Proteoglycans Enhance the Therapeutic Effect of BMSC Transplantation on Osteoarthritis
Abstract
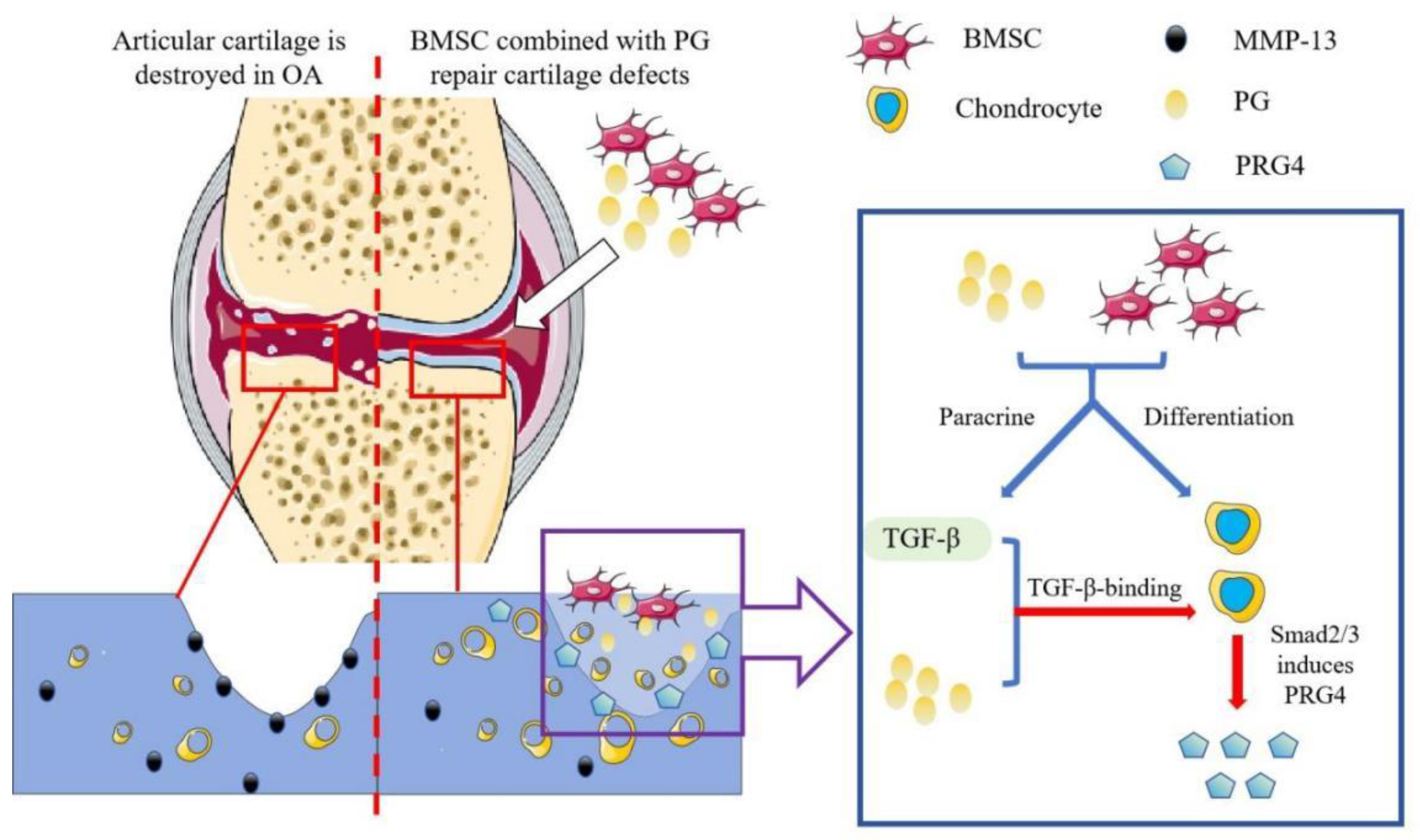
1. Introduction
2. Materials and Methods
