Abstract
Obstructive Sleep Apnea (OSA), a sleep disorder with high prevalence, is normally accompanied by affective, autonomic, and cognitive abnormalities, and is deemed to be linked to functional brain alterations. To investigate alterations in brain functional connectivity properties in patients with OSA, a comparative analysis of global and local topological properties of brain networks was conducted between patients with OSA and healthy controls (HCs), utilizing functional near-infrared spectroscopy (fNIRS) imaging. A total of 148 patients with OSA and 150 healthy individuals were involved. Firstly, quantitative alterations in blood oxygen concentration, changes in functional connectivity, and variations in graph theory-based network topological characteristics were assessed. Then, with Mann–Whitney statistics, this study compared whether there are significant differences in the above characteristics between patients with OSA and HCs. Lastly, the study further examined the correlation between the altered characteristics and the apnea hypopnea index (AHI) using linear regression. Results revealed a higher mean and standard deviation of hemoglobin concentration in the superior temporal gyrus among patients with OSA compared to HCs. Resting-state functional connectivity (RSFC) exhibited a slight increase between the superior temporal gyrus and other specific areas in patients with OSA. Notably, neither patients with OSA nor HCs demonstrated significant small-world network properties. Patients with OSA displayed an elevated clustering coefficient (p < 0.05) and local efficiency (p < 0.05). Additionally, patients with OSA exhibited a tendency towards increased nodal betweenness centrality (p < 0.05) and degree centrality (p < 0.05) in the right supramarginal gyrus, as well as a trend towards higher betweenness centrality (p < 0.05) in the right precentral gyrus. The results of multiple linear regressions indicate that the influence of the AHI on RSFC between the right precentral gyrus and right superior temporal gyrus (p < 0.05), as well as between the right precentral gyrus and right supramarginal gyrus (p < 0.05), are statistically significant. These findings suggest that OSA may compromise functional brain connectivity and network topological properties in affected individuals, serving as a potential neurological mechanism underlying the observed abnormalities in brain function associated with OSA.
1. Introduction
Obstructive Sleep Apnea (OSA) is a sleep disorder primarily characterized by repeated apnea or hypopnea during sleep. Apnea during sleep is usually due to relaxation or collapse of the throat’s tissue, resulting in an airway obstruction that prevents air from flowing into the lungs [1]. Patients with OSA often wake up during the night, even though they may not be aware of it, which can lead to poor sleep quality and cause daytime fatigue and lethargy. As a result of poor sleep quality, individuals with OSA may find it challenging to concentrate and even experience mental health issues such as depression and anxiety [2]. In addition, OSA can lead to a variety of health problems, including hypertension, heart disease, stroke, diabetes, and Alzheimer’s disease (AD) [3,4,5,6,7].
As OSA is normally accompanied by affective, autonomic, and cognitive abnormalities, numerous research studies have explored the structural changes or functional changes in the brain [8,9]. For structural brain alterations, it has been found that OSA can result in changes to white or gray matter (the two basic tissue types that make up the brain and spinal cord), which may affect the transfer of information and communication between brain regions [10,11,12]. In addition, a diffusion tensor imaging (DTI) study showed that white matter exhibited extensive alterations in individuals with OSA, affecting axonal connections among key structures within the limbic system, and frontal, temporal, and parietal cortices [13]. Studies indicate a significant correlation between the apnea hypopnea index (AHI) and white matter changes, as well as white matter hyperintensities, across different populations [14,15]. OSA leads to intermittent hypoxemia and fragmented sleep, both of which may have negative effects on gray and white matter in various regions of the brain. These adverse effects are potential contributors to memory impairments and executive function deficits, as well as dysregulation of the autonomic nervous system and respiratory control observed in individuals with OSA [10,16]. With continued treatment, these effects and patterns may be altered. Several studies have indicated that continuous positive airway pressure (CPAP) treatment can lead to adaptive changes in the neurocognitive structure of individuals with OSA [17,18]. The above investigations of structural brain alterations in patients with OSA commonly employ structural magnetic resonance imaging (sMRI). However, sMRI, applying static images, lacks the capacity to directly furnish spatiotemporal dynamic insights into brain function.
To investigate the spatiotemporal dynamics of the brain, functional brain alterations, especially abnormalities in brain functional connectivity, have been explored. Functional connectivity, where temporal correlation or synergistic activity between different functionally related brain regions at a specific period can be quantified, has been widely explored in many neuropsychiatric disorders, such as schizophrenia, depression, cognitive disorders, etc. [19,20,21]. OSA may impair cognitive function, including attention, memory, and executive function. Thus, OSA is deemed to be linked by affecting functional brain connectivity in different regions, such as the prefrontal lobes, insula, and hippocampus [22,23,24,25]. The aberrant functional connectivity (FC) observed in the sensorimotor network and default mode network (DMN) is closely associated with the neurocognitive impairments observed in individuals with OSA [22,23]. The results of abnormal FC in the hippocampus and amygdala subregions also provide a new neuroimaging perspective for further understanding cognitive and emotional disturbances related to OSA [24,25]. Several functional magnetic resonance imaging (fMRI) studies suggested that during specific tasks (e.g., visual stimulation and executive functioning tasks), patients with OSA may exhibit different functional brain activity from healthy controls, which may involve multiple regions, such as the prefrontal, parietal, and temporal lobes [26,27,28]. A study of cortical activation patterns during a visuospatial N-back task reveals that both the task-positive network and DMN are affected in individuals with OSA [28]. In addition, the severity of OSA may be associated with neural activation during working memory tasks, suggesting potential compensatory neural responses [26]. A study related to executive function and emotional processing indicates that children’s cognitive control, conflict monitoring, and attention allocation are influenced by OSA [27].
