First- vs. Second-Generation Autologous Platelet Concentrates and Their Implications for Wound Healing: Differences in Proteome and Secretome
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of APC
2.1.1. Generation of Platelet-Rich Fibrin (PRF)
2.1.2. Isolation of Platelet-Rich Plasma (PRP)
2.1.3. Preparation of Platelet-Free Plasma (PFP)
2.1.4. Cell Count Analysis
2.2. Macrophage Assays
2.2.1. Isolation and Polarization of Human Monocyte-Derived Macrophages
2.2.2. Flow Cytometry
2.3. Proteomic Analysis
2.3.1. Protein Extraction
2.3.2. One-Dimensional Gel Electrophoresis (1DE) SDS-PAGE
2.3.3. Mass Spectrometry (MS)-Based Proteome Analysis
2.3.4. Label-Free Quantitative Proteomic Analysis
2.4. In Vivo Analysis
YOLK SAC Membrane (YSM) Assay
2.5. Statistical Analysis
3. Results
3.1. First- and Second-Generation Platelet Concentrates Demonstrate Differences in Cell Content
3.2. First- and Second-Generation Autologous Platelet Concentrate as Well as Platelet-Free Plasma Polarize M0-Macrophages into a More “M0/M2-like” Phenotype
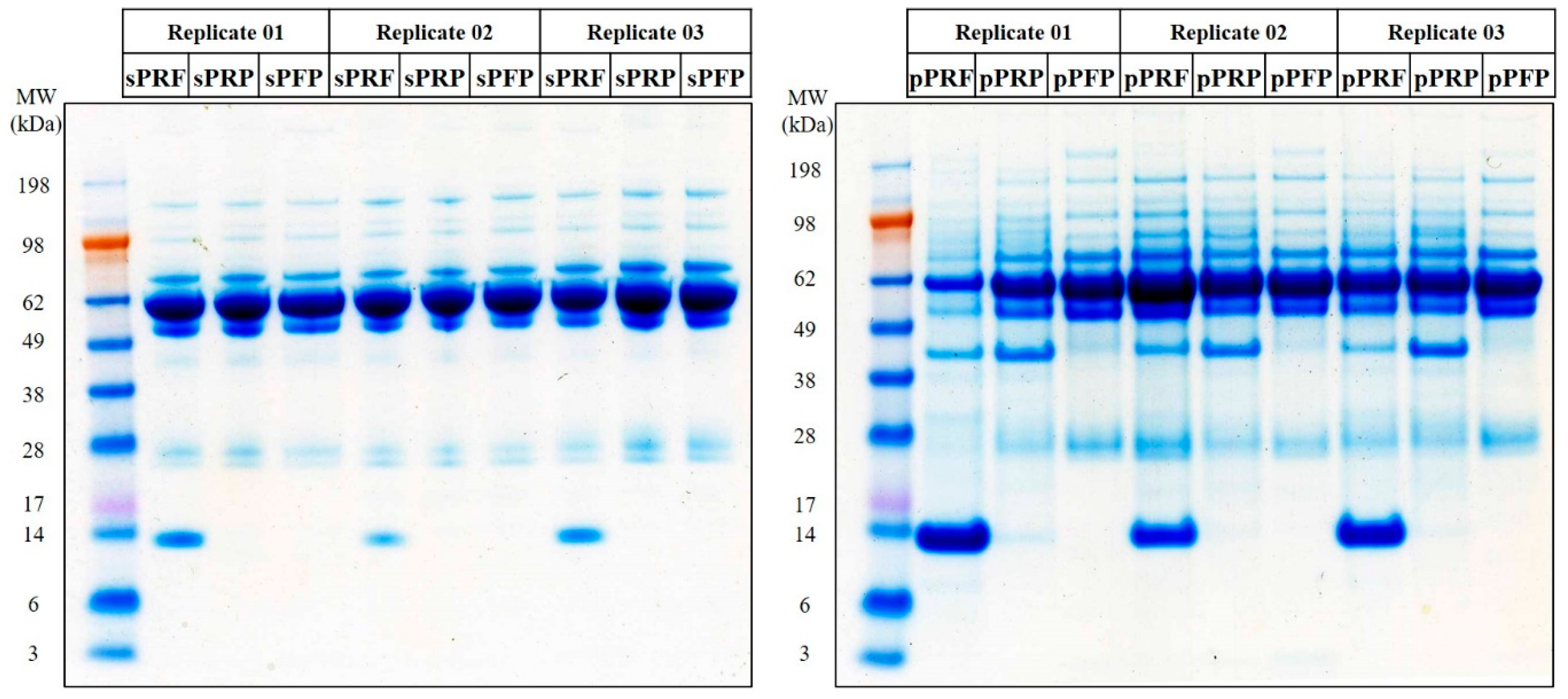
3.3. Proteome and Secretome Map of the APC Sub-Classes
3.4. Second-Generation Autologous Platelet Concentrates Demonstrate Increased Pro-Angiogenic Potential
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blatt, S.; Burkhardt, V.; Kämmerer, P.W.; Pabst, A.M.; Sagheb, K.; Heller, M.; Al-Nawas, B.; Schiegnitz, E. Biofunctionalization of porcine-derived collagen matrices with platelet rich fibrin: Influence on angiogenesis in vitro and in vivo. Clin. Oral Investig. 2020, 24, 3425–3436. [Google Scholar] [CrossRef] [PubMed]
- Puricelli, C.; Boggio, E.; Gigliotti, C.L.; Stoppa, I.; Sutti, S.; Giordano, M.; Dianzani, U.; Rolla, R. Platelets, Protean Cells with All-Around Functions and Multifaceted Pharmacological Applications. Int. J. Mol. Sci. 2023, 24, 4565. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, L.; Ríordáin, R.N. Autologous platelet concentrates in oral surgery: Protocols, properties, and clinical applications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Del Corso, M.; Vervelle, A.; Simonpieri, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; Dohan Ehrenfest, D.M. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 1: Periodontal and dentoalveolar surgery. Curr. Pharm. Biotechnol. 2012, 13, 1207–1230. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, R.; Patro, B.P.; Jeyaraman, N.; Gangadaran, P.; Rajendran, R.L.; Nallakumarasamy, A.; Jeyaraman, M.; Ramani, P.; Ahn, B.C. Evolution and Clinical Advances of Platelet-Rich Fibrin in Musculoskeletal Regeneration. Bioengineering 2023, 10, 58. [Google Scholar] [CrossRef]
- Caruana, A.; Savina, D.; Macedo, J.P.; Soares, S.C. From Platelet-Rich Plasma to Advanced Platelet-Rich Fibrin: Biological Achievements and Clinical Advances in Modern Surgery. Eur. J. Dent. 2019, 13, 280–286. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Bielecki, T.; Jimbo, R.; Barbé, G.; Del Corso, M.; Inchingolo, F.; Sammartino, G. Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte- and platelet-rich fibrin (L-PRF). Curr. Pharm. Biotechnol. 2012, 13, 1145–1152. [Google Scholar] [CrossRef]
