Novel Subperiosteal Device Geometry and Investigation of Efficacy on Surrounding Bone Formation and Bone-Bonding Strength
Abstract
1. Introduction
2. Materials and Methods
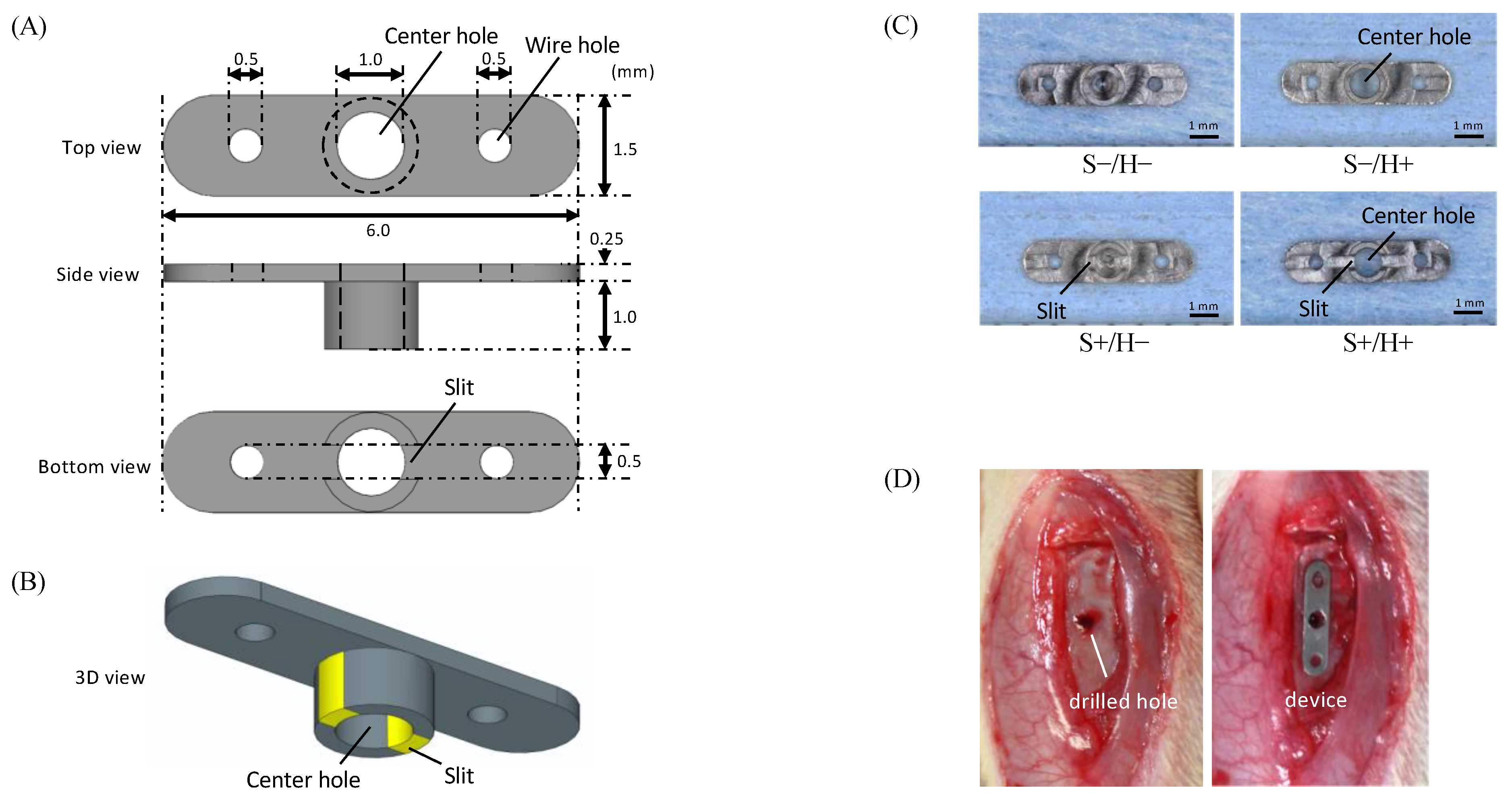
2.1. Preparation of the Specimen
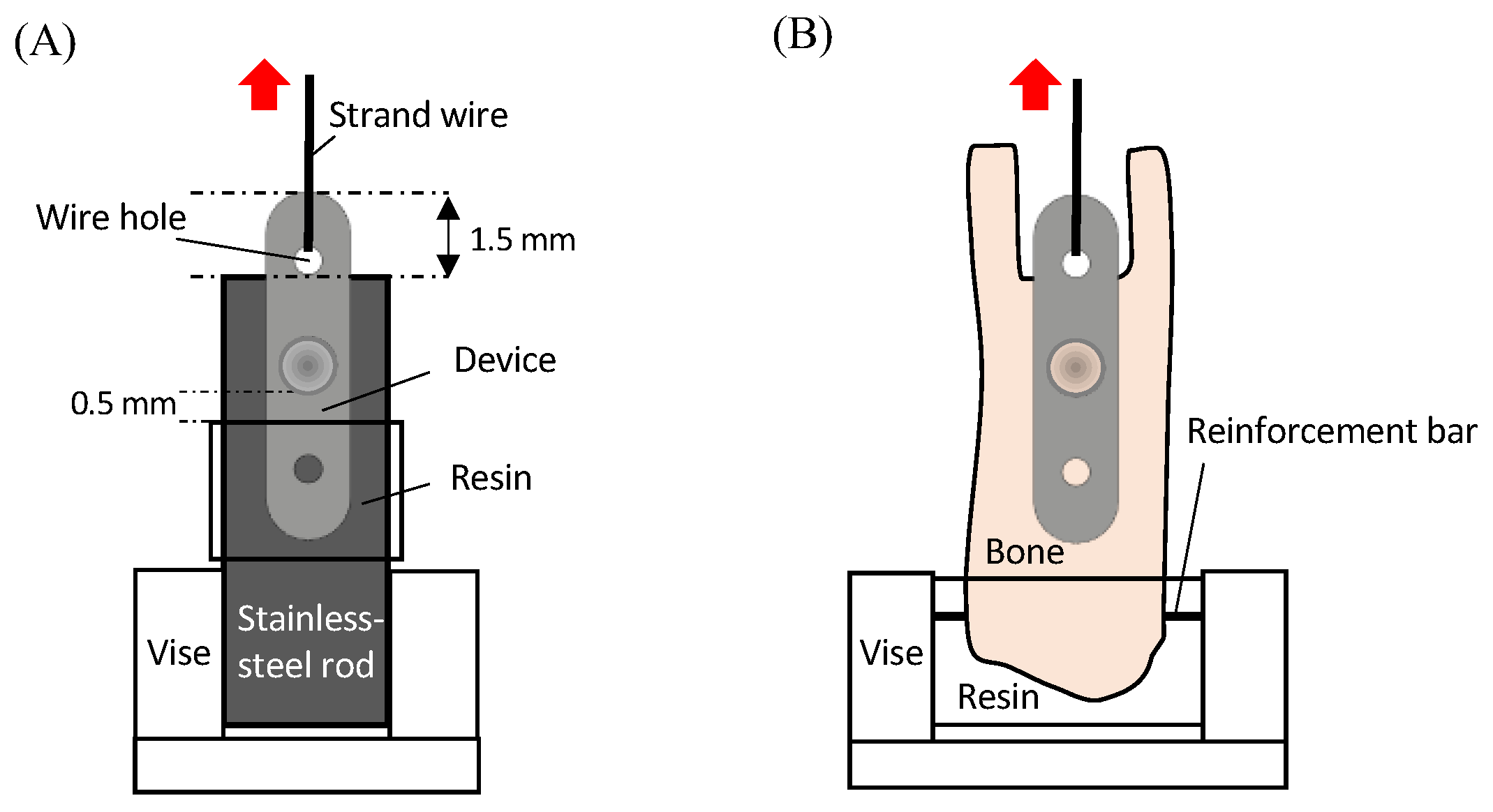
2.2. Testing the Mechanical Properties of the Specimen
2.3. Animal Experiment
2.4. Evaluation
2.4.1. Bone-Bonding Strength
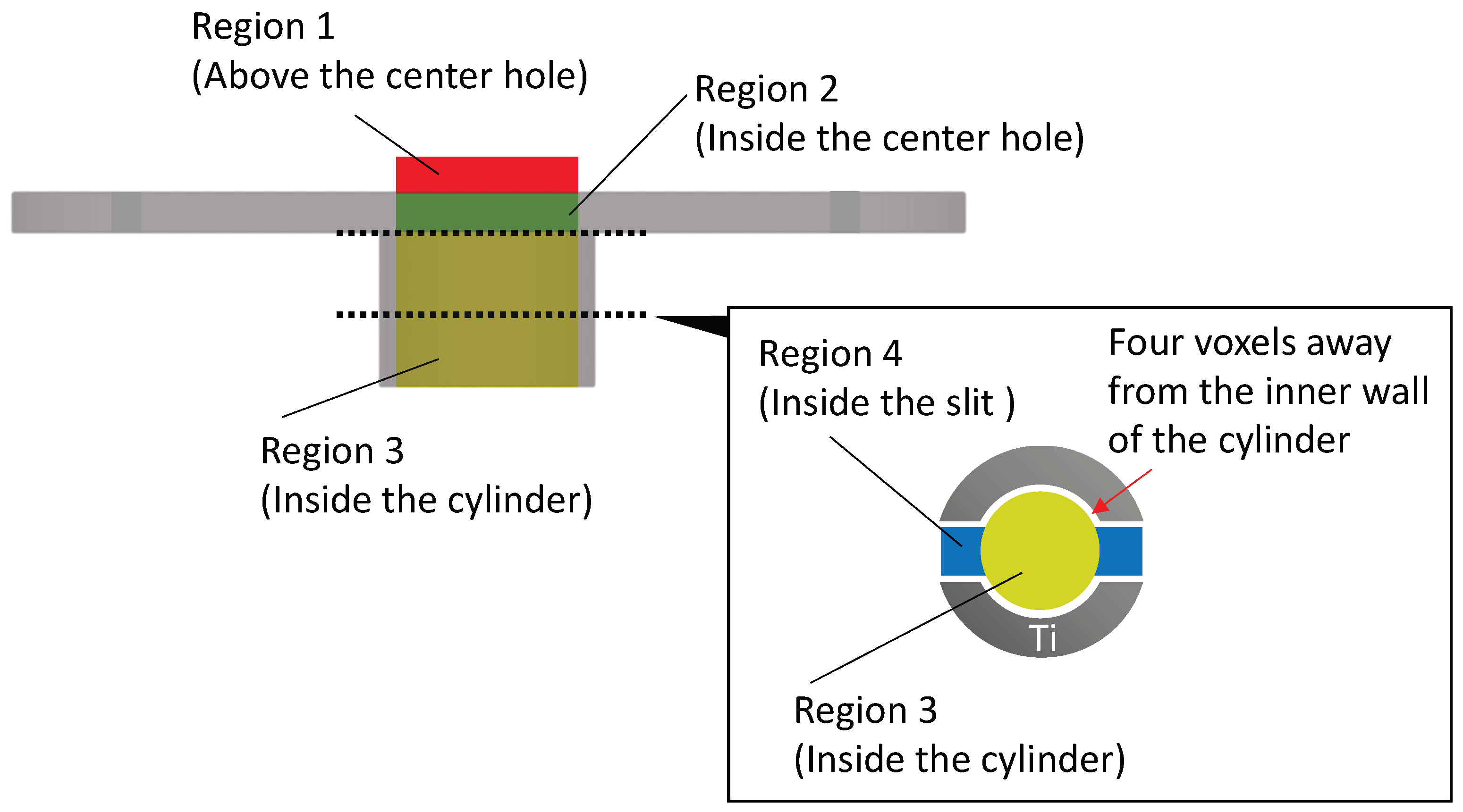
2.4.2. μCT Image Analysis
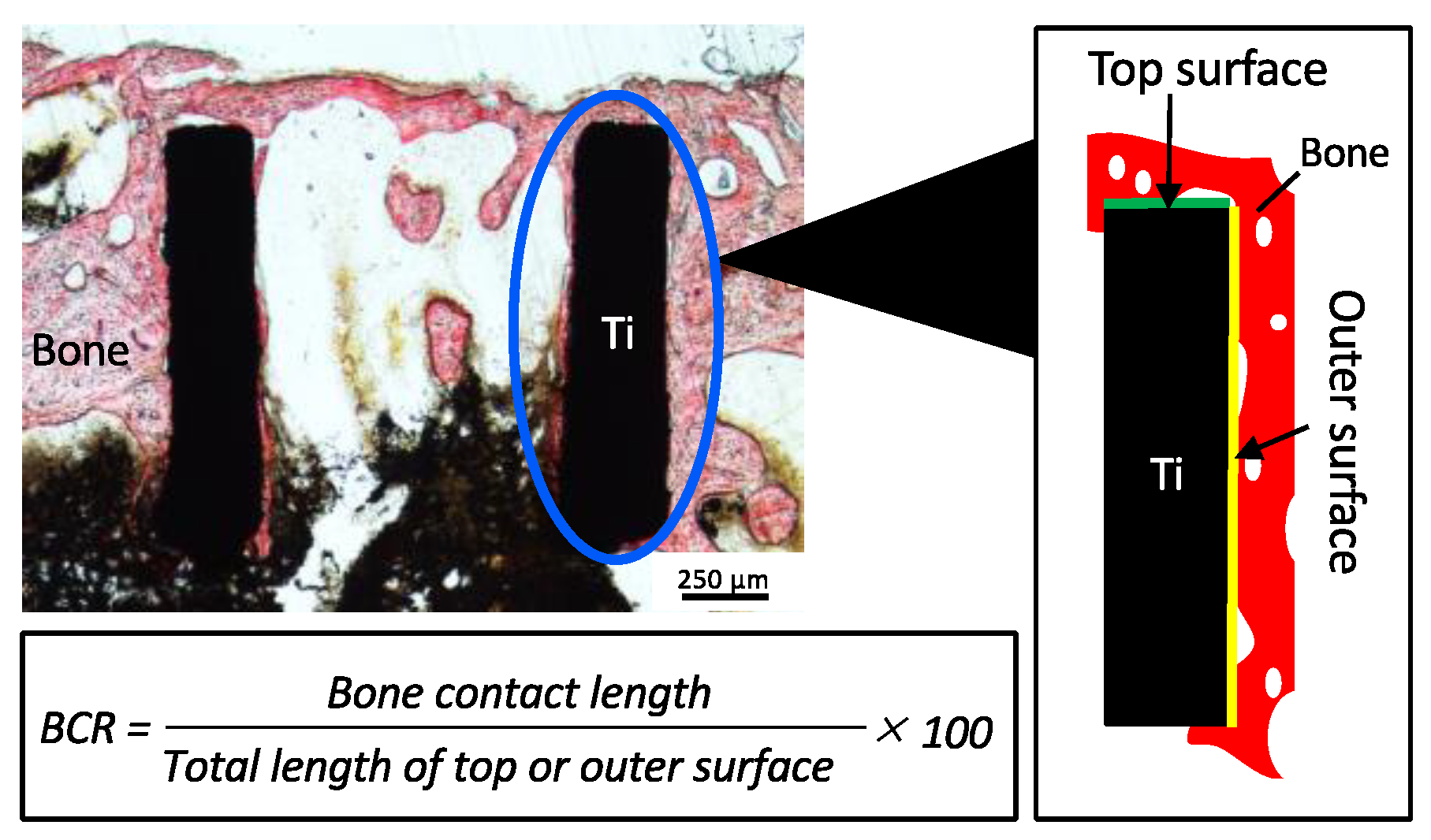
2.4.3. Histological Observation
2.5. Statistical Analysis
3. Results
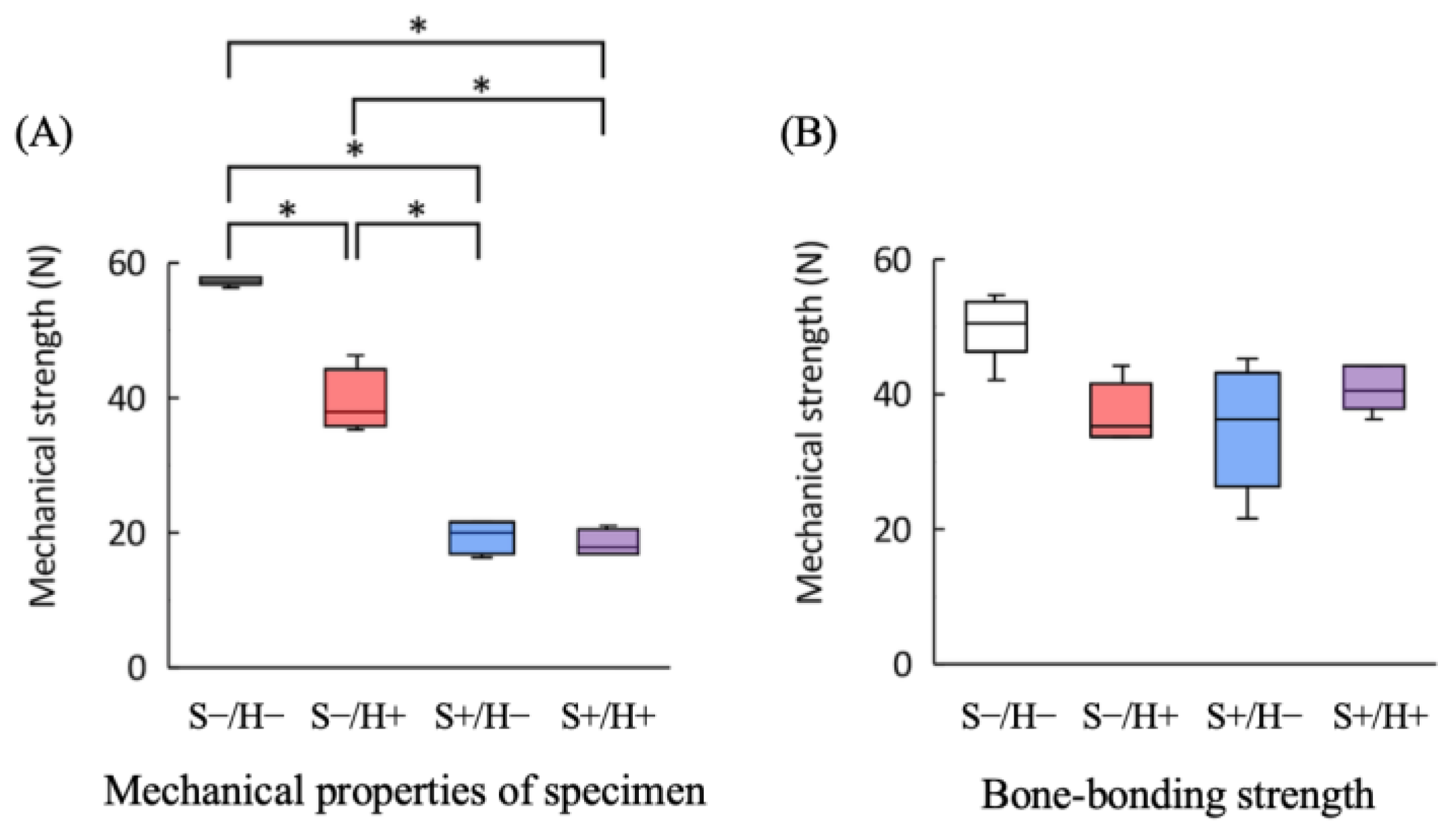
3.1. Mechanical Properties of the Specimen
3.2. Bone-Bonding Strength Tests
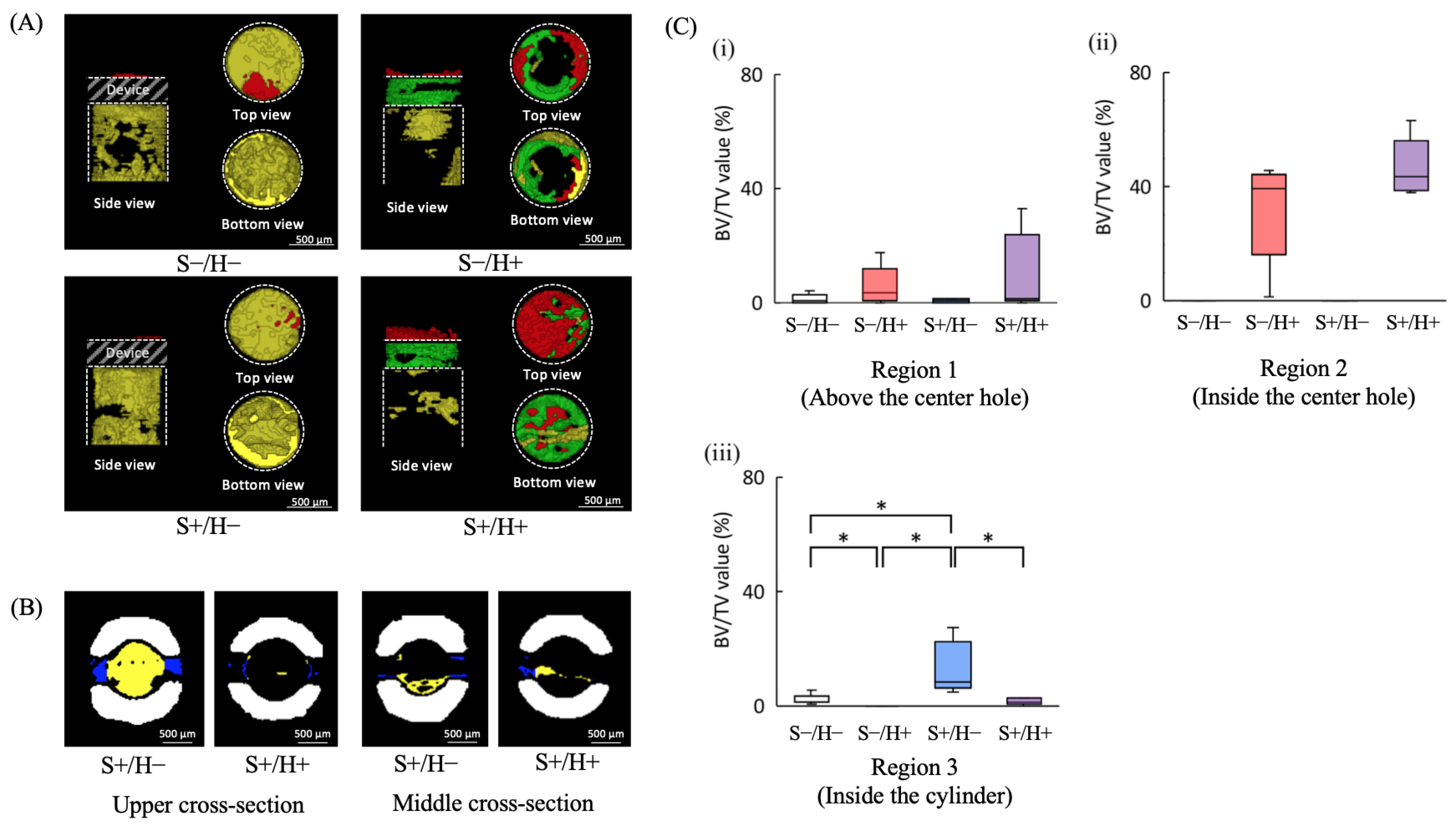
3.3. μCT Image Analysis
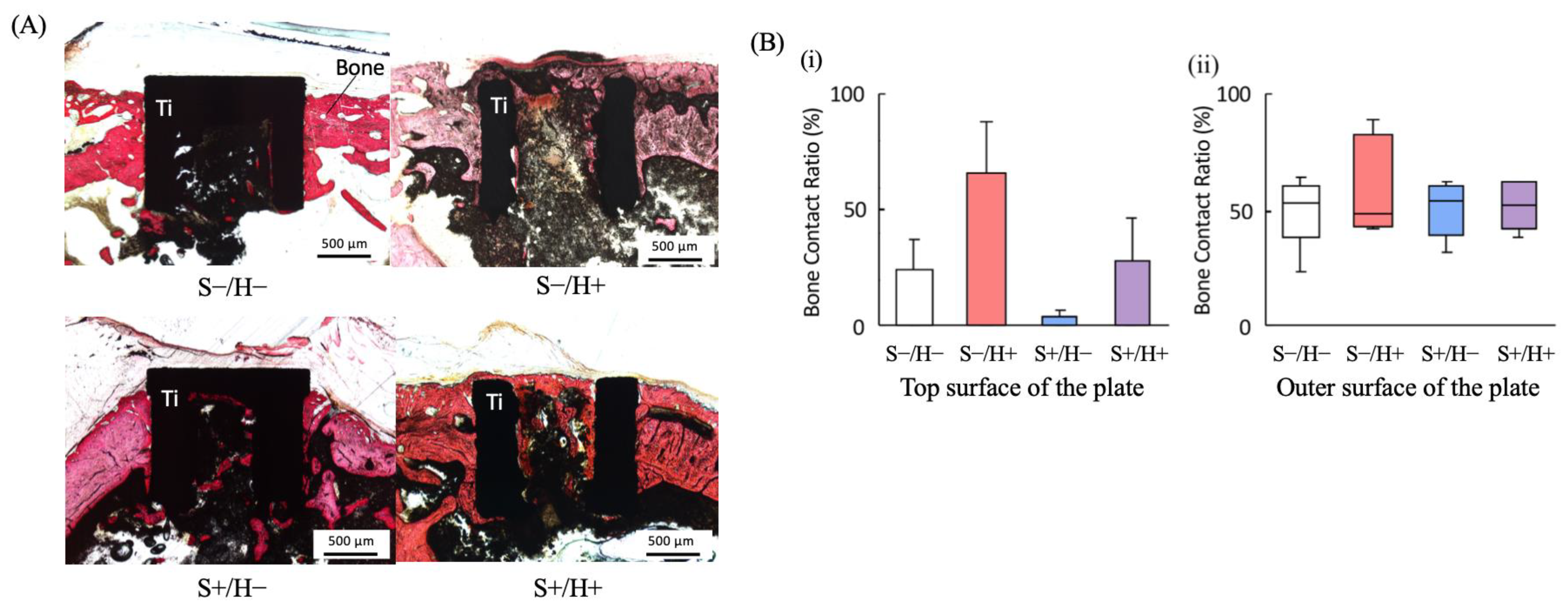
3.4. Histological Observations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, S.K.; Chowdhary, R.; Chrcanovic, B.R.; Brånemark, P.I. Osseoperception in dental implants: A systematic review. J. Prosthodont. 