2.1. Cell Culture
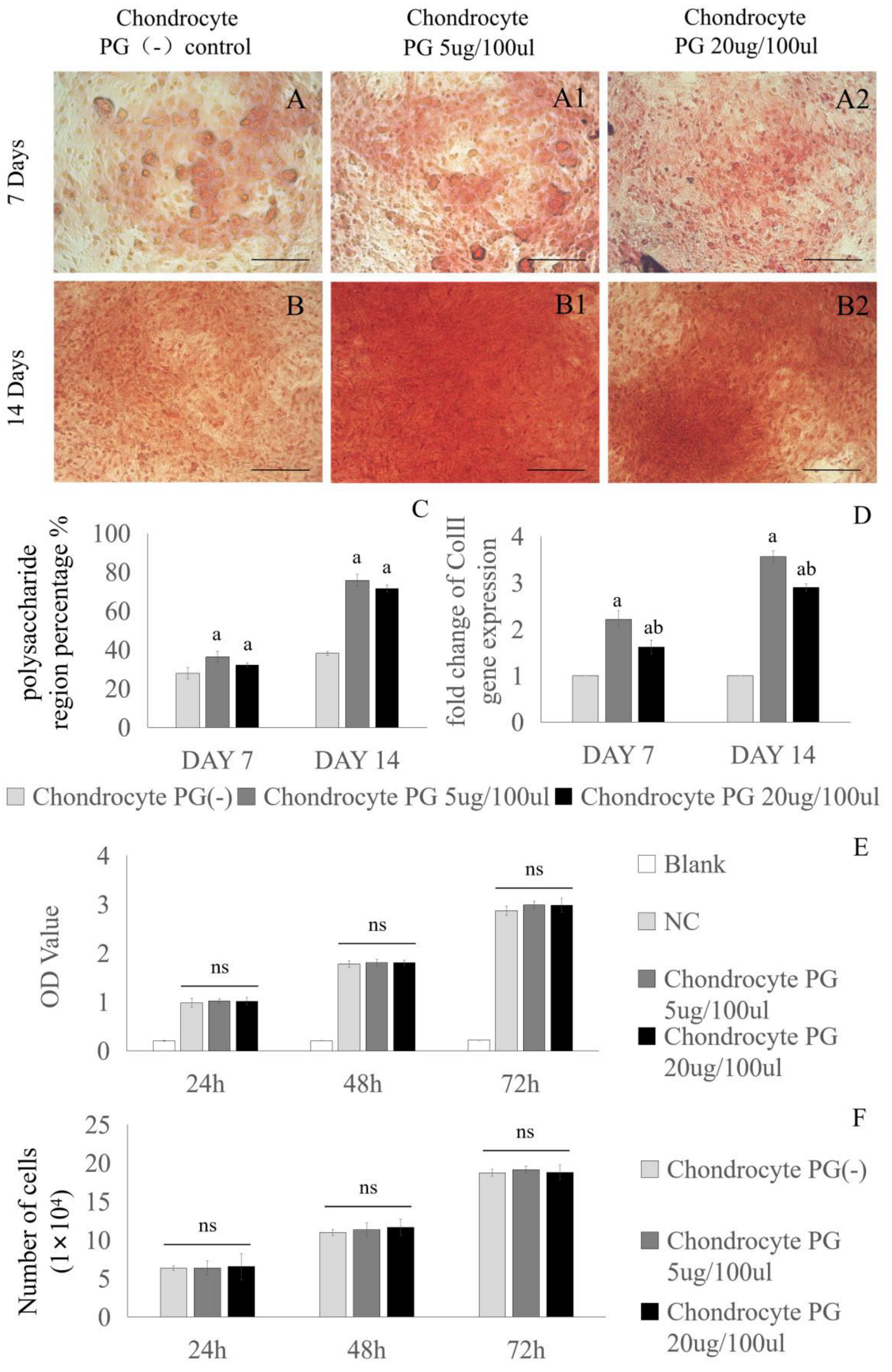
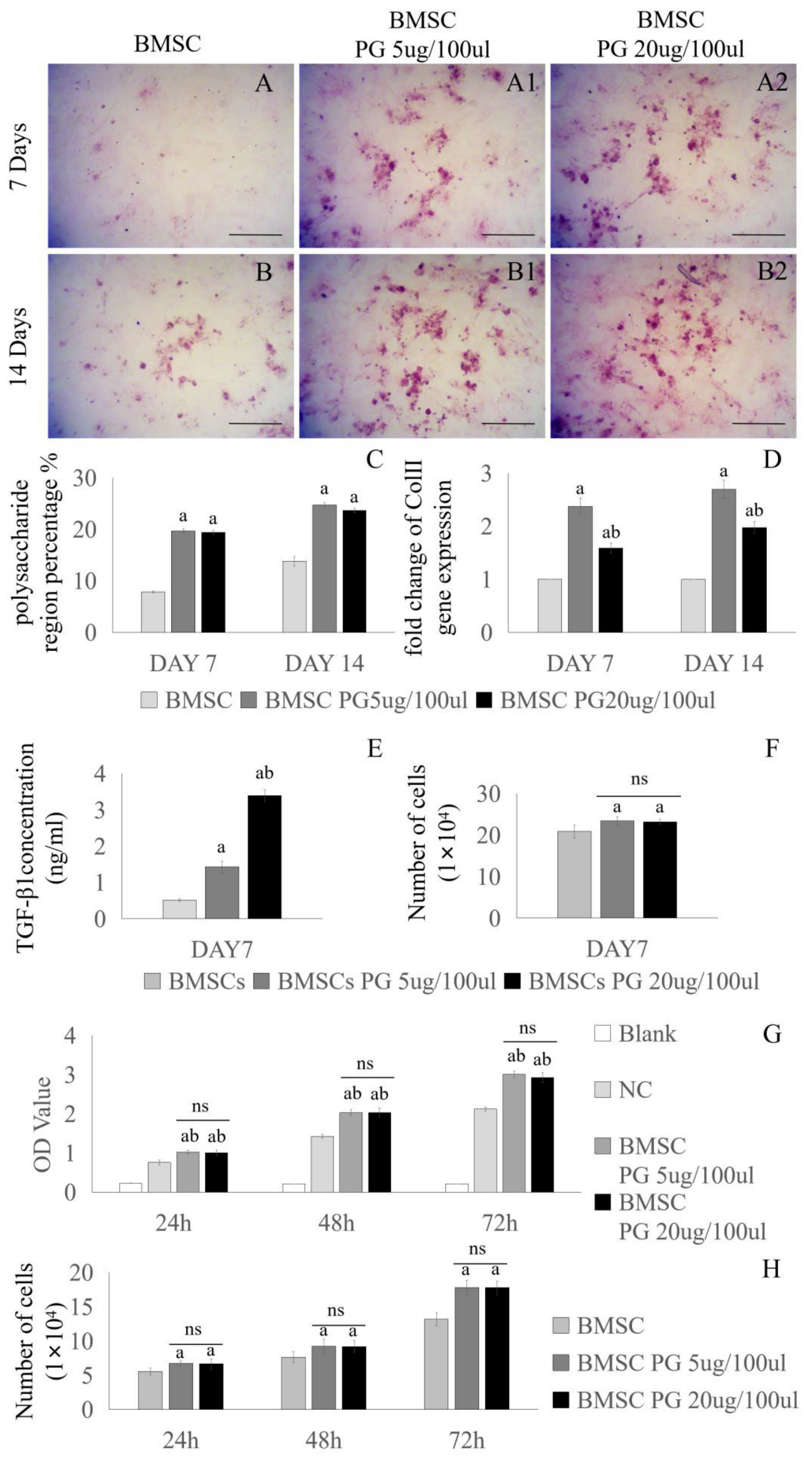
2.2. Safranin O Staining of Cells
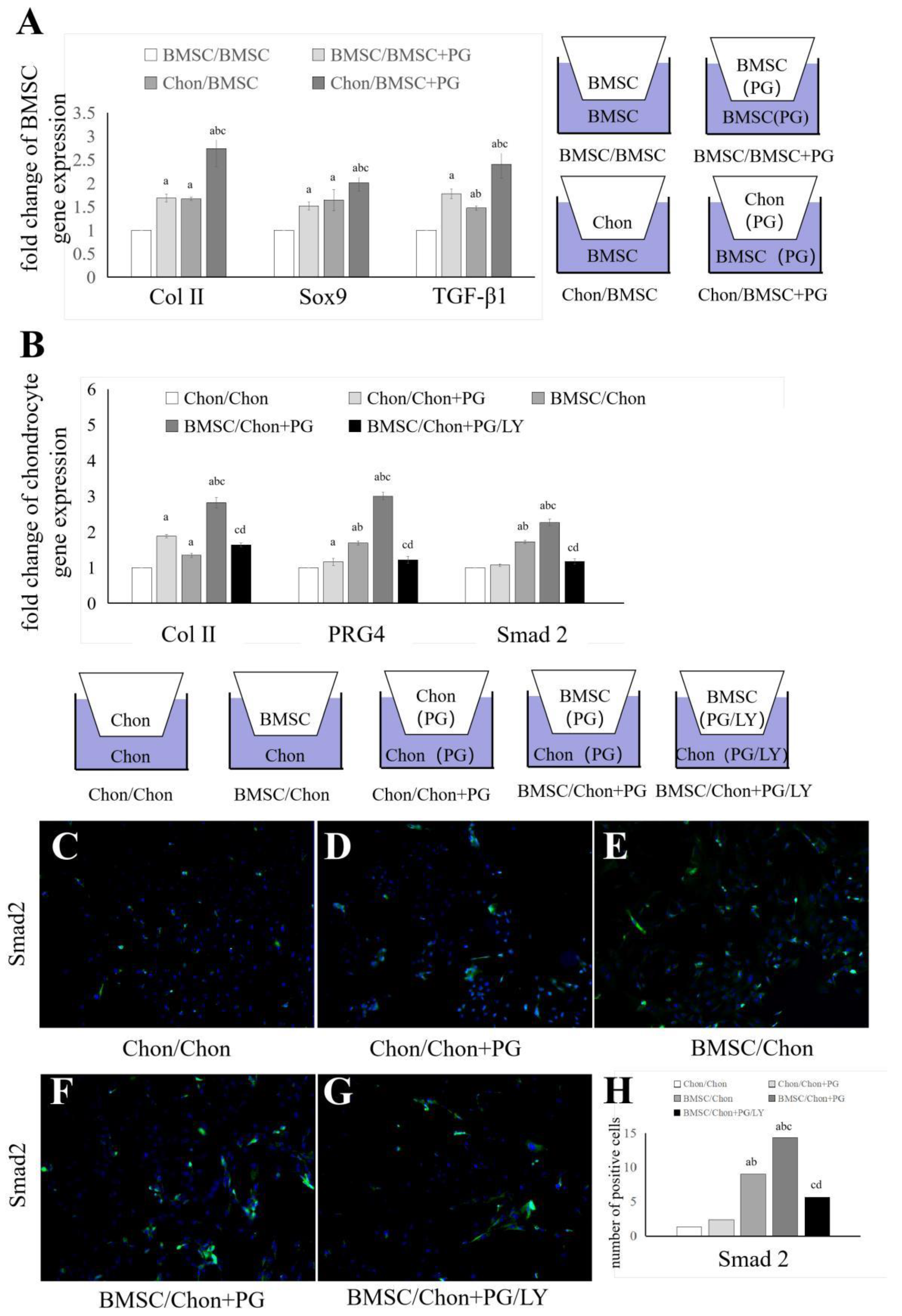
2.3. Real-Time Quantitative PCR Analysis of Chondrocytes and BMSCs
- Col II: F-GTCCTTCTGGAGATCAGGGTACT, R-ATTCCATTAGAGCCATCTTTGCC;
- PRG4: F-GTGCCCATCAAAGCCTCTTATCA, R-CAGTGTTATCGCGGAAGTAACGA;
- Sox 9: F-TGACCCGTTGATGTCCACTT, R-TCCACGAAGGGTCTCTTCTCG;
- TGF-β1: F-CTGCTGACCCCCACTGATAC, R-TGACCCGTTGATGTCCACTT;
- Smad2: F-TCCATCTTGCCATTCAC, R-TTCTTCCTGCCCATTCT.
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Cell Counting Kit-8 Proliferation Assay
2.6. Cell Counting Protocol
2.7. Transwell Coculture System
2.8. Cellular Immunofluorescence Staining
2.9. Establishment of the Animal OA Model and Intra-Articular Injection of Treatments
2.10. Safranin O–Fast Green Staining of Paraffin Sections
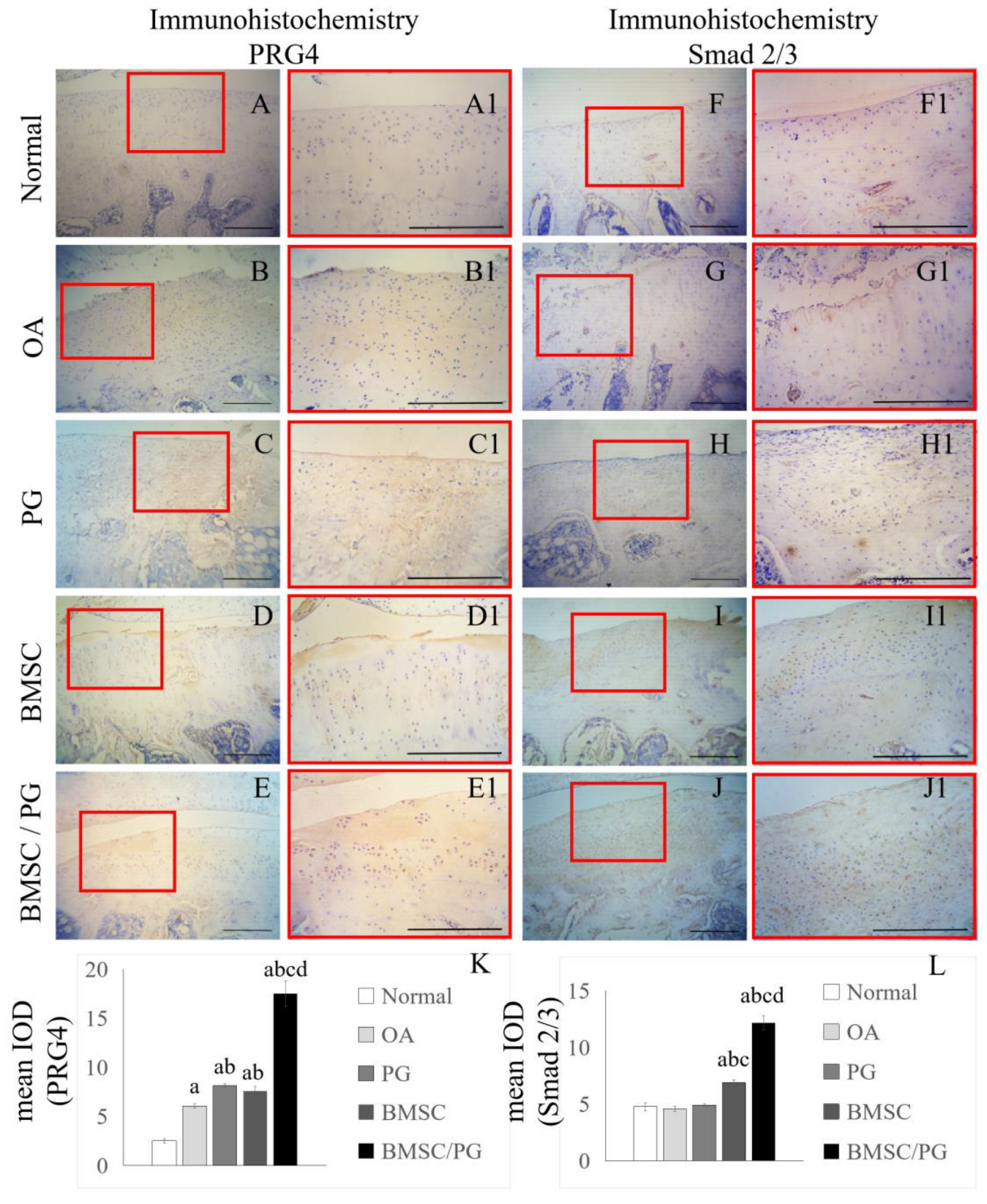
2.11. Detection of Protein Levels Using Immunohistochemistry
2.12. Statistical Analysis
3. Results
3.1. Chondrogenic Effect of Proteoglycans on Cartilage Cells
3.2. Effect of Proteoglycans on the Chondrogenic Differentiation of BMSCs
3.3. PG Affects Intercellular Communication Between BMSCs and Chondrocytes Through the TGF-β Signaling Pathway
3.4. BMSCs Combined with PGs Have a Better Protective Effect on the Articular Cartilage of Rats with OA
3.5. BMSCs Combined with PGs Promoted the Production of PRG4 by Increasing the Level of Smad2 in Rat Chondrocytes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Narcisi, R.; Quarto, R.; Ulivi, V.; Muraglia, A.; Molfetta, L.; Giannoni, P. TGF β-1 administration during ex vivo expansion of human articular chondrocytes in a serum-free medium redirects the cell phenotype toward hypertrophy. J. Cell. Physiol. 2012, 227, 3282–3290. [Google Scholar] [CrossRef] [PubMed]
- Sebbag, E.; Felten, R.; Sagez, F.; Sibilia, J.; Devilliers, H.; Arnaud, L. The world-wide burden of musculoskeletal diseases: A sys-tematic analysis of the World Health Organization Burden of Diseases Database. Ann. Rheum. Dis. 2019, 78, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; He, T.; Bajpayee, A.G. Recent advances in targeted drug delivery for treatment of osteoarthritis. Curr. Opin. Rheumatol. 2021, 33, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Maudens, P.; Jordan, O.; Allémann, E. Recent advances in intra-articular drug delivery systems for osteoarthritis therapy. Drug Discov. Today 2018, 23, 1761–1775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, T.; Ran, C.; Wang, W.; Piao, F.; Yang, J.; Tian, S.; Li, L.; Zhao, D. Immunoregulatory paracrine effect of mesenchymal stem cells and mechanism in the treatment of osteoarthritis. Front. Cell Dev. Biol. 2024, 12, 1411507. [Google Scholar] [CrossRef]
- Roseti, L.; Desando, G.; Cavallo, C.; Petretta, M.; Grigolo, B. Articular Cartilage Regeneration in Osteoarthritis. Cells 2019, 8, 1305. [Google Scholar] [CrossRef]
- Filardo, G.; Perdisa, F.; Roffi, A.; Marcacci, M.; Kon, E. Stem cells in articular cartilage regeneration. J. Orthop. Surg. Res. 2016, 11, 42. [Google Scholar] [CrossRef]
- Kim, K.-I.; Lee, M.C.; Lee, J.H.; Moon, Y.-W.; Lee, W.-S.; Lee, H.-J.; Hwang, S.-C.; In, Y.; Shon, O.-J.; Bae, K.-C.; et al. Clinical Efficacy and Safety of the Intra-articular Injection of Autologous Adipose-Derived Mesenchymal Stem Cells for Knee Osteoarthritis: A Phase III, Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Sports Med. 2023, 51, 2243–2253. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Lei, J.; Deng, H.; Ran, Y.; Lv, Y.; Amhare, A.F.; Wang, L.; Guo, X.; Han, J.; Lammi, M.J. Altered Expression of Aggrecan, FAM20B, B3GALT6, and EXTL2 in Patients with Osteoarthritis and Kashin-Beck Disease. Cartilage 2021, 13 (Suppl. 1), 818S–828S. [Google Scholar] [CrossRef]