- Kobayashi, E.; Flückiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef]
- Schär, M.O.; Diaz-Romero, J.; Kohl, S.; Zumstein, M.A.; Nesic, D. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin. Orthop. Relat. Res. 2015, 473, 1635–1643. [Google Scholar] [CrossRef]
- Neal, T.W.; Schlieve, T. Autologous platelet concentrates in oral surgery: A problem of reporting. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 134, 407. [Google Scholar] [CrossRef] [PubMed]
- Hermida-Nogueira, L.; Barrachina, M.N.; Morán, L.A.; Bravo, S.; Diz, P.; García, Á.; Blanco, J. Deciphering the secretome of leukocyte-platelet rich fibrin: Towards a better understanding of its wound healing properties. Sci. Rep. 2020, 10, 14571. [Google Scholar] [CrossRef] [PubMed]
- Al-Sharabi, N.; Gruber, R.; Sanz, M.; Mohamed-Ahmed, S.; Kristoffersen, E.K.; Mustafa, K.; Shanbhag, S. Proteomic Analysis of Mesenchymal Stromal Cells Secretome in Comparison to Leukocyte- and Platelet-Rich Fibrin. Int. J. Mol. Sci. 2023, 24, 13057. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Ghanaati, S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients’ own inflammatory cells, platelets and growth factors: The first introduction to the low speed centrifugation concept. Eur. J. Trauma Emerg. Surg. 2018, 44, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Jurk, K.; Lahav, J.; Van Aken, H.; Brodde, M.F.; Nofer, J.R.; Kehrel, B.E. Extracellular protein disulfide isomerase regulates feedback activation of platelet thrombin generation via modulation of coagulation factor binding. J. Thromb. Haemost. 2011, 9, 2278–2290. [Google Scholar] [CrossRef]
- Dohrmann, M.; Makhoul, S.; Gross, K.; Krause, M.; Pillitteri, D.; von Auer, C.; Walter, U.; Lutz, J.; Volf, I.; Kehrel, B.E.; et al. CD36-fibrin interaction propagates FXI-dependent thrombin generation of human platelets. FASEB J. 2020, 34, 9337–9357. [Google Scholar] [CrossRef]
- Dahlen, B.; Schulz, A.; Göbel, S.; Tröbs, S.O.; Schwuchow-Thonke, S.; Spronk, H.M.; Prochaska, J.H.; Arnold, N.; Lackner, K.J.; Gori, T.; et al. The impact of platelet indices on clinical outcome in heart failure: Results from the MyoVasc study. ESC Heart Fail. 2021, 8, 2991–3001. [Google Scholar] [CrossRef]
- Trzeciak, E.R.; Zimmer, N.; Kammerer, P.W.; Thiem, D.; Al-Nawas, B.; Tuettenberg, A.; Blatt, S. GARP Regulates the Immune Capacity of a Human Autologous Platelet Concentrate. Biomedicines 2022, 10, 3136. [Google Scholar] [CrossRef]
- Kotecha, N.; Krutzik, P.O.; Irish, J.M. Web-based analysis and publication of flow cytometry experiments. Curr. Protoc. Cytom. 2010, 53, 10.17.1–10.17.24. [Google Scholar] [CrossRef]
- Perumal, N.; Straßburger, L.; Herzog, D.P.; Müller, M.B.; Pfeiffer, N.; Grus, F.H.; Manicam, C. Bioenergetic shift and actin cytoskeleton remodelling as acute vascular adaptive mechanisms to angiotensin II in murine retina and ophthalmic artery. Redox Biol. 2020, 34, 101597. [Google Scholar] [CrossRef]
- Perumal, N.; Straßburger, L.; Schmelter, C.; Gericke, A.; Pfeiffer, N.; Grus, F.H.; Manicam, C. Sample preparation for mass-spectrometry-based proteomics analysis of ocular microvessels. JoVE J. Vis. Exp. 2019, 144, e59140. [Google Scholar] [CrossRef]
