Diagnosis of Forme Fruste Keratoconus Using Corvis ST Sequences with Digital Image Correlation and Machine Learning
Abstract
1. Introduction
2. Material and Methods
2.1. Participants and Procedures
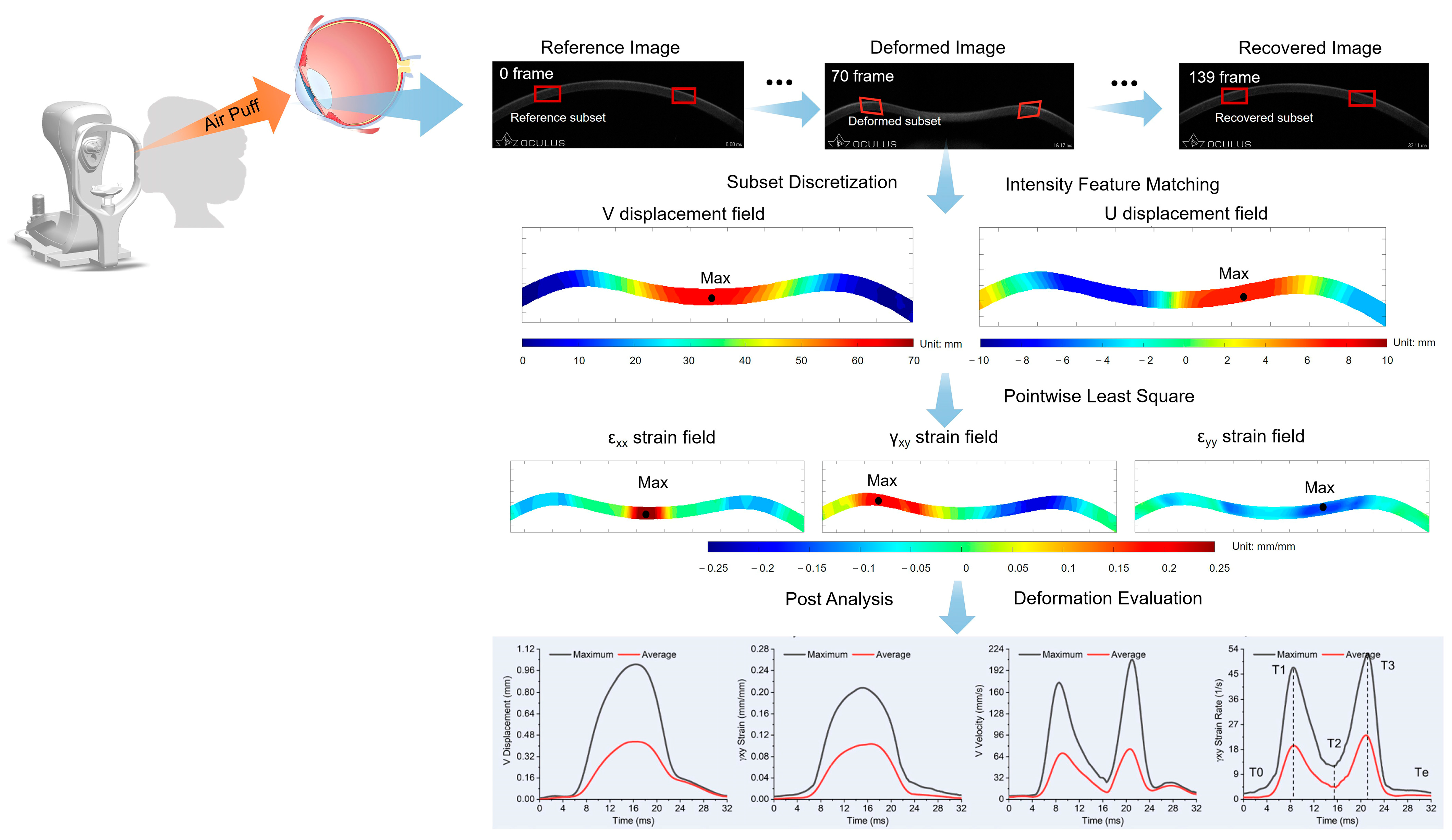
2.2. Incremental DIC Method
2.3. Feature Extraction and Model Construction
2.4. Experimental Setting and Performance Metrics
2.5. Statistical Analysis
3. Results
3.1. The Demographic Data of the Participants
3.2. Initial Model Construction
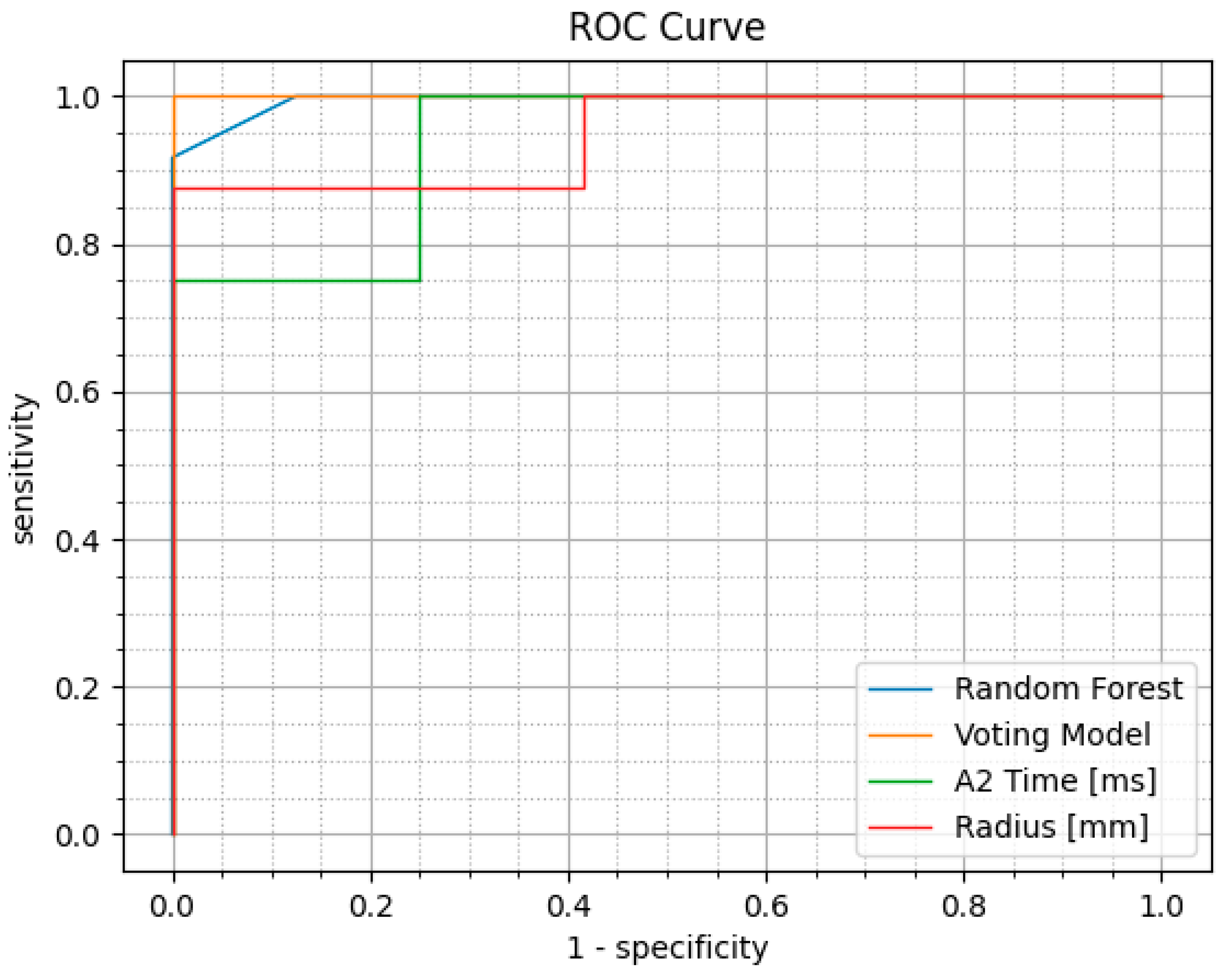
3.3. Performance of the Machine Learning Models and Voting Classifier Model
3.4. Performance Comparison with Existing CVS Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Terminology
| FFKC | Forme fruste keratoconus, an early form of keratoconus. |
| KC | Keratoconus, a non-inflammatory eye condition where the cornea progressively thins and bulges into a cone-like shape. |
| DIC | Digital Image Correlation, an optical method used to measure deformation, strain, and motion on a wide range of materials and structures. |
| CVS | Corvis ST, a non-contact tonometer equipped with an ultra-high speed Scheimpflug camera used to measure intraocular pressure and assess corneal biomechanics by analyzing the dynamic deformation response of the cornea to an air puff. |
| AUC | Area under the ROC curve, which plots the true positive rate against the false positive rate. |
| ROC | Receiver Operating Characteristic, a graphical plot that illustrates the performance of a binary classification model across various threshold settings. |
| ORA | Ocular Response Analyzer, a device used to measure the biomechanical properties of the cornea, such as corneal hysteresis and corneal resistance factor. |
| CBI | Corvis Biomechanical Index, a parameter derived from Corvis ST. |
| cCBI | Corvis Biomechanical Index for Chinese people. |
| AI | Artificial Intelligence, the simulation of human intelligence in machines programmed to think and learn. |
| ML | Machine Learning, a subset of AI that enables computers to learn from data and make predictions or decisions without being explicitly programmed. |
| NB | Naïve Bayes, a probabilistic machine learning algorithm based on Bayes’s theorem with an assumption of independence between features. |
| RF | Random Forest, an ensemble learning method that combines multiple decision trees to improve predictive accuracy and control overfitting. |
| TPRK | Trans-Epithelial Photorefractive Keratectomy, a type of laser eye surgery that corrects vision by reshaping the cornea without manually removing the epithelial layer. |
| LASIK | Laser-Assisted In Situ Keratomileusis, a popular refractive surgery procedure that reshapes the cornea using an excimer laser to correct myopia, hyperopia, and astigmatism. |
| SMILE | Small Incision Lenticule Extraction, a minimally invasive refractive surgery technique that uses a femtosecond laser to create a lenticule within the cornea, which is then removed through a small incision to correct refractive errors without creating a corneal flap. |
References
- Santodomingo-Rubido, J.; Carracedo, G.; Suzaki, A.; Villa-Collar, C.; Vincent, S.J.; Wolffsohn, J.S. Keratoconus: An updated review. Contact Lens Anterior Eye 2022, 45, 101559. [Google Scholar] [CrossRef] [PubMed]
- Krachmer, J.H.; Feder, R.S.; Belin, M.W. Keratoconus and related noninflammatory corneal thinning disorders. Surv. Ophthalmol. 1984, 28, 293–322. [Google Scholar] [CrossRef]
- Bahar, I.; Kaiserman, I.; Srinivasan, S.; Slomovic, A.R.; Rootman, D.S. Comparison of Three Different Techniques of Corneal Transplantation for Keratoconus. Am. J. Ophthalmol. 2008, 146, 905–912. [Google Scholar] [CrossRef]
- Lee, L.R.; Hirst, L.W.; Readshaw, G. Clinical detection of unilateral keratoconus. Aust. N. Z. J. Ophthalmol. 1995, 23, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, Y.S.; Nesburn, A.B.; McDonnell, P.J. Videokeratography of the Fellow Eye in Unilateral Keratoconus. Ophthalmology 1993, 100, 181–186. [Google Scholar] [CrossRef]
- Pniakowska, Z.; Jurowski, P. Detection of the early keratoconus based on corneal biomechanical properties in the refractive surgery candidates. Indian J. Ophthalmol. 2016, 64, 109–113. [Google Scholar] [CrossRef]
- Fernández Pérez, J.; Valero Marcos, A.; Martínez Peña, F.J. Early diagnosis of keratoconus: What difference is it making? Br. J. Ophthalmol. 2014, 98, 1465–1466. [Google Scholar] [CrossRef]
- Shetty, R.; Rao, H.; Khamar, P.; Sainani, K.; Vunnava, K.; Jayadev, C.; Kaweri, L. Keratoconus Screening Indices and Their Diagnostic Ability to Distinguish Normal From Ectatic Corneas. Am. J. Ophthalmol. 2017, 181, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, C.; Wang, R.; Liu, Z.; Wang, M.; Tan, H.; Wu, Y.; Liu, X.; Sun, H.; Yang, R.; et al. Annotation-efficient deep learning for automatic medical image segmentation. Nat. Commun. 2021, 12, 5915. [Google Scholar] [CrossRef]
