Exploring Electrospun Scaffold Innovations in Cardiovascular Therapy: A Review of Electrospinning in Cardiovascular Disease
Abstract
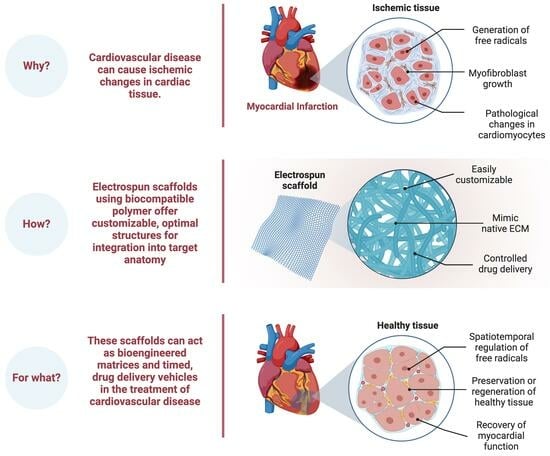
1. Introduction
2. Fundamentals of Electrospinning
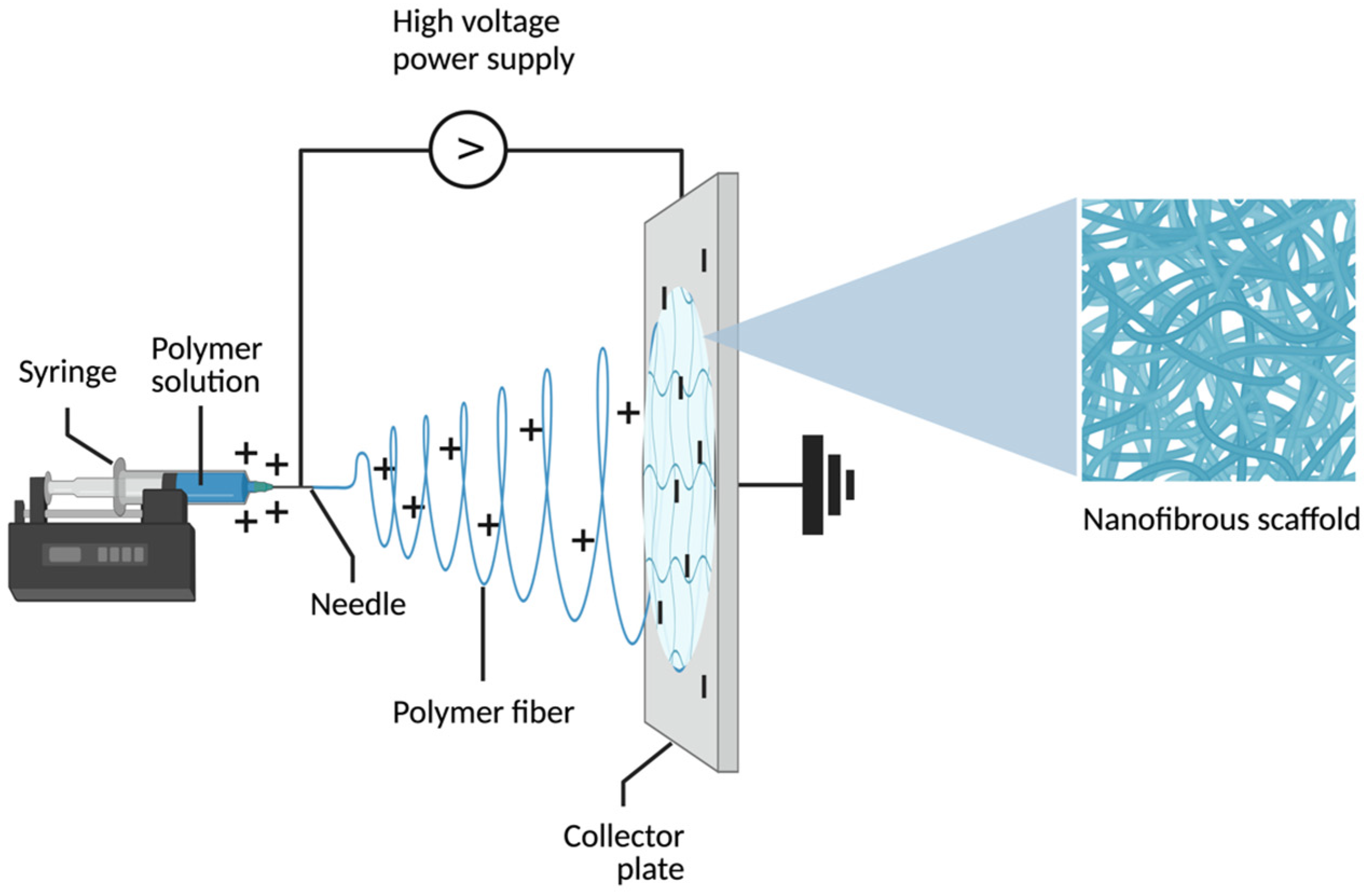
2.1. Definition and Principles of Electrospinning
2.2. Electrospinning Process and Parameters
2.3. Selection of Polymers and Nanofibers in Cardiac Tissue Engineering
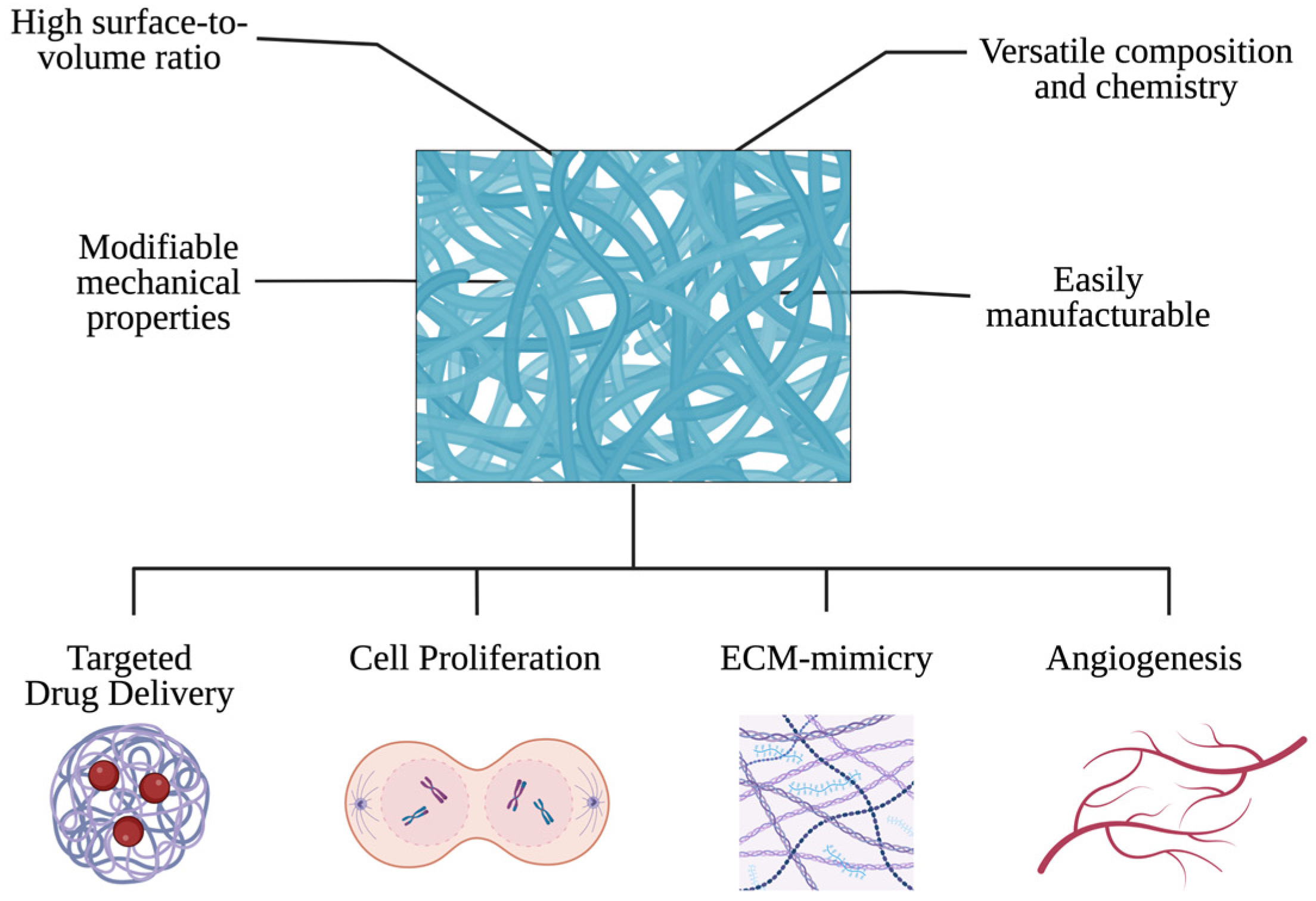
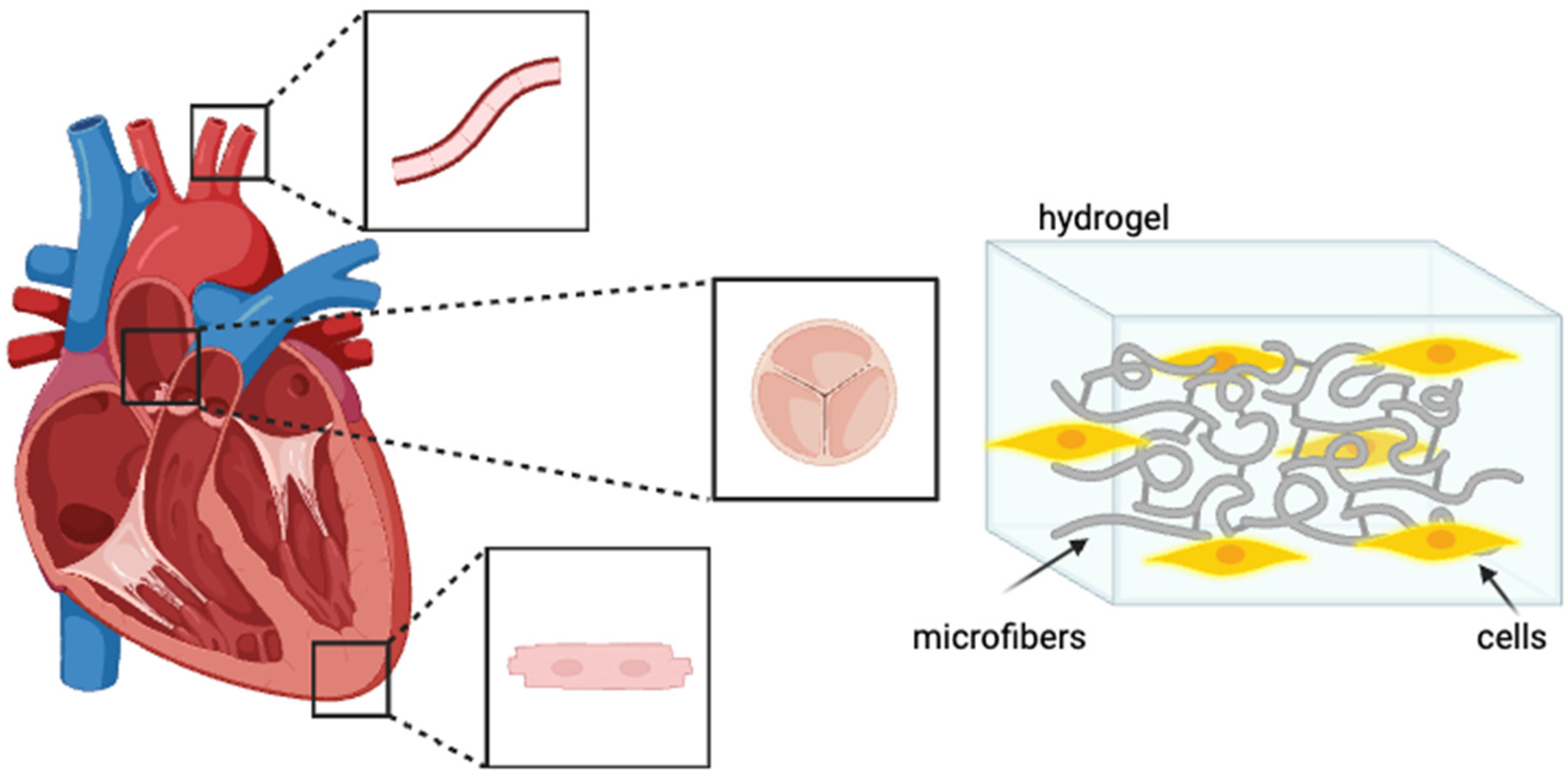
3. Electrospinning in Cardiac Tissue Engineering
3.1. Electrospun Nanofibers for Myocardial Regeneration
3.1.1. Enhancing Cell Adhesion and Proliferation
3.1.2. Stimulating Angiogenesis
3.1.3. Improving the Mechanical Properties of Scaffolds
3.2. Electrospun Scaffolds for Heart Valve Replacement
3.2.1. Challenges in Heart Valve Tissue Engineering
3.2.2. Applications of Electrospinning in Valve Tissue Engineering
3.3. Electrospun Patch for Infarct Repair
3.3.1. Functionalizing the Patch for Controlled Drug Delivery
3.3.2. Integration of Patch with Native Tissue
3.4. Electrospinning and Drug Delivery in Heart Disease
3.4.1. Incorporation of Therapeutic Agents into Electrospun Nanofibers
3.4.2. Controlled Release Systems for Cardiac Drug Delivery
3.4.3. Electrospun Nanofiber-Based Drug Delivery for Atherosclerosis Treatment
3.4.4. Alternative Bioengineered Patch Strategies
4. Challenges and Future Perspectives
4.1. Scalability and Commercialization of Electrospinning Technology
4.2. Improving Mechanical Strength and Degradation Rate
4.3. Multifunctional Electrospun Materials for Personalized Medicine
4.4. Integration of Electrospun Scaffolds with Cardiac Cells and Tissues
5. Comparative Analysis with Other Tissue Engineering Approaches
5.1. Electrospinning vs. 3D Printing in Cardiac Tissue Engineering
5.2. Electrospinning vs. Decellularized Scaffolds for Heart Regeneration
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 27 November 2023).