2016, 25, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Pham Dang, N.; Barthélémy, I.; Bekara, F. From rigid bone plate fixation to stable dynamic osteosynthesis in mandibular and craniomaxillo-facial surgery: Historical evolution of concepts and technical developments. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Umalkar, S.S.; Jadhav, V.V.; Paul, P.; Reche, A. Modern anchorage systems in orthodontics. Cureus 2022, 14, e31476. [Google Scholar] [CrossRef] [PubMed]
- Murat, S.; Kamburoğlu, K.; Kılıç, C.; Ozen, T.; Gurbuz, A. Nerve damage assessment following implant placement in human cadaver jaws: An ex vivo comparative study. J. Oral Implantol. 2014, 40, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Park, W.B.; Kim, Y.J.; Han, J.Y.; Park, J.S.; Kang, P. Radiographic and nasal endoscopic observation of accidentally perforated anterior nasal cavity with dental implants: Case reports with 5–23 years of follow-up. J. Oral Implantol. 2021, 47, 492–497. [Google Scholar] [CrossRef]
- Inchingolo, A.M.; Malcangi, G.; Costa, S.; Fatone, M.C.; Avantario, P.; Campanelli, M.; Piras, F.; Patano, A.; Ferrara, I.; Di Pede, C.; et al. Tooth complications after orthodontic miniscrews insertion. Int. J. Environ. Res. Public Health 2023, 20, 1562. [Google Scholar] [CrossRef]