- Steyaert, S.; Pizurica, M.; Nagaraj, D.; Khandelwal, P.; Hernandez-Boussard, T.; Gentles, A.J.; Gevaert, O. Multimodal data fusion for cancer biomarker discovery with deep learning. Nat. Mach. Intell. 2023, 5, 351–362. [Google Scholar] [CrossRef]
- Placido, D.; Yuan, B.; Hjaltelin, J.X.; Zheng, C.; Haue, A.D.; Chmura, P.J.; Yuan, C.; Kim, J.; Umeton, R.; Antell, G.; et al. A deep learning algorithm to predict risk of pancreatic cancer from disease trajectories. Nat. Med. 2023, 29, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Hidalgo, I.; Rodriguez, P.; Rozema, J.J.; Ní Dhubhghaill, S.; Zakaria, N.; Tassignon, M.-J.; Koppen, C. Evaluation of a machine-learning classifier for keratoconus detection based on scheimpflug tomography. Cornea 2016, 35, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Kovács, I.; Miháltz, K.; Kránitz, K.; Juhász, É.; Takács, Á.; Dienes, L.; Gergely, R.; Nagy, Z.Z. Accuracy of machine learning classifiers using bilateral data from a Scheimpflug camera for identifying eyes with preclinical signs of keratoconus. J. Cataract. Refract. Surg. 2016, 42, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Ambrósio, R.; Machado, A.P.; Leão, E.; Lyra, J.M.G.; Salomão, M.Q.; Esporcatte, L.G.P.; Filho, J.B.d.F.; Ferreira-Meneses, E.; Sena, N.B.; Haddad, J.S.; et al. Optimized Artificial Intelligence for Enhanced Ectasia Detection Using Scheimpflug-Based Corneal Tomography and Biomechanical Data. Am. J. Ophthalmol. 2023, 251, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Chen, X.; Li, K.; Liu, Y.; Cao, H.; Li, J.; Jhanji, V.; Zou, H.; Liu, F.; Wang, R.; et al. Artificial Intelligence–Based Diagnostic Model for Detecting Keratoconus Using Videos of Corneal Force Deformation. Transl. Vis. Sci. Technol. 2022, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.-J.; Elsheikh, A.; Rozema, J.J.; Hafezi, N.; Aslanides, I.M.; Hillen, M.; Eckert, D.; Funck, C.; Koppen, C.; Cui, L.-L.; et al. Combining Spectral-Domain OCT and Air-Puff Tonometry Analysis to Diagnose Keratoconus. J. Refract. Surg. 2022, 38, 374–380. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, V.S.; Dupps, W.J. Biomechanical diagnostics of the cornea. Int. Ophthalmol. Clin. 2017, 57, 75–86. [Google Scholar] [CrossRef]
- Roberts, C.J.; Mahmoud, A.M.; Bons, J.P.; Hossain, A.; Elsheikh, A.; Vinciguerra, R.; Vinciguerra, P.; Ambrósio, R., Jr. Introduction of two novel stiffness parameters and interpretation of air puff–induced biomechanical deformation parameters with a dynamic Scheimpflug analyzer. J. Refract. Surg. 2017, 33, 266–273. [Google Scholar] [CrossRef]
- Vinciguerra, R.; Ambrósio, R.; Elsheikh, A.; Roberts, C.J.; Lopes, B.; Morenghi, E.; Azzolini, C.; Vinciguerra, P. Detection of keratoconus with a new biomechanical index. J. Refract. Surg. 2016, 32, 803–810. [Google Scholar] [CrossRef]
- Salouti, R.; Bagheri, M.; Shamsi, A.; Zamani, M.; Ghoreyshi, M.; Hossein Nowroozzadeh, M. Corneal parameters in healthy subjects assessed by Corvis ST. J. Ophthalmic Vis. Res. 2020, 15, 24–31. [Google Scholar] [CrossRef]
- Elsheikh, A.; Wang, D.; Pye, D. Determination of the modulus of elasticity of the human cornea. J. Refract. Surg. 2007, 23, 808–818. [Google Scholar] [CrossRef]
- Vol, B. Strain-rate sensitivity of porcine and ovine corneas. Acta Bioeng. Biomech. 2011, 13, 25–36. [Google Scholar]
- Maczynska, E.; Karnowski, K.; Szulzycki, K.; Malinowska, M.; Dolezyczek, H.; Cichanski, A.; Wojtkowski, M.; Kaluzny, B.; Grulkowski, I. Assessment of the influence of viscoelasticity of cornea in animal ex vivo model using air-puff optical coherence tomography and corneal hysteresis. J. Biophotonics 2019, 12, e201800154. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, L.; Zheng, Y. Determining in vivo elasticity and viscosity with dynamic Scheimpflug imaging analysis in keratoconic and healthy eyes. J. Biophotonics 2016, 463, 454–463. [Google Scholar] [CrossRef]
- Wang, B.; Yang, L.; Cheng, J.; Wang, J.; Mei, Y. In-vivo high-speed biomechanical imaging of the cornea using Corvis ST and digital image correlation. Comput. Biol. Med. 2023, 153, 106540. [Google Scholar] [CrossRef]
- Bing, P.; Wu, D.; Xia, Y. Incremental calculation for large deformation measurement using reliability-guided digital image correlation. Opt. Lasers Eng. 2012, 50, 586–592. [Google Scholar]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Smadja, D.; Touboul, D.; Cohen, A.; Doveh, E.; Santhiago, M.R.; Mello, G.R.; Krueger, R.R.; Colin, J. Detection of subclinical keratoconus using an automated decision tree classification. Am. J. Ophthalmol. 2013, 156, 237–246.e1. [Google Scholar] [CrossRef]