As research progresses, fMRI has demonstrated its importance in examining brain functional connectivity and networks. However, despite its remarkable achievements, fMRI also has some noteworthy drawbacks. Firstly, its high cost makes it a financial consideration in many studies. Additionally, fMRI needs to be conducted in a relatively confined environment, which may impact the comfort and participation of participants. Most importantly, compared to some other brain imaging techniques, fMRI has relatively low temporal resolution, making it challenging to capture detailed dynamic changes in brain function. Functional Near Infrared Spectroscopy (fNIRS), a non-invasive brain imaging technique, offers better temporal resolution and a relatively high tolerance to head and body movements compared to fMRI. Due to its advantages of being lightweight, low-cost, and its ability to be used in relatively natural environments, it has been used to study the underlying pathogenesis of a variety of neurological disorders, such as central nervous system disorders (including AD, depression, etc.). A few studies have successfully used fNIRS to explore brain activity in patients with OSA. To illustrate, Z. Mingming et al. used fNIRS in conjunction with graph theory metrics to assess brain network abnormalities in the prefrontal lobe of patients with OSA [29]. The study observed that patients with OSA exhibited a reduced number of connecting edges between the right central prefrontal cortex and other right hemisphere regions as well as lower global efficiency, local efficiency, and clustering coefficients compared to the HC group [29]. Z. Zhang et al. studied the dynamics of cerebral hemodynamics during nocturnal CPAP therapy [30]. They found that cyclic oscillations in oxyhemoglobin concentration, deoxyhemoglobin concentration, tissue oxygenation index, and blood volume associated with cyclic apneic events were eliminated in all patients with OSA after CPAP treatment [30]. Additionally, S. Baillieul et al. employed fNIRS to assess the effect of CPAP on gait control in patients with severe OSA syndrome [31]. However, their trial revealed that eight weeks of CPAP treatment did not improve gait control in nonobese patients with OSA [31]. These studies demonstrated the feasibility of fNIRS to study brain activity in patients with OSA. Patients with OSA may experience cognitive decline during the daytime, such as diminished attention, memory, and decision-making skills. The temporoparietal lobe plays a key role in processing cognitive functions, such as memory, attention, and perception [7,32]. Studying daytime fNIRS signals in the temporoparietal lobes of patients with OSA is important for understanding the effects of OSA on brain function. Resting-state studies, which are not contingent on subjects performing specific tasks, offer a more reflective portrayal of the brain’s spontaneity. This helps capture the intrinsic activity of the brain without introducing external interference. Therefore, our experiments will be conducted in the resting state. It is noteworthy that no study has used resting-state fNIRS to study the functional connectivity and topological properties of the temporal parietal lobe in patients with OSA.
This study aims to investigate alterations in brain functional connectivity properties in the temporoparietal lobe of patients with OSA using fNIRS techniques. In our study, we utilized resting-state functional connectivity (RSFC) combined with graph theory methods to explore connectivity changes in the temporoparietal regions and analyze the topological properties of the functional network. Subsequently, we compared intergroup differences in RSFC and multiple network parameters in the temporoparietal regions between patients with OSA and healthy controls (HCs). Additionally, we assessed the relationship between abnormal characteristic changes and the AHI score using linear regression. This study examines the influence of OSA from the perspective of brain function, and investigates alterations in brain functional connectivity properties using fNIRS.
2. Materials and Methods
2.1. Participants
After recruiting subjects, we ensured in the informed consent form that the subjects did not have any mental or neurological disorders before performing the test. In addition to this, we informed the subjects in advance not to consume beverages such as coffee and tea for 12 h prior to the test. During the 10–15 min resting-state fNIRS experiment, participants were asked to remain as still as possible, with their bodies naturally relaxed and eyes closed, and not to fall asleep or to think any particular things. The light and temperature in the laboratory remained constant and comfortable throughout the experiment.
A total of 980 Chinese subjects participated in this experiment in Shanghai, and we used the coefficient of variation to exclude 186 subjects’ data. The CV is expressed as a percentage, calculated by the formula CV (%) = 100 × standard deviation of the data/mean of the data. The signal quality of all individual channels was assessed through the CV, with channels exceeding a certain threshold (CV > 15%) indicating the presence of non-physiological noise and, therefore, being excluded from further processing [33,34,35]. We monitored the subjects’ sleep prior to the experiment and obtained the subjects’ AHI. Subjects with an AHI > 5 were defined as patients with OSA, and those with an AHI < 5 were defined as healthy individuals. Then, from the 792 subjects, two groups were selected that were balanced in terms of gender and age, that is, the group with OSA and the healthy control group. A total of 298 subjects (185 males and 103 females) participated in the data analysis of this study.
The study protocol was approved by the ethics committee of the School of Life Sciences of Fudan University, and informed consent was obtained from all subjects.
2.2. fNIRS Recording
A multichannel fNIRS instrument (NirScout, NIRx Medizintechnik GmbH, Berlin, Germany) with a sampling rate of 8.928571 Hz measured relative changes in hemoglobin concentration in the temporoparietal area cortex using infrared light at two wavelengths, 690 and 830 nm. The seven sources and seven detectors formed a total of 16 channels [36]. Each channel consisted of a source–detector pair at a distance of 3–4 cm and was placed with reference to a 10–20 system. Optode placement is shown in Figure 1.
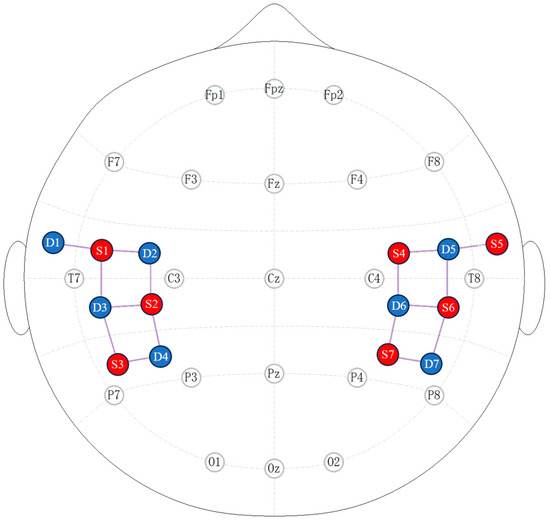
Figure 1.
Two-dimensional view of optode placement. Red denotes the source and blue signifies the detector. Seven sources and seven detectors make up a total of 16 channels.
