Introduction of AI Technology for Objective Physical Function Assessment
Abstract
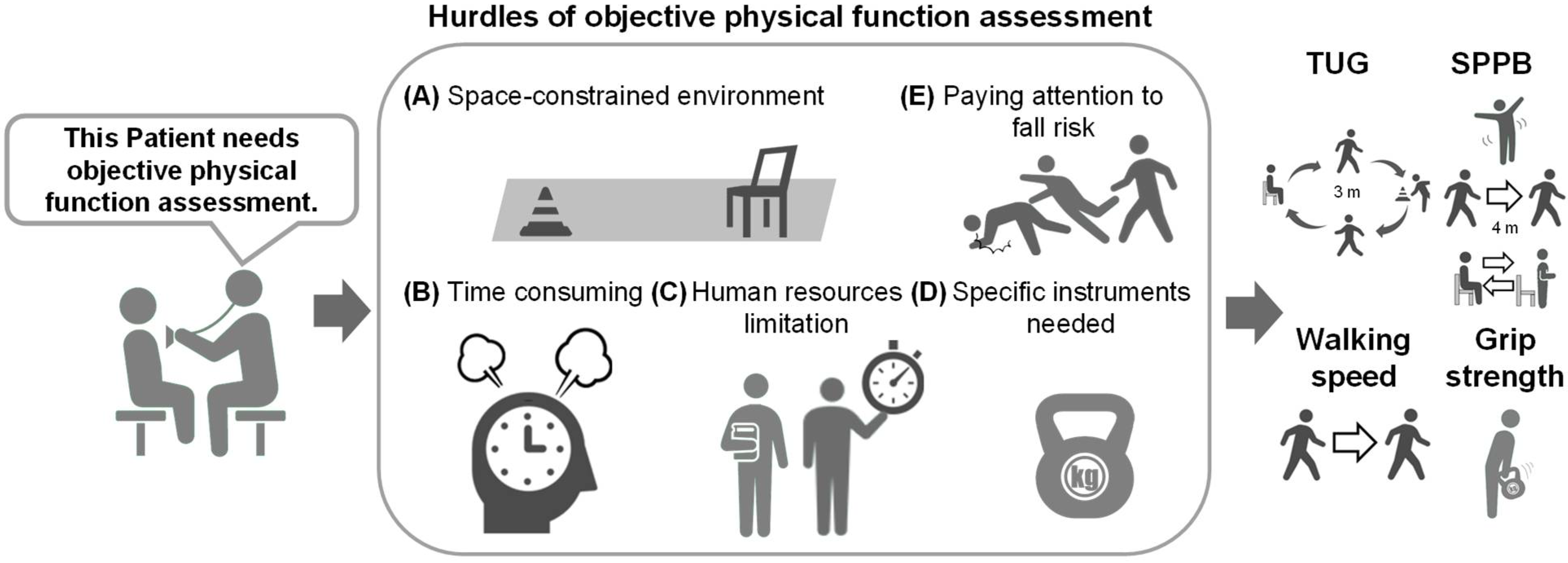
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Selection Criteria
3. Results
3.1. Overall Trends in AI-Based Objective Physical Function Assessment Research
3.2. Characteristics of Studies Setting TUG/SPPB as an Output Label
3.3. Characteristics of Studies Setting Grip Strength an Output Label
3.4. Characteristics of Studies Setting Walking Speed an Output Label
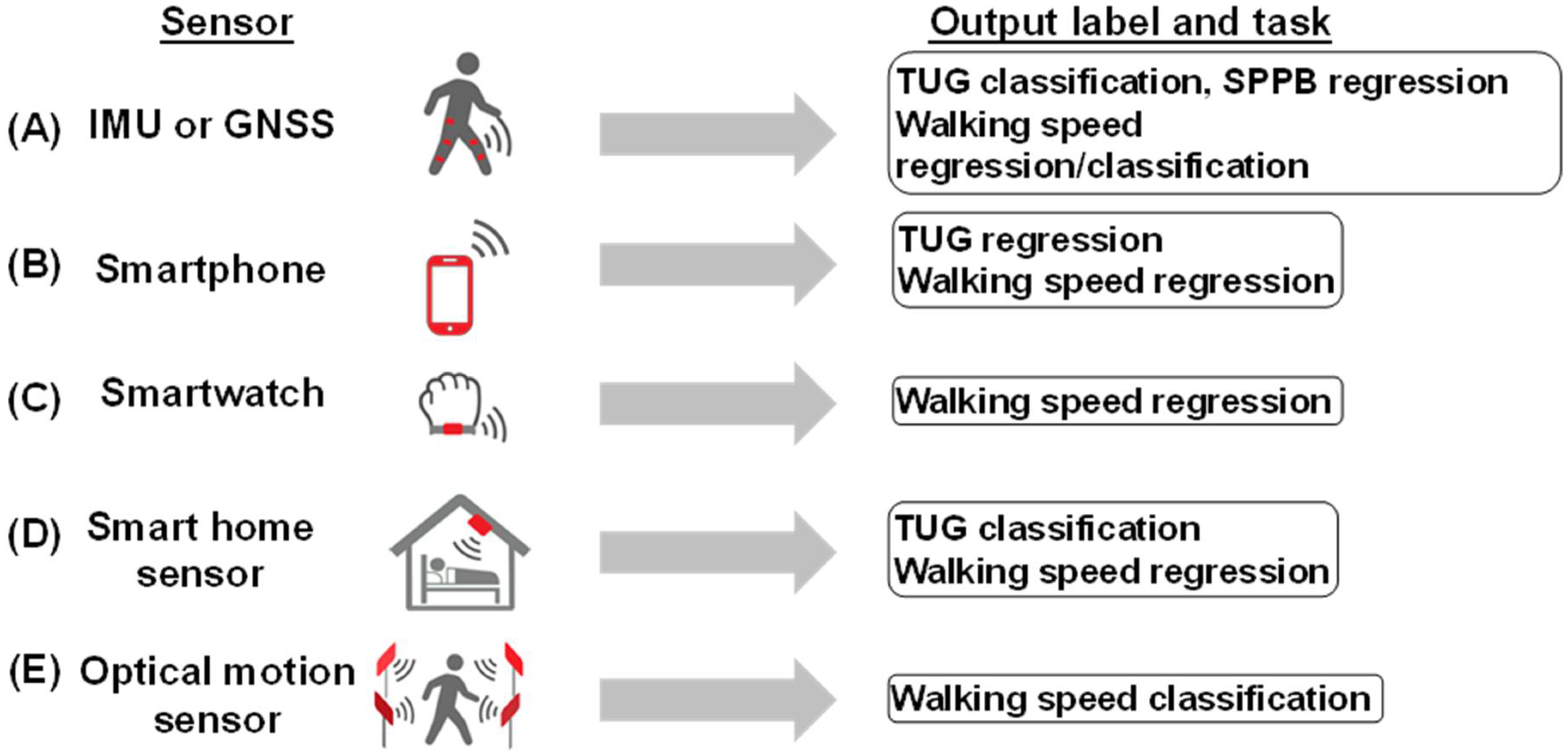
3.5. Summary of Sensor Data Acquisition
3.6. Studies with Image Input Data
3.7. Studies with Video Input Data
3.8. Studies with Tabular Input Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakker, E.A.; Zoccali, C.; Dekker, F.W.; Eijsvogels, T.M.H.; Jager, K.J. Assessing physical activity and function in patients with chronic kidney disease: A narrative review. Clin. Kidney J. 2021, 14, 768–779. [Google Scholar] [CrossRef]
- Toyoshima, K.; Seino, S.; Tamura, Y.; Ishikawa, J.; Chiba, Y.; Ishizaki, T.; Fujiwara, Y.; Shinkai, S.; Kitamura, A.; Araki, A. Difference between "Physical Fitness Age" Based on Physical Function and Chronological Age Is Associated with Obesity, Hyperglycemia, Depressive Symptoms, and Low Serum Albumin. J. Nutr. Health Aging 2022, 26, 501–509. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Survey on the Inclusion of Cancer Care in Health-Benefit Packages, 2020–2021; World Health Organization: Geneva, Switzerland, 2024.
- Longevity, T.L.H. Older patients with cancer: Evidence-based care needs evidence. Policy Rev. 2021, 2, e678. [Google Scholar]
- Zhao, J.; Xu, L.; Sun, J.; Song, M.; Wang, L.; Yuan, S.; Zhu, Y.; Wan, Z.; Larsson, S.C.; Tsilidis, K.K. The global trends in incidence, death, burden and risk factors of early-onset cancer from 1990 to 2019. BMJ Oncol. 2023, 2, e000049. [Google Scholar] [CrossRef]
- Given, B.; Given, C.W. Older adults and cancer treatment. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2008, 113, 3505–3511. [Google Scholar] [CrossRef]
- Cheung, M.C.; Earle, C.C.; Rangrej, J.; Ho, T.H.; Liu, N.; Barbera, L.; Saskin, R.; Porter, J.; Seung, S.J.; Mittmann, N. Impact of aggressive management and palliative care on cancer costs in the final month of life. Cancer 2015, 121, 3307–3315. [Google Scholar] [CrossRef]
- West, H.J.; Jin, J.O. JAMA Oncology Patient Page. Performance Status in Patients With Cancer. JAMA Oncol. 2015, 1, 998. [Google Scholar] [CrossRef]
- Scott, J.M.; Stene, G.; Edvardsen, E.; Jones, L.W. Performance status in cancer: Not broken, but time for an upgrade? J. Clin. Oncol. 2020, 38, 2824. [Google Scholar] [CrossRef]
- Kim, Y.J.; Hui, D.; Zhang, Y.; Park, J.C.; Chisholm, G.; Williams, J.; Bruera, E. Differences in Performance Status Assessment Among Palliative Care Specialists, Nurses, and Medical Oncologists. J. Pain Symptom Manag. 2015, 49, 1050–1058.e2. [Google Scholar] [CrossRef]
- Neeman, E.; Gresham, G.; Ovasapians, N.; Hendifar, A.; Tuli, R.; Figlin, R.; Shinde, A. Comparing Physician and Nurse Eastern Cooperative Oncology Group Performance Status (ECOG-PS) Ratings as Predictors of Clinical Outcomes in Patients with Cancer. Oncologist 2019, 24, e1460–e1466. [Google Scholar] [CrossRef]
- Schnadig, I.D.; Fromme, E.K.; Loprinzi, C.L.; Sloan, J.A.; Mori, M.; Li, H.; Beer, T.M. Patient-physician disagreement regarding performance status is associated with worse survivorship in patients with advanced cancer. Cancer 2008, 113, 2205–2214. [Google Scholar] [CrossRef]
- Agaronnik, N.; Lindvall, C.; El-Jawahri, A.; He, W.; Iezzoni, L. Use of natural language processing to assess frequency of functional status documentation for patients newly diagnosed with colorectal cancer. JAMA Oncol. 2020, 6, 1628–1630. [Google Scholar] [CrossRef]
- Muhandiramge, J.; Orchard, S.G.; Warner, E.T.; van Londen, G.J.; Zalcberg, J.R. Functional decline in the cancer patient: A review. Cancers 2022, 14, 1368. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Simonsick, E.M.; Salive, M.E.; Wallace, R.B. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N. Engl. J. Med. 1995, 332, 556–562. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Ezzatvar, Y.; Ramírez-Vélez, R.; Sáez de Asteasu, M.L.; Martínez-Velilla, N.; Zambom-Ferraresi, F.; Izquierdo, M.; García-Hermoso, A. Physical function and all-cause mortality in older adults diagnosed with cancer: A systematic review and meta-analysis. J. Gerontol. Ser. A 2021, 76, 1447–1453. [Google Scholar] [CrossRef]
- Nakano, J.; Fukushima, T.; Tanaka, T.; Fu, J.B.; Morishita, S. Physical function predicts mortality in patients with cancer: A systematic review and meta-analysis of observational studies. Support. Care Cancer 2021, 29, 5623–5634. [Google Scholar] [CrossRef]
- Gill, T.M.; Becher, R.D.; Murphy, T.E.; Gahbauer, E.A.; Leo-Summers, L.; Han, L. Factors associated with days away from home in the year after major surgery among community-living older persons. Ann. Surg. 2023, 278, e13–e19. [Google Scholar] [CrossRef]
- Hori, K.; Usuba, K.; Sakuyama, A.; Adachi, Y.; Hirakawa, K.; Nakayama, A.; Nagayama, M.; Shimokawa, T.; Takanashi, S.; Isobe, M. Hospitalization-Associated Disability After Cardiac Surgery in Elderly Patients―Exploring the Risk Factors Using Machine Learning Algorithms―. Circ. Rep. 2021, 3, 423–430. [Google Scholar] [CrossRef]
- Sliwinski, S.; Faqar-Uz-Zaman, S.F.; Heil, J.; Mohr, L.; Detemble, C.; Dreilich, J.; Zmuc, D.; Bechstein, W.O.; Becker, S.; Chun, F. Predictive value of a novel digital risk calculator to determine early patient outcomes after major surgery: A proof-of-concept pilot study. Patient Saf. Surg. 2024, 18, 13. [Google Scholar] [CrossRef]
- Hendriks, S.; Huisman, M.G.; Ghignone, F.; Vigano, A.; de Liguori Carino, N.; Farinella, E.; Girocchi, R.; Audisio, R.A.; van Munster, B.; de Bock, G.H. Timed up and go test and long-term survival in older adults after oncologic surgery. BMC Geriatr. 2022, 22, 934. [Google Scholar] [CrossRef]
- Almugbel, F.A.; Timilshina, N.; Papadopoulos, E.; Al-Showbaki, L.; Alibhai, S.M. The role of grip strength and short physical performance battery test in predicting chemotherapy-related outcomes in older adults with cancer. J. Geriatr. Oncol. 2022, 13, 318–324. [Google Scholar] [CrossRef]
- Papadopoulos, E.; Helal, A.A.; Jin, R.; Monginot, S.; Berger, A.; Romanovsky, L.; Alibhai, S.M. The impact of pre-treatment muscle strength and physical performance on treatment modification in older adults with cancer following comprehensive geriatric assessment. Age Ageing 2022, 51, afac152. [Google Scholar] [CrossRef]
- Sourdet, S.; Brechemier, D.; Steinmeyer, Z.; Gerard, S.; Balardy, L. Impact of the comprehensive geriatric assessment on treatment decision in geriatric oncology. BMC Cancer 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Ai, D.; Ding, N.; Wu, H. The impact of sarcopenia on nutritional status in elderly patients with gastrointestinal tumors. Sci. Rep. 2023, 13, 10308. [Google Scholar] [CrossRef]
- Tanaka, K.; Taoda, A.; Kashiwagi, H. The associations between nutritional status, physical function and skeletal muscle mass of geriatric patients with colorectal cancer. Clin. Nutr. ESPEN 2021, 41, 318–324. [Google Scholar] [CrossRef]
- Farrugia, M.; Erickson, K.; Wendel, E.; Platek, M.E.; Ji, W.; Attwood, K.; Ma, S.J.; Gu, F.; Singh, A.K.; Ray, A.D. Change in physical performance correlates with decline in quality of life and frailty status in head and neck cancer patients undergoing radiation with and without chemotherapy. Cancers 2021, 13, 1638. [Google Scholar] [CrossRef]
- Wooten, S.V.; Fleming, R.D.; Wolf, J.S., Jr.; Stray-Gundersen, S.; Bartholomew, J.B.; Mendoza, D.; Stanforth, P.R.; Stanforth, D.; Hernandez, L.M.; Tanaka, H. Prehabilitation program composed of blood flow restriction training and sports nutrition improves physical functions in abdominal cancer patients awaiting surgery. Eur. J. Surg. Oncol. 2021, 47, 2952–2958. [Google Scholar] [CrossRef]
- Lafaro, K.J.; Raz, D.J.; Kim, J.Y.; Hite, S.; Ruel, N.; Varatkar, G.; Erhunmwunsee, L.; Melstrom, L.; Lee, B.; Singh, G. Pilot study of a telehealth perioperative physical activity intervention for older adults with cancer and their caregivers. Support. Care Cancer 2020, 28, 3867–3876. [Google Scholar] [CrossRef]
- Veronese, N.; Stubbs, B.; Volpato, S.; Zuliani, G.; Maggi, S.; Cesari, M.; Lipnicki, D.M.; Smith, L.; Schofield, P.; Firth, J. Association between gait speed with mortality, cardiovascular disease and cancer: A systematic review and meta-analysis of prospective cohort studies. J. Am. Med. Dir. Assoc. 2018, 19, 981–988.e7. [Google Scholar] [CrossRef]
- Perracini, M.R.; Mello, M.; de Oliveira Máximo, R.; Bilton, T.L.; Ferriolli, E.; Lustosa, L.P.; da Silva Alexandre, T. Diagnostic accuracy of the short physical performance battery for detecting frailty in older people. Phys. Ther. 2020, 100, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Dotan, E.; Walter, L.C.; Browner, I.S.; Clifton, K.; Cohen, H.J.; Extermann, M.; Gross, C.; Gupta, S.; Hollis, G.; Hubbard, J. NCCN guidelines® insights: Older adult oncology, version 1.2021: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2021, 19, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.A.; Lewis, C.A.; Escalon, M.X. Inpatient Rehabilitation Issues Related to COVID-19. Phys. Med. Rehabil. Clin. N. Am. 2023, 34, 513–522. [Google Scholar] [CrossRef]
- Wilson, R.D.; Lewis, S.A.; Murray, P.K. Trends in the rehabilitation therapist workforce in underserved areas: 1980–2000. J. Rural. Health 2009, 25, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Soubra, R.; Mourad-Chehade, F.; Chkeir, A. Automation of the timed up and go test using a doppler radar system for gait and balance analysis in elderly people. J. Healthc. Eng. 2023, 2023, 2016262. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, H.H.C.; Ong, E.H.; Heyzer, L.; Tan, C.N.; Ghazali, F.; Yang, D.Z.; Jung, H.-W.; Ismail, N.H.; Lim, W.S. Validation of a multi-sensor-based kiosk in the use of the Short Physical Performance Battery in older adults attending a fall and balance clinic. Ann. Geriatr. Med. Res. 2022, 26, 125. [Google Scholar] [CrossRef]
- Duncan, L.; Gulati, P.; Giri, S.; Ostadabbas, S.; Mirbozorgi, S.A. Camera-based human gait speed monitoring and tracking for performance assessment of elderly patients with cancer. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Guadalajara, Mexico, 1–5 November 2021; IEEE: New York, NY, USA, 2021; pp. 3522–3525. [Google Scholar]
- Ponciano, V.; Pires, I.M.; Ribeiro, F.R.; Marques, G.; Garcia, N.M.; Pombo, N.; Spinsante, S.; Zdravevski, E. Is The Timed-Up and Go Test Feasible in Mobile Devices? A Systematic Review. Electronics 2020, 9, 528. [Google Scholar] [CrossRef]
- Hamamoto, R.; Suvarna, K.; Yamada, M.; Kobayashi, K.; Shinkai, N.; Miyake, M.; Takahashi, M.; Jinnai, S.; Shimoyama, R.; Sakai, A.; et al. Application of Artificial Intelligence Technology in Oncology: Towards the Establishment of Precision Medicine. Cancers 2020, 12, 3532. [Google Scholar] [CrossRef]
- Hamamoto, R.; Komatsu, M.; Takasawa, K.; Asada, K.; Kaneko, S. Epigenetics Analysis and Integrated Analysis of Multiomics Data, Including Epigenetic Data, Using Artificial Intelligence in the Era of Precision Medicine. Biomolecules 2020, 10, 62. [Google Scholar] [CrossRef]
- Yamada, M.; Saito, Y.; Imaoka, H.; Saiko, M.; Yamada, S.; Kondo, H.; Takamaru, H.; Sakamoto, T.; Sese, J.; Kuchiba, A.; et al. Development of a real-time endoscopic image diagnosis support system using deep learning technology in colonoscopy. Sci. Rep. 2019, 9, 14465. [Google Scholar] [CrossRef]
- Jinnai, S.; Yamazaki, N.; Hirano, Y.; Sugawara, Y.; Ohe, Y.; Hamamoto, R. The Development of a Skin Cancer Classification System for Pigmented Skin Lesions Using Deep Learning. Biomolecules 2020, 10, 1123. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Sakai, A.; Komatsu, R.; Matsuoka, R.; Yasutomi, S.; Shozu, K.; Dozen, A.; Machino, H.; Hidaka, H.; Arakaki, T.; et al. Detection of Cardiac Structural Abnormalities in Fetal Ultrasound Videos Using Deep Learning. Appl. Sci. 2021, 11, 371. [Google Scholar] [CrossRef]
- Takahashi, S.; Takahashi, M.; Kinoshita, M.; Miyake, M.; Kawaguchi, R.; Shinojima, N.; Mukasa, A.; Saito, K.; Nagane, M.; Otani, R.; et al. Fine-Tuning Approach for Segmentation of Gliomas in Brain Magnetic Resonance Images with a Machine Learning Method to Normalize Image Differences among Facilities. Cancers 2021, 13, 1415. [Google Scholar] [CrossRef] [PubMed]
- Asada, K.; Kobayashi, K.; Joutard, S.; Tubaki, M.; Takahashi, S.; Takasawa, K.; Komatsu, M.; Kaneko, S.; Sese, J.; Hamamoto, R. Uncovering Prognosis-Related Genes and Pathways by Multi-Omics Analysis in Lung Cancer. Biomolecules 2020, 10, 524. [Google Scholar] [CrossRef]
- Kobayashi, K.; Bolatkan, A.; Shiina, S.; Hamamoto, R. Fully-Connected Neural Networks with Reduced Parameterization for Predicting Histological Types of Lung Cancer from Somatic Mutations. Biomolecules 2020, 10, 1249. [Google Scholar] [CrossRef]
- Takahashi, S.; Asada, K.; Takasawa, K.; Shimoyama, R.; Sakai, A.; Bolatkan, A.; Shinkai, N.; Kobayashi, K.; Komatsu, M.; Kaneko, S.; et al. Predicting Deep Learning Based Multi-Omics Parallel Integration Survival Subtypes in Lung Cancer Using Reverse Phase Protein Array Data. Biomolecules 2020, 10, 1460. [Google Scholar] [CrossRef]
- Hamamoto, R. Application of Artificial Intelligence for Medical Research. Biomolecules 2021, 11, 90. [Google Scholar] [CrossRef]
- Hamamoto, R.; Koyama, T.; Kouno, N.; Yasuda, T.; Yui, S.; Sudo, K.; Hirata, M.; Sunami, K.; Kubo, T.; Takasawa, K.; et al. Introducing AI to the molecular tumor board: One direction toward the establishment of precision medicine using large-scale cancer clinical and biological information. Exp. Hematol. Oncol. 2022, 11, 82. [Google Scholar] [CrossRef]
- FDA, Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices. Available online: https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-aiml-enabled-medical-devices (accessed on 13 May 2024).
- Hamamoto, R.; Takasawa, K.; Machino, H.; Kobayashi, K.; Takahashi, S.; Bolatkan, A.; Shinkai, N.; Sakai, A.; Aoyama, R.; Yamada, M.; et al. Application of non-negative matrix factorization in oncology: One approach for establishing precision medicine. Brief. Bioinform. 2022, 23, bbac246. [Google Scholar] [CrossRef]
- Polus, J.S.; Bloomfield, R.A.; Vasarhelyi, E.M.; Lanting, B.A.; Teeter, M.G. Machine learning predicts the fall risk of total hip arthroplasty patients based on wearable sensor instrumented performance tests. J. Arthroplast. 2021, 36, 573–578. [Google Scholar] [CrossRef]
- Friedrich, B.; Lau, S.; Elgert, L.; Bauer, J.M.; Hein, A. A deep learning approach for TUG and SPPB score prediction of (pre-) frail older adults on real-life IMU data. Healthcare 2021, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, R.A.; Broberg, J.S.; Williams, H.A.; Lanting, B.A.; McIsaac, K.A.; Teeter, M.G. Machine learning and wearable sensors at preoperative assessments: Functional recovery prediction to set realistic expectations for knee replacements. Med. Eng. Phys. 2021, 89, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Zhuparris, A.; Maleki, G.; Koopmans, I.; Doll, R.J.; Voet, N.; Kraaij, W.; Cohen, A.; van Brummelen, E.; De Maeyer, J.H.; Groeneveld, G.J. Smartphone and Wearable Sensors for the Estimation of Facioscapulohumeral Muscular Dystrophy Disease Severity: Cross-sectional Study. JMIR Form. Res. 2023, 7, e41178. [Google Scholar] [CrossRef] [PubMed]
- Dubois, A.; Bihl, T.; Bresciani, J.-P. Identifying fall risk predictors by monitoring daily activities at home using a depth sensor coupled to machine learning algorithms. Sensors 2021, 21, 1957. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Mizokami, F.; Kameya, Y.; Hayakawa, Y.; Watanabe, T.; Matsui, Y. Machine learning versus binomial logistic regression analysis for fall risk based on SPPB scores in older adult outpatients. Digit. Health 2023, 9, 20552076231219438. [Google Scholar] [CrossRef]
- Kraus, M.; Stumpf, U.C.; Keppler, A.M.; Neuerburg, C.; Böcker, W.; Wackerhage, H.; Baumbach, S.F.; Saller, M.M. Development of a Machine Learning-Based Model to Predict Timed-Up-and-Go Test in Older Adults. Geriatrics 2023, 8, 99. [Google Scholar] [CrossRef]
- Sasani, K.; Catanese, H.N.; Ghods, A.; Rokni, S.A.; Ghasemzadeh, H.; Downey, R.J.; Shahrokni, A. Gait speed and survival of older surgical patient with cancer: Prediction after machine learning. J. Geriatr. Oncol. 2019, 10, 120–125. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, P.; Zhang, Y.; Miyazaki, K. Gait analysis using stereo camera in daily environment. Berlin, Germany, 23–27 July 2019. IEEE: New York City, NY, USA, 2019; pp. 1471–1475. [Google Scholar]
- Hwang, J.; Lee, J.; Lee, K.-S. A deep learning-based method for grip strength prediction: Comparison of multilayer perceptron and polynomial regression approaches. PLoS ONE 2021, 16, e0246870. [Google Scholar] [CrossRef]
- Bae, J.-H.; Li, X.; Kim, T.; Bang, H.-S.; Lee, S.; Seo, D.Y. Prediction models of grip strength in adults above 65 years using Korean National Physical Fitness Award Data from 2009 to 2019. Eur. Geriatr. Med. 2023, 14, 1059–1064. [Google Scholar] [CrossRef]
- Supratak, A.; Datta, G.; Gafson, A.R.; Nicholas, R.; Guo, Y.; Matthews, P.M. Remote monitoring in the home validates clinical gait measures for multiple sclerosis. Front. Neurol. 2018, 9, 399303. [Google Scholar] [CrossRef]
- Soltani, A.; Dejnabadi, H.; Savary, M.; Aminian, K. Real-world gait speed estimation using wrist sensor: A personalized approach. IEEE J. Biomed. Health Inform. 2019, 24, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Dobkin, B.H.; Xu, X.; Batalin, M.; Thomas, S.; Kaiser, W. Reliability and validity of bilateral ankle accelerometer algorithms for activity recognition and walking speed after stroke. Stroke 2011, 42, 2246–2250. [Google Scholar] [CrossRef] [PubMed]
- Mannini, A.; Sabatini, A.M. Walking speed estimation using foot-mounted inertial sensors: Comparing machine learning and strap-down integration methods. Med. Eng. Phys. 2014, 36, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, R.S.; Mahadevan, N.; Moon, Y.; Seagers, K.; Sheth, N.; Wright, J.A., Jr.; DiCristofaro, S.; Silva, I.; Jortberg, E.; Ceruolo, M. A machine learning approach for gait speed estimation using skin-mounted wearable sensors: From healthy controls to individuals with multiple sclerosis. PLoS ONE 2017, 12, e0178366. [Google Scholar] [CrossRef]
- Aziz, W.; Hussain, L.; Khan, I.R.; Alowibdi, J.S.; Alkinani, M.H. Machine learning based classification of normal, slow and fast walking by extracting multimodal features from stride interval time series. Math. Biosci. Eng. 2021, 18, 495–517. [Google Scholar] [CrossRef]
- Atrsaei, A.; Dadashi, F.; Mariani, B.; Gonzenbach, R.; Aminian, K. Toward a remote assessment of walking bout and speed: Application in patients with multiple sclerosis. IEEE J. Biomed. Health Inform. 2021, 25, 4217–4228. [Google Scholar] [CrossRef]
- Juen, J.; Cheng, Q.; Schatz, B. A natural walking monitor for pulmonary patients using mobile phones. IEEE J. Biomed. Health Inform. 2015, 19, 1399–1405. [Google Scholar] [CrossRef]
- Aziz, O.; Zihajehzadeh, S.; Park, A.; Tae, C.-G.; Park, E.J. Improving energy expenditure estimation through activity classification and walking speed estimation using a smartwatch. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; IEEE: New York, NY, USA, 2020; pp. 3940–3944. [Google Scholar]
- Lee, D.W.; Han, H.-S.; Lee, M.C.; Ro, D.H. Prediction of Postoperative Gait Speed Change after Bilateral Primary Total Knee Arthroplasty in Female Patients Using a Machine Learning Algorithm. Orthop. Traumatol. Surg. Res. 2024, 110, 103842. [Google Scholar] [CrossRef]
- Davis, J.R.C.; Knight, S.P.; Donoghue, O.A.; Hernández, B.; Rizzo, R.; Kenny, R.A.; Romero-Ortuno, R. Comparison of gait speed reserve, usual gait speed, and maximum gait speed of adults aged 50+ in Ireland using explainable machine learning. Front. Netw. Physiol. 2021, 1, 754477. [Google Scholar] [CrossRef]
- Sikandar, T.; Rabbi, M.F.; Ghazali, K.H.; Altwijri, O.; Alqahtani, M.; Almijalli, M.; Altayyar, S.; Ahamed, N.U. Using a deep learning method and data from two-dimensional (2D) marker-less video-based images for walking speed classification. Sensors 2021, 21, 2836. [Google Scholar] [CrossRef]
- Chen, H.-C.; Sunardi; Liau, B.-Y.; Lin, C.-Y.; Akbari, V.B.H.; Lung, C.-W.; Jan, Y.-K. Estimation of various walking intensities based on wearable plantar pressure sensors using artificial neural networks. Sensors 2021, 21, 6513. [Google Scholar] [CrossRef] [PubMed]
- Kidziński, Ł.; Yang, B.; Hicks, J.L.; Rajagopal, A.; Delp, S.L.; Schwartz, M.H. Deep neural networks enable quantitative movement analysis using single-camera videos. Nat. Commun. 2020, 11, 4054. [Google Scholar] [CrossRef] [PubMed]
- Lonini, L.; Moon, Y.; Embry, K.; Cotton, R.J.; McKenzie, K.; Jenz, S.; Jayaraman, A. Video-based pose estimation for gait analysis in stroke survivors during clinical assessments: A proof-of-concept study. Digit. Biomark. 2022, 6, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Carl von Ossietzky Universität Oldenburg, O.T.A.G.O. Available online: https://uol.de/en/amt/research/projects/otago (accessed on 5 September 2024).
- Merriaux, P.; Dupuis, Y.; Boutteau, R.; Vasseur, P.; Savatier, X. A study of vicon system positioning performance. Sensors 2017, 17, 1591. [Google Scholar] [CrossRef]
- Donoghue, O.A.; McGarrigle, C.A.; Foley, M.; Fagan, A.; Meaney, J.; Kenny, R.A. Cohort profile update: The Irish longitudinal study on ageing (TILDA). Int. J. Epidemiol. 2018, 47, 1398–1398l. [Google Scholar] [CrossRef]
- Makihara, Y.; Mannami, H.; Tsuji, A.; Hossain, M.A.; Sugiura, K.; Mori, A.; Yagi, Y. The OU-ISIR gait database comprising the treadmill dataset. IPSJ Trans. Comput. Vis. Appl. 2012, 4, 53–62. [Google Scholar] [CrossRef]
- Stone, E.; Skubic, M.; Rantz, M.; Abbott, C.; Miller, S. Average in-home gait speed: Investigation of a new metric for mobility and fall risk assessment of elders. Gait Posture 2015, 41, 57–62. [Google Scholar] [CrossRef]
- Friedrich, B.; Steen, E.-E.; Fudickar, S.; Hein, A. Analysing the Correlation of Geriatric Assessment Scores and Activity in Smart Homes. arXiv preprint 2021, arXiv:2103.05971. [Google Scholar] [CrossRef]
- Bohannon, R.W. Minimal clinically important difference for grip strength: A systematic review. J. Phys. Ther. Sci. 2019, 31, 75–78. [Google Scholar] [CrossRef]
- Pavasini, R.; Guralnik, J.; Brown, J.C.; di Bari, M.; Cesari, M.; Landi, F.; Vaes, B.; Legrand, D.; Verghese, J.; Wang, C.; et al. Short Physical Performance Battery and all-cause mortality: Systematic review and meta-analysis. BMC Med. 2016, 14, 215. [Google Scholar] [CrossRef]
- Robinson, T.N.; Wu, D.S.; Sauaia, A.; Dunn, C.L.; Stevens-Lapsley, J.E.; Moss, M.; Stiegmann, G.V.; Gajdos, C.; Cleveland, J.C., Jr.; Inouye, S.K. Slower walking speed forecasts increased postoperative morbidity and 1-year mortality across surgical specialties. Ann. Surg. 2013, 258, 582–588; discussion 588–590. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Shojima, K.; Tamaki, K.; Mori, T.; Kusunoki, H.; Onishi, M.; Tsuji, S.; Matsuzawa, R.; Nagai, K.; Sano, K. Association Between Timed Up-and-Go Test and Future Changes in the Frailty Status in a Longitudinal Study of Japanese Community-Dwelling Older Adults. Clin. Interv. Aging 2023, 18, 1191–1200. [Google Scholar] [CrossRef] [PubMed]

| Reference | Main Device to Obtain Input Data | Details of Input Variable or Device | Label Setting/Label Measurement Method | Output Label/Sort of Task | ML Technology | Validation Method | Metrics from Best Model | Baseline Characteristics | Concept | |
|---|---|---|---|---|---|---|---|---|---|---|
| Polus et al. [53] | IMU | 4 sensors during TUG: above and below each knee before and 2 weeks after THA | TUG > 14(6 weeks after THA) | TUG/classification | LDA, SVM | 10-fold CV | LDA: Accuracy 0.87 | 72 patients undergoing THA | Preventing falls by predicting their risk based on TUG | |
| Friedrich et al. [54] | IMU | Single sensor on the right side of the hip | SPPB: score itself TUG: <10, 11–19, 20–29 | SPPB/regression TUG/classification | LSTM+CNN | Train–val–test | Accuracy (TUG) 95.9% Accuracy (SPPB) 94.3% | 20 older patients (OTAGO study) | Predicting TUG on real-life IMU data | |
| Bloomfield et al. [55] | IMU+EHR | ·4 sensors: above and below each knee during TUG ·Clinical information ·Patient-reported subjective measures | (Preoperative TUG—postoperative) >2.27 | TUG/classification | SVM, NB, RF, | 10-fold CV | RF: Accuracy 0.80 | 82 patients undergoing TKA | Predicting functional recovery for appropriately adjusting patient expectations | |
| Zhuparris et al. [56] | Smartphone | ·Health-related data from smartphone ·Sensor in smartphone | TUG score itself | TUG/regression | Elastic Net, RF, xgBoost | 5-fold CV | Elastic Net: R2 0.59 | 38 patients with FSHD | Quantifying FSHD progression with TUG | |
| Dubois et al. [57] | Depth sensor | Kinect V2 placed in each room of the rehabilitation center | TUG ≥ 13.5 s | TUG/classification | AdaBoost, NB, KNN, SVM, RF, NN | Leave-one-out CV | KNN, NN: Accuracy 1.0 | 30 older patients in a rehabilitation center | Preventing fall with home-sensor data | |
| Hasegawa et al. [58] | EHR | ·Clinical information mainly from EHR ·Physical measurements | SPPB ≤ 6(men)/≤9(women) as fall risk | SPPB/classification | Prediction One. Ver3.0.1.3 (SONY) BLRA | Train–test split | Prediction One: Accuracy 0.74 | 797 older patients at frailty outpatient service | Comparing model performance of predicting fall risk based on SPPB | |
| Kraus et al. [59] | EHR | Clinical information from HER | TUG score itself | TUG/regression | GLM, SVM, RF, xgBoost | 5-fold CV | RF: MAE 2.7 | 103 orthogeriatric patients | Predicting TUG without mobility data | |
| Sasani et al. [60] | Tabular data | Components of GA | TUG < 10 s, TUG ≥ 10 s, uncertain | TUG/classification | Decision Tree Classifier | None | Decision Tree Classifier: Accuracy 78% | 1901 old patients undergoing cancer surgery | Predicting accurately TUG score with ML | |
| Li et al. [61] | Video | Stereo camera | TUG score itself | TUG/regression | Mask R-CNN+ polynomial regression | None | RE <0.1 (20 participants in 40) | 40 older adults in a daycare facility | Assessing the health status of the older patients with TUG | |
| Hwang et al. [62] | Tabular data | Variables from physical profile and body part measurements (not from EHR) | Grip strength score itself | Grip strength/regression | MLP regression and different polynomial regressions | K-fold CV | MLP regression: correlation 0.88 | 164 healthy young volunteers | Predicting grip strength accurately to reduce the risk of upper extremity disorder | |
| Bae et al. [63] | Big Data | Tabular data from Korean National Fitness Award Data from 2009 to 2019 | Grip strength score itself | Grip strength/regression | LR, LASSO, Ridge, RF, xGBoost, Light GBM, CatBoost | 5-fold CV | CatBoost: MSE 16.6 | 107,290 participants aged over 65 | Predicting grip strength without measuring | |
| Supratak et al. [64] | IMU | Single sensor on the lower back | 25-foot walking test in clinic | Walking speed/ regression | SVR | Correlation | Correlation 0.98 | 32 young patients with MS | Validating gait speed at home against a 25-foot walking test | |
| Soltani et al. [65] | IMU+GNSS | 2 sensors: on each wrist | Walking speed measured by GNSS | Walking speed/ regression | LASSO (feature extraction) | CV | RMSE 0.05 | 40 healthy young volunteers | Estimating walking speed with personalization | |
| Dobkin et al. [66] | IMU | 2 sensors: above each ankle | Walking speed measured by stopwatch | Walking speed/ regression | Sensor system (Medical Daily Activity Wireless Network algorithm) | Correlation | Correlation 0.98 | 12 patients with stroke 6 healthy participants | Acquiring quantitative data on daily performance | |
| Mannini et al. [67] | IMU | Single sensor on the right shoe | Walking speed manually measured | Walking speed/ regression | ·Hidden Markov model ·Strap-down integration ·LR | Leave-one-out CV | R2 0.96 | 23 healthy adults | Exploring the ML method to predict walking speed | |
| McGinnis et al. [68] | IMU | 5 sensors: on sacrum, bilateral thigh, and bilateral shank | 6 min walking test on a treadmill | Walking speed/ regression | SVR | Leave-one-out CV | RMSE 0.12 (patients with MS) | 17 healthy participants 30 patients with MS | Resolving the hurdle of assessing walking speed | |
| Aziz et al. [69] | IMU | Single sensor inside one shoe | Slow/normal/fast speed | Walking speed/ classification | RF, xgBoost, SVM | Train–test split | RF: Accuracy 1.0 | 10 healthy men | Analyzing gait patterns of aged people | |
| Atrsaei et al. [70] | IMU | Single sensor on the waist | 10 m walk test | Walking speed/ regression | GPR | Leave-one-out CV | RMSE 1.10 | 35 participants with MS | Predicting walking speed at home with IMU | |
| Juen et al. [71] | Smartphone | Smartphone in waist belt at L3 | 6 min walking test | Walking speed/ regression | SVM, GPR | Leave-one-out CV | SVM: Error 3.23 | 28 patients with pulmonary disease 10 healthy participants | Monitoring individual health status continuously | |
| Aziz et al. [72] | Smartwatch | Smartwatch on the right wrist | Speed during treadmill walking: 0.5, 0.75, 1.0, 1.25, 1.5, 1.75 m/s | Walking speed/ regression | GPR | None | MAPE 4% (best, 1.0 m/s) | 10 healthy young adults | Assessing walking speed for preventing chronic diseases | |
| Lee et al. [73] | Optical motion capture+ EHR | ·Clinical information from EHR ·Variables extracted from optical motion capture | The difference between post/pre-operative gait speed | Walking speed/ classification | GBM | 10-fold CV | AUC 0.86 | 128 female patients undergoing bilateral TKA | Predicting postoperative walking speed by preoperative clinical variable | |
| Davis et al. [74] | Big Data | Tabular data | GSR = MGS—UGS | Walking speed/ regression | HGBR | 5-fold CV | R2 0.21 | 3925 participants from TILDA wave3 | Predicting gait speed from population statistical data | |
| Sikandar et al. [75] | Image | 5 ratio-based body measurement from marker free video images | Slow (2 to 3 km/h), normal (4 to 5 km/h), and fast (6 to 7 km/h) | Walking speed/ classification | BiLSTM | 17-fold CV | Accuracy 92.79% | 34 participants (OU-ISIR dataset A) | Classifying walking speed with body measurements | |
| Chen et al. [76] | Image | Plantar region pressure images | (0.8, 1.6, 2.4 m/s) and (10, 20 min) | Walking speed/ classification | ROI+CNN | Train–test split | F1-score: 1.00 (first toe, 2.4 m/s for 10 min) | 12 healthy young participants | Detecting appropriate exercise intensity | |
| Kidzinski et al. [77] | Video | Timeline keypoint data derived from OpenPose | Walking speed measured by the VICON system | Walking speed/ regression | OpenPose+ (CNN/RF/Ridge) | Train–val–test | OpenPose+CNN: Correlation 0.73 | 1026 pediatric patients with cerebral palsy | Simplifying the quantitative gait assessment | |
| Lonini et al. [78] | Video | Below-waist videos of patients recorded by normal camera | Walking speed measured by GAITRite | Walking speed/ regression | DeepLabCut(ResNet based) | Leave-one-out CV | Correlation 0.92 | eight patients with stroke | Predicting the walking speed of patients with stroke without expensive instrument | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouno, N.; Takahashi, S.; Komatsu, M.; Sakaguchi, Y.; Ishiguro, N.; Takeda, K.; Fujioka, K.; Matsuoka, A.; Fujimori, M.; Hamamoto, R. Introduction of AI Technology for Objective Physical Function Assessment. Bioengineering 2024, 11, 1154. https://doi.org/10.3390/bioengineering11111154
Kouno N, Takahashi S, Komatsu M, Sakaguchi Y, Ishiguro N, Takeda K, Fujioka K, Matsuoka A, Fujimori M, Hamamoto R. Introduction of AI Technology for Objective Physical Function Assessment. Bioengineering. 2024; 11(11):1154. https://doi.org/10.3390/bioengineering11111154
Chicago/Turabian StyleKouno, Nobuji, Satoshi Takahashi, Masaaki Komatsu, Yusuke Sakaguchi, Naoaki Ishiguro, Katsuji Takeda, Kyoko Fujioka, Ayumu Matsuoka, Maiko Fujimori, and Ryuji Hamamoto. 2024. "Introduction of AI Technology for Objective Physical Function Assessment" Bioengineering 11, no. 11: 1154. https://doi.org/10.3390/bioengineering11111154
APA StyleKouno, N., Takahashi, S., Komatsu, M., Sakaguchi, Y., Ishiguro, N., Takeda, K., Fujioka, K., Matsuoka, A., Fujimori, M., & Hamamoto, R. (2024). Introduction of AI Technology for Objective Physical Function Assessment. Bioengineering, 11(11), 1154. https://doi.org/10.3390/bioengineering11111154