- Chandapura, R.; Salomão, M.Q.; Ambrósio, R., Jr.; Swarup, R.; Shetty, R.; Roy, A.S. Bowman’s topography for improved detection of early ectasia. J. Biophotonics 2019, 12, e201900126. [Google Scholar] [CrossRef]
- Lu, N.-J.; Koppen, C.; Hafezi, F.; Dhubhghaill, S.N.; Aslanides, I.M.; Wang, Q.-M.; Cui, L.-L.; Rozema, J.J. Combinations of Scheimpflug tomography, ocular coherence tomography and air-puff tonometry improve the detection of keratoconus. Contact Lens Anterior Eye 2023, 46, 101840. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.J.; Dupps, W.J.D., Jr. Biomechanics of corneal ectasia and biomechanical treatments. J. Cataract. Refract. Surg. 2014, 40, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Shen, Y.; Jhanji, V.; Zhou, X.; Zhao, J.; Zhao, Y.; Zhou, X. Comparison of Corneal Biomechanics in Post-SMILE, Post-LASEK, and Keratoconic Eyes. Front. Med. 2021, 8, 695697. [Google Scholar] [CrossRef] [PubMed]
- Mikula, E.; Winkler, M.; Juhasz, T.; Brown, D.J.; Shoa, G.; Tran, S.; Kenney, M.C.; Jester, J.V. Axial mechanical and structural characterization of keratoconus corneas. Exp. Eye Res. 2018, 175, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Labiris, G.; Gatzioufas, Z.; Sideroudi, H.; Giarmoukakis, A.; Kozobolis, V.; Seitz, B. Biomechanical diagnosis of keratoconus: Evaluation of the keratoconus match index and the keratoconus match probability. Acta Ophthalmol. 2013, 91, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, R.; Ambrosio, R.; Wang, Y.; Zhang, F.; Zhou, X.; Bai, J.; Yu, K.; Chen, S.; Fang, X.; Vinciguerra, P. Detection of Keratoconus with a New Corvis Biomechanical Index Optimized for Chinese Populations. Am. J. Ophthalmol. 2023, 252, 182–187. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, L.; Guo, L.; Qin, X.; Zhang, D.; Li, L.; Jie, Y.; Zhang, H. Comprehensive evaluation of corneas from normal, forme fruste keratoconus and clinical keratoconus patients using morphological and biomechanical properties. Int. Ophthalmol. 2021, 41, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.-L.; Tian, L.; Cao, K.; Li, Y.-X.; Li, N.; Yang, W.-Q.; Jie, Y. Comparison of the morphological and biomechanical characteristics of keratoconus, forme fruste keratoconus, and normal corneas. Semin. Ophthalmol. 2021, 36, 671–678. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, D.; Guo, L.; Qin, X.; Zhang, H.; Zhang, H.; Jie, Y.; Li, L. Comparisons of corneal biomechanical and tomographic parameters among thin normal cornea, forme fruste keratoconus, and mild keratoconus. Eye Vis. 2021, 8, 44. [Google Scholar] [CrossRef]