The positions of all NIRS channels were located using a 3D digitizing system that identified the positions of 14 NIRS photodiodes based on the origin and four reference points (Nz, AR, AL, Cz). The coordinates of the fNIRS channels based on source and detector positions were calculated using the MATLAB toolbox AtlasViewer [37]. The estimated average positions of the 16 channels were obtained based on the anatomical information of the Brodmann Areas. The MNI coordinates and label names are showed in Table 1. In this study, the data acquisition used NIRStar software (Version 15.2).

Table 1.
Channel-projection-to-cortex labels.
2.3. Data Analysis
2.3.1. Preprocessing
The fNIRS data were preprocessed using Matlab package Homer2. Subjects typically required several minutes to transition into a quiet state from the commencement of data acquisition. To ensure a sufficiently reliable signal for analysis, we excluded the initial few minutes of data from the fNIRS dataset and trimmed the final data length to 10 min. The raw light intensity signals were first converted to changes in optical density, after which the processed fNIRS time series were corrected for motion artifacts. Motion artifact correction was followed by filtering out instrumental noise and physiological noise, such as low-frequency drift, heartbeat, and respiration, using bandpass filters with cutoff frequencies of 0.01 Hz and 0.1 Hz, respectively. Subsequently, the preprocessed optical density data at both wavelengths were converted to relative changes in the concentration of oxyhemoglobin (HbO2) and deoxyhemoglobin (HbR) by a modified Beer–Lambert law.
2.3.2. Functional Connectivity
RSFC is used to study the functional associations between different regions of the brain when subjects are not performing a specific task and are at rest. Functional connectivity strength is a measure of the strength or interrelationships of connections between different regions in a brain network. For each selected channel, the correlation between its time series and those of all other brain regions was calculated to represent the strength of RSFC. In the present study, only HbO2 was used to calculate functional connectivity because a previous study has shown that HbO2 is more sensitive to blood flow and oxygen transport than HbR [38]. After calculating the Pearson correlation coefficients between channels, a correlation matrix was generated for each participant’s data.
2.3.3. Graph Theory Analysis
In brain networks, channels are usually defined as nodes and the correlation coefficients between any channel pairs are defined as edges. We used sparsity thresholding to binarize the correlation matrix and compute graph-theoretic metrics. The sparsity S can be expressed as the percentage of connections retained in the network [39]. If S is 0.2, the sorted connections with connection weights in the top 20% will be retained. By adjusting the sparsity, the density of the network can be controlled so that the connections retained in the network are more prominent or important [40,41]. When S is too large, it indicates that the network is relatively dense and retains more connections. This can lead to an overly complex network that contains noise or less important connections. An excessively small S may lead to a loss of information and make the network structure less complete. Using sparsity thresholding, we computed each graph theory parameter along a range of edge densities of 0.2 < S < 0.8 in 0.05 increments. Then, we used MATLAB software (Version R2018b) to compute global and local network properties in binarized networks. Global network properties include global efficiency (Eg), local efficiency (Eloc), clustering coefficient (Cp), shortest path length (Lp), and small-world properties (σ, γ, λ). Regional network properties include nodal betweenness centrality (BC) and degree centrality (DC). A sparsity threshold of around 0.35 preserves important topological information in the network while keeping the network less complex, so we mainly analyzed the network characteristics at a sparsity threshold of 0.35.
2.3.4. Statistical Analysis
Statistical analysis was performed using RStudio (v4.2.3). A simple t-test was used for body mass index (BMI) differences. Gender differences were statistically quantified using a chi-square test. Age, AHI, RSFC, and graph-based indexes between the two groups were assessed using the Mann–Whitney U test. Subsequently, we conducted multiple linear regression analyses to assess the potential association between the extracted features and the clinical evaluation AHI. The analysis involved calculating the regression between the z-value of the feature and the AHI, while considering age, BMI, and gender as covariates. Statistical significance for each independent variable was determined with a significance threshold set at p < 0.05 after applying the Benjamini–Hochberg (BH) correction.
3. Results
3.1. Demographic Characteristics
The demographic information of subjects is detailed in Table 2. There was no significant difference between patients with OSA and controls in terms of age (p = 0.784) and gender (p = 0.451). The BMI (p < 0.01) and AHI (p < 0.01) were significantly higher in patients with OSA than in the control group. Other indicators of anxiety, depression, and sleep quality were not significantly different between the two groups.

Table 2.
Demographic information and AHI of OSA patients and HCs.
3.2. Changes in HbO2 Concentration between Patients with OSA and HCs
In the present study, the mean (p = 0.023) and standard deviation (p = 0.020) of HbO2 concentration in Ch14 (the right superior temporal gyrus) were significantly higher in patients with OSA compared to HCs. The mean and standard deviation of hemoglobin concentration in Ch14 were extracted, as shown in Figure 2.
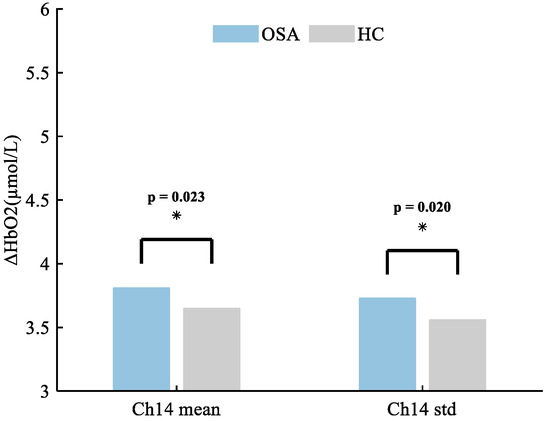
Figure 2.
The altered brain regions in patients with OSA exhibit distinctive characteristics in mean and variance of HbO2 concentration, with the most prominent changes observed in channel 14, corresponding to the right superior temporal gyrus. Specifically, the mean HbO2 concentration demonstrates a significant difference, with values of 3.81 ± 1.54 compared to 3.65 ± 1.51 in individuals without OSA. Additionally, there is a notable distinction in the standard deviation (std) of HbO2 concentration, showing values of 3.73 ± 1.03 for patients with OSA versus 3.56 ± 1.53 for HCs. * p < 0.05.
3.3. RSFC within the Temporoparietal Network between Patients with OSA and HCs
In both groups, all brain regions showed similar patterns of RSFC. The mean correlation RSFC matrix within the temporoparietal network is shown in Figure 3. The RSFC between Ch1 (the left superior temporal gyrus) and Ch10 (the right precentral gyrus) (p = 0.049), and between Ch1 and Ch13 (the right supramarginal gyrus) (p = 0.033) were significantly higher in patients with OSA compared to HCs. In addition to this, patients with OSA showed significantly increased RSFC between Ch11 (the right superior temporal gyrus) and Ch10 (p = 0.039), and between Ch11 and Ch8 (the left angular gyrus) (p = 0.021). Abnormal connections are shown in Figure 4.
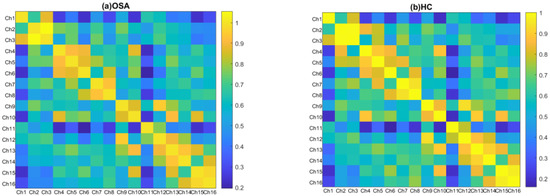
Figure 3.
Group-averaged correlation matrix plots of temporoparietal regions for individuals with OSA (a) and HCs (b). Each pixel in the correlation matrix plot represents the z-value of the Pearson correlation coefficient after Fisher z-transformation for the corresponding channel pair.
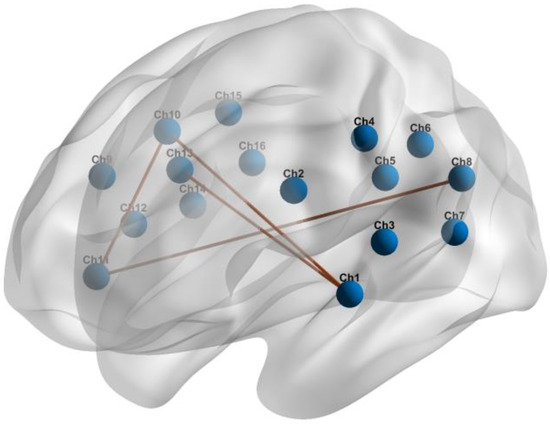
Figure 4.
Abnormal RSFC within the temporoparietal brain network in individuals with OSA compared to HCs.
3.4. Differences in Global Network Metrics of the Temporoparietal Network
Neither patients with OSA nor HCs exhibited an effective small-world topology (σ > 1, γ >> 1, λ ≈ 1) over a wide range of sparsity thresholds defined as 0.2–0.8 (see Figure 5), suggesting that the temporoparietal network does not have typical small-world properties. Cp was higher (p = 0.038) and Eloc was higher (p = 0.049) in patients with OSA compared to HCs. The differences in Lp (p = 0.997) and Eg (p = 0.972) were not statistically significant. The global network metrics are illustrated in Figure 6.
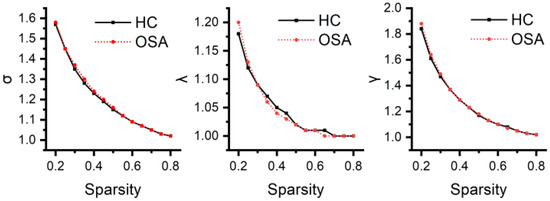
Figure 5.
The small-world characteristics of the temporoparietal network for both patients with OSA and HCs across the defined wide threshold range. In the figure, it is evident that the average shortest path length ratio (λ) is approximately equal to one, and the small-world index (σ) is greater than one. However, the clustering coefficient ratio (γ) is not significantly greater than one, indicating that neither patients with OSA nor HCs exhibit a typical small-world topology.
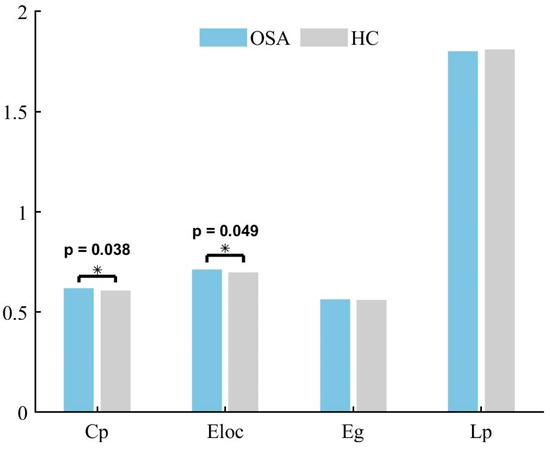
Figure 6.
The between-group differences in global network metrics for the temporoparietal network in individuals with OSA and HCs. There is a noticeable trend suggesting a distinction among patients with OSA, with an elevated Cp (p < 0.05) and an increased Eloc (p < 0.05). * p < 0.05.
3.5. Differences in Regional Network Metrics of the Temporoparietal Network
Patients with OSA showed abnormal BC and DC in certain regions. Compared to HCs, BC values were higher in Ch9 (the right precentral gyrus) and Ch13, with higher DC values in Ch13 (p < 0.05). Other regions did not show any node metrics that differed between groups. Regional network measures are shown in Table 3.

Table 3.
The between-group variations in BC and DC of the temporoparietal network among individuals with OSA and HCs.
3.6. Correlations between RSFC and AHI
To further investigate the relationship between the temporoparietal network and the diagnostic index of OSA, namely the AHI, linear regression analysis was conducted on selected brain network features and the AHI. Following BH correction, the analysis revealed a significant positive correlation between the AHI and RSFC from Ch10 to Ch11 (coefficient estimate β = 0.017, p = 0.040). The negative sign of the regression coefficient for the AHI indicates a direct negative relationship with RSFC between Ch10 and Ch15 (coefficient estimate β = −0.015, p = 0.037). This suggests that higher values of the AHI are associated with lower values of RSFC between Ch10 and Ch15.
4. Discussion
The aim of this study was to investigate differences in temporoparietal neural activity between patients with OSA and healthy controls. To this end, we compared RSFC as well as graph theory-based network metrics between the two groups. Several studies have shown that there are significant gender and age differences in some brain regions in patients with OSA [42,43,44]. To avoid the influence of these confounders, we selected patients with OSA and healthy individuals with similar age distributions and sex ratios in this study, which can help to confirm that our findings are mainly due to the influence of OSA rather than age or sex differences. To the best of our knowledge, our study is the first resting-state fNIRS study to explore intergroup differences in the temporoparietal lobe between patients with OSA and HCs.
We found that the mean and standard deviation of HbO2 concentration in the right superior temporal gyrus were higher in patients with OSA than in healthy individuals. In the resting state, both patients with OSA and healthy people showed regular patterns of changes in blood oxygen concentration, but the right superior temporal gyrus demonstrated an intergroup difference, which indicated that the right and left hemispheres of patients with OSA were differently differentiated. Jing Gao et al. found that the right brainstem, left dorsolateral superior frontal gyrus, and superior temporal gyrus of the group with OSA were atrophied compared to HCs, which suggested that OSA affects the structure of the brain, and the effects are laterally biased [45]. De-Chang Peng et al. found that regional homogeneity was significantly higher in the right posterior cerebellar lobe and right cingulate gyrus in patients with OSA [46]. In our study, the differences in HbO2 concentration occurred mainly in the right hemisphere, suggesting that having OSA may lead to significant structural and functional effects on one side of the brain.
The interesting finding in our study was that RSFC between the left superior temporal gyrus and the right precentral gyrus as well as the right supramarginal gyrus was higher in the group with OSA than in the healthy control group. In addition, RSFC between the right superior temporal gyrus and the right precentral gyrus as well as the left angular gyrus was also increased in patients with OSA. The results we found suggest that OSA has a specific effect on RSFC between the superior temporal gyrus and other regions. The superior temporal gyrus plays a variety of important roles in the human brain involving perception, memory, and a number of higher cognitive functions. The superior temporal gyrus is an important part of the default mode network, interconnecting with other core regions of the network. Several studies have shown that increased FC in OSA is commonly seen in the temporoparietal network [7,23,32,47]. The precentral gyrus and supramarginal gyrus are part of the sensorimotor network, which focuses on processing sensory information and motor control. Several studies have shown that OSA selectively impairs RSFC within DMN subregions and also specifically affects RSFC in cognitive and sensorimotor-related brain networks [32,48,49]. The increase in RSFC between the superior temporal gyrus and other regions can be interpreted as a hyper-reflexive or compensatory mechanism under specific conditions and may indicate an adaptive compensatory response of RSFC to cognitive impairment [8].
Several studies have shown that patients with OSA exhibit abnormal small-world organization in either functional or structural brain networks such as the DMN [50,51,52]. However, in the present study, neither patients with OSA nor HCs showed effective small-world organization properties. The small-world networks have a topology with high clustering coefficients and small shortest path lengths, which is not consistent with the results we obtained. The reason is that even though some regions of the temporal and parietal lobes in our study are involved in the DMN, the properties are not exactly equivalent to the DMN. Distinct brain networks may exhibit different properties at different scales. Cp and Eloc are measures of local connectivity and transmission efficiency of the network, respectively. Contrary to the findings of some studies, our results found increased Cp and Eloc in patients with OSA [50,51]. There are also findings in agreement with our results [52]; however, it is worth noting that we studied different regions of the brain network. OSA may lead to changes in brain structure, including changes in gray and white matter. These changes may affect the connectivity patterns of the brain networks and thus the clustering coefficients and local efficiencies. Two opposing patterns may be observed in neuroimaging studies of patients with OSA, one of which may indicate cellular damage while the other may reflect compensatory structural changes [10]. Therefore, the results may vary depending on factors such as the study region or the methodology used.
In the current study, the topological organization of the region between the two groups is compared using BC and DC. DC and BC are two network centrality metrics commonly used in social network analysis, which are used to measure the importance of nodes and their mediations, respectively. Specifically, we observed higher BC and DC in the right supramarginal gyrus and higher DC in the right precentral gyrus in the group with OSA compared with HCs. The consistent trend of increased regional findings may be related to the neuropathologic features of OSA. Our findings generally show more noticeable changes in the right hemisphere of patients with OSA, which is mutually supportive with the results of a previous fMRI study [53]. Similar to the high RSFC in some regions mentioned above, the high BC in the right supramarginal gyrus and the right precentral gyrus may reflect pathological changes or compensatory mechanisms in disease states. Furthermore, since these two regions are components of the default mode network (DMN), our findings support and confirm the perspective of DMN abnormalities in patients with OSA as proposed in previous studies [48,52,54].
The results of the multiple linear regression analysis highlight the impact of the AHI on RSFC between the right precentral gyrus and right superior temporal gyrus, as well as between the right precentral gyrus and right supramarginal gyrus. The AHI, an index used to assess sleep apnea and hypopnea, may be associated with abnormalities inducing physiological and metabolic changes within the brain, thereby affecting functional connectivity among different brain regions [3,22]. Respiratory control and neuromodulation may influence the connections between brain regions, and the onset of sleep disorders could potentially trigger adjustments in these connections. Patients with OSA often experience respiratory control dysfunction, which may be related to morphological differences in certain gray matter regions of the brain [46]. Linear regression results suggest that RSFC between the right precentral gyrus and other brain regions deserves particular attention. This reveals that the function of the right precentral gyrus may be influenced in patients with OSA. The influence received may manifest as changes related to motor control, respiratory regulation, or other neural functions, as the right precentral gyrus is a crucial component of the sensorimotor network [32]. These findings offer a new imaging perspective for a more in-depth understanding of brain functional connectivity disruptions associated with OSA. Significantly, the relative results observed on the right side of the brain underscore a lateralizing effect of OSA on cerebral function.
This study has several limitations. First, the proportion of patients with severe OSA in our study was relatively small, and more patients with severe OSA should be included in future studies. Second, the results of RSFC and network parameters were uncorrected. So, our results should be interpreted with caution and need to be validated by future studies. Compared with previous studies of patients with OSA, we expanded our sample size; however, studies with larger samples are still needed to confirm our results. The chosen focus area for the study is the temporal parietal region, and the research is deemed necessary and lacking in this specific domain, making it worthwhile to investigate OSA pathology in this region. Nevertheless, a comprehensive analysis covering the entire brain may have the potential to reveal deeper insights into the pathology of OSA, encouraging a more thorough exploration of this sleep disorder. Subsequent studies will consider the entire cerebral cortex. In this study, we did not analyze the severity of the cognitive consequences of OSA on individuals and the extent to which OSA affects cognitive function in relation to brain activity patterns, but we plan to conduct such an analysis in the future. The last limitation of this study is the lack of follow-up data. A longitudinal study including pre-treatment data and post-treatment data at different periods is necessary for patients with OSA.
