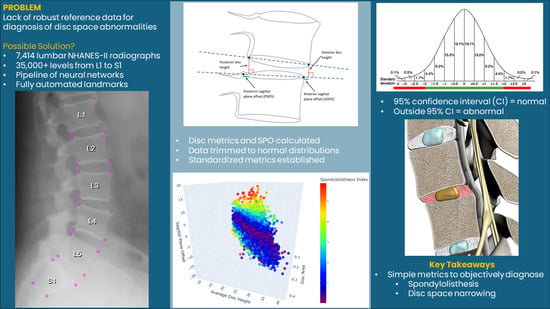
Reference Data for Diagnosis of Spondylolisthesis and Disc Space Narrowing Based on NHANES-II X-rays
Abstract
1. Introduction
- Determine if objective disc metrics can be developed that account for (1) the variability due to patient positioning, (2) the normal variability between patients, and (3) the variability between levels, using the database of over 7000 lumbar spine radiographs from the NHANES-II study, supplemented with a collection of flexion-extension radiographs to better understand the importance of disc angle and load bearing.
- Develop reference data for diagnosis of abnormal disc metrics based on the NHANES-II radiographs by excluding degenerated discs and outliers in the data distributions.
- Document the errors that can occur in disc metrics due to variability in radiographic projection using precisely calculated landmarks obtained from variable, digitally reconstructed radiographic projections.
2. Materials and Methods
- Method 1
- ○
- Anterior disc height (ADH);
- ○
- Posterior disc height (PDH);
- ○
- Disc angle (DA);
- ○
- Anterior SPO (ASPO);
- ○
- Posterior SPO (PSPO).
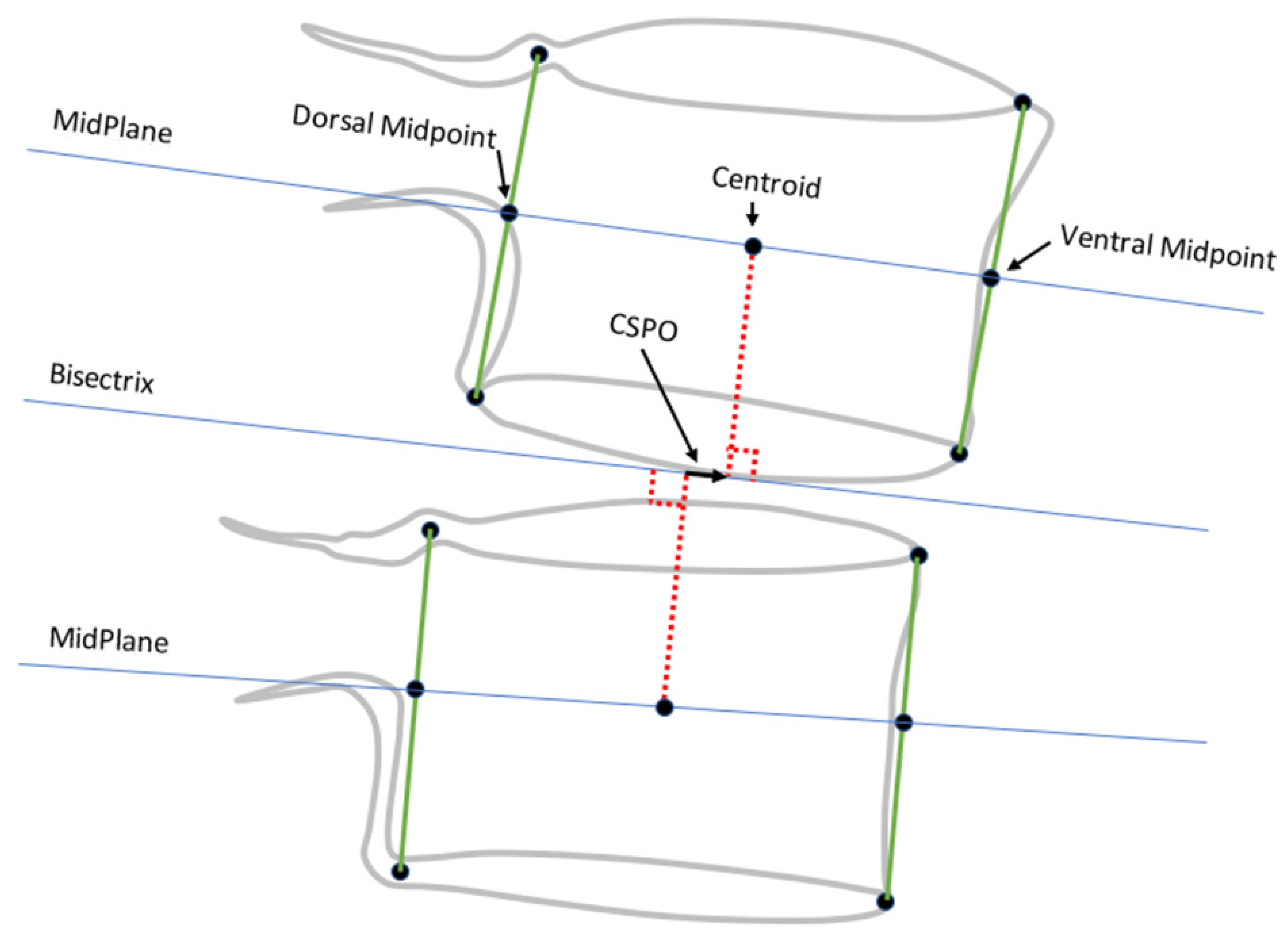
- Method 2
- ○
- Ventral disc height (VDH);
- ○
- Dorsal disc height (DDH);
- ○
- Mid-plane angle (MPA);
- ○
- Centroid SPO (CSPO).
3. Results
3.1. Summary of Data Analyzed
3.2. Establishing Normal Disc Metrics
3.3. Correlations between Disc and SPO Metrics
3.4. Analysis of Sources of Variance in SPO Metrics
3.5. Applicability of NHANES-II Reference Data to Flexion-Extension X-rays
3.6. Applicability of a SpondyIndex Based on Flexion-Extension X-rays to NHANES-II X-rays
3.7. Prevalence of Abnormalities in the NHANES-II Lumbar Spine Radiographs
3.8. Associations with Back Pain
3.9. Descriptive Statistics Disc Metrics Measured from Standing Neutral-Lateral X-rays
4. Discussion
- (1)
- Poor representation of some races, nations of origin, and sex in some age groups. In particular, females are under-represented. By design of the NHANES-II study, lumbar X-rays were not intended to be obtained for pregnant women or women under 50 [79]. An additional limitation relates to the data on race and nation of origin recorded in the NHANES-II data. Of the NHANES-II participants, 86.9% were “white”, 11.2% “black”, and the rest “other”. A more uniform representation of races would likely be needed to fully understand the importance of race. The same is true for the “nation of origin” data in NHANES-II. The ages of the participants were also biased toward older ages.
- (2)
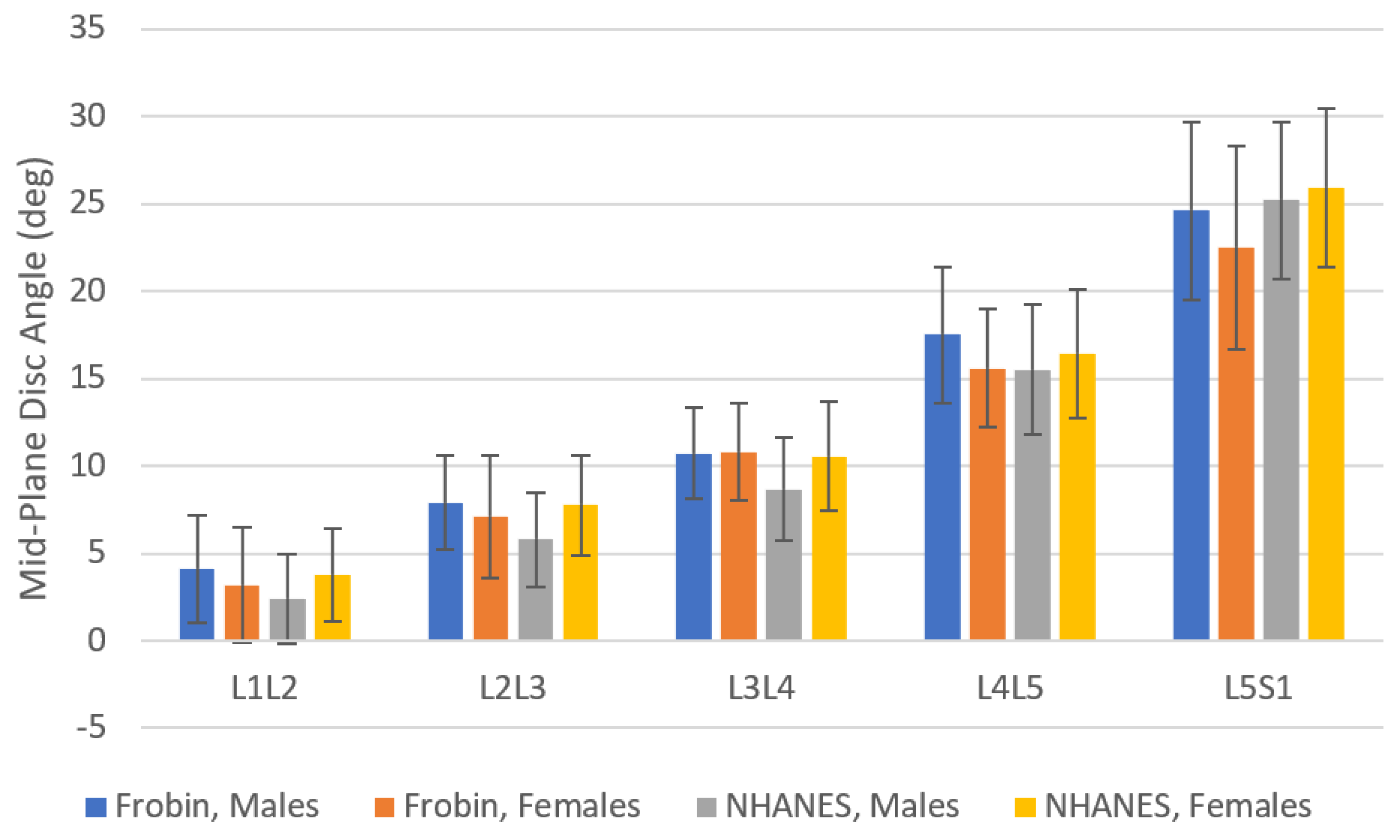
- NHANES-II lumbar spine X-rays were obtained with participants lying on their sides. Disc metrics in Supplementary File S5 may not be directly and precisely applicable to X-rays taken in other positions. Using all the NHANES data, the L1-S1 angle was 51.3 (SD 12.8). Table 3 provides data from three prior studies reporting L1-S1 angles measured from X-rays obtained with participants standing [80,81,82]. The lordosis data from the NHANES-II study was very similar to lordosis measured from standing X-rays in three other studies. Figure 5 shows mid-plane angles from the NHANES-II study, excluding levels where any disc or SPO metric was abnormal, compared to mid-plane angles reported by Frobin et al. from X-rays obtained with participants standing [13]. There are some differences, but also some similarities. These comparative data can help in deciding whether to use NHANES-II reference data with standing or other X-rays. With respect to external validity of the disc height measurements, comparable reference data were hard to find. There are multiple publications reporting lumbar intervertebral disc height reference data in units of millimeters, frequently from MRI or CT exams [60,63,83,84]. Since a scaling device was not included in the NHANES-II X-rays, these prior publications cannot be used as comparative data. Data for a large collection of radiographs obtained in other positions and analyzed using the same methods would be required to understand the applicability of the NHANES-II data to other protocols for obtaining lateral lumbar spine X-rays. It was assumed that the average disc height data would be the most universally applicable of the NHANES-II disc height reference data, since average disc height should minimize differences due to disc angle changes from variability in patient positioning.