- Cao, K.; Verspoor, K.; Sahebjada, S.; Baird, P.N. Evaluating the performance of various machine learning algorithms to detect subclinical keratoconus. Transl. Vis. Sci. Technol. 2020, 9, 24. [Google Scholar] [CrossRef]
- Cao, K.; Verspoor, K.; Chan, E.; Daniell, M.; Sahebjada, S.; Baird, P.N. Machine learning with a reduced dimensionality representation of comprehensive Pentacam tomography parameters to identify subclinical keratoconus. Comput. Biol. Med. 2021, 138, 104884. [Google Scholar] [CrossRef] [PubMed]
- Castro-Luna, G.; Jiménez-Rodríguez, D.; Castaño-Fernández, A.B.; Pérez-Rueda, A. Diagnosis of subclinical keratoconus based on machine learning techniques. J. Clin. Med. 2021, 10, 4281. [Google Scholar] [CrossRef] [PubMed]
- Kundu, G.; Shetty, R.; Khamar, P.; Mullick, R.; Gupta, S.; Nuijts, R.; Roy, A.S. Universal architecture of corneal segmental tomography biomarkers for artificial intelligence-driven diagnosis of early keratoconus. Br. J. Ophthalmol. 2023, 107, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Alturki, N.; Umer, M.; Ishaq, A.; Abuzinadah, N.; Alnowaiser, K.; Mohamed, A.; Saidani, O.; Ashraf, I. Combining CNN Features with Voting Classifiers for Optimizing Performance of Brain Tumor Classification. Cancers 2023, 15, 1767. [Google Scholar] [CrossRef] [PubMed]
- Umer, M.; Naveed, M.; Alrowais, F.; Ishaq, A.; Al Hejaili, A.; Alsubai, S.; Eshmawi, A.A.; Mohamed, A.; Ashraf, I. Breast Cancer Detection Using Convoluted Features and Ensemble Machine Learning Algorithm. Cancers 2022, 14, 6015. [Google Scholar] [CrossRef]
- Yoo, T.K.; Ryu, I.H.; Lee, G.; Kim, Y.; Kim, J.K.; Lee, I.S.; Kim, J.S.; Rim, T.H. Adopting machine learning to automatically identify candidate patients for corneal refractive surgery. npj Digit. Med. 2019, 2, 59. [Google Scholar] [CrossRef]
| Group | Gender (Male/Female) | Age | CCT | bIOP |
|---|---|---|---|---|
| Normal | 35/15 | 20.08 ± 4.19 | 558.58 ± 26.55 | 16.63 ± 2.88 |
| FFKC | 39/11 | 19.74 ± 5.09 | 530.12 ± 26.51 | 13.70 ± 2.01 |
| Statistic Value | X2 = 0.83 | t = 1.12 | t = 5.36 | t = 5.90 |
| p value | 0.36 | 0.26 | <0.001 | <0.001 |
| Dataset | ML Models | Accuracy (%) | Precision | Recall | F1-Score | Sensitivity | Specificity | AUC |
|---|---|---|---|---|---|---|---|---|
