Efficacy of Autologous Micrografting Technology in Managing Osteoarthritis Pain: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Study and Patients
2.2. Eligibility Criteria
2.3. Rigenera® Protocol
2.4. Assessment
2.5. Statistical Analysis
3. Results
3.1. Pain Management
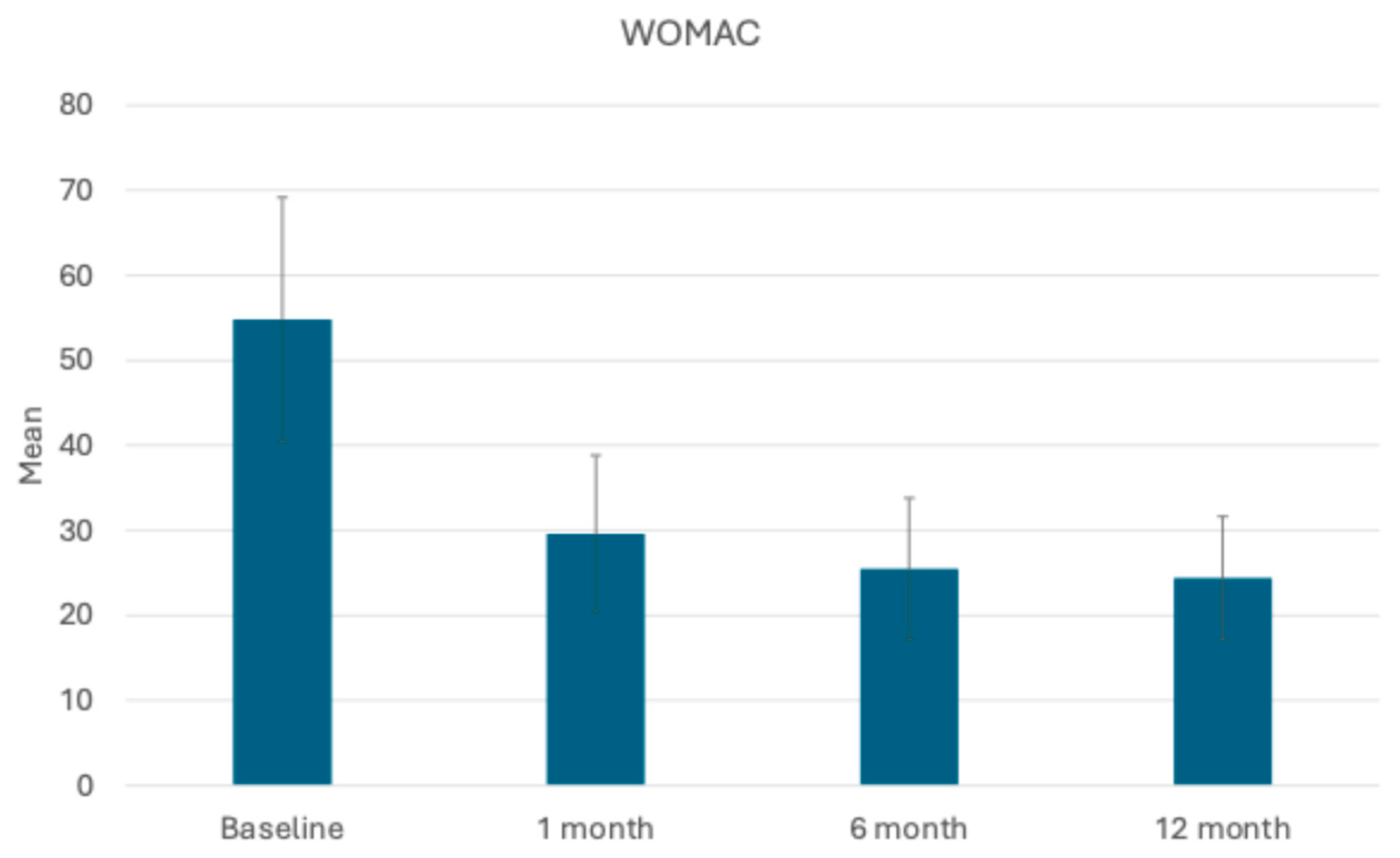
3.2. Assessment of Joint Function and Symptoms: KOOS and WOMAC Scores
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Peleteiro, B.; Araújo, J.; Branco, J.; Santos, R.A.; Ramos, E. The effect of osteoarthritis definition on prevalence and incidence estimates: A systematic review. Osteoarthr. Cartil. 2011, 19, 1270–1285. [Google Scholar] [CrossRef] [PubMed]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef]
- Vina, E.R.; Kwoh, C.K. Epidemiology of osteoarthritis: Literature update. Curr. Opin. Rheumatol. 2018, 30, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef]
- Coaccioli, S.; Sarzi-Puttini, P.; Zis, P.; Rinonapoli, G.; Varrassi, G. Osteoarthritis: New Insight on Its Pathophysiology. J. Clin. Med. 2022, 11, 6013. [Google Scholar] [CrossRef]
- Altman, R.D. Criteria for the classification of osteoarthritis of the knee and hip. Scand. J. Rheumatol. 1987, 16 (Suppl. 65), 31–39. [Google Scholar] [CrossRef]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res.® 2016, 474, 1886–1893. [Google Scholar] [CrossRef]
- Veronese, N.; Cooper, C.; Bruyère, O.; Al-Daghri, N.M.; Branco, J.; Cavalier, E.; Cheleschi, S.; da Silva Rosa, M.C.; Conaghan, P.G.; Dennison, E.M.; et al. Multimodal Multidisciplinary Management of Patients with Moderate to Severe Pain in Knee Osteoarthritis: A Need to Meet Patient Expectations. Drugs 2022, 82, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Baumbach, L.; Roos, E.M.; Lykkegaard, J.; Thomsen, K.S.; Kristensen, P.L.; Christensen, A.I.; Thorlund, J.B. Patients with osteoarthritis are least likely to receive lifestyle advice compared with patients with diabetes and hypertension: A national health survey study from Denmark. Osteoarthr. Cartil. Open 2020, 2, 100067. [Google Scholar] [CrossRef] [PubMed]
- Primorac, D.; Molnar, V.; Matišić, V.; Hudetz, D.; Jeleč, Ž.; Rod, E.; Čukelj, F.; Vidović, D.; Vrdoljak, T.; Dobričić, B.; et al. Comprehensive Review of Knee Osteoarthritis Pharmacological Treatment and the Latest Professional Societies’ Guidelines. Pharmaceuticals 2021, 14, 205. [Google Scholar] [CrossRef] [PubMed]
- Bruyère, O.; Cooper, C.; Pelletier, J.P.; Maheu, E.; Rannou, F.; Branco, J.; Luisa Brandi, M.; Kanis, J.A.; Altman, R.D.; Hochberg, M.C.; et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis-From evidence-based medicine to the real-life setting. Semin. Arthritis Rheum. 2016, 45, S3–S11. [Google Scholar] [CrossRef]