5. Conclusions
In summary, the current study examined the resting-state brain function network in patients with OSA. We found that patients with OSA had higher HbO2 concentrations in the superior temporal gyrus compared to healthy controls. We also found slightly higher RSFC between the superior temporal gyrus and some other regions, such as the right premolar gyrus, compared to healthy controls. These findings suggest that OSA may lead to abnormal resting-state function in some regions of the brain. Clustering coefficients and local efficiencies of the temporoparietal network in patients with OSA showed an increasing trend. In addition, the betweenness centrality and degree centrality of the right supramarginal gyrus and the meso-centrality of the right precentral gyrus similarly showed an increasing trend. The results of multiple linear regressions demonstrated that the influence of the AHI on RSFC among specific regions in the right temporal and parietal lobes was notably significant. The present study provides some insights into the potential neuropathological mechanisms underlying abnormal brain function in patients with OSA.
Author Contributions
Writing—original draft preparation, F.X.; writing—review and editing, M.L., Y.W., L.Z., J.L., C.C. and W.C.; supervision, Y.W., C.C. and W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Shanghai Municipal Science and Technology International R&D Collaboration Project (Grant No. 20510710500).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the School of Life Sciences of Fudan University (FE231841, 4 July 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets presented in this article are not readily available because the data are part of an ongoing study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Young, T. Risk Factors for Obstructive Sleep Apnea in Adults. JAMA 2004, 291, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, S.; Bardwell, W.A.; Guglielmi, O.; Chiorri, C.; Bonanni, E.; Magnavita, N. Association of Anxiety and Depression in Obstructive Sleep Apnea Patients: A Systematic Review and Meta-Analysis. Behav. Sleep Med. 2020, 18, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A.; Lindberg, E. Obstructive Sleep Apnea Is a Common Disorder in the Population—A Review on the Epidemiology of Sleep Apnea. J. Thorac. Dis. 2015, 7, 1311–1322. [Google Scholar] [PubMed]
- Kario, K. Obstructive Sleep Apnea Syndrome and Hypertension: Ambulatory Blood Pressure. Hypertens. Res. 2009, 32, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Somers, V.K.; White, D.P.; Amin, R.; Abraham, W.T.; Costa, F.; Culebras, A.; Daniels, S.; Floras, J.S.; Hunt, C.E.; Olson, L.J.; et al. Sleep Apnea and Cardiovascular Disease: An American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing in Collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008, 118, 1080–1111. [Google Scholar] [CrossRef] [PubMed]
- Bubu, O.M.; Andrade, A.G.; Umasabor-Bubu, O.Q.; Hogan, M.M.; Turner, A.D.; de Leon, M.J.; Ogedegbe, G.; Ayappa, I.; Jean-Louis G, G.; Jackson, M.L.; et al. Obstructive Sleep Apnea, Cognition and Alzheimer’s Disease: A Systematic Review Integrating Three Decades of Multidisciplinary Research. Sleep Med. Rev. 2020, 50, 101250. [Google Scholar] [CrossRef] [PubMed]
- Caporale, M.; Palmeri, R.; Corallo, F.; Muscarà, N.; Romeo, L.; Bramanti, A.; Marino, S.; Lo Buono, V. Cognitive Impairment in Obstructive Sleep Apnea Syndrome: A Descriptive Review. Sleep Breath. 2021, 25, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tang, S.; Lyu, X.; Yang, C.; Chen, X. Structural and Functional Brain Alterations in Obstructive Sleep Apnea: A Multimodal Meta-Analysis. Sleep Med. 2019, 54, 195–204. [Google Scholar] [CrossRef]
- Tahmasian, M.; Rosenzweig, I.; Eickhoff, S.B.; Sepehry, A.A.; Laird, A.R.; Fox, P.T.; Morrell, M.J.; Khazaie, H.; Eickhoff, C.R. Structural and Functional Neural Adaptations in Obstructive Sleep Apnea: An Activation Likelihood Estimation Meta-Analysis. Neurosci. Biobehav. Rev. 2016, 65, 142–156. [Google Scholar] [CrossRef]
- Baril, A.-A.; Martineau-Dussault, M.-È.; Sanchez, E.; André, C.; Thompson, C.; Legault, J.; Gosselin, N. Obstructive Sleep Apnea and the Brain: A Focus on Gray and White Matter Structure. Curr. Neurol. Neurosci. Rep. 2021, 21, 11. [Google Scholar] [CrossRef]
- Taylor, K.S.; Millar, P.J.; Murai, H.; Haruki, N.; Kimmerly, D.S.; Bradley, T.D.; Floras, J.S. Cortical Autonomic Network Gray Matter and Sympathetic Nerve Activity in Obstructive Sleep Apnea. Sleep 2018, 41, zsx208. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-L.; Lu, C.-H.; Lin, H.-C.; Chen, P.-C.; Chou, K.-H.; Lin, W.-M.; Tsai, N.-W.; Su, Y.-J.; Friedman, M.; Lin, C.-P.; et al. White Matter Damage and Systemic Inflammation in Obstructive Sleep Apnea. Sleep 2015, 38, 361–370. [Google Scholar] [CrossRef]
- Brain Structural Changes in Obstructive Sleep Apnea. Sleep 2008, 31, 967–977. [CrossRef]