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Hamedani, Y.; Teixeira, R.B.; Karbasiafshar, C.; Wipf, P.; Bhowmick, S.; Abid, M.R. Delivery of a mitochondria-targeted antioxidant from biocompatible, polymeric nanofibrous scaffolds. FEBS Open Bio 2021, 11, 35–47. [Google Scholar] [CrossRef]
- Chinnappan, B.A.; Krishnaswamy, M.; Xu, H.; Hoque, M.E. Electrospinning of Biomedical Nanofibers/Nanomembranes: Effects of Process Parameters. Polymers 2022, 14, 3719. [Google Scholar] [CrossRef]
- Torres-Martínez, E.J.; Bravo, J.M.C.; Medina, A.S.; González, G.L.P.; Gómez, L.J.V. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef]
- Tucker, N.; Stanger, J.J.; Staiger, M.P.; Razzaq, H.; Hofman, K. The History of the Science and Technology of Electrospinning from 1600 to 1995. J. Eng. Fibers Fabr. 2012, 7, 155892501200702. [Google Scholar] [CrossRef]
- Hong, J.; Yeo, M.; Yang, G.H.; Kim, G. Cell-Electrospinning and Its Application for Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 6208. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef]
- Bhattarai, R.S.; Bachu, R.D.; Boddu, S.H.S.; Bhaduri, S. Biomedical Applications of Electrospun Nanofibers: Drug and Nanoparticle Delivery. Pharmaceutics 2018, 11, 5. [Google Scholar] [CrossRef]
- Herrero-Herrero, M.; Gómez-Tejedor, J.A.; Vallés-Lluch, A. Role of Electrospinning Parameters on Poly(Lactic-co-Glycolic Acid) and Poly(Caprolactone-co-Glycolic acid) Membranes. Polymers 2021, 13, 695. [Google Scholar] [CrossRef]
- Opalkova Siskova, A.; Sacarescu, L.; Opalek, A.; Mosnacek, J.; Peptu, C. Electrospinning of Cyclodextrin–Oligolactide Derivatives. Biomolecules 2023, 13, 203. [Google Scholar] [CrossRef]
- Mokhtari, F.; Latifi, M.; Shamshirsaz, M. Applying the Genetic Algorithm for Determination Electrospinning Parameters of Poly Vinylidene Fluoride (PVDF) Nano Fibers: Theoretical & Experimental Analysis. J. Text. Eng. Fash. Technol. 2017, 3. [Google Scholar] [CrossRef][Green Version]
- Tarus, B.; Fadel, N.; Al-Oufy, A.; El-Messiry, M. Effect of polymer concentration on the morphology and mechanical characteristics of electrospun cellulose acetate and poly (vinyl chloride) nanofiber mats. Alex. Eng. J. 2016, 55, 2975–2984. [Google Scholar] [CrossRef]
- Koski, A.; Yim, K.; Shivkumar, S. Effect of molecular weight on fibrous PVA produced by electrospinning. Mater. Lett. 2004, 58, 493–497. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Hong, Y.; Zhao, Y.; Qiu, S.; Wang, C.; Wei, Y. Influence of solvents on the formation of ultrathin uniform poly(vinyl pyrrolidone) nanofibers with electrospinning. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3721–3726. [Google Scholar] [CrossRef]
- Megelski, S.; Stephens, J.S.; Chase, D.B.; Rabolt, J.F. Micro- and Nanostructured Surface Morphology on Electrospun Polymer Fibers. Macromolecules 2002, 35, 8456–8466. [Google Scholar] [CrossRef]
- Mazoochi, T.; Hamadanian, M.; Ahmadi, M.; Jabbari, V. Investigation on the morphological characteristics of nanofiberous membrane as electrospun in the different processing parameters. Int. J. Ind. Chem. 2012, 3, 2. [Google Scholar] [CrossRef]
- Mit-uppatham, C.; Nithitanakul, M.; Supaphol, P. Ultrafine Electrospun Polyamide-6 Fibers: Effect of Solution Conditions on Morphology and Average Fiber Diameter. Macromol. Chem. Phys. 2004, 205, 2327–2338. [Google Scholar] [CrossRef]
- Szewczyk, P.K.; Stachewicz, U. The impact of relative humidity on electrospun polymer fibers: From structural changes to fiber morphology. Adv. Colloid. Interface Sci. 2020, 286, 102315. [Google Scholar] [CrossRef]
- Zulkifli, M.Z.A.; Nordin, D.; Shaari, N.; Kamarudin, S.K. Overview of Electrospinning for Tissue Engineering Applications. Polymers 2023, 15, 2418. [Google Scholar] [CrossRef]
- Boroumand, S.; Haeri, A.; Nazeri, N.; Rabbani, S. Review Insights In Cardiac Tissue Engineering: Cells, Scaffolds, and Pharmacological Agents. Iran. J. Pharm. Res. 2021, 20, 467–496. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Conducting Polymers for Tissue Engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S. A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef]
- Nguyen, P.K.; Rhee, J.-W.; Wu, J.C. Adult stem cell therapy and heart failure, 2000 to 2016: A systematic review. JAMA Cardiol. 2016, 1, 831–841. [Google Scholar] [CrossRef]
- Sencadas, V.; Correia, D.M.; Ribeiro, C.; Moreira, S.; Botelho, G.; Gómez Ribelles, J.L.; Lanceros-Mendez, S. Physical-chemical properties of cross-linked chitosan electrospun fiber mats. Polym. Test. 2012, 31, 1062–1069. [Google Scholar] [CrossRef]
- Hussain, A.; Collins, G.; Yip, D.; Cho, C.H. Functional 3-D cardiac co-culture model using bioactive chitosan nanofiber scaffolds. Biotechnol. Bioeng. 2013, 110, 637–647. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Sakib, M.N.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications—A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Alhalafi, M.H.; Emam, E.-A.M.; Ibrahim, H.; Mosaad, R.M. A Review of Chitosan and Chitosan Nanofiber: Preparation, Characterization, and Its Potential Applications. Polymers 2023, 15, 2820. [Google Scholar] [CrossRef]
- Abadi, B.; Goshtasbi, N.; Bolourian, S.; Tahsili, J.; Adeli-Sardou, M.; Forootanfar, H. Electrospun hybrid nanofibers: Fabrication, characterization, and biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 986975. [Google Scholar] [CrossRef]
- Ndlovu, S.P.; Ngece, K.; Alven, S.; Aderibigbe, B.A. Gelatin-Based Hybrid Scaffolds: Promising Wound Dressings. Polymers 2021, 13, 2959. [Google Scholar] [CrossRef]
- Li, T.; Sun, M.; Wu, S. State-of-the-Art Review of Electrospun Gelatin-Based Nanofiber Dressings for Wound Healing Applications. Nanomaterials 2022, 12, 784. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Said, N.S.; Yosri, N.; Hawash, H.B.; El-Sherif, D.M.; Abouzid, M.; Abdel-Daim, M.M.; Yaseen, M.; Omar, H.; Shou, Q.; et al. Gelatin nanofibers: Recent insights in synthesis, bio-medical applications and limitations. Heliyon 2023, 9, e16228. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.Z.; Ramakrishna, S.; Lim, C.T. Electrospinning and mechanical characterization of gelatin nanofibers. Polymer 2004, 45, 5361–5368. [Google Scholar] [CrossRef]
- Hernández-Rangel, A.; Martin-Martinez, E.S. Collagen based electrospun materials for skin wounds treatment. J. Biomed. Mater. Res. A 2021, 109, 1751–1764. [Google Scholar] [CrossRef]
- Law, J.X.; Liau, L.L.; Saim, A.; Yang, Y.; Idrus, R. Electrospun Collagen Nanofibers and Their Applications in Skin Tissue Engineering. Tissue Eng. Regen. Med. 2017, 14, 699–718. [Google Scholar] [CrossRef] [PubMed]
- Rho, K.S.; Jeong, L.; Lee, G.; Seo, B.-M.; Park, Y.J.; Hong, S.-D.; Roh, S.; Cho, J.J.; Park, W.H.; Min, B.-M. Electrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials 2006, 27, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Mbese, Z.; Alven, S.; Aderibigbe, B.A. Collagen-Based Nanofibers for Skin Regeneration and Wound Dressing Applications. Polymers 2021, 13, 4368. [Google Scholar] [CrossRef] [PubMed]