- Lin, F.; Zhang, X.; Cui, C. Mesenchymal stem cells and platelet rich plasma therapy for knee osteoarthritis: An umbrella review of systematic reviews with meta-analysis. Ann. Saudi Med. 2024, 44, 195–211. [Google Scholar] [CrossRef]
- Grässel, S.; Muschter, D. Recent advances in the treatment of osteoarthritis. F1000Research 2020, 9, 325. [Google Scholar] [CrossRef]
- De Girolamo, L.; Kon, E.; Filardo, G.; Marmotti, A.G.; Soler, F.; Peretti, G.M.; Vannini, F.; Madry, H.; Chubinskaya, S. Regenerative approaches for the treatment of early OA. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1826–1835. [Google Scholar] [CrossRef]
- Giaccone, M.; Brunetti, M.; Camandona, M.; Trovato, L.; Graziano, A. A New Medical Device, Based on Rigenera Protocol, in the Management of Complex Wounds. J. Stem Cells Res. Rev. Rep. 2014, 1, 3. [Google Scholar]
- Purpura, V.; Bondioli, E.; Graziano, A.; Trovato, L.; Melandri, D.; Ghetti, M.; Marchesini, A.; Cusella De Angelis, M.G.; Benedetti, L.; Ceccarelli, G.; et al. Tissue Characterization after a New Disaggregation Method for Skin Micro-Grafts Generation. JoVE (J. Vis. Exp.) 2016, 109, e53579. [Google Scholar] [CrossRef]
- Mummolo, S.; Mancini, L.; Quinzi, V.; D’Aquino, R.; Marzo, G.; Marchetti, E. Rigenera® Autologous Micrografts in Oral Regeneration: Clinical, Histological, and Radiographical Evaluations. Appl. Sci. 2020, 10, 5084. [Google Scholar] [CrossRef]
- De Francesco, F.; Gravina, P.; Busato, A.; Farinelli, L.; Soranzo, C.; Vidal, L.; Zingaretti, N.; Zavan, B.; Sbarbati, A.; Riccio, M.; et al. Stem Cells in Autologous Microfragmented Adipose Tissue: Current Perspectives in Osteoarthritis Disease. Int. J. Mol. Sci. 2021, 22, 10197. [Google Scholar] [CrossRef] [PubMed]
- Tsoukas, D.; Muntean, I.; Simos, C.; Sabido-Vera, R. Prospective Observational Study of a Non-Arthroscopic Autologous Cartilage Micrografting Technology for Knee Osteoarthritis. Bioengineering 2023, 10, 1294. [Google Scholar] [CrossRef] [PubMed]
- Roos, E.M.; Roos, H.P.; Lohmander, L.S.; Ekdahl, C.; Beynnon, B.D. Knee Injury and Osteoarthritis Outcome Score (KOOS)--development of a self-administered outcome measure. J. Orthop. Sports Phys. Ther. 1998, 28, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988, 15, 1833–1840. [Google Scholar]
- Boonstra, A.M.; Schiphorst Preuper, H.R.; Reneman, M.F.; Posthumus, J.B.; Stewart, R.E. Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Int. J. Rehabil. Res. 2008, 31, 165–169. [Google Scholar] [CrossRef]
- McCormack, H.M.; Horne, D.J.; Sheather, S. Clinical applications of visual analogue scales: A critical review. Psychol. Med. 1988, 18, 1007–1019. [Google Scholar] [CrossRef]
