Comparative Analysis of In Vitro Pumps Used in Cardiovascular Investigations: Focus on Flow Generation Principles and Characteristics of Generated Flows
Abstract
:1. Introduction
2. Types of Pumps Used for In Vitro Cardiovascular Investigations
3. Non-Contact Pumps
3.1. Peristaltic Pumps


3.2. Osmosis Pumps
3.3. Centrifugal Pumps
4. Contact Pumps
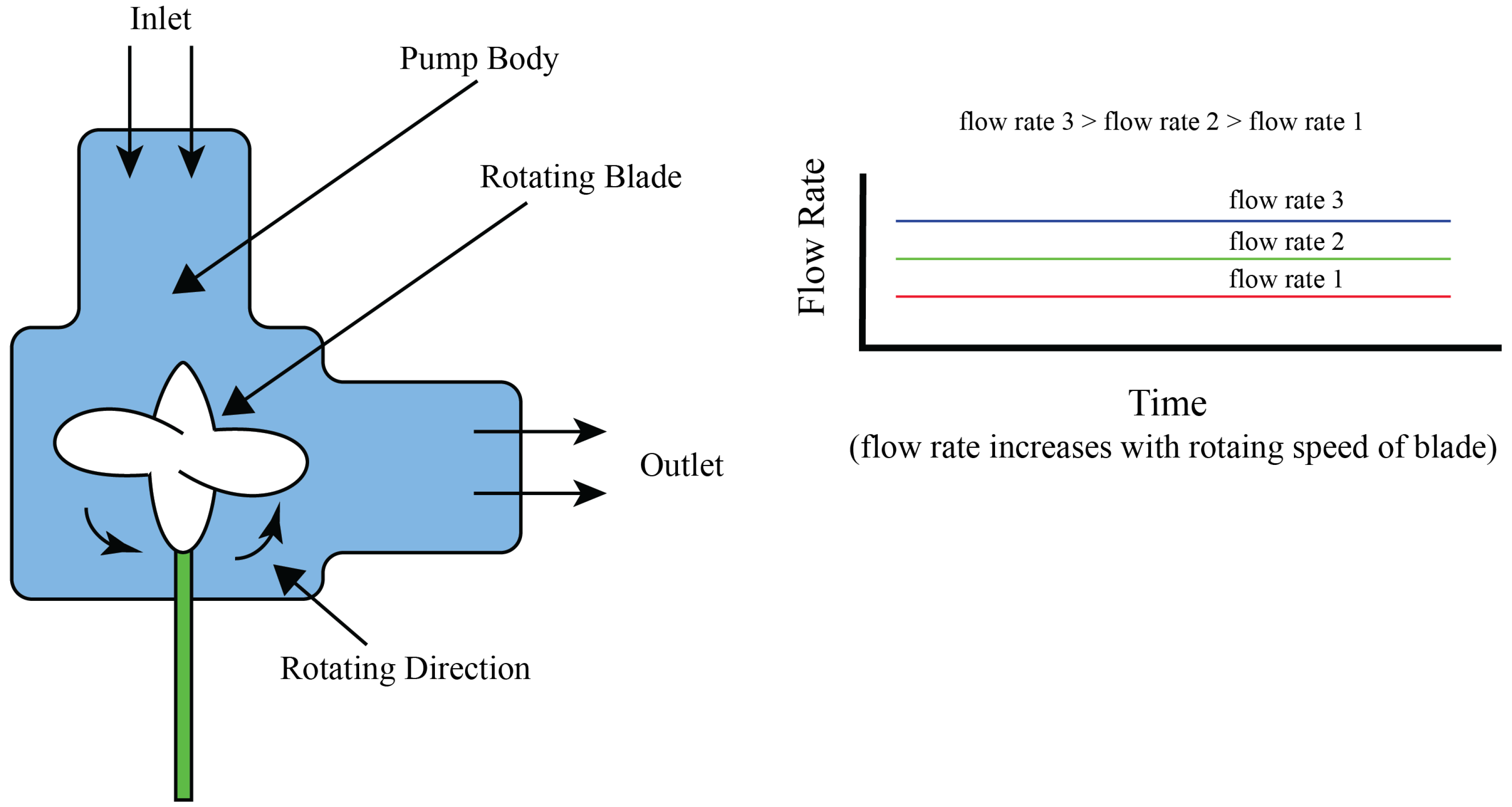
4.1. Centrifugal Pumps
4.2. Piston Pumps

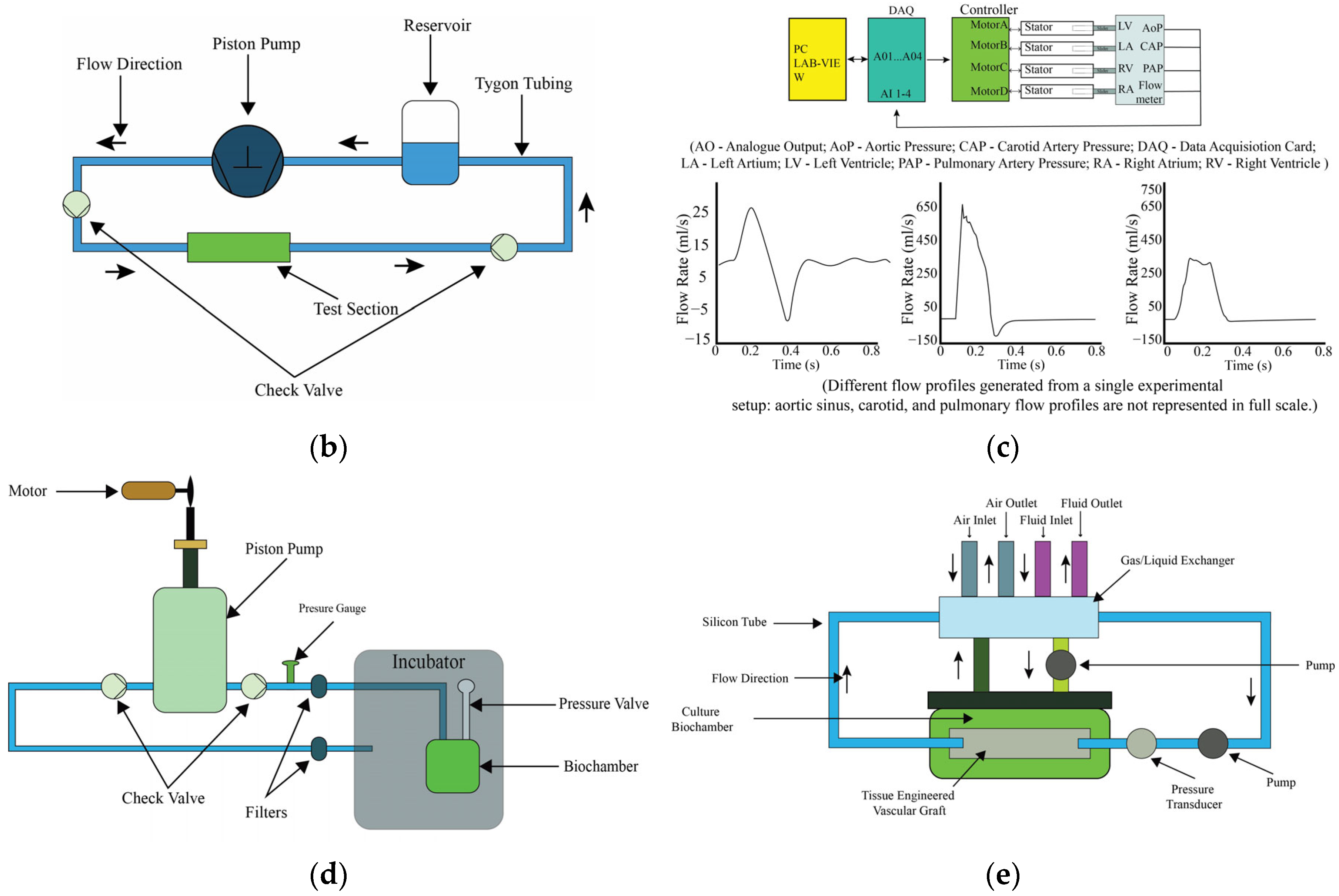
4.3. Diaphragm Pumps
4.4. Syringe Pumps

4.5. Vaccum Pumps
4.6. Gear Pumps
5. Emerging Pump Systems
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 1 September 2024).
- Bouhrira, N.; DeOre, B.J.; Galie, P.A. Implementation and characterization of a physiologically relevant flow waveform in a 3D microfluidic model of the blood–brain barrier. Biotechnol. Bioeng. 2021, 118, 2411–2421. [Google Scholar] [CrossRef] [PubMed]
- Rezaienia, M.A.; Rahideh, A.; Rothman, M.T.; Sell, S.A.; Mitchell, K.; Korakianitis, T. In Vitro Comparison of Two Different Mechanical Circulatory Support Devices Installed in Series and in Parallel. Artif. Organs 2014, 38, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Helms, F.; Lau, S.; Klingenberg, M.; Aper, T.; Haverich, A.; Wilhelmi, M.; Böer, U. Complete Myogenic Differentiation of Adipogenic Stem Cells Requires Both Biochemical and Mechanical Stimulation. Ann. Biomed. Eng. 2020, 48, 913–926. [Google Scholar] [CrossRef] [PubMed]
- Eoh, J.H.; Shen, N.; Burke, J.A.; Hinderer, S.; Xia, Z.; Schenke-Layland, K.; Gerecht, S. Enhanced elastin synthesis and maturation in human vascular smooth muscle tissue derived from induced-pluripotent stem cells. Acta Biomater. 2017, 52, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhu, X.; Futai, N.; Cho, B.S.; Takayama, S. Computerized microfluidic cell culture using elastomeric channels and Braille displays. Proc. Natl. Acad. Sci. USA 2004, 101, 15861–15866. [Google Scholar] [CrossRef]
- Chong, A.; Sun, Z.; van de Velde, L.; Jansen, S.; Versluis, M.; Reijnen, M.M.P.J.; Jebbink, E.G. A novel roller pump for physiological flow. Artif. Organs 2020, 44, 818–826. [Google Scholar] [CrossRef]
- Balagaddé, F.K.; You, L.; Hansen, C.L.; Arnold, F.H.; Quake, S.R. Long-Term Monitoring of Bacteria Undergoing Programmed Population Control in a Microchemostat. Science 2005, 309, 137–140. [Google Scholar] [CrossRef]
- Gómez-Sjöberg, R.; Leyrat, A.A.; Pirone, D.M.; Chen, C.S.; Quake, S.R. Versatile, fully automated, microfluidic cell culture system. Anal. Chem. 2007, 79, 8557–8563. [Google Scholar] [CrossRef]
- Ju, J.; Ko, J.-M.; Cha, H.-C.; Park, J.Y.; Im, C.-H.; Lee, S.-H. An electrofusion chip with a cell delivery system driven by surface tension. J. Micromech. Microeng. 2008, 19, 015004. [Google Scholar] [CrossRef]
- Ju, J.; Park, J.Y.; Kim, K.C.; Kim, H.; Berthier, E.; Beebe, D.J.; Lee, S.-H. Backward flow in a surface tension driven micropump. J. Micromech. Microeng. 2008, 18, 087002. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, S.-K.; Woo, D.-H.; Lee, E.-J.; Kim, J.-H.; Lee, S.-H. Differentiation of Neural Progenitor Cells in a Microfluidic Chip-Generated Cytokine Gradient. Stem Cells 2009, 27, 2646–2654. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Hwang, C.M.; Lee, S.H.; Lee, S.-H. Gradient generation by an osmotic pump and the behavior of human mesenchymal stem cells under the fetal bovine serum concentration gradient. Lab A Chip 2007, 7, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.W.; Taylor, A.M.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. External force-assisted cell positioning inside microfluidic devices. Biomed. Microdevices 2007, 9, 15–23. [Google Scholar] [CrossRef]
- Ren, Y.; Chow, L.M.-C.; Leung, W.W.-F. Cell culture using centrifugal microfluidic platform with demonstration on Pichia pastoris. Biomed. Microdevices 2013, 15, 321–337. [Google Scholar] [CrossRef]
- Huang, S.B.; Wu, M.-H.; Cui, Z.; Cui, Z.; Lee, G.-B. A membrane-based serpentine-shape pneumatic micropump with pumping performance modulated by fluidic resistance. J. Micromech. Microeng. 2008, 18, 045008. [Google Scholar] [CrossRef]
- Law, Y.F.; Cobbold, R.S.C.; Johnston, K.W.; Bascom, P.A.J. Computer-controlled pulsatile pump system for physiological flow simulation. Med. Biol. Eng. Comput. 1987, 25, 590–595. [Google Scholar] [CrossRef]
- Hoenicka, M.; Wiedemann, L.; Puehler, T.; Hirt, S.; Birnbaum, D.E.; Schmid, C. Effects of Shear Forces and Pressure on Blood Vessel Function and Metabolism in a Perfusion Bioreactor. Ann. Biomed. Eng. 2010, 38, 3706–3723. [Google Scholar] [CrossRef]
- Wang, J.; Heo, J.; Hua, S.Z. Spatially resolved shear distribution in microfluidic chip for studying force transduction mechanisms in cells. Lab A Chip 2010, 10, 235–239. [Google Scholar] [CrossRef]
- Farcas, M.A.; Rouleau, L.; Fraser, R.; Leask, R.L. The development of 3-D, in vitro, endothelial culture models for the study of coronary artery disease. Biomed. Eng. OnLine 2009, 8, 30. [Google Scholar] [CrossRef]
- Ota, H.; Yamamoto, R.; Deguchi, K.; Tanaka, Y.; Kazoe, Y.; Sato, Y.; Miki, N. Three-dimensional spheroid-forming lab-on-a-chip using micro-rotational flow. Sens. Actuators B Chem. 2010, 147, 359–365. [Google Scholar] [CrossRef]
- Futai, N.; Gu, W.; Song, J.W.; Takayama, S. Handheld recirculation system and customized media for microfluidic cell culture. Lab A Chip 2006, 6, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.S.; Cabrera, L.M.; Song, J.W.; Futai, N.; Tung, Y.-C.; Smith, G.D.; Takayama, S. Characterization and resolution of evaporation-mediated osmolality shifts that constrain microfluidic cell culture in poly(dimethylsiloxane) devices. Anal. Chem. 2007, 79, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Mehta, G.; Mehta, K.; Sud, D.; Song, J.W.; Bersano-Begey, T.; Futai, N.; Heo, Y.S.; Mycek, M.-A.; Linderman, J.J.; Takayama, S. Quantitative measurement and control of oxygen levels in microfluidic poly(dimethylsiloxane) bioreactors during cell culture. Biomed. Microdevices 2007, 9, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Shinjo, M.; Hirakawa, S.; Nishinaka, M.; Tanaka, Y.; Mawatari, K.; Kitamori, T.; Sato, K. A palmtop-sized microfluidic cell culture system driven by a miniaturized infusion pump. Electrophoresis 2012, 33, 1729–1735. [Google Scholar] [CrossRef]
- Unger, M.A.; Chou, H.-P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef]
- Wu, M.H.; Huang, S.B.; Cui, Z.; Cui, Z.; Lee, G.B. A high throughput perfusion-based microbioreactor platform integrated with pneumatic micropumps for three-dimensional cell culture. Biomed. Microdevices 2008, 10, 309–319. [Google Scholar] [CrossRef]
- Wolf, F.; González, D.M.R.; Steinseifer, U.; Obdenbusch, M.; Herfs, W.; Brecher, C.; Jockenhoevel, S.; Mela, P.; Schmitz-Rode, T. VascuTrainer: A Mobile and Disposable Bioreactor System for the Conditioning of Tissue-Engineered Vascular Grafts. Ann. Biomed. Eng. 2018, 46, 616–626. [Google Scholar] [CrossRef]
- Diamantouros, S.E.; Hurtado-Aguilar, L.G.; Schmitz-Rode, T.; Mela, P.; Jockenhoevel, S. Pulsatile Perfusion Bioreactor System for Durability Testing and Compliance Estimation of Tissue Engineered Vascular Grafts. Ann. Biomed. Eng. 2013, 41, 1979–1989. [Google Scholar] [CrossRef]
- Chaudhury, R.A.; Atlasman, V.; Pathangey, G.; Pracht, N.; Adrian, R.J.; Frakes, D.H. A High Performance Pulsatile Pump for Aortic Flow Experiments in 3-Dimensional Models. Cardiovasc. Eng. Technol. 2016, 7, 148–158. [Google Scholar] [CrossRef]
- Shaikh, F.M.; O’Brien, T.P.; Callanan, A.; Kavanagh, E.G.; Burke, P.E.; Grace, P.A.; McGloughlin, T.M. New Pulsatile Hydrostatic Pressure Bioreactor for Vascular Tissue-engineered Constructs. Artif. Organs 2010, 34, 153–158. [Google Scholar] [CrossRef]
- Song, L.; Zhou, Q.; Duan, P.; Guo, P.; Li, D.; Xu, Y.; Li, S.; Luo, F.; Zhang, Z. Successful Development of Small Diameter Tissue-Engineering Vascular Vessels by Our Novel Integrally Designed Pulsatile Perfusion-Based Bioreactor. PLoS ONE 2012, 7, e42569. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.; Rezaienia, M.A.; Rahideh, A.; Keeble, T.R.; Rothman, M.T.; Korakianitis, T. In Vitro Cardiovascular System Emulator (Bioreactor) for the Simulation of Normal and Diseased Conditions with and Without Mechanical Circulatory Support. Artif. Organs 2013, 37, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Vignali, E.; Gasparotti, E.; Mariotti, A.; Haxhiademi, D.; Ait-Ali, L.; Celi, S. High-Versatility Left Ventricle Pump and Aortic Mock Circulatory Loop Development for Patient-Specific Hemodynamic In Vitro Analysis. ASAIO J. Am. Soc. Artif. Intern. Organs 2022, 68, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Kizilski, S.B.; Zhang, X.; Kneier, N.E.; Lizarraga, M.D.C.; Schulz, N.E.; Hammer, P.E.; Hoganson, D.M. An In Vitro Circulatory Loop Model of the Pediatric Right Ventricular Outflow Tract as a Platform for Valve Evaluation. Cardiovasc. Eng. Technol. 2023, 14, 217–229. [Google Scholar] [CrossRef]
- Fanni, B.M.; Gasparotti, E.; Vignali, E.; Capelli, C.; Positano, V.; Celi, S. An integrated in-vitro and in-silico workflow to study the pulmonary bifurcation hemodynamics. Comput. Fluids 2023, 260, 105912. [Google Scholar] [CrossRef]
- Kaasi, A.; Cestari, I.A.; Stolf, N.A.; Leirner, A.A.; Hassager, O.; Cestari, I.N. A new approach to heart valve tissue engineering: Mimicking the heart ventricle with a ventricular assist device in a novel bioreactor. J. Tissue Eng. Regen. Med. 2011, 5, 292–300. [Google Scholar] [CrossRef]
- Kado, Y.; Miyamoto, T.; Horvath, D.J.; Gao, S.; Fukamachi, K.; Karimov, J.H. Development of a circulatory mock loop for biventricular device testing with various heart conditions. Int. J. Artif. Organs 2020, 43, 600–605. [Google Scholar] [CrossRef]
- Ruel, J.; Lachance, G. Mathematical modeling and experimental testing of three bioreactor configurations based on windkessel models. Heart Int. 2010, 5, e1. [Google Scholar] [CrossRef]
- Hung, P.J.; Lee, P.J.; Sabounchi, P.; Aghdam, N.; Lin, R.; Lee, L.P. A novel high aspect ratio microfluidic design to provide a stable and uniform microenvironment for cell growth in a high throughput mammalian cell culture array. Lab A Chip 2005, 5, 44. [Google Scholar] [CrossRef]
- Yoshino, D.; Sato, K.; Sato, M. Endothelial Cell Response Under Hydrostatic Pressure Condition Mimicking Pressure Therapy. Cell. Mol. Bioeng. 2015, 8, 296–303. [Google Scholar] [CrossRef]
- Baeckert, M.; Batliner, M.; Grass, B.; Buehler, P.K.; Daners, M.S.; Meboldt, M.; Weiss, M. Performance of modern syringe infusion pump assemblies at low infusion rates in the perioperative setting. Br. J. Anaesth. 2020, 124, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Tung, Y.-C.; Wei, H.-H.; Grotberg, J.B.; Skerlos, S.J.; Kurabayashi, K.; Takayama, S. Use of Air-Liquid Two-Phase Flow in Hydrophobic Microfluidic Channels for Disposable Flow Cytometers. Biomed. Microdevices 2002, 4, 141–149. [Google Scholar] [CrossRef]
- King, K.R.; Wang, S.; Jayaraman, A.; Yarmush, M.L.; Toner, M. Microfluidic flow-encoded switching for parallel control of dynamic cellular microenvironments. Lab A Chip 2007, 8, 107–116. [Google Scholar] [CrossRef]
- Tsai, W.; Savaş, Ö. Flow pumping system for physiological waveforms. Med. Biol. Eng. Comput. 2010, 48, 197–201. [Google Scholar] [CrossRef]
- Choi, J.-W.; Choe, J.H.; Jung, S.Y.; Park, H.; Ha, H. Fabrication of affordable pulse duplication system for the in-vitro cardiovascular experiments based on gear pump and orifice flowmeter. J. Mech. Sci. Technol. 2019, 33, 3927–3932. [Google Scholar] [CrossRef]
- Chodzyński, K.J.; Boudjeltia, K.Z.; Lalmand, J.; Aminian, A.; Vanhamme, L.; de Sousa, D.R.; Gremmo, S.; Bricteux, L.; Renotte, C.; Courbebaisse, G.; et al. An in vitro test bench reproducing coronary blood flow signals. Biomed. Eng. Online 2015, 14, 77. [Google Scholar] [CrossRef]
- Drost, S.; de Kruif, B.J.; Newport, D. Arduino control of a pulsatile flow rig. Med. Eng. Phys. 2018, 51, 67–71. [Google Scholar] [CrossRef]
- Lee, H.; Rho, Y.; Park, C.; Hwang, C.; Kim, W.; Sun, K.; Choi, M.; Lee, K.; Cheong, J.; Shim, E.; et al. Application of the moving-actuator type pump as a ventricular assist device: In vitro and in vivo studies. Int. J. Artif. Organs 2002, 25, 556–561. [Google Scholar] [CrossRef]
- Mechoor, R.R.; Schmidt, T.; Kung, E. A Real-Time Programmable Pulsatile Flow Pump for In Vitro Cardiovascular Experimentation. J. Biomech. Eng. 2016, 138, 111002. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, E.; Adam, A.; Salik, M.; Gareso, P. Programmable Syringe Pump for Selective Micro Droplet Deposition. J. Elektron. dan Telekomun. 2019, 19, 75. [Google Scholar] [CrossRef]
- Kassis, T.; Perez, P.M.; Yang, C.J.W.; Soenksen, L.R.; Trumper, D.L.; Griffith, L.G. PiFlow: A biocompatible low-cost programmable dynamic flow pumping system utilizing a Raspberry Pi Zero and commercial piezoelectric pumps. HardwareX 2018, 4, e00034. [Google Scholar] [CrossRef]
- Chen, S.; Qian, C.; Cheng, W.; Kan, J.; Ji, J.; Zhang, Z.; Wang, J. A low frequency driven piezoelectric pump with flexible valve. Sens. Actuators Phys. 2021, 319, 112567. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Shen, Y.; Chen, S.; Yang, Z. A Resonant Piezoelectric Diaphragm Pