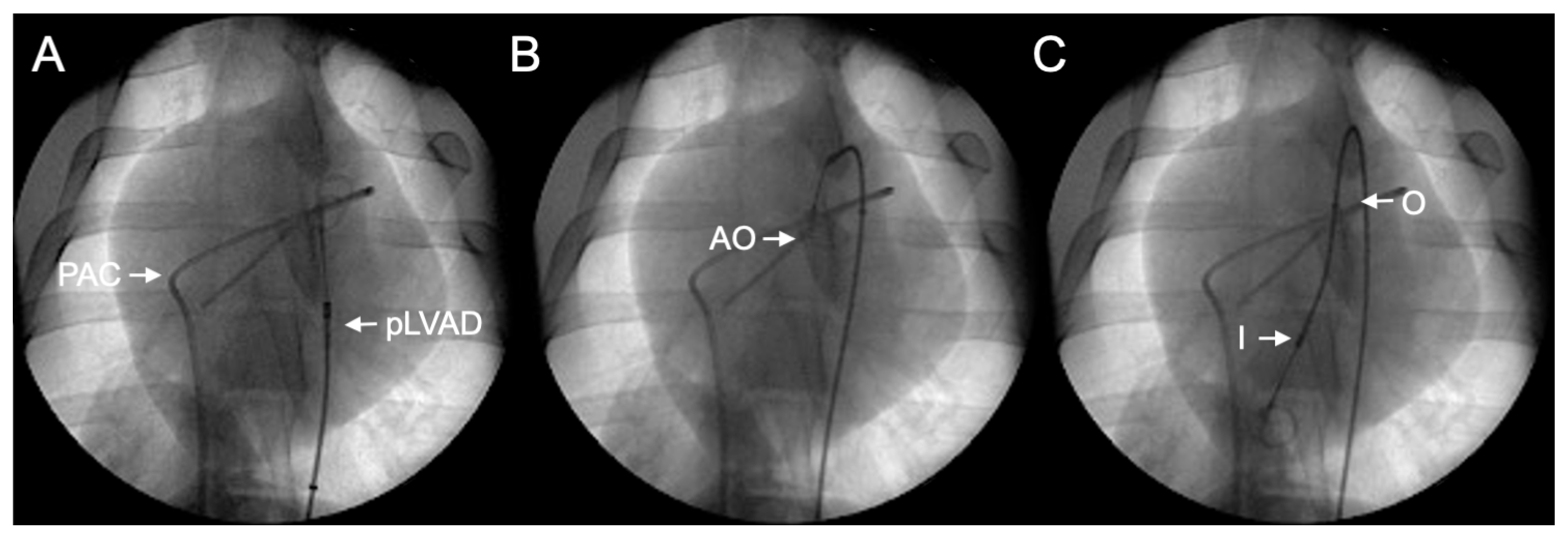
| Parameter | Value ± SD |
|---|---|
| Vascular access complications [n] | 0 |
| pLVAD implantation time [s] | 59 ± 28 |
| Implantation success [%] | 100 |
| Defibrillation attempts [n] | 3.5 ± 2 |
| Time to ROSC [min] | 11.33 ± 2.07 |
| Norepinephrine [mg] | 1.8 ± 0.4 |
| Survival [n] [%] | 6/8; 75% |
| BL (n = 8) | PR 10 (n = 6) | PR30 (n = 6) | PR 120 (n = 6) | PR 300 (n = 6) | PR 360 (n = 6) | |
|---|---|---|---|---|---|---|
| HR [bpm] | 73 ± 15 | 144 ± 33 | 134 ± 43 | 86 ± 10 | 67 ± 11 | 70 ± 17 |
| MAP [mmHg] | 86 ± 12 | 94 ± 19 | 57 ± 20 | 77 ± 15 | 80 ± 9 | 79 ± 8 |
| MPAP [mmHg] | 21 ± 1 | 23 ± 5 | 21 ± 4 | 21 ± 3 | 23 ± 2 | 22 ± 2 |
| CVP [mmHg] | 10 ± 2 | 11 ± 2 | 12 ± 4 | 11 ± 2 | 12 ± 2 | 11 ± 2 |
| PCWP [mmHg] | 14 ± 3 | 15 ± 3 | 15 ± 2 | 15 ± 2 | 14 ± 2 | 13 ± 2 |
| CO [L/min] | 6.9 ± 1.1 | 10.0 ± 1.6 | 7.8 ± 2.0 | 6.1 ± 0.7 | 5.1 ± 0.4 | 5.1 ± 0.9 |
| pLVAD flow [L/min] | 0 ± 0 | 2.9 ± 0.5 | 3.3 ± 0.9 | 3.0 ± 0.8 | 3.0 ± 0.6 | 0 ± 0 |
| paO2 [mmHg] | 144 ± 20 | 472 ± 94 | 173 ± 38 | 161 ± 10 | 167 ± 12 | 171 ± 14 |
| paCO2 [mmHg] | 38 ± 2 | 42 ± 3 | 40 ± 4 | 37 ± 2 | 37 ± 1 | 38 ± 1 |
| SvO2 [%] | 62 ± 8 | 82 ± 4 | 53 ± 16 | 53 ± 9 | 52 ± 6 | 54 ± 9 |
| pH | 7.47 ± 0.04 | 7.3 ± 0.05 | 7.34 ± 0.02 | 7.45 ± 0.04 | 7.49 ± 0.00 | 7.49 ± 0.01 |
| Hb [g/dL] | 9.3 ± 0.7 | 11.4 ± 0.7 | 10.1 ± 0.9 | 9.5 ± 0.6 | 8.9 ± 0.9 | 8.8 ± 0.9 |
| Lactate [mmol/L] | 1.7 ± 2.3 | 8.1 ± 2.4 | 8.9 ± 1.7 | 4.8 ± 1.2 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| Glucose [mg/dL] | 111 ± 20 | 185 ± 56 | 151 ± 71 | 129 ± 29 | 111 ± 12 | 104 ± 10 |
| PLT/nL [n] | 280 ± 65 | 204 ± 37 | ||||
| WBC/nL [n] | 18.9 ± 4.4 | 13.3 ± 5.3 |