- Zacharias, H.U.; Weihs, A.; Habes, M.; Wittfeld, K.; Frenzel, S.; Rashid, T.; Stubbe, B.; Obst, A.; Szentkirályi, A.; Bülow, R.; et al. Association between Obstructive Sleep Apnea and Brain White Matter Hyperintensities in a Population-Based Cohort in Germany. JAMA Netw. Open 2021, 4, e2128225. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yun, C.-H.; Thomas, R.J.; Lee, S.H.; Seo, H.S.; Cho, E.R.; Lee, S.K.; Yoon, D.W.; Suh, S.; Shin, C. Obstructive Sleep Apnea as a Risk Factor for Cerebral White Matter Change in a Middle-Aged and Older General Population. Sleep 2013, 36, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Joo, E.Y.; Tae, W.S.; Lee, M.J.; Kang, J.W.; Park, H.S.; Lee, J.Y.; Suh, M.; Hong, S.B. Reduced Brain Gray Matter Concentration in Patients with Obstructive Sleep Apnea Syndrome. Sleep 2010, 33, 235–241. [Google Scholar] [CrossRef]
- Rosenzweig, I.; Glasser, M.; Crum, W.R.; Kempton, M.J.; Milosevic, M.; McMillan, A.; Leschziner, G.D.; Kumari, V.; Goadsby, P.; Simonds, A.K.; et al. Changes in Neurocognitive Architecture in Patients with Obstructive Sleep Apnea Treated with Continuous Positive Airway Pressure. EBioMedicine 2016, 7, 221–229. [Google Scholar] [CrossRef]
- Canessa, N.; Castronovo, V.; Cappa, S.F.; Aloia, M.S.; Marelli, S.; Falini, A.; Alemanno, F.; Ferini-Strambi, L. Obstructive Sleep Apnea: Brain Structural Changes and Neurocognitive Function before and after Treatment. Am. J. Respir. Crit. Care Med. 2011, 183, 1419–1426. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, G.; Dong, Q.; Niu, H. Applications of Resting-State fNIRS in the Developing Brain: A Review from the Connectome Perspective. Front. Neurosci. 2020, 14, 476. [Google Scholar] [CrossRef]
- Li, S.; Hu, N.; Zhang, W.; Tao, B.; Dai, J.; Gong, Y.; Tan, Y.; Cai, D.; Lui, S. Dysconnectivity of Multiple Brain Networks in Schizophrenia: A Meta-Analysis of Resting-State Functional Connectivity. Front. Psychiatry 2019, 10, 482. [Google Scholar] [CrossRef]
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P.W. The Present and Future Use of Functional Near-infrared Spectroscopy (fNIRS) for Cognitive Neuroscience. Ann. N. Y. Acad. Sci. 2020, 1464, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Li, H.; Shu, Y.; Liu, X.; Li, P.; Li, K.; Xie, W.; Zeng, Y.; Peng, D. Aberrant Resting-State Functional Brain Connectivity of Insular Subregions in Obstructive Sleep Apnea. Front. Neurosci. 2022, 15, 765775. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, L.; Duan, W.; Li, H.; Kong, L.; Shu, Y.; Li, P.; Li, K.; Xie, W.; Zeng, Y.; et al. Abnormal Functional Connectivity of Hippocampal Subdivisions in Obstructive Sleep Apnea: A Resting-State Functional Magnetic Resonance Imaging Study. Front. Neurosci. 2022, 16, 850940. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, L.; Li, H.; Xin, H.; Zhang, J.; Wei, Z.; Peng, D. Abnormal Resting-State Functional Connectivity of Amygdala Subregions in Patients with Obstructive Sleep Apnea. Neuropsychiatr. Dis. Treat. 2019, 15, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, G.; Luo, H.; Li, H.; Peng, Y.; Zong, D.; Ouyang, R. Aberrant Hippocampal Network Connectivity Is Associated with Neurocognitive Dysfunction in Patients with Moderate and Severe Obstructive Sleep Apnea. Front. Neurol. 2020, 11, 580408. [Google Scholar] [CrossRef] [PubMed]
- Archbold, K.H.; Borghesani, P.R.; Mahurin, R.K.; Kapur, V.K.; Landis, C.A. Neural Activation Patterns during Working Memory Tasks and OSA Disease Severity: Preliminary Findings. J. Clin. Sleep Med. 2009, 5, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kheirandish-Gozal, L.; Yoder, K.; Kulkarni, R.; Gozal, D.; Decety, J. Preliminary Functional MRI Neural Correlates of Executive Functioning and Empathy in Children with Obstructive Sleep Apnea. Sleep 2014, 37, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Prilipko, O.; Huynh, N.; Schwartz, S.; Tantrakul, V.; Kim, J.H.; Peralta, A.R.; Kushida, C.; Paiva, T.; Guilleminault, C. Task Positive and Default Mode Networks during a Parametric Working Memory Task in Obstructive Sleep Apnea Patients and Healthy Controls. Sleep 2011, 34, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, W.; Mo, X.; Yang, J.; Liu, H.; Shi, L.; Ma, H.; Jiang, Z. Abnormal Prefrontal Functional Network in Adult Obstructive Sleep Apnea: A Resting-state fNIRS Study. J. Sleep Res. 2023, 33, e14033. [Google Scholar] [CrossRef]
- Zhang, Z.; Schneider, M.; Fritschi, U.; Lehner, I.; Khatami, R. Near-Infrared Spectroscopy (NIRS) as a Useful Tool to Evaluate the Treatment Efficacy of Positive Airways Pressure Therapy in Patients with Obstructive Sleep Apnea Syndrome (OSAS): A Pilot Study. J. Innov. Opt. Health Sci. 2014, 7, 1450014. [Google Scholar] [CrossRef]
- Baillieul, S.; Wuyam, B.; Pérennou, D.; Tamisier, R.; Bailly, S.; Benmerad, M.; Piscicelli, C.; Le Roux-Mallouf, T.; Vergès, S.; Pépin, J.-L. A Randomized Sham-Controlled Trial on the Effect of Continuous Positive Airway Pressure Treatment on Gait Control in Severe Obstructive Sleep Apnea Patients. Sci. Rep. 2021, 11, 9329. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, D.; Qin, W.; Li, Q.; Chen, B.; Zhang, Y.; Yu, C. Altered Resting-State Brain Activity in Obstructive Sleep Apnea. Sleep 2013, 36, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, S.B.; Yücel, M.A.; Akın, A. Analysis of Task-Evoked Systemic Interference in fNIRS Measurements: Insights from fMRI. NeuroImage 2014, 87, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Hocke, L.; Oni, I.; Duszynski, C.; Corrigan, A.; Frederick, B.; Dunn, J. Automated Processing of fNIRS Data—A Visual Guide to the Pitfalls and Consequences. Algorithms 2018, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Seidel, O.; Carius, D.; Roediger, J.; Rumpf, S.; Ragert, P. Changes in Neurovascular Coupling during Cycling Exercise Measured by Multi-Distance fNIRS: A Comparison between Endurance Athletes and Physically Active Controls. Exp. Brain Res. 2019, 237, 2957–2972. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xiao, F.; Shen, N.; Wang, Y.; Tang, J.; Luo, J.; Chen, W.; Chen, C. Protocol for Simultaneous EEG and fNIRS Measurement in Characterizing Brain State. Phenomics 2024. [Google Scholar] [CrossRef]
- Aasted, C.M.; Yücel, M.A.; Cooper, R.J.; Dubb, J.; Tsuzuki, D.; Becerra, L.; Petkov, M.P.; Borsook, D.; Dan, I.; Boas, D.A. Anatomical Guidance for Functional Near-Infrared Spectroscopy: AtlasViewer Tutorial. Neurophoton 2015, 2, 020801. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Mottola, L.; Quaresima, V. Principles, Techniques, and Limitations of Near Infrared Spectroscopy. Can. J. Appl. Physiol. 2004, 29, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, Z.; Zhao, C.; Duan, L.; Gong, Y.; Li, Z.; Zhu, C. NIRS-KIT: A MATLAB Toolbox for Both Resting-State and Task fNIRS Data Analysis. Neurophoton. 2021, 8, 010802. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Liu, X.; Biswal, B.B.; Niu, H. Effect of Resting-State fNIRS Scanning Duration on Functional Brain Connectivity and Graph Theory Metrics of Brain Network. Front. Neurosci. 2017, 11, 392. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, Z.; Niu, H. Reliability Evaluation on Weighted Graph Metrics of fNIRS Brain Networks. Quant. Imaging Med. Surg. 2019, 9, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Li, X.; Chen, J.; Ren, X.; Mei, L.; Qiu, Y.; Zhang, J.; Wang, S.; Xu, Z.; Li, H.; et al. Brain Function in Children with Obstructive Sleep Apnea: A Resting-State fMRI Study. Sleep 2021, 44, zsab047. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Mao, M.; Hu, Y.; Yi, X.; Zheng, Y.; Ying, Z.; Cheng, D.; Rao, Y.; Zhang, J.; Mu, X.; et al. Gender-Specific Association between Obstructive Sleep Apnea and Cognitive Impairment among Adults. Sleep Med. 2022, 98, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Torres, Z.; Jiménez-Correa, U.; Montes-Rodríguez, C.J. Sex Differences in Brain Oscillatory Activity during Sleep and Wakefulness in Obstructive Sleep Apnea. J. Sleep Res. 2020, 29, e12977. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Cao, J.; Chen, J.; Wu, D.; Luo, K.; Shen, G.; Fang, Y.; Zhang, W.; Huang, G.; Su, X.; et al. Brain Morphology and Functional Connectivity Alterations in Patients with Severe Obstructive Sleep Apnea. Sleep Med. 2023, 111, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Peng, D.-C.; Gong, H.-H.; Li, H.-J.; Nie, X.; Zhang, W. Altered Intrinsic Regional Brain Activity in Male Patients with Severe Obstructive Sleep Apnea: A Resting-State Functional Magnetic Resonance Imaging Study. Neuropsychiatr. Dis. Treat. 2014, 10, 1819. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, Z.; He, Y.; Wu, Q. Relationship between Blood Amyloid A and Resting Magnetic Resonance Functional Brain Connections in Patients with Obstructive Sleep Apnea–Hypopnea Syndrome. Sleep Breath. 2023, 27, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-J.; Nie, X.; Gong, H.; Zhang, W.; Nie, S.; Peng, D.-C. Abnormal Resting-State Functional Connectivity within the Default Mode Network Subregions in Male Patients with Obstructive Sleep Apnea. Neuropsychiatr. Dis. Treat. 2016, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fan, X.; Li, H.; Ye, C.; Yu, H.; Gong, H.; Zeng, X.; Peng, D.; Yan, L. Topological Reorganization of the Default Mode Network in Severe Male Obstructive Sleep Apnea. Front. Neurol. 2018, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Y.; Zhao, D.; Liu, B.; Zhang, H.; Huang, Z.; Babourina-Brooks, B.; Peet, A.C.; Zhang, L.; Feng, Y.; et al. Small-World Properties of the Whole-Brain Functional Networks in Patients with Obstructive Sleep Apnea-hypopnea Syndrome. Sleep Med. 2019, 62, 53–58. [Google Scholar] [CrossRef]
- Chen, L.-T.; Fan, X.-L.; Li, H.-J.; Nie, S.; Gong, H.-H.; Zhang, W.; Zeng, X.-J.; Long, P.; Peng, D.-C. Disrupted Small-World Brain Functional Network Topology in Male Patients with Severe Obstructive Sleep Apnea Revealed by Resting-State fMRI. Neuropsychiatr. Dis. Treat. 2017, 13, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cao, C.; Hu, B.; Li, D.; Sun, Y.; Wu, J.; Zhang, Q. Topological Regularization of Networks in Adult Patients with Moderate-to-Severe Obstructive Sleep Apnea-Hypopnea Syndrome: A Structural MRI Study. Nat. Sci. Sleep 2020, 12, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, D.; Liu, K.; Weng, J.; Guan, Y.; Chan, K.C.C.; Chu, W.C.W.; Shi, L. Brain Structure Network Analysis in Patients with Obstructive Sleep Apnea. PLoS ONE 2015, 10, e0139055. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-T.; Fan, X.; Li, H.-J.; Ye, C.-L.; Yu, H.-H.; Xin, H.; Gong, H.; Peng, D.-C.; Yan, L. Aberrant Brain Functional Connectome in Patients with Obstructive Sleep Apnea. Neuropsychiatr. Dis. Treat. 2018, 14, 1059–1070. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).