- Wojcieszek, A.; Kurowska, A.; Majda, A.; Liszka, H.; Gądek, A. The Impact of Chronic Pain, Stiffness and Difficulties in Performing Daily Activities on the Quality of Life of Older Patients with Knee Osteoarthritis. Int. J. Environ. Res. Public Health 2022, 19, 16815. [Google Scholar] [CrossRef]
- Thirumaran, A.J.; Deveza, L.A.; Atukorala, I.; Hunter, D.J. Assessment of Pain in Osteoarthritis of the Knee. J. Pers. Med. 2023, 13, 1139. [Google Scholar] [CrossRef]
- Helito, C.P.; Moreira, F.S.; Santiago, M.A.M.; Medeiros, L.F.B.; Giglio, P.N.; da Silva, A.G.M.; Gobbi, R.G.; Pécora, J.R. Prevalence and interference of neuropathic pain in the quality of life in patients with knee osteoarthritis. Clinics 2023, 78, 100287. [Google Scholar] [CrossRef]
- Hasan, M.; Shuckett, R. Clinical features and pathogenetic mechanisms of osteo arthritis of the hips and knees. BC Med. J. 2010, 52, 8. [Google Scholar]
- Yunus, M.H.M.; Nordin, A.; Kamal, H. Pathophysiological Perspective of Osteoarthritis. Medicina 2020, 56, 614. [Google Scholar] [CrossRef] [PubMed]
- Houard, X.; Goldring, M.B.; Berenbaum, F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr. Rheumatol. Rep. 2013, 15, 375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Mezey, G.A.; Máté, Z.; Paulik, E. Factors Influencing Pain Management of Patients with Osteoarthritis: A Cross-Sectional Study. J. Clin. Med. 2022, 11, 1352. [Google Scholar] [CrossRef]
- Kawde, K.; Pisulkar, G.; Salwan, A.; Jayasoorya, A.; Jadawala, V.H.; Taywade, S. A Comprehensive Review of Current Management Trends in Medial Compartment Arthritis of the Knee Joint. Cureus 2024, 16, e56666. [Google Scholar] [CrossRef]
- Balli, M.; Vitali, F.; Janiszewski, A.; Caluwé, E.; Cortés-Calabuig, A.; Carpentier, S.; Duelen, R.; Ronzoni, F.; Marcelis, L.; Bosisio, F.M.; et al. Autologous micrograft accelerates endogenous wound healing response through ERK-induced cell migration. Cell Death Differ. 2020, 27, 1520–1538. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Gentile, P.; Marcarelli, M.; Balli, M.; Ronzoni, F.L.; Benedetti, L.; Cusella De Angelis, M.G. In Vitro and In Vivo Studies of Alar-Nasal Cartilage Using Autologous Micro-Grafts: The Use of the Rigenera® Protocol in the Treatment of an Osteochondral Lesion of the Nose. Pharmaceuticals 2017, 10, 53. [Google Scholar] [CrossRef]
- Marcarelli, M.; Zappia, M.; Rissolio, L.; Baroni, C.; Astarita, C.; Trovato, L.; Graziano, A. Cartilage Micrografts as a Novel Non-Invasive and Non-Arthroscopic Autograft Procedure for Knee Chondropathy: Three-Year Follow-Up Study. J. Clin. Med. 2021, 10, 322. [Google Scholar] [CrossRef]