| BL (n = 7) | PR 30 (n = 5) | PR 300 (n = 5) | PR 360 (n = 5) | |
|---|---|---|---|---|
| LV-EF [%] | 59 ± 7 | 45 ± 9 | 66 ± 12 | 59 ± 19 |
| LV-GLS [%] | −24.2 ± 3.3 | −11.9 ± 5.8 | −23.4 ± 3.8 | −20.1 ± 4.1 |
| RV-GLS [%] | −26.2 ± 4.4 | −14.0 ± 5.9 | −23.7 ± 3.2 | −18.6 ± 6.2 |
| RVD basal [mm] | 28 ± 5 | 30 ± 7 | 30 ± 6 | 29 ± 4 |
| TASV [cm/s] | 10.8 ± 2.2 | 7.0 ± 3.3 | 8.4 ± 1.5 | 9.4 ± 1.5 |
| E/A | 1.8 ± 0.8 | 1.8 ± 1.2 | 1.7 ± 0.5 | 1.3 ± 0.2 |
| DT MV [ms] | 253 ± 151 | 130 ± 80 | 191 ± 77 | 213 ± 67 |
| E/E’ | 7.1 ± 2.3 | 8.8 ± 3.5 | 5.6 ± 2.7 | 7.5 ± 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Billig, S.; Zayat, R.; Yelenski, S.; Nix, C.; Bennek-Schoepping, E.; Hochhausen, N.; Derwall, M. The Self-Expandable Impella CP (ECP) as a Mechanical Resuscitation Device. Bioengineering 2024, 11, 456. https://doi.org/10.3390/bioengineering11050456
Billig S, Zayat R, Yelenski S, Nix C, Bennek-Schoepping E, Hochhausen N, Derwall M. The Self-Expandable Impella CP (ECP) as a Mechanical Resuscitation Device. Bioengineering. 2024; 11(5):456. https://doi.org/10.3390/bioengineering11050456
Chicago/Turabian StyleBillig, Sebastian, Rachad Zayat, Siarhei Yelenski, Christoph Nix, Eveline Bennek-Schoepping, Nadine Hochhausen, and Matthias Derwall. 2024. "The Self-Expandable Impella CP (ECP) as a Mechanical Resuscitation Device" Bioengineering 11, no. 5: 456. https://doi.org/10.3390/bioengineering11050456
APA StyleBillig, S., Zayat, R., Yelenski, S., Nix, C., Bennek-Schoepping, E., Hochhausen, N., & Derwall, M. (2024). The Self-Expandable Impella CP (ECP) as a Mechanical Resuscitation Device. Bioengineering, 11(5), 456. https://doi.org/10.3390/bioengineering11050456