- Mehana, E.-S.E.; Khafaga, A.F.; El-Blehi, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef] [PubMed]
- Damen, A.; van Donkelaar, C.; Cardinaels, R.; Brandt, J.-M.; Schmidt, T.; Ito, K. Proteoglycan 4 reduces friction more than other synovial fluid components for both cartilage-cartilage and cartilage-metal articulation. Osteoarthr. Cartil. 2021, 29, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Tham, M.; Ramasamy, S.; Gan, H.T.; Ramachandran, A.; Poonepalli, A.; Yu, Y.H.; Ahmed, S. CSPG is a secreted factor that stimulates neural stem cell survival possibly by enhanced EGFR signaling. PLoS ONE 2010, 5, e15341. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lai, J.H.; Han, L.H.; Tong, X.; Yang, F. Modulating stem cell-chondrocyte interactions for cartilage repair using com-binatorial extracellular matrix-containing hydrogels. J. Mater. Chem. B 2016, 4, 7641–7650. [Google Scholar] [CrossRef] [PubMed]
- Sirko, S.; Akita, K.; Von Holst, A.; Faissner, A. Structural and functional analysis of chondroitin sulfate proteoglycans in the neural stem cell niche. Methods Enzymol. 2010, 479, 37–71. [Google Scholar]
- David-Raoudi, M.; Deschrevel, B.; Leclercq, S.; Galéra, P.; Boumediene, K.; Pujol, J. Chondroitin sulfate increases hyaluronan production by human synoviocytes through differential regulation of hyaluronan synthases: Role of p38 and Akt. Arthritis Rheum. 2009, 60, 760–770. [Google Scholar] [CrossRef]
- Zhen, G.; Wen, C.; Jia, X.; Li, Y.; Crane, J.L.; Mears, S.C.; Askin, F.B.; Frassica, F.J.; Chang, W.; Yao, J.; et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 2013, 19, 704–712. [Google Scholar] [CrossRef]
- Wu, M.; Wu, S.; Chen, W.; Li, Y.P. The roles and regulatory mechanisms of TGF-beta and BMP signaling in bone and cartilage development, homeostasis and disease. Cell Res. 2024, 34, 101–123. [Google Scholar] [CrossRef]
- Yunus, M.H.M.; Nordin, A.; Kamal, H. Pathophysiological Perspective of Osteoarthritis. Medicina 2020, 56, 614. [Google Scholar] [CrossRef]
- Aaron, R.K.; Racine, J.; Dyke, J.P. Contribution of Circulatory Disturbances in Subchondral Bone to the Pathophysiology of Osteoarthritis. Curr. Rheumatol. Rep. 2017, 19, 49. [Google Scholar] [CrossRef]
- Johnson, K.; Zhu, S.; Tremblay, M.S.; Payette, J.N.; Wang, J.; Bouchez, L.C.; Meeusen, S.; Althage, A.; Cho, C.Y.; Wu, X.; et al. A stem cell-based approach to cartilage repair. Science 2012, 336, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.; Chevalier, X.; Henrotin, Y.; Hunter, D.; Uebelhart, D. Symptom and structure modification in osteoarthritis with pharmaceutical-grade chondroitin sulfate: What’s the evidence? Curr. Med. Res. Opin. 2013, 29, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Monfort, J.; Pelletier, J.P.; Garcia-Giralt, N.; Martel-Pelletier, J. Biochemical basis of the effect of chondroitin sulphate on oste-oarthritis articular tissues. Ann. Rheum. Dis. 2008, 67, 735–740. [Google Scholar] [CrossRef] [PubMed]
- López-Senra, E.; Casal-Beiroa, P.; López-Álvarez, M.; Serra, J.; González, P.; Valcarcel, J.; Vázquez, J.A.; Burguera, E.F.; Blanco, F.J.; Magalhães, J. Impact of Prevalence Ratios of Chondroitin Sulfate (CS)- 4 and -6 Isomers Derived from Marine Sources in Cell Proliferation and Chondrogenic Differentiation Processes. Mar. Drugs 2020, 18, 94. [Google Scholar] [CrossRef]
- Krawetz, R.J.; Wu, Y.E.; Bertram, K.L.; Shonak, A.; Masson, A.O.; Ren, G.; Leonard, C.; Kapoor, M.; Matyas, J.R.; Salo, P.T. Synovial mesenchymal progenitor derived aggrecan regulates cartilage homeostasis and endogenous repair capacity. Cell Death Dis. 2022, 13, 470. [Google Scholar] [CrossRef]
- Bachvarova, V.; Dierker, T.; Esko, J.; Hoffmann, D.; Kjellen, L.; Vortkamp, A. Chondrocytes respond to an altered heparan sul-fate composition with distinct changes of heparan sulfate structure and increased levels of chondroitin sulfate. Matrix Biol. 2020, 93, 43–59. [Google Scholar] [CrossRef]
- Lamoureux, F.; Baud’Huin, M.; Duplomb, L.; Heymann, D.; Rédini, F. Proteoglycans: Key partners in bone cell biology. BioEssays 2007, 29, 758–771. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Gialeli, C.; Bouris, P.; Giannopoulou, E.; Skandalis, S.S.; Aletras, A.J.; Iozzo, R.V.; Karamanos, N.K. Cell–matrix interactions: Focus on proteoglycan–proteinase interplay and pharmacological targeting in cancer. FEBS J. 2014, 281, 5023–5042. [Google Scholar] [CrossRef]
- Sorensen, H.P.; Vives, R.R.; Manetopoulos, C.; Albrechtsen, R.; Lydolph, M.C.; Jacobsen, J.; Couchman, J.R.; Wewer, U.M. Hepa-ran sulfate regulates ADAM12 through a molecular switch mechanism. J. Biol. Chem. 2008, 283, 31920–31932. [Google Scholar] [CrossRef]
- Malla, N.; Berg, E.; Theocharis, A.D.; Svineng, G.; Uhlin-Hansen, L.; Winberg, J. In vitro reconstitution of complexes between pro-matrix metalloproteinase-9 and the proteoglycans serglycin and versican. FEBS J. 2013, 280, 2870–2887. [Google Scholar] [CrossRef]
- Alcaide-Ruggiero, L.; Cugat, R.; Domínguez, J.M. Proteoglycans in Articular Cartilage and Their Contribution to Chondral Injury and Repair Mechanisms. Int. J. Mol. Sci. 2023, 24, 10824. [Google Scholar] [CrossRef] [PubMed]
- Ruan, M.Z.C.; Erez, A.; Guse, K.; Dawson, B.; Bertin, T.; Chen, Y.; Jiang, M.-M.; Yustein, J.; Gannon, F.; Lee, B.H.L. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci. Transl. Med. 2013, 5, 176ra34. [Google Scholar] [CrossRef] [PubMed]
- Hwang, N.S.; Varghese, S.; Puleo, C.; Zhang, Z.; Elisseeff, J. Morphogenetic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. J. Cell. Physiol. 2007, 212, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, X.; He, X.; Wu, B.; Xu, M.; Chang, H.; Zhang, X.; Xing, Z.; Jing, X.; Kong, D.; et al. Delivery of the Sox9 gene promotes chondrogenic differentiation of human umbilical cord blood-derived mesenchymal stem cells in an in vitro model. Braz. J. Med. Biol. Res. 2014, 47, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, G.; Wang, W.; Ran, C.; Piao, F.; Ma, Z.; Zhang, Z.; Zheng, G.; Cao, F.; Xie, H.; et al. Bone marrow mesenchymal stem cells paracrine TGF-β1 to mediate the biological activity of osteoblasts in bone repair. Cytokine 2023, 164, 156139. [Google Scholar] [CrossRef]