| Patient Number | Age | Sex | BMI (kg/m2) | Diagnosis | Kellgren and Lawrence Scale Mean Score | Past Relevant Medications—Type and Treatment Period |
|---|---|---|---|---|---|---|
| 1 | 64 | F | 31 | Primary Osteoarthritis | 3 | Anti-Inflammatory Drugs, Corticoid Infiltration (1 time), HA Infiltration (2 times) |
| 2 | 59 | M | 28 | Post-Traumatic Osteoarthritis | 4 | Anti-Inflammatory Drugs, Corticoid Infiltration (3 times), HA Infiltration (3 times) |
| 3 | 39 | F | 35 | Patello-femoral Pain/Chondral Injuries | 2 | Anti-Inflammatory Drugs, Corticoid Infiltration (1 time), HA Infiltration (4 times) |
| 4 | 66 | F | 25 | Primary Osteoarthritis | 3 | Anti-Inflammatory Drugs, Corticoid Infiltration (5 times), HA Infiltration (4 times) |
| 5 | 45 | M | 24 | Post-Traumatic Osteoarthritis | 3 | Anti-Inflammatory Drugs, Corticoid Infiltration (3 times), HA Infiltration (3 times) |
| 6 | 54 | M | 34 | Primary Osteoarthritis | 4 | Anti-Inflammatory Drugs, Corticoid Infiltration (2 times), HA Infiltration (4 times) |
| 7 | 64 | F | 32 | Primary Osteoarthritis | 3 | Anti-Inflammatory Drugs, Corticoid Infiltration (1 time), HA Infiltration (1 time) |
| 8 | 47 | F | 30 | Patello-femoral Pain/Chondral Injuries | 2 | Anti-Inflammatory Drugs, Corticoid Infiltration (3 times), HA Infiltration (2 times), Genicular Nerve Block |
| 9 | 55 | M | 28 | Primary Osteoarthritis | 3 | Anti-Inflammatory Drugs, Corticoid Infiltration (3 times), HA Infiltration (2 times) |
| 10 | 63 | M | 31 | Primary Osteoarthritis | 3 | Anti-Inflammatory Drugs, Corticoid Infiltration (2 times), HA Infiltration (3 times), Genicular Nerve Block |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helito, C.P.; Pessei, V.; Zaniboni, C.; Muntean, I. Efficacy of Autologous Micrografting Technology in Managing Osteoarthritis Pain: A Pilot Study. Bioengineering 2024, 11, 1119. https://doi.org/10.3390/bioengineering11111119
Helito CP, Pessei V, Zaniboni C, Muntean I. Efficacy of Autologous Micrografting Technology in Managing Osteoarthritis Pain: A Pilot Study. Bioengineering. 2024; 11(11):1119. https://doi.org/10.3390/bioengineering11111119
Chicago/Turabian StyleHelito, Camilo Partezani, Valeria Pessei, Cecilia Zaniboni, and Ilie Muntean. 2024. "Efficacy of Autologous Micrografting Technology in Managing Osteoarthritis Pain: A Pilot Study" Bioengineering 11, no. 11: 1119. https://doi.org/10.3390/bioengineering11111119
APA StyleHelito, C. P., Pessei, V., Zaniboni, C., & Muntean, I. (2024). Efficacy of Autologous Micrografting Technology in Managing Osteoarthritis Pain: A Pilot Study. Bioengineering, 11(11), 1119. https://doi.org/10.3390/bioengineering11111119