- (3)
- The accuracy study using simulated X-rays (Supplementary File S4) revealed potentially large errors in disc and SPO metrics when the radiographic projection was very poor. It would be valuable to establish a neural network or other method to either make a correction in the metrics when large out-of-plane imaging is encountered, or to abstain from reporting data when vertebrae are poorly imaged. Nevertheless, a certain level of error must be expected in SPO and other metrics. This may be a particularly significant issue with large amounts of frontal plane spinal curvature. This study does not provide clear guidance on this issue and additional data are needed. Caution should be used when interpreting disc metrics and SPO from lateral X-rays where the vertebrae are poorly imaged.
- (4)
- Trimming the data such that only truly normal discs were used to define “normal” disc and SPO metrics was a challenge. Abnormal disc heights, angles, and SPO can be expected in the NHANES-II study (since there was no attempt to exclude spine abnormalities). It was assumed that normal disc heights, disc angles, and SPO would have a Gaussian distribution. The NHANES data were also analyzed with what are referred to as “robust” statistics [27,28]. Standardized scores were generated from the robust statistics, but these were nearly the same as the standard Z scores generated using means and standard deviations. Since the standard Z scores are easier to explain in diagnostic tests, robust statistics were not used. In addition, in a normal disc, the resting position of the vertebrae, when the radiograph was obtained, would be expected to be in the neutral zone. Within the neutral zone, little force is required to produce sagittal plane movements [85,86]. It can be hypothesized that the amount of SPO may change slightly, but remain within the neutral zone, every time a person assumes a “neutral” position. That would be expected to contribute to a normal distribution of SPO within a large population. There is currently no gold standard method that is validated for classifying discs as normal versus abnormal. MRI exams with appropriate imaging sequences would have provided a more robust assessment of disc health, but they were not available. In the current study, an attempt was made to trim data so as to achieve a Gaussian distribution. This approach has been used by other authors [87,88,89]. A better strategy may be possible for assuring that only truly normal discs are used to define normative reference data. For example, a formal optimization scheme might be used to obtain the best possible Gaussian distribution, though justification for such an optimization is not well developed.