- Lee, S.Y.; Nakagawa, T.; Reddi, A.H. Induction of chondrogenesis and expression of superficial zone protein (SZP)/lubricin by mesenchymal progenitors in the infrapatellar fat pad of the knee joint treated with TGF-beta1 and BMP-7. Biochem. Biophys. Res. Commun. 2008, 376, 148–153. [Google Scholar] [CrossRef]
- Takahata, Y.; Hagino, H.; Kimura, A.; Urushizaki, M.; Yamamoto, S.; Wakamori, K.; Murakami, T.; Hata, K.; Nishimura, R. Regu-latory Mechanisms of Prg4 and Gdf5 Expression in Articular Cartilage and Functions in Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 4672. [Google Scholar] [CrossRef]
- Schmidt, T.A.; Gastelum, N.S.; Han, E.H.; Nugent-Derfus, G.E.; Schumacher, B.L.; Sah, R.L. Differential regulation of proteoglycan 4 metabolism in cartilage by IL-1alpha, IGF-I, and TGF-beta1. Osteoarthr. Cartil. 2008, 16, 90–97. [Google Scholar] [CrossRef]
- Panahipour, L.; Omerbasic, A.; Nasirzade, J.; Gruber, R. TGF-β Activity of a Demineralized Bone Matrix. Int. J. Mol. Sci. 2021, 22, 664. [Google Scholar] [CrossRef]
- Markmann, A.; Hausser, H.; Schönherr, E.; Kresse, H. Influence of decorin expression on transforming growth fac-tor-beta-mediated collagen gel retraction and biglycan induction. Matrix Biol. 2000, 19, 631–636. [Google Scholar] [CrossRef]
- Couchman, J.R.; Pataki, C.A. An introduction to proteoglycans and their localization. J. Histochem. Cytochem. 2012, 60, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, U. A personal voyage through the proteoglycan field. Matrix Biol. 2014, 35, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Lin, X. Shaping morphogen gradients by proteoglycans. Cold Spring Harb. Perspect. Biol. 2009, 1, a002493. [Google Scholar] [CrossRef] [PubMed]
- Clayton, S.W.; Ban, G.I.; Liu, C.; Serra, R. Canonical and noncanonical TGF-β signaling regulate fibrous tissue differentiation in the axial skeleton. Sci. Rep. 2020, 10, 21364. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, C.; Liu, T.; Bao, Y.; Wang, W.; Xue, D.; Yin, G.; Zhang, X.; Zhao, D. Proteoglycans Enhance the Therapeutic Effect of BMSC Transplantation on Osteoarthritis. Bioengineering 2024, 11, 1167. https://doi.org/10.3390/bioengineering11111167
Ran C, Liu T, Bao Y, Wang W, Xue D, Yin G, Zhang X, Zhao D. Proteoglycans Enhance the Therapeutic Effect of BMSC Transplantation on Osteoarthritis. Bioengineering. 2024; 11(11):1167. https://doi.org/10.3390/bioengineering11111167
Chicago/Turabian StyleRan, Chunxiao, Tianhao Liu, Yongming Bao, Weidan Wang, Dongling Xue, Guangxiao Yin, Xiuzhi Zhang, and Dewei Zhao. 2024. "Proteoglycans Enhance the Therapeutic Effect of BMSC Transplantation on Osteoarthritis" Bioengineering 11, no. 11: 1167. https://doi.org/10.3390/bioengineering11111167
APA StyleRan, C., Liu, T., Bao, Y., Wang, W., Xue, D., Yin, G., Zhang, X., & Zhao, D. (2024). Proteoglycans Enhance the Therapeutic Effect of BMSC Transplantation on Osteoarthritis. Bioengineering, 11(11), 1167. https://doi.org/10.3390/bioengineering11111167