- Perumal, N.; Herfurth, A.; Pfeiffer, N.; Manicam, C. Short-Term Omega-3 Supplementation Modulates Novel Neurovascular and Fatty Acid Metabolic Proteome Changes in the Retina and Ophthalmic Artery of Mice with Targeted Cyp2c44 Gene Deletion. Cells 2022, 11, 3494. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Luber, C.A.; Cox, J.; Lauterbach, H.; Fancke, B.; Selbach, M.; Tschopp, J.; Akira, S.; Wiegand, M.; Hochrein, H.; O’Keeffe, M.; et al. Quantitative proteomics reveals subset-specific viral recognition in dendritic cells. Immunity 2010, 32, 279–289. [Google Scholar] [CrossRef]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote) omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Del Corso, M.; Diss, A.; Mouhyi, J.; Charrier, J.B. Three-dimensional architecture and cell composition of a Choukroun’s platelet-rich fibrin clot and membrane. J. Periodontol. 2010, 81, 546–555. [Google Scholar] [CrossRef]
- Everts, P.A.; Malanga, G.A.; Paul, R.V.; Rothenberg, J.B.; Stephens, N.; Mautner, K.R. Assessing clinical implications and perspectives of the pathophysiological effects of erythrocytes and plasma free hemoglobin in autologous biologics for use in musculoskeletal regenerative medicine therapies. A review. Regen. Ther. 2019, 11, 56–64. [Google Scholar] [CrossRef]
- Bielecki, T.; Dohan Ehrenfest, D.M.; Everts, P.A.; Wiczkowski, A. The role of leukocytes from L-PRP/L-PRF in wound healing and immune defense: New perspectives. Curr. Pharm. Biotechnol. 2012, 13, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Cl, K.; Jeyaraman, M.; Jeyaraman, N.; Ramasubramanian, S.; Khanna, M.; Yadav, S. Antimicrobial Effects of Platelet-Rich Plasma and Platelet-Rich Fibrin: A Scoping Review. Cureus 2023, 15, e51360. [Google Scholar] [CrossRef] [PubMed]
- Yaprak, E.; Kasap, M.; Akpinar, G.; Islek, E.E.; Sinanoglu, A. Abundant proteins in platelet-rich fibrin and their potential contribution to wound healing: An explorative proteomics study and review of the literature. J. Dent. Sci. 2018, 13, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, J.H.; Wang, K. The role of fibronectin in mediating cell migration. Am. J. Physiol. Cell Physiol. 2024, 326, C1212–C1225. [Google Scholar] [CrossRef] [PubMed]
- Kyriakides, T.R.; Maclauchlan, S. The role of thrombospondins in wound healing, ischemia, and the foreign body reaction. J. Cell Commun. Signal 2009, 3, 215–225. [Google Scholar] [CrossRef]
- Al-Ostoot, F.H.; Salah, S.; Khamees, H.A.; Khanum, S.A. Tumor angiogenesis: Current challenges and therapeutic opportunities. Cancer Treat. Res. Commun. 2021, 28, 100422. [Google Scholar] [CrossRef]
- Kaur, S.; Bronson, S.M.; Pal-Nath, D.; Miller, T.W.; Soto-Pantoja, D.R.; Roberts, D.D. Functions of Thrombospondin-1 in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 4570. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Reddy, P.; Lent-Schochet, D.; Ramakrishnan, N.; McLaughlin, M.; Jialal, I. Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clin. Chim. Acta 2019, 496, 35–44. [Google Scholar] [CrossRef]
- Dohle, E.; Parkhoo, K.; Bennardo, F.; Schmeinck, L.; Sader, R.; Ghanaati, S. Immunomodulation of Cancer Cells Using Autologous Blood Concentrates as a Patient-Specific Cell Culture System: A Comparative Study on Osteosarcoma and Fibrosarcoma Cell Lines. Bioengineering 2024, 11, 303. [Google Scholar] [CrossRef]
- Giannotti, L.; Di Chiara Stanca, B.; Spedicato, F.; Nitti, P.; Damiano, F.; Demitri, C.; Calabriso, N.; Carluccio, M.A.; Palermo, A.; Siculella, L.; et al. Progress in Regenerative Medicine: Exploring Autologous Platelet Concentrates and Their Clinical Applications. Genes 2023, 14, 1669. [Google Scholar] [CrossRef] [PubMed]
- Blatt, S.; Thiem, D.G.E.; Kyyak, S.; Pabst, A.; Al-Nawas, B.; Kämmerer, P.W. Possible Implications for Improved Osteogenesis? The Combination of Platelet-Rich Fibrin with Different Bone Substitute Materials. Front. Bioeng. Biotechnol. 2021, 9, 640053. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kawase, T.; Okuda, K.; Wolff, L.F.; Yoshie, H. In vitro immunological and biological evaluations of the angiogenic potential of platelet-rich fibrin preparations: A standardized comparison with PRP preparations. Int. J. Implant. Dent. 2015, 1, 31. [Google Scholar] [CrossRef] [PubMed]








| Parameter | Full Blood (EDTA) | PRF | PRP | PFP | Statistical Significance (p-Value) |
|---|---|---|---|---|---|
| Platelet Count (×109/L) | 261.3 ± 12.5 | 345.7 ± 20.3 | 369.7 ± 15.2 | 0 | p < 0.01 |
| Erythrocyte Count (×109/L) | 4503 ± 300 | 23.33 ± 1.5 | 20.00 ± 1.3 | 0 | p < 0.05 |
| Leukocyte Count (×109/L) | 6.853 ± 0.4 | 1.333 ± 0.2 | 0.003 ± 0.001 | 0 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stiller, H.L.; Perumal, N.; Manicam, C.; Trzeciak, E.R.; Todt, J.; Jurk, K.; Tuettenberg, A.; Schumann, S.; Schiegnitz, E.; Blatt, S. First- vs. Second-Generation Autologous Platelet Concentrates and Their Implications for Wound Healing: Differences in Proteome and Secretome. Bioengineering 2024, 11, 1171. https://doi.org/10.3390/bioengineering11111171
Stiller HL, Perumal N, Manicam C, Trzeciak ER, Todt J, Jurk K, Tuettenberg A, Schumann S, Schiegnitz E, Blatt S. First- vs. Second-Generation Autologous Platelet Concentrates and Their Implications for Wound Healing: Differences in Proteome and Secretome. Bioengineering. 2024; 11(11):1171. https://doi.org/10.3390/bioengineering11111171
Chicago/Turabian StyleStiller, Hanna L., Natarajan Perumal, Caroline Manicam, Emily R. Trzeciak, Julia Todt, Kerstin Jurk, Andrea Tuettenberg, Sven Schumann, Eik Schiegnitz, and Sebastian Blatt. 2024. "First- vs. Second-Generation Autologous Platelet Concentrates and Their Implications for Wound Healing: Differences in Proteome and Secretome" Bioengineering 11, no. 11: 1171. https://doi.org/10.3390/bioengineering11111171
APA StyleStiller, H. L., Perumal, N., Manicam, C., Trzeciak, E. R., Todt, J., Jurk, K., Tuettenberg, A., Schumann, S., Schiegnitz, E., & Blatt, S. (2024). First- vs. Second-Generation Autologous Platelet Concentrates and Their Implications for Wound Healing: Differences in Proteome and Secretome. Bioengineering, 11(11), 1171. https://doi.org/10.3390/bioengineering11111171