- (5)
- Although Supplementary File S5 provides data to calculate disc and SPO metrics as number of SD from average normal, a clinically meaningful threshold must be validated for use with these reference data that can classify a metric as normal vs abnormal. Even though a metric that is two SD from average would be technically outside of the 95% confidence interval used to define “normal”, that may not be clinically significant. Until well-designed clinical trials are completed, the threshold level for the standardized score that is clinically efficacious will not be known.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Timon, S.J.; Gardner, M.J.; Wanich, T.; Poynton, A.; Pigeon, R.; Widmann, R.F.; Rawlins, B.A.; Burke, S.W. Not all spondylolisthesis grading instruments are reliable. Clin. Orthop. Relat. Res. 2005, 434, 157–162. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.E.; Mehlman, C.T.; Tamai, J.; Do, T.T.; Crawford, A.H.; Klein, G. Spondylolisthesis: Intraobserver and interobserver reliability with regard to the measurement of slip percentage. J. Pediatr. Orthop. 2009, 29, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Wall, M.S.; Oppenheim, W.L. Measurement error of spondylolisthesis as a function of radiographic beam angle. J. Pediatr. Orthop. 1995, 15, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Niggemann, P.; Kuchta, J.; Grosskurth, D.; Beyer, H.; Hoeffer, J.; Delank, K. Spondylolysis and isthmic spondylolisthesis: Impact of vertebral hypoplasia on the use of the Meyerding classification. Br. J. Radiol. 2012, 85, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Galbusera, F.; Casaroli, G.; Bassani, T. Artificial intelligence and machine learning in spine research. Jor Spine 2019, 2, e1044. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Canseco, J.A.; Nicholson, K.J.; Patel, N.; Vaccaro, A.R. The role of machine learning in spine surgery: The future is now. Front. Surg. 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Yu, K.; Xie, Z.; Wang, P.; Zhang, W.; Huang, Y.; Wang, Y.; Wu, X. Current Applications of Machine Learning in Spine: From Clinical View. Glob. Spine J. 2022, 12, 1827–1840. [Google Scholar] [CrossRef] [PubMed]
- Alini, M.; Diwan, A.D.; Erwin, W.M.; Little, C.B.; Melrose, J. An update on animal models of intervertebral disc degeneration and low back pain: Exploring the potential of artificial intelligence to improve research analysis and development of prospective therapeutics. JOR Spine 2023, 6, e1230. [Google Scholar] [CrossRef] [PubMed]
- McDowell, A. Plan and Operation of the Second National Health and Nutrition Examination Survey, 1976–1980. Available online: https://stacks.cdc.gov/view/cdc/13005 (accessed on 26 March 2024).
- Hipp, J.A.; Grieco, T.F.; Newman, P.; Reitman, C.A. Definition of Normal Vertebral Morphometry Using NHANES-II Radiographs. JBMR Plus 2022, 6, e10677. [Google Scholar] [CrossRef]
- Hurxthal, L.M. Measurement of anterior vertebral compressions and biconcave vertebrae. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1968, 103, 635–644. [Google Scholar] [CrossRef]
- Diacinti, D.; Guglielmi, G. Vertebral morphometry. Radiol. Clin. North. Am. 2010, 48, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Frobin, W.; Brinckmann, P.; Biggemann, M.; Tillotson, M.; Burton, K. Precision measurement of disc height, vertebral height and sagittal plane displacement from lateral radiographic views of the lumbar spine. Clin. Biomech. 1997, 12 (Suppl. 1), S1–S63. [Google Scholar] [CrossRef] [PubMed]
- Keynan, O.; Fisher, C.G.; Vaccaro, A.; Fehlings, M.G.; Oner, F.C.; Dietz, J.; Kwon, B.; Rampersaud, R.; Bono, C.; France, J.; et al. Radiographic measurement parameters in thoracolumbar fractures: A systematic review and consensus statement of the spine trauma study group. Spine 2006, 31, E156–E165. [Google Scholar] [CrossRef] [PubMed]
- Quint, D.J.; Tuite, G.F.; Stern, J.D.; Doran, S.E.; Papadopoulos, S.M.; McGillicuddy, J.E.; Lundquist, C.A. Computer-assisted measurement of lumbar spine radiographs. Acad. Radiol. 1997, 4, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Y.; Cheung, J.P.Y.; Dokos, S.; Wong, K.-Y.K. Learning-based coronal spine alignment prediction using smartphone-acquired scoliosis radiograph images. IEEE Access 2021, 9, 38287–38295. [Google Scholar] [CrossRef]