- Wilk, S.; Benko, A. Advances in Fabricating the Electrospun Biopolymer-Based Biomaterials. J. Funct. Biomater. 2021, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Mokhena, T.C.; Mochane, M.J.; Mtibe, A.; John, M.J.; Sadiku, E.R.; Sefadi, J.S. Electrospun Alginate Nanofibers Toward Various Applications: A Review. Materials 2020, 13, 934. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; He, A.; Zheng, J.; Xu, S.; Li, J.; Han, C.C. Effects of Chain Conformation and Entanglement on the Electrospinning of Pure Alginate. Biomacromolecules 2008, 9, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Aljabali, A.A.A.; Mishra, V.; Tambuwala, M.M.; Serrano-Aroca, Á. Alginate: Enhancement Strategies for Advanced Applications. Int. J. Mol. Sci. 2022, 23, 4486. [Google Scholar] [CrossRef]
- Taemeh, M.A.; Shiravandi, A.; Korayem, M.A.; Daemi, H. Fabrication challenges and trends in biomedical applications of alginate electrospun nanofibers. Carbohydr. Polym. 2020, 228, 115419. [Google Scholar] [CrossRef]
- Saini, P.; Arora, M.; Kumar, M.N.V.R. Poly(lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.Y.; Chan, V.; Li, C.; Hsieh, J.H.; Lin, P.H.; Tsai, Y.-H.; Chen, Y. Fabrication of polylactic acid/paclitaxel nano fibers by electrospinning for cancer therapeutics. BMC Chem. 2020, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-Based Composites for Various Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.R.; Steffens, D.; Santi, B.; Quintiliano, K.; Steffen, N.; Pilger, D.A.; Pranke, P. Development of VEGF-loaded PLGA matrices in association with mesenchymal stem cells for tissue engineering. Braz. J. Med. Biol. Res. 2017, 50, e5648. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Augustine, R.; Kalarikkal, N.; Thomas, S.; Seantier, B.; Grohens, Y. Recent advances in electrospun polycaprolactone based scaffolds for wound healing and skin bioengineering applications. Mater. Today Commun. 2019, 19, 319–335. [Google Scholar] [CrossRef]
- Salim, S.A.; Badawi, N.M.; EL-Moslamy, S.H.; Kamoun, E.A.; Daihom, B.A. Novel long-acting brimonidine tartrate loaded-PCL/PVP nanofibers for versatile biomedical applications: Fabrication, characterization and antimicrobial evaluation. RSC Adv. 2023, 13, 14943–14957. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Choi, S.M. Advances in Waterborne Polyurethane-Based Biomaterials for Biomedical Applications. In Novel Biomaterials for Regenerative Medicine; Chun, H.J., Park, K., Kim, C.-H., Khang, G., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; pp. 251–283. ISBN 9789811309472. [Google Scholar]
- Xu, C.; Hong, Y. Rational design of biodegradable thermoplastic polyurethanes for tissue repair. Bioact. Mater. 2021, 15, 250–271. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, M.; Kędra, K. Biomedical Polyurethanes for Anti-Cancer Drug Delivery Systems: A Brief, Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 8181. [Google Scholar] [CrossRef]
- Xu, C.; Huang, Y.; Wu, J.; Tang, L.; Hong, Y. Triggerable Degradation of Polyurethanes for Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2015, 7, 20377–20388. [Google Scholar] [CrossRef]
- Bazgir, M.; Saeinasab, M.; Zhang, W.; Zhang, X.; Min Tsui, K.; Maasoumi Sarvestani, A.; Nawaz, S.; Coates, P.; Youseffi, M.; Elies, J.; et al. Investigation of Cell Adhesion and Cell Viability of the Endothelial and Fibroblast Cells on Electrospun PCL, PLGA and Coaxial Scaffolds for Production of Tissue Engineered Blood Vessel. J. Funct. Biomater. 2022, 13, 282. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, X.; Zhang, F.; Diekwisch, T.G.H.; Luan, X.; Yang, J. Triple PLGA/PCL scaffold modification including silver-impregnation, collagen-coating, and electrospinning significantly improve biocompatibility, antimicrobial, and osteogenic properties for oro-facial tissue regeneration. ACS Appl. Mater. Interfaces 2019, 11, 37381–37396. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of Collagen Nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef]
- Zamani, F.; Amani-Tehran, M.; Latifi, M.; Shokrgozar, M.A. The influence of surface nanoroughness of electrospun PLGA nanofibrous scaffold on nerve cell adhesion and proliferation. J. Mater. Sci. Mater. Med. 2013, 24, 1551–1560. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Wu, Q.; Bao, F.; Yang, H.; Qiu, X.; Chang, J. Chitosan/Calcium Silicate Cardiac Patch Stimulates Cardiomyocyte Activity and Myocardial Performance after Infarction by Synergistic Effect of Bioactive Ions and Aligned Nanostructure. ACS Appl. Mater. Interfaces 2019, 11, 1449–1468. [Google Scholar] [CrossRef]
- Chung, H.-J.; Kim, J.-T.; Kim, H.-J.; Kyung, H.-W.; Katila, P.; Lee, J.-H.; Yang, T.-H.; Yang, Y.-I.; Lee, S.-J. Epicardial delivery of VEGF and cardiac stem cells guided by 3-dimensional PLLA mat enhancing cardiac regeneration and angiogenesis in acute myocardial infarction. J. Control. Release Off. J. Control. Release Soc. 2015, 205, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Spadaccio, C.; Nappi, F.; De Marco, F.; Sedati, P.; Taffon, C.; Nenna, A.; Crescenzi, A.; Chello, M.; Trombetta, M.; Gambardella, I.; et al. Implantation of a Poly-l-Lactide GCSF-Functionalized Scaffold in a Model of Chronic Myocardial Infarction. J. Cardiovasc. Transl. Res. 2017, 10, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Bertuoli, P.T.; Ordoño, J.; Armelin, E.; Pérez-Amodio, S.; Baldissera, A.F.; Ferreira, C.A.; Puiggalí, J.; Engel, E.; del Valle, L.J.; Alemán, C. Electrospun Conducting and Biocompatible Uniaxial and Core–Shell Fibers Having Poly(lactic acid), Poly(ethylene glycol), and Polyaniline for Cardiac Tissue Engineering. ACS Omega 2019, 4, 3660–3672. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Truong, M.; Li, Y.V.; Wang, Z. Mechanical Considerations of Electrospun Scaffolds for Myocardial Tissue and Regenerative Engineering. Bioengineering 2020, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Kai, D.; Prabhakaran, M.P.; Jin, G.; Ramakrishna, S. Guided orientation of cardiomyocytes on electrospun aligned nanofibers for cardiac tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98, 379–386. [Google Scholar] [CrossRef]
- Stassen, O.M.J.A.; Muylaert, D.E.P.; Bouten, C.V.C.; Hjortnaes, J. Current Challenges in Translating Tissue-Engineered Heart Valves. Curr. Treat. Options Cardiovasc. Med. 2017, 19, 71. [Google Scholar] [CrossRef]
- Fioretta, E.S.; Dijkman, P.E.; Emmert, M.Y.; Hoerstrup, S.P. The future of heart valve replacement: Recent developments and translational challenges for heart valve tissue engineering. J. Tissue Eng. Regen. Med. 2018, 12, e323–e335. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Natural Polymers in Heart Valve Tissue Engineering: Strategies, Advances and Challenges. Biomedicines 2022, 10, 1095. [Google Scholar] [CrossRef]