- Block, M.S.; Hoffman, D.R. A new device for absolute anchorage for orthodontics. Am. J. Orthod. Dentofac. Orthop. 1995, 107, 251–258. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; He, H.; Peng, C.; Zhang, T.; Ngan, P. Osseointegration and biomechanical properties of the onplant system. Am. J. Orthod. Dentofac. Orthop. 2007, 132, 278.e1–278.e6. [Google Scholar] [CrossRef]
- Feldmann, I.; Bondemark, L. Anchorage capacity of osseointegrated and conventional anchorage systems: A randomized controlled trial. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 339.e19–339.e28. [Google Scholar] [CrossRef]
- Uezono, M.; Takakuda, K.; Kikuchi, M.; Suzuki, S.; Moriyama, K. Hydroxyapatite/collagen nanocomposite-coated titanium rod for achieving rapid osseointegration onto bone surface. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1031–1038. [Google Scholar] [CrossRef]
- Bai, L.; Du, Z.; Du, J.; Yao, W.; Zhang, J.; Weng, Z.; Liu, S.; Zhao, Y.; Liu, Y.; Zhang, X.; et al. A multifaceted coating on titanium dictates osteoimmunomodulation and osteo/angio-genesis towards ameliorative osseointegration. Biomaterials 2018, 162, 154–169. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X.; Hsu, Y.; He, Y.; Wang, F.; Yang, F.; Yan, F.; Xia, D.; Liu, Y. Surface modification of titanium implants with Mg-containing coatings to promote osseointegration. Acta Biomater. 2023, 169, 19–44. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.C.; Lojudice, F.H.; Halcsik, E.; Navarro, R.D.; Sogayar, M.C.; Granjeiro, J.M. Bone morphogenetic proteins: Facts, challenges, and future perspectives. J. Dent. Res. 2014, 93, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Raina, D.B.; Larsson, D.; Sezgin, E.A.; Isaksson, H.; Tägil, M.; Lidgren, L. Biomodulation of an implant for enhanced bone-implant anchorage. Acta Biomater. 2019, 96, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, Y.; Wang, A.; Zhu, Z.; Li, Y.; Zhu, C.; Che, Z.; Liu, T.; Liu, H.; Huang, L. Application of BMP in bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 810880. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Chen, J.; Yan, F.; Xiao, Y. Effects of simvastatin on bone healing around titanium implants in osteoporotic rats. Clin. Oral Implants Res. 2009, 20, 145–150. [Google Scholar] [CrossRef]
- Sendyk, D.I.; Deboni, M.C.; Pannuti, C.M.; Naclério-Homem, M.G.; Wennerberg, A. The influence of statins on osseointegration: A systematic review of animal model studies. J. Oral Rehabil. 2016, 43, 873–882. [Google Scholar] [CrossRef]
- Zidrou, C.; Kapetanou, A.; Rizou, S. The effect of drugs on implant osseointegration- A narrative review. Injury 2023, 54, 110888. [Google Scholar] [CrossRef]
- Hunziker, E.B.; Jovanovic, J.; Horner, A.; Keel, M.J.; Lippuner, K.; Shintani, N. Optimisation of BMP-2 dosage for the osseointegration of porous titanium implants in an ovine model. Eur. Cell. Mater. 2016, 32, 241–256. [Google Scholar] [CrossRef]
- Wychowański, P.; Starzyńska, A.; Adamska, P.; Słupecka-Ziemilska, M.; Sobocki, B.K.; Chmielewska, A.; Wysocki, B.; Alterio, D.; Marvaso, G.; Jereczek-Fossa, B.A.; et al. Methods of topical administration of drugs and biological active substances for dental implants-A narrative review. Antibiotics 2021, 10, 919. [Google Scholar] [CrossRef]