- Galbusera, F.; Bassani, T.; Costa, F.; Brayda-Bruno, M.; Zerbi, A.; Wilke, H.-J. Artificial neural networks for the recognition of vertebral landmarks in the lumbar spine. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2016, 6, 447–452. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Weng, C.-H.; Huang, Y.-J.; Fu, C.-J.; Tsai, T.-T.; Yeh, C.-Y. Deep learning approach for automatic landmark detection and alignment analysis in whole-spine lateral radiographs. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yao, H.; Ma, C.; Chen, X.; Wang, W.; Ji, H.; He, L.; Luo, M.; Guo, Y. Artificial intelligence X-ray measurement technology of anatomical parameters related to lumbosacral stability. Eur. J. Radiol. 2021, 110071. [Google Scholar] [CrossRef] [PubMed]
- Cina, A.; Bassani, T.; Panico, M.; Luca, A.; Masharawi, Y.; Brayda-Bruno, M.; Galbusera, F. 2-step deep learning model for landmarks localization in spine radiographs. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Al Arif, S.M.R.; Gundry, M.; Knapp, K.; Slabaugh, G. Improving an active shape model with random classification forest for segmentation of cervical vertebrae. In Proceedings of the International Workshop on Computational Methods and Clinical Applications for Spine Imaging, Athens, Greece, 17 October 2016; pp. 3–15. [Google Scholar]
- Frobin, W.; Brinckmann, P.; Leivseth, G.; Biggemann, M.; Reikeras, O. Precision measurement of segmental motion from flexion-extension radiographs of the lumbar spine. Clin.Biomech. 1996, 11, 457–465. [Google Scholar] [CrossRef]
- Ravi, B.; Rampersaud, R. Clinical magnification error in lateral spinal digital radiographs. Spine 2008, 33, E311–E316. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, H.; Koizumi, M.; Yoneda, M.; Iida, J.; Oshima, T.; Tanaka, Y. Magnification error in digital radiographs of the cervical spine against magnetic resonance imaging measurements. Asian Spine J. 2013, 7, 267–272. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lunt, M.; Gowin, W.; Johnell; Armbrecht, G.; Felsenberg, D.; EPOS Study Group. A statistical method to minimize magnification errors in serial vertebral radiographs. Osteoporos. Int. 2001, 12, 909–913. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Hodges, J.L., Jr.; Lehmann, E.L. Estimates of location based on rank tests. In Selected Works of EL Lehmann; Springer: Berlin/Heidelberg, Germany, 2011; pp. 287–300. [Google Scholar]
- Rousseeuw, P.J.; Croux, C. Alternatives to the median absolute deviation. J. Am. Stat. Assoc. 1993, 88, 1273–1283. [Google Scholar] [CrossRef]
- Staub, B.N.; Holman, P.J.; Reitman, C.A.; Hipp, J. Sagittal plane lumbar intervertebral motion during seated flexion-extension radiographs of 658 asymptomatic nondegenerated levels. J. Neurosurg. Spine 2015, 23, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, P.; Sansone, M.; Cesarelli, M.; Allen, R.; Bracale, M. Estimation of out-of-plane vertebra rotations on radiographic projections using CT data: A simulation study. Med. Eng. Phys. 2002, 24, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Andersson, G.; Schultz, A.; Nathan, A.; Irstam, L. Roentgenographic measurement of lumbar intervertebral disc height. Spine 1981, 6, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Danielson, B.; Frennered, K.; Irstam, L. Roentgenologic assessment of spondylolisthesis: I. A study of measurement variations. Acta Radiol. 1988, 29, 345–351. [Google Scholar] [CrossRef]
- Pearson, A.M.; Spratt, K.F.; Genuario, J.; McGough, W.; Kosman, K.; Lurie, J.; Sengupta, D.K. Precision of lumbar intervertebral measurements: Does a computer-assisted technique improve reliability? Spine 2011, 36, 572–580. [Google Scholar] [CrossRef]
- Watters, W.C., 3rd; Bono, C.M.; Gilbert, T.J.; Kreiner, D.S.; Mazanec, D.J.; Shaffer, W.O.; Baisden, J.; Easa, J.E.; Fernand, R.; Ghiselli, G.; et al. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. Spine J. 2009, 9, 609–614. [Google Scholar] [CrossRef]
- Matz, P.G.; Meagher, R.; Lamer, T.; Tontz, W.L., Jr.; Annaswamy, T.M.; Cassidy, R.C.; Cho, C.H.; Dougherty, P.; Easa, J.E.; Enix, D.E. Guideline summary review: An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. Spine J. 2016, 16, 439–448. [Google Scholar] [CrossRef]
- Mummaneni, P.V.; Bisson, E.F.; Kerezoudis, P.; Glassman, S.; Foley, K.; Slotkin, J.R.; Potts, E.; Shaffrey, M.; Shaffrey, C.I.; Coric, D. Minimally invasive versus open fusion for Grade I degenerative lumbar spondylolisthesis: Analysis of the Quality Outcomes Database. Neurosurg. Focus 2017, 43, E11. [Google Scholar] [CrossRef]
- Câmara, J.R.; Keen, J.R.; Asgarzadie, F. Functional radiography in examination of spondylolisthesis. Am. J. Roentgenol. 2015, 204, W461–W469. [Google Scholar] [CrossRef]