- Jana, S.; Lerman, A. In vivo tissue engineering of a trilayered leaflet-shaped tissue construct. Regen. Med. 2020, 15, 1177–1192. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Q.; Wang, H.; Gang, H.; Zhou, Y.; Gu, S.; Zhang, R.; Xu, W.; Yang, H. Biomechanical Scaffolds of Decellularized Heart Valves Modified by Electrospun Polylactic Acid. Appl. Biochem. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Motiwale, S.; Russell, M.D.; Conroy, O.; Carruth, J.; Wancura, M.; Robinson, A.; Cosgriff-Hernandez, E.; Sacks, M.S. Anisotropic elastic behavior of a hydrogel-coated electrospun polyurethane: Suitability for heart valve leaflets. J. Mech. Behav. Biomed. Mater. 2022, 125, 104877. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, K.; Weghofer, A.; Urbanczyk, M.; Maulana, T.I.; Loskill, P.; Jones, P.D.; Schenke-Layland, K. Development of a bi-layered cryogenic electrospun polylactic acid scaffold to study calcific aortic valve disease in a 3D co-culture model. Acta Biomater. 2022, 140, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Prabhakaran, M.P.; Ramakrishna, S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int. J. Nanomed. 2013, 8, 2997–3017. [Google Scholar] [CrossRef]
- Shafiq, M.; Zhang, Y.; Zhu, D.; Zhao, Z.; Kim, D.-H.; Kim, S.H.; Kong, D. In situ cardiac regeneration by using neuropeptide substance P and IGF-1C peptide eluting heart patches. Regen. Biomater. 2018, 5, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Walthall, J.M.; Venkataraman, R.; Crowder, S.W.; Jung, D.K.; Yu, S.S.; Feaster, T.K.; Wang, X.; Giorgio, T.D.; Hong, C.C.; et al. Combinatorial Polymer Electrospun Matrices Promote Physiologically-Relevant Cardiomyogenic Stem Cell Differentiation. PLoS ONE 2011, 6, e28935. [Google Scholar] [CrossRef]
- Gu, X.; Matsumura, Y.; Tang, Y.; Roy, S.; Hoff, R.; Wang, B.; Wagner, W.R. Sustained viral gene delivery from a micro-fibrous, elastomeric cardiac patch to the ischemic rat heart. Biomaterials 2017, 133, 132–143. [Google Scholar] [CrossRef]
- Rai, R.; Tallawi, M.; Frati, C.; Falco, A.; Gervasi, A.; Quaini, F.; Roether, J.A.; Hochburger, T.; Schubert, D.W.; Seik, L.; et al. Bioactive Electrospun Fibers of Poly(glycerol sebacate) and Poly(ε-caprolactone) for Cardiac Patch Application. Adv. Healthc. Mater. 2015, 4, 2012–2025. [Google Scholar] [CrossRef] [PubMed]
- Katz, B.; Rosenberg, A.; Frishman, W.H. Controlled-release drug delivery systems in cardiovascular medicine. Am. Heart J. 1995, 129, 359–368. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Kundrat, V.; Cernekova, N.; Kovalcik, A.; Enev, V.; Marova, I. Drug Release Kinetics of Electrospun PHB Meshes. Materials 2019, 12, 1924. [Google Scholar] [CrossRef]
- Tabakoglu, S.; Kołbuk, D.; Sajkiewicz, P. Multifluid electrospinning for multi-drug delivery systems: Pros and cons, challenges, and future directions. Biomater. Sci. 2022, 11, 37–61. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Yang, J.; Zheng, X.; Wang, M.; Liu, Y.; Yu, D.-G. A nanofiber-based drug depot with high drug loading for sustained release. Int. J. Pharm. 2020, 583, 119397. [Google Scholar] [CrossRef]
- Atanasio, G.D.N.; Ferrari, P.F.; Campardelli, R.; Perego, P.; Palombo, D. Innovative nanotools for vascular drug delivery: The atherosclerosis case study. J. Mater. Chem. B 2021, 9, 8558–8568. [Google Scholar] [CrossRef]
- Nazarkina, Z.K.; Chelobanov, B.P.; Kuznetsov, K.A.; Shutov, A.V.; Romanova, I.V.; Karpenko, A.A.; Laktionov, P.P. Influence of Elongation of Paclitaxel-Eluting Electrospun-Produced Stent Coating on Paclitaxel Release and Transport through the Arterial Wall after Stenting. Polymers 2021, 13, 1165. [Google Scholar] [CrossRef]
- Kuznetsov, K.A.; Murashov, I.S.; Chernonosova, V.S.; Chelobanov, B.P.; Stepanova, A.O.; Sergeevichev, D.S.; Karpenko, A.A.; Laktionov, P.P. Vascular Stents Coated with Electrospun Drug-Eluting Material: Functioning in Rabbit Iliac Artery. Polymers 2020, 12, 1741. [Google Scholar] [CrossRef]
- Son, Y.J.; Kim, H.S.; Choi, D.H.; Yoo, H.S. Multilayered electrospun fibrous meshes for restenosis-suppressing metallic stents. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 628–635. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Jiang, X.; Dai, B.; Zhang, L.; Zhu, Y. Decellularized extracellular matrix materials for treatment of ischemic cardiomyopathy. Bioact. Mater. 2024, 33, 460–482. [Google Scholar] [CrossRef]
- Hamsho, K.; Broadwin, M.; Stone, C.R.; Sellke, F.W.; Abid, M.R. The Current State of Extracellular Matrix Therapy for Ischemic Heart Disease. Med. Sci. 2024, 12, 8. [Google Scholar] [CrossRef]
- Vetter, V.C.; Bouten, C.V.C.; van der Pol, A. Hydrogels for Cardiac Restorative Support: Relevance of Gelation Mechanisms for Prospective Clinical Use. Curr. Heart Fail. Rep. 2023, 20, 519–529. [Google Scholar] [CrossRef]
- Huang, K.; Ozpinar, E.W.; Su, T.; Tang, J.; Shen, D.; Qiao, L.; Hu, S.; Li, Z.; Liang, H.; Mathews, K.; et al. An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci. Transl. Med. 2020, 12, eaat9683. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed]
- Fadil, F.; Affandi, N.D.N.; Misnon, M.I.; Bonnia, N.N.; Harun, A.M.; Alam, M.K. Review on Electrospun Nanofiber-Applied Products. Polymers 2021, 13, 2087. [Google Scholar] [CrossRef] [PubMed]
- Omer, S.; Forgách, L.; Zelkó, R.; Sebe, I. Scale-up of Electrospinning: Market Overview of Products and Devices for Pharmaceutical and Biomedical Purposes. Pharmaceutics 2021, 13, 286. [Google Scholar] [CrossRef] [PubMed]
- Kenry; Lim, C.T. Nanofiber technology: Current status and emerging developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar] [CrossRef]
- Vass, P.; Szabó, E.; Domokos, A.; Hirsch, E.; Galata, D.; Farkas, B.; Démuth, B.; Andersen, S.K.; Vigh, T.; Verreck, G.; et al. Scale-up of electrospinning technology: Applications in the pharmaceutical industry. WIREs Nanomed. Nanobiotechnology 2020, 12, e1611. [Google Scholar] [CrossRef]
- Huang, Y.; Song, J.; Yang, C.; Long, Y.; Wu, H. Scalable manufacturing and applications of nanofibers. Mater. Today 2019, 28, 98–113. [Google Scholar] [CrossRef]
- Angkawinitwong, U.; Awwad, S.; Khaw, P.T.; Brocchini, S.; Williams, G.R. Electrospun formulations of bevacizumab for sustained release in the eye. Acta Biomater. 2017, 64, 126–136. [Google Scholar] [CrossRef]
- Varesano, A.; Rombaldoni, F.; Mazzuchetti, G.; Tonin, C.; Comotto, R. Multi-jet nozzle electrospinning on textile substrates: Observations on process and nanofibre mat deposition. Polym. Int. 2010, 59, 1606–1615. [Google Scholar] [CrossRef]
- Kakoria, A.; Sinha-Ray, S. A Review on Biopolymer-Based Fibers via Electrospinning and Solution Blowing and Their Applications. Fibers 2018, 6, 45. [Google Scholar] [CrossRef]
- Jirsak, O.; Sanetrnik, F.; Lukas, D.; Kotek, V.; Martinova, L.; Chaloupek, J. A Method of Nanofibres Production from a Polymer Solution Using Electrostatic Spinning and a Device for Carrying out the Method. 2005. Available online: https://patents.google.com/patent/WO2005024101A1/en (accessed on 22 December 2023).