| Training | Naïve Bayes | 86.25 | 0.82 | 0.95 | 0.88 | 0.95 | 0.76 | 0.95 |
| Random Forest | 100.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| Voting Classifier | 86.25 | 0.82 | 0.95 | 0.88 | 0.95 | 0.76 | 0.99 | |
| Logistic Regression | 90.00 | 0.89 | 0.93 | 0.91 | 0.93 | 0.87 | 0.97 | |
| Validation | Naïve Bayes | 85.00 | 0.73 | 1.00 | 0.84 | 1.00 | 0.75 | 0.92 |
| Random Forest | 95.00 | 0.89 | 1.00 | 0.94 | 1.00 | 0.92 | 0.99 | |
| Voting Classifier | 85.00 | 0.73 | 1.00 | 0.84 | 1.00 | 0.75 | 1.00 | |
| Logistic Regression | 95.00 | 0.89 | 1.00 | 1.00 | 1.00 | 0.92 | 0.94 |
| Variable | AUC | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Radius [mm] | 0.948 | 100.000 | 87.500 |
| A2 Time [ms] | 0.938 | 75.000 | 100.000 |
| Max Inverse Radius [mm] | 0.932 | 83.330 | 100.000 |
| SP A1 | 0.927 | 83.330 | 100.000 |
| cCBI | 0.927 | 91.670 | 100.000 |
| CBI | 0.917 | 91.670 | 100.000 |
| SSI2 | 0.906 | 91.670 | 87.500 |
| A1 Time [ms] | 0.896 | 83.330 | 100.000 |
| SP HC | 0.896 | 91.670 | 87.500 |
| Integrated Radius [mm] | 0.865 | 75.000 | 100.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Qi, K.; Zhang, P.; Cheng, J.; Soha, H.; Jin, Y.; Ci, H.; Zheng, X.; Wang, B.; Mei, Y.; et al. Diagnosis of Forme Fruste Keratoconus Using Corvis ST Sequences with Digital Image Correlation and Machine Learning. Bioengineering 2024, 11, 429. https://doi.org/10.3390/bioengineering11050429
Yang L, Qi K, Zhang P, Cheng J, Soha H, Jin Y, Ci H, Zheng X, Wang B, Mei Y, et al. Diagnosis of Forme Fruste Keratoconus Using Corvis ST Sequences with Digital Image Correlation and Machine Learning. Bioengineering. 2024; 11(5):429. https://doi.org/10.3390/bioengineering11050429
Chicago/Turabian StyleYang, Lanting, Kehan Qi, Peipei Zhang, Jiaxuan Cheng, Hera Soha, Yun Jin, Haochen Ci, Xianling Zheng, Bo Wang, Yue Mei, and et al. 2024. "Diagnosis of Forme Fruste Keratoconus Using Corvis ST Sequences with Digital Image Correlation and Machine Learning" Bioengineering 11, no. 5: 429. https://doi.org/10.3390/bioengineering11050429
APA StyleYang, L., Qi, K., Zhang, P., Cheng, J., Soha, H., Jin, Y., Ci, H., Zheng, X., Wang, B., Mei, Y., Chen, S., & Wang, J. (2024). Diagnosis of Forme Fruste Keratoconus Using Corvis ST Sequences with Digital Image Correlation and Machine Learning. Bioengineering, 11(5), 429. https://doi.org/10.3390/bioengineering11050429