- Bourassa-Moreau, É.; Mac-Thiong, J.-M.; Labelle, H. Redefining the technique for the radiologic measurement of slip in spondylolisthesis. Spine 2010, 35, 1401–1405. [Google Scholar] [CrossRef]
- Danielson, B.; Frennered, K.; Selvik, G.; Irstam, L. Roentgenologic Assessment of Spondylolisthesis: II. An Evaluation of Progression. Acta Radiol. 1989, 30, 65–68. [Google Scholar] [CrossRef]
- Tallroth, K.; Ylikoski, M.; Landtman, M.; Santavirta, S. Reliability of radiographical measurements of spondylolisthesis and extension-flexion radiographs of the lumbar spine. Eur. J. Radiol. 1994, 18, 227–231. [Google Scholar] [CrossRef]
- Mac-Thiong, J.M.; Duong, L.; Parent, S.; Hresko, M.T.; Dimar, J.R.; Weidenbaum, M.; Labelle, H. Reliability of the Spinal Deformity Study Group classification of lumbosacral spondylolisthesis. Spine 2012, 37, E95–E102. [Google Scholar] [CrossRef]
- Bao, H.; Yan, P.; Zhu, W.; Bao, M.; Li, Y.; Zhang, Z.; Qiu, Y.; Zhu, F. Validation and reliability analysis of the spinal deformity study group classification for L5-S1 lumbar spondylolisthesis. Spine 2015, 40, E1150–E1154. [Google Scholar] [CrossRef]
- Denard, P.J.; Holton, K.F.; Miller, J.; Fink, H.A.; Kado, D.M.; Yoo, J.U.; Marshall, L.M. Lumbar spondylolisthesis among elderly men: Prevalence, correlates and progression. Spine 2010, 35, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Austevoll, I.M.; Gjestad, R.; Brox, J.I.; Solberg, T.K.; Storheim, K.; Rekeland, F.; Hermansen, E.; Indrekvam, K.; Hellum, C. The effectiveness of decompression alone compared with additional fusion for lumbar spinal stenosis with degenerative spondylolisthesis: A pragmatic comparative non-inferiority observational study from the Norwegian Registry for Spine Surgery. Eur. Spine J. 2017, 26, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Wiltse, L.L.; Newman, P.H.; Macnab, I. Classification of spondylolisis and spondylolisthesis. Clin. Orthop. Relat. Res. 1976, 117, 23–29. [Google Scholar] [CrossRef]
- Wiltse, L.L. Classification, terminology and measurements in spondylolisthesis. Iowa Orthop. J. 1981, 1, 52. [Google Scholar]
- Newman, P.H.; Stone, K.H. The etiology of spondylolisthesis. J. Bone Jt. Surg. Br. Vol. 1963, 45, 39–59. [Google Scholar] [CrossRef]
- Fujiwara, A.; Tamai, K.; An, H.S.; Kurihashi, T.; Lim, T.H.; Yoshida, H.; Saotome, K. The relationship between disc degeneration, facet joint osteoarthritis, and stability of the degenerative lumbar spine. J. Spinal Disord. 2000, 13, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Chaput, C.; Padon, D.; Rush, J.; Lenehan, E.; Rahm, M. The significance of increased fluid signal on magnetic resonance imaging in lumbar facets in relationship to degenerative spondylolisthesis. Spine 2007, 32, 1883–1887. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.F.; Miller, J.E. Hypoplasia of the lumbar vertebral body simulating spondylolisthesis. Radiology 1979, 133, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Legaye, J. Radiographic analysis of the listhesis associated with lumbar isthmic spondylolysis. Orthop. Traumatol. Surg. Res. 2018, 104, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Mac-Thiong, J.-M.; Labelle, H.; Parent, S.; Hresko, M.T.; Deviren, V.; Weidenbaum, M. Reliability and development of a new classification of lumbosacral spondylolisthesis. Scoliosis 2008, 3, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.X.J.; Kaplar, Z.; Deng, M.; Leung, J.C. Lumbar degenerative spondylolisthesis epidemiology: A systematic review with a focus on gender-specific and age-specific prevalence. J. Orthop. Transl. 2017, 11, 39–52. [Google Scholar] [CrossRef]
- Kalichman, L.; Kim, D.H.; Li, L.; Guermazi, A.; Berkin, V.; Hunter, D.J. Spondylolysis and spondylolisthesis: Prevalence and association with low back pain in the adult community-based population. Spine 2009, 34, 199–205. [Google Scholar] [CrossRef]
- Ishimoto, Y.; Cooper, C.; Ntani, G.; Yamada, H.; Hashizume, H.; Nagata, K.; Muraki, S.; Tanaka, S.; Yoshida, M.; Yoshimura, N. Is radiographic lumbar spondylolisthesis associated with occupational exposures? Findings from a nested case control study within the Wakayama spine study. BMC Musculoskelet. Disord. 2019, 20, 1–8. [Google Scholar] [CrossRef]
- Chen, I.-R.; Wei, T.-S. Disc height and lumbar index as independent predictors of degenerative spondylolisthesis in middle-aged women with low back pain. Spine 2009, 34, 1402–1409. [Google Scholar] [CrossRef]
- Martin, J.T.; Oldweiler, A.B.; Kosinski, A.S.; Spritzer, C.E.; Soher, B.J.; Erickson, M.M.; Goode, A.P.; DeFrate, L.E. Lumbar intervertebral disc diurnal deformations and T2 and T1rho relaxation times vary by spinal level and disc region. Eur. Spine J. 2022, 31, 746–754. [Google Scholar] [CrossRef]