- Jana, S.; Franchi, F.; Lerman, A. Trilayered tissue structure with leaflet-like orientations developed through in vivo tissue engineering. Biomed. Mater. 2019, 15, 015004. [Google Scholar] [CrossRef] [PubMed]
- Machado-Paula, M.M.; Corat, M.A.F.; Lancellotti, M.; Mi, G.; Marciano, F.R.; Vega, M.L.; Hidalgo, A.A.; Webster, T.J.; Lobo, A.O. A comparison between electrospinning and rotary-jet spinning to produce PCL fibers with low bacteria colonization. Mater. Sci. Eng. C 2020, 111, 110706. [Google Scholar] [CrossRef] [PubMed]
- Rogalski, J.; Bastiaansen, C.; Peijs, T. PA6 Nanofibre Production: A Comparison between Rotary Jet Spinning and Electrospinning. Fibers 2018, 6, 37. [Google Scholar] [CrossRef]
- Rogalski, J.J.; Bastiaansen, C.W.M.; Peijs, T. Rotary jet spinning review—A potential high yield future for polymer nanofibers. Nanocomposites 2017, 3, 97–121. [Google Scholar] [CrossRef]
- Badrossamay, M.R.; McIlwee, H.A.; Goss, J.A.; Parker, K.K. Nanofiber Assembly by Rotary Jet-Spinning. Nano Lett. 2010, 10, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, G.M.; MacQueen, L.A.; Lind, J.U.; Fitzgibbons, S.A.; Chantre, C.O.; Huggler, I.; Golecki, H.M.; Goss, J.A.; Parker, K.K. Production of Synthetic, Para-Aramid and Biopolymer Nanofibers by Immersion Rotary Jet-Spinning. Macromol. Mater. Eng. 2017, 302, 1600365. [Google Scholar] [CrossRef]
- Kaur, S.; Sundarrajan, S.; Rana, D.; Sridhar, R.; Gopal, R.; Matsuura, T.; Ramakrishna, S. Review: The characterization of electrospun nanofibrous liquid filtration membranes. J. Mater. Sci. 2014, 49, 6143–6159. [Google Scholar] [CrossRef]
- Nauman, S.; Lubineau, G.; Alharbi, H.F. Post Processing Strategies for the Enhancement of Mechanical Properties of ENMs (Electrospun Nanofibrous Membranes): A Review. Membranes 2021, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Khalf, A.; Singarapu, K.; Madihally, S.V. Influence of solvent characteristics in triaxial electrospun fiber formation. React. Funct. Polym. 2015, 90, 36–46. [Google Scholar] [CrossRef]
- He, J.-H.; Wan, Y.-Q.; Yu, J.-Y. Effect of concentration on electrospun polyacrylonitrile (PAN) nanofibers. Fibers Polym. 2008, 9, 140–142. [Google Scholar] [CrossRef]
- Barber, J.G.; Handorf, A.M.; Allee, T.J.; Li, W.-J. Braided Nanofibrous Scaffold for Tendon and Ligament Tissue Engineering. Tissue Eng. Part A 2013, 19, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Petrigliano, F.A.; Arom, G.A.; Nazemi, A.N.; Yeranosian, M.G.; Wu, B.M.; McAllister, D.R. In Vivo Evaluation of Electrospun Polycaprolactone Graft for Anterior Cruciate Ligament Engineering. Tissue Eng. Part A 2015, 21, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xu, Y.; Zhang, S.; Li, T.; Ramakrishna, S.; Liu, Y. Progress of Improving Mechanical Strength of Electrospun Nanofibrous Membranes. Macromol. Mater. Eng. 2020, 305, 2000230. [Google Scholar] [CrossRef]
- Abhari, R.E.; Mouthuy, P.-A.; Zargar, N.; Brown, C.; Carr, A. Effect of annealing on the mechanical properties and the degradation of electrospun polydioxanone filaments. J. Mech. Behav. Biomed. Mater. 2017, 67, 127–134. [Google Scholar] [CrossRef]
- He, B.; Tian, L.; Li, J.; Pan, Z. Effect of hot-stretching on morphology and mechanical properties of electrospun PMIA nanofibers. Fibers Polym. 2013, 14, 405–408. [Google Scholar] [CrossRef]
- Roman, J.; Neri, W.; Derré, A.; Poulin, P. Electrospun lignin-based twisted carbon nanofibers for potential microelectrodes applications. Carbon 2019, 145, 556–564. [Google Scholar] [CrossRef]
- Salimbeigi, G.; Cahill, P.A.; McGuinness, G.B. Solvent system effects on the physical and mechanical properties of electrospun Poly(ε-caprolactone) scaffolds for in vitro lung models. J. Mech. Behav. Biomed. Mater. 2022, 136, 105493. [Google Scholar] [CrossRef]
- Yang, L.; Fitié, C.F.C.; Van Der Werf, K.O.; Bennink, M.L.; Dijkstra, P.J.; Feijen, J. Mechanical properties of single electrospun collagen type I fibers. Biomaterials 2008, 29, 955–962. [Google Scholar] [CrossRef]
- Spearman, S.S.; Irin, F.; Rivero, I.V.; Green, M.J.; Abidi, N. Effect of dsDNA wrapped single-walled carbon nanotubes on the thermal and mechanical properties of polycaprolactone and polyglycolide fiber blend composites. Polymer 2015, 56, 476–481. [Google Scholar] [CrossRef]
- Liao, X.; Dulle, M.; De Souza E Silva, J.M.; Wehrspohn, R.B.; Agarwal, S.; Förster, S.; Hou, H.; Smith, P.; Greiner, A. High strength in combination with high toughness in robust and sustainable polymeric materials. Science 2019, 366, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, M.; Liu, L. Fabrication and Degradation of Electrospun Scaffolds from L-Tyrosine-Based Polyurethane Blends for Tissue Engineering Applications. ISRN Nanotechnol. 2012, 2012, 627420. [Google Scholar] [CrossRef]
- Thao, N.T.T.; Lee, S.; Shin, G.R.; Kang, Y.; Choi, S.; Kim, M.S. Preparation of Electrospun Small Intestinal Submucosa/Poly(caprolactone-co-Lactide-co-glycolide) Nanofiber Sheet as a Potential Drug Carrier. Pharmaceutics 2021, 13, 253. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Mukundan, S.; Karaca, E.; Dolatshahi-Pirouz, A.; Patel, A.; Rangarajan, K.; Mihaila, S.M.; Iviglia, G.; Zhang, H.; Khademhosseini, A. Nanoclay-Enriched Poly(ɛ-caprolactone) Electrospun Scaffolds for Osteogenic Differentiation of Human Mesenchymal Stem Cells. Tissue Eng. Part A 2014, 20, 2088–2101. [Google Scholar] [CrossRef]
- Kim, K.; Yu, M.; Zong, X.; Chiu, J.; Fang, D.; Seo, Y.-S.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Control of degradation rate and hydrophilicity in electrospun non-woven poly(d,l-lactide) nanofiber scaffolds for biomedical applications. Biomaterials 2003, 24, 4977–4985. [Google Scholar] [CrossRef]
- Modifying Biodegradation Rate of Electrospun Fibers. Available online: http://electrospintech.com/degradmodification.html (accessed on 22 December 2023).