- Danesh-Sani, S.A.; Tarnow, D.; Yip, J.K.; Mojaver, R. The influence of cortical bone perforation on guided bone regeneration in humans. Int. J. Oral Maxillofac. Surg. 2017, 46, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T. In vivo studies of bone grafts. The possibility of vascular anastomoses in healing bone. Acta Orthop. Scand. 1980, 51, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.G.; Kim, K.H.; Jo, Y.; Lee, J.Y.; Park, Y.J.; Chung, C.P.; Seol, Y.J.; Han, J.S. Bone regeneration into side openings and hollow inner channel of a dental implant. J. Mech. Behav. Biomed. Mater. 2020, 101, 103416. [Google Scholar] [CrossRef] [PubMed]
- Mathey, E.; Pelletier, M.H.; Walsh, W.R.; Gall, K.; Carpenter, D. Implant Strength Contributes to the Osseointegration Strength of Porous Metallic Materials. J. Biomech. Eng. 2024, 146, 101005. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanyjavadpour, F.; Kazemi, P.; Moradinezhad, M.; Rakhshan, V. Distribution and amount of stresses caused by insertion or removal of orthodontic miniscrews into the maxillary bone: A finite element analysis. Int. Orthod. 2019, 17, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A.E.; Ekici, R.; Karakaya, M.; Alkan, A. Bone stress and damage distributions during dental implant insertion: A novel dynamic FEM analysis. Comput. Methods Biomech. Biomed. Eng. 2022, 25, 1381–1392. [Google Scholar] [CrossRef]
- Chen, C.M.; Wu, J.H.; Lu, P.C.; Wang, H.C.; Lee, H.E.; Wang, C.H.; Du, J.K. Horizontal pull-out strength of orthodontic infrazygomatic mini-implant: An in vitro study. Implant Den. 2011, 20, 139–145. [Google Scholar] [CrossRef]
- Olsen, B.R.; Reginato, A.M.; Wang, W. Bone development. Annu. Rev. Cell Dev. Biol. 2000, 16, 191–220. [Google Scholar] [CrossRef]
- Zhang, N.; Barrell, W.B.; Liu, K.J. Identification of distinct subpopulations of Gli1-lineage cells in the mouse mandible. J. Anat. 2023, 243, 90–99. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaisaka, Y.; Uezono, M.; Inoue, M.; Takakuda, K.; Moriyama, K. Novel Subperiosteal Device Geometry and Investigation of Efficacy on Surrounding Bone Formation and Bone-Bonding Strength. Bioengineering 2024, 11, 1122. https://doi.org/10.3390/bioengineering11111122
Kaisaka Y, Uezono M, Inoue M, Takakuda K, Moriyama K. Novel Subperiosteal Device Geometry and Investigation of Efficacy on Surrounding Bone Formation and Bone-Bonding Strength. Bioengineering. 2024; 11(11):1122. https://doi.org/10.3390/bioengineering11111122
Chicago/Turabian StyleKaisaka, Yoshiya, Masayoshi Uezono, Masaki Inoue, Kazuo Takakuda, and Keiji Moriyama. 2024. "Novel Subperiosteal Device Geometry and Investigation of Efficacy on Surrounding Bone Formation and Bone-Bonding Strength" Bioengineering 11, no. 11: 1122. https://doi.org/10.3390/bioengineering11111122
APA StyleKaisaka, Y., Uezono, M., Inoue, M., Takakuda, K., & Moriyama, K. (2024). Novel Subperiosteal Device Geometry and Investigation of Efficacy on Surrounding Bone Formation and Bone-Bonding Strength. Bioengineering, 11(11), 1122. https://doi.org/10.3390/bioengineering11111122