- Meadows, K.D.; Peloquin, J.M.; Newman, H.R.; Cauchy, P.J.; Vresilovic, E.J.; Elliott, D.M. MRI-based measurement of in vivo disc mechanics in a young population due to flexion, extension, and diurnal loading. JOR Spine 2023, 6, e1243. [Google Scholar] [CrossRef]
- Shao, Z.; Rompe, G.; Schiltenwolf, M. Radiographic changes in the lumbar intervertebral discs and lumbar vertebrae with age. Spine 2002, 27, 263–268. [Google Scholar] [CrossRef]
- Amonoo-Kuofi, H.S. Morphometric changes in the heights and anteroposterior diameters of the lumbar intervertebral discs with age. J. Anat. 1991, 175, 159. [Google Scholar]
- Twomey, L.T.; Taylor, J.R. Age changes in lumbar vertebrae and intervertebral discs. Clin. Orthop. Relat. Res. 1987, 224, 97–104. [Google Scholar] [CrossRef]
- Videman, T.; Battié, M.C.; Gibbons, L.E.; Gill, K. Aging changes in lumbar discs and vertebrae and their interaction: A 15-year follow-up study. Spine J. 2014, 14, 469–478. [Google Scholar] [CrossRef]
- Bach, K.; Ford, J.; Foley, R.; Januszewski, J.; Murtagh, R.; Decker, S.; Uribe, J.S. Morphometric analysis of lumbar intervertebral disc height: An imaging study. World Neurosurg. 2019, 124, e106–e118. [Google Scholar] [CrossRef]
- Demir, M.; Emre, A.; Seringeç, N.; Yoldaş, A.; Çiçek, M.; Ertoğrul, R.; Güneri, B. Intervertebral disc heights and concavity index of the lumbar spine in young healthy adults. Anatomy 2018, 12, 34–37. [Google Scholar] [CrossRef][Green Version]
- Leone, A.; Cassar-Pullicino, V.N.; Guglielmi, G.; Bonomo, L. Degenerative lumbar intervertebral instability: What is it and how does imaging contribute? Skelet. Radiol. 2009, 38, 529–533. [Google Scholar] [CrossRef]
- Lattig, F.; Fekete, T.F.; Grob, D.; Kleinstück, F.S.; Jeszenszky, D.; Mannion, A.F. Lumbar facet joint effusion in MRI: A sign of instability in degenerative spondylolisthesis? Eur. Spine J. 2012, 21, 276–281. [Google Scholar] [CrossRef]
- Simmonds, A.M.; Rampersaud, Y.R.; Dvorak, M.F.; Dea, N.; Melnyk, A.D.; Fisher, C.G. Defining the inherent stability of degenerative spondylolisthesis: A systematic review. J. Neurosurg. Spine 2015, 23, 178–189. [Google Scholar] [CrossRef]
- Hasegawa, K.; Kitahara, K.; Shimoda, H.; Ishii, K.; Ono, M.; Homma, T.; Watanabe, K. Lumbar degenerative spondylolisthesis is not always unstable: Clinicobiomechanical evidence. Spine 2014, 39, 2127–2135. [Google Scholar] [CrossRef]
- Wang, D.; Yuan, H.; Liu, A.; Li, C.; Yang, K.; Zheng, S.; Wang, L.; Wang, J.C.; Buser, Z. Analysis of the relationship between the facet fluid sign and lumbar spine motion of degenerative spondylolytic segment using Kinematic MRI. Eur. J. Radiol. 2017, 94, 6–12. [Google Scholar] [CrossRef]
- Nizard, R.S.; Wybier, M.; Laredo, J.D. Radiologic assessment of lumbar intervertebral instability and degenerative spondylolisthesis. Radiol. Clin. N. Am. 2001, 39, 55–71. [Google Scholar] [CrossRef]
- Leone, A.; Guglielmi, G.; Cassar-Pullicino, V.N.; Bonomo, L. Lumbar intervertebral instability: A review. Radiology 2007, 245, 62–77. [Google Scholar] [CrossRef]
- Izzo, R.; Guarnieri, G.; Guglielmi, G.; Muto, M. Biomechanics of the spine. Part I: Spinal stability. Eur. J. Radiol. 2013, 82, 118–126. [Google Scholar] [CrossRef]
- Evans, N.; McCarthy, M. Management of symptomatic degenerative low-grade lumbar spondylolisthesis. EFORT Open Rev. 2018, 3, 620–631. [Google Scholar] [CrossRef]
- Wang, M.; Luo, X.J.; Ye, Y.J.; Zhang, Z. Does Concomitant Degenerative Spondylolisthesis Influence the Outcome of Decompression Alone in Degenerative Lumbar Spinal Stenosis? A Meta-Analysis of Comparative Studies. World Neurosurg. 2019, 123, 226–238. [Google Scholar] [CrossRef]
- Reitman, C.A.; Cho, C.H.; Bono, C.M.; Ghogawala, Z.; Glaser, J.; Kauffman, C.; Mazanec, D.; O’Brien Jr, D.; O’Toole, J.; Prather, H. Management of degenerative spondylolisthesis: Development of appropriate use criteria. Spine J. 2021, 21, 1256–1267. [Google Scholar] [CrossRef]
- Mannion, A.; Pittet, V.; Steiger, F.; Vader, J.-P.; Becker, H.-J.; Porchet, F. Development of appropriateness criteria for the surgical treatment of symptomatic lumbar degenerative spondylolisthesis (LDS). Eur. Spine J. 2014, 23, 1903–1917. [Google Scholar] [CrossRef]
- Even, J.L.; Chen, A.F.; Lee, J.Y. Imaging characteristics of “dynamic” versus “static” spondylolisthesis: Analysis using magnetic resonance imaging and flexion/extension films. Spine J. 2014, 14, 1965–1969. [Google Scholar] [CrossRef]
- Inose, H.; Kato, T.; Onuma, H.; Morishita, S.; Matsukura, Y.; Yuasa, M.; Hirai, T.; Yoshii, T.; Okawa, A. Predictive Factors Affecting Surgical Outcomes in Patients with Degenerative Lumbar Spondylolisthesis. Spine 2021, 46, 610–616. [Google Scholar] [CrossRef]
- Antani, S.; Cheng, J.; Long, J.; Long, L.R.; Thoma, G.R. Medical validation and CBIR of spine X-ray images over the Internet. In Internet Imaging VII; SPIE: Bellingham, WA, USA, 2006; p. 60610J. [Google Scholar]
- Korovessis, P.; Repantis, T.; Papazisis, Z.; Iliopoulos, P. Effect of Sagittal Spinal Balance, Levels of Posterior Instrumentation, and Length of Follow-up on Low Back Pain in Patients Undergoing Posterior Decompression and Instrumented Fusion for Degenerative Lumbar Spine Disease: A Multifactorial Analysis. Spine 2010, 35, 898–905. [Google Scholar] [CrossRef]
- Lord, M.J.; Small, J.M.; Dinsay, J.M.; Watkins, R.G. Lumbar lordosis. Effects of sitting and standing. Spine 1997, 22, 2571–2574. [Google Scholar] [CrossRef]
- Been, E.; Barash, A.; Pessah, H.; Peleg, S. A new look at the geometry of the lumbar spine. Spine 2010, 35, E1014–E1017. [Google Scholar] [CrossRef]
- Zhou, S.H.; McCarthy, I.D.; McGregor, A.H.; Coombs, R.R.; Hughes, S.P. Geometrical dimensions of the lower lumbar vertebrae--analysis of data from digitised CT images. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2000, 9, 242–248. [Google Scholar] [CrossRef]
- Shrestha, P.; Maskey, S.; Sharma, K.; Karki, S.; Kisiju, T. Morphometric Study of Lumbar Intervertebral Spaces (discs) by Using MRI. J. Lumbini Med. Coll. 2020, 8, 10–16. [Google Scholar]
- Crawford, N.R.; Peles, J.D.; Dickman, C.A. The spinal lax zone and neutral zone: Measurement techniques and parameter comparisons. J. Spinal Disord. 1998, 11, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, M.M. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J. Spinal Disord. 1992, 5, 390–396; discussion 397. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Cummings, S.R.; Stone, K.; Hudes, E.; Palermo, L.; Steiger, P. A new approach to defining normal vertebral dimensions. J. Bone Miner. Res. 1991, 6, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Rea, J.; Steiger, P.; Blake, G.; Potts, E.; Smith, I.; Fogelman, I. Morphometric X-ray absorptiometry: Reference data for vertebral dimensions. J. Bone Miner. Res. 1998, 13, 464–474. [Google Scholar]
- O’Neill, T.W.; Varlow, J.; Felsenberg, D.; Johnell, O.; Weber, K.; Marchant, F.; Delmas, P.D.; Cooper, C.; Kanis, J.; Silman, A.J. Variation in vertebral height ratios in population studies. European Vertebral Osteoporosis Study Group. J. Bone Miner. Res. 1994, 9, 1895–1907. [Google Scholar] [CrossRef]


| Sex | No. | Age | BMI |
|---|---|---|---|
| Male | 4582 | 50.9 [15.3] | 25.5 [4.0] |
| Female | 2830 | 63.3 [6.4] | 26.4 [5.5] |
| ADH | PDH | Avg DH | DA | ASPO | PSPO | VDH | DDH | MPA | CSPO | Disc Area | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ADH | 1 | ||||||||||
| PDH | 0.48 | 1 | |||||||||
| AvgDH | 0.9 | 0.81 | 1 | ||||||||
| DA | 0.7 | −0.29 | 0.32 | 1 | |||||||
| ASPO | −0.33 | 0.19 | −0.12 | −0.53 | 1 | ||||||
| PSPO | 0.29 | −0.07 | 0.16 | 0.36 | −0.78 | 1 | |||||
| VDH | 0.99 | 0.45 | 0.88 | 0.72 | −0.4 | 0.29 | 1 | ||||
| DDH | 0.5 | 0.99 | 0.82 | −0.26 | 0.12 | −0.05 | 0.48 | 1 | |||
| MPA | 0.52 | −0.31 | 0.19 | 0.82 | −0.37 | 0.2 | 0.54 | −0.29 | 1 | ||
| CSPO | −0.17 | 0.11 | −0.06 | −0.28 | 0.82 | −0.86 | −0.21 | 0.06 | −0.19 | 1 | |
| Disc Area | 0.91 | 0.79 | 1 | 0.34 | −0.15 | 0.22 | 0.88 | 0.8 | 0.21 | −0.11 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hipp, J.; Grieco, T.; Newman, P.; Patel, V.; Reitman, C. Reference Data for Diagnosis of Spondylolisthesis and Disc Space Narrowing Based on NHANES-II X-rays. Bioengineering 2024, 11, 360. https://doi.org/10.3390/bioengineering11040360
Hipp J, Grieco T, Newman P, Patel V, Reitman C. Reference Data for Diagnosis of Spondylolisthesis and Disc Space Narrowing Based on NHANES-II X-rays. Bioengineering. 2024; 11(4):360. https://doi.org/10.3390/bioengineering11040360
Chicago/Turabian StyleHipp, John, Trevor Grieco, Patrick Newman, Vikas Patel, and Charles Reitman. 2024. "Reference Data for Diagnosis of Spondylolisthesis and Disc Space Narrowing Based on NHANES-II X-rays" Bioengineering 11, no. 4: 360. https://doi.org/10.3390/bioengineering11040360
APA StyleHipp, J., Grieco, T., Newman, P., Patel, V., & Reitman, C. (2024). Reference Data for Diagnosis of Spondylolisthesis and Disc Space Narrowing Based on NHANES-II X-rays. Bioengineering, 11(4), 360. https://doi.org/10.3390/bioengineering11040360