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact. Mater. 2021, 6, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, R.J.; Steger, A.L.; Blakney, A.K.; Woodrow, K.A. In pursuit of functional electrospun materials for clinical applications in humans. Ther. Deliv. 2016, 7, 387–409. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, Y.; Li, Z.; Zhou, P.; Mao, Y. Electrospun nanofibrous membrane for biomedical application. SN Appl. Sci. 2022, 4, 172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ramakrishna, S.; Liu, X. Electrospinning and emerging healthcare and medicine possibilities. APL Bioeng. 2020, 4, 030901. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Cui, Y.; Zheng, L.; Kah-Hoe Chow, P.; Wang, C.-H. Development of a gene/drug dual delivery system for brain tumor therapy: Potent inhibition via RNA interference and synergistic effects. Biomaterials 2013, 34, 7483–7494. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Han, Y.; Wang, X.; Qin, C.; Zhai, D.; Yi, Z.; Chang, J.; Xiao, Y.; Wu, C. Copper Silicate Hollow Microspheres-Incorporated Scaffolds for Chemo-Photothermal Therapy of Melanoma and Tissue Healing. ACS Nano 2018, 12, 2695–2707. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Li, Y.; Tan, Y.; Liu, Y.; Pan, W.; Tan, G. Stimuli-responsive electrospun nanofibers for drug delivery, cancer therapy, wound dressing, and tissue engineering. J. Nanobiotechnology 2023, 21, 237. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, G.; Wei, J.; Wu, Q.; Li, X. Cardiomyocyte coculture on layered fibrous scaffolds assembled from micropatterned electrospun mats. Mater. Sci. Eng. C 2017, 81, 500–510. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Hu, T.; Guo, B.; Ma, P.X. Electrospun conductive nanofibrous scaffolds for engineering cardiac tissue and 3D bioactuators. Acta Biomater. 2017, 59, 68–81. [Google Scholar] [CrossRef]
- Masoumi, N.; Annabi, N.; Assmann, A.; Larson, B.L.; Hjortnaes, J.; Alemdar, N.; Kharaziha, M.; Manning, K.B.; Mayer, J.E.; Khademhosseini, A. Tri-layered elastomeric scaffolds for engineering heart valve leaflets. Biomaterials 2014, 35, 7774–7785. [Google Scholar] [CrossRef] [PubMed]
- Izadpanah, P.; Golchin, A.; Firuzyar, T.; Najafi, M.; Jangjou, A.; Hashemi, S. The effect of shear stress on cardiac differentiation of mesenchymal stem cells. Mol. Biol. Rep. 2022, 49, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, P.; Tandon, I.; Balachandran, K. Effect of Cyclic Uniaxial Mechanical Strain on Endothelial Progenitor Cell Differentiation. Cardiovasc. Eng. Technol. 2022, 13, 872–885. [Google Scholar] [CrossRef]
- Ghosh, A.; Orasugh, J.T.; Ray, S.S.; Chattopadhyay, D. Integration of 3D Printing–Coelectrospinning: Concept Shifting in Biomedical Applications. ACS Omega 2023, 8, 28002–28025. [Google Scholar] [CrossRef] [PubMed]
- Vyas, C.; Ates, G.; Aslan, E.; Hart, J.; Huang, B.; Bartolo, P. Three-Dimensional Printing and Electrospinning Dual-Scale Polycaprolactone Scaffolds with Low-Density and Oriented Fibers to Promote Cell Alignment. 3D Print. Addit. Manuf. 2020, 7, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Keit, E.; Chen, S.; Wang, H.; Xie, J. Expansion of Two-dimension Electrospun Nanofiber Mats into Three-dimension Scaffolds. J. Vis. Exp. 2019, 143, e58918. [Google Scholar] [CrossRef]
- Kim, Y.S.; Majid, M.; Melchiorri, A.J.; Mikos, A.G. Applications of decellularized extracellular matrix in bone and cartilage tissue engineering. Bioeng. Transl. Med. 2019, 4, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Santschi, M.; Vernengo, A.; Eglin, D.; D’Este, M.; Wuertz-Kozak, K. Decellularized matrix as a building block in bioprinting and electrospinning. Curr. Opin. Biomed. Eng. 2019, 10, 116–122. [Google Scholar] [CrossRef]
- Shi, J.; Teng, Y.; Li, D.; He, J.; Midgley, A.C.; Guo, X.; Wang, X.; Yang, X.; Wang, S.; Feng, Y.; et al. Biomimetic tri-layered small-diameter vascular grafts with decellularized extracellular matrix promoting vascular regeneration and inhibiting thrombosis with the salidroside. Mater. Today Bio 2023, 21, 100709. [Google Scholar] [CrossRef]
- Smoak, M.M.; Han, A.; Watson, E.; Kishan, A.; Grande-Allen, K.J.; Cosgriff-Hernandez, E.; Mikos, A.G. Fabrication and Characterization of Electrospun Decellularized Muscle-Derived Scaffolds. Tissue Eng. Part C Methods 2019, 25, 276–287. [Google Scholar] [CrossRef]
- Masaeli, E.; Karamali, F.; Loghmani, S.; Eslaminejad, M.B.; Nasr-Esfahani, M.H. Bio-engineered electrospun nanofibrous membranes using cartilage extracellular matrix particles. J. Mater. Chem. B 2017, 5, 765–776. [Google Scholar] [CrossRef]
- Schoen, B.; Avrahami, R.; Baruch, L.; Efraim, Y.; Goldfracht, I.; Elul, O.; Davidov, T.; Gepstein, L.; Zussman, E.; Machluf, M. Electrospun Extracellular Matrix: Paving the Way to Tailor-Made Natural Scaffolds for Cardiac Tissue Regeneration. Adv. Funct. Mater. 2017, 27, 1700427. [Google Scholar] [CrossRef]
- Magaz, A.; Li, X.; Gough, J.E.; Blaker, J.J. Graphene oxide and electroactive reduced graphene oxide-based composite fibrous scaffolds for engineering excitable nerve tissue. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111632. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef] [PubMed]
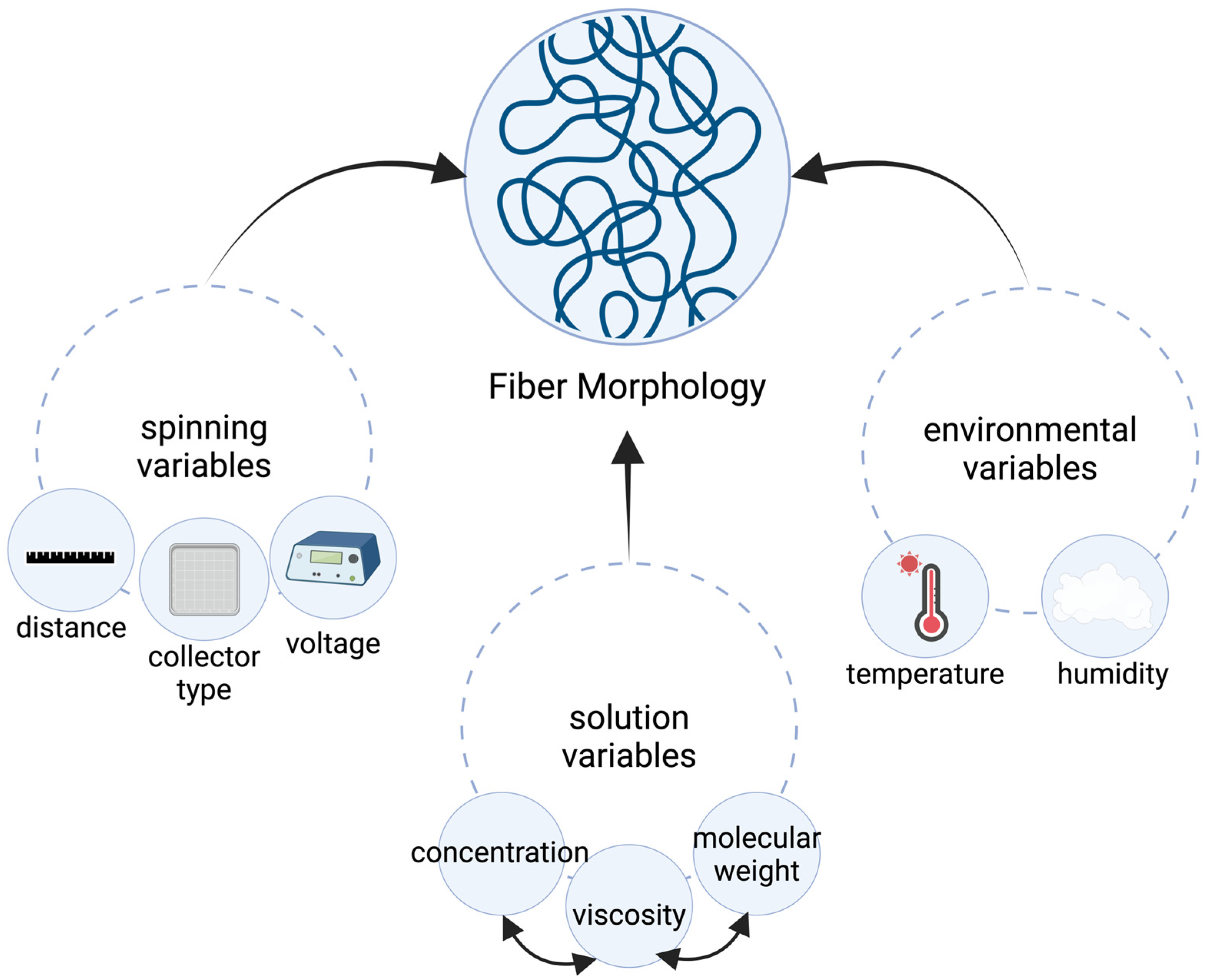

| Parameter | Effect of Fiber Morphology | References |
|---|---|---|
| Solution Parameters | ||
| Concentration | Fiber diameter increases with polymer solution concentration. Beads form at low viscosity, and microribbons form at extremely high viscosity | [4,8,10,11,12,13] |
| Polymer Molecular Weight | Fiber diameter increases with molecular weight | [14] |
| Surface Tension | Occurrence of beads decreases with a decrease in surface tension | [4,8] |
| Conductivity | Occurrence of beads decreases with a decrease in conductivity | [15] |
| Process Parameters | ||
| Flow Rate | Increasing flow rates are associated with an increase in fiber diameter | [4,8,9,16] |
| Voltage | Relationship may vary based on polymer formulation | [8,16,17] |
| Separation Distance | Beads form at large distances | [4,8] |
| Ambient Parameters | ||
| Temperature | Increasing temperatures result in a decrease in fiber diameter | [18] |
| Humidity | High relative humidity causes varied fiber diameters and morphologies | [19] |
| Material | Characteristics | Fiber Diameter * (nm) | Biomedical Applications | References |
|---|---|---|---|---|
| Natural Polymers | ||||
| Chitosan |
| 50–450 |
| [25,26,27,28] |
| Gelatin |
| 100–340 |
| [29,30,31,32,33] |
| Collagen |
| 100–1200 |
| [29,34,35,36,37] |
| Alginate |
| 120–300 |
| [29,38,39,40,41,42] |
| Synthetic Polymers | ||||
| Polylactide (PLA) |
| 360–430 |
| [29,43,44] |
| Poly(lactic-co-glycolic acid) (PLGA) |
| 100–600 |
| [45,46] |
| Polycaprolactone (PCL) |
| 320–1550 |
| [29,47,48] |
| Polyurethane (PU) |
| 456–1043 |
| [29,49,50,51,52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broadwin, M.; Imarhia, F.; Oh, A.; Stone, C.R.; Sellke, F.W.; Bhowmick, S.; Abid, M.R. Exploring Electrospun Scaffold Innovations in Cardiovascular Therapy: A Review of Electrospinning in Cardiovascular Disease. Bioengineering 2024, 11, 218. https://doi.org/10.3390/bioengineering11030218
Broadwin M, Imarhia F, Oh A, Stone CR, Sellke FW, Bhowmick S, Abid MR. Exploring Electrospun Scaffold Innovations in Cardiovascular Therapy: A Review of Electrospinning in Cardiovascular Disease. Bioengineering. 2024; 11(3):218. https://doi.org/10.3390/bioengineering11030218
Chicago/Turabian StyleBroadwin, Mark, Frances Imarhia, Amy Oh, Christopher R. Stone, Frank W. Sellke, Sankha Bhowmick, and M. Ruhul Abid. 2024. "Exploring Electrospun Scaffold Innovations in Cardiovascular Therapy: A Review of Electrospinning in Cardiovascular Disease" Bioengineering 11, no. 3: 218. https://doi.org/10.3390/bioengineering11030218
APA StyleBroadwin, M., Imarhia, F., Oh, A., Stone, C. R., Sellke, F. W., Bhowmick, S., & Abid, M. R. (2024). Exploring Electrospun Scaffold Innovations in Cardiovascular Therapy: A Review of Electrospinning in Cardiovascular Disease. Bioengineering, 11(3), 218. https://doi.org/10.3390/bioengineering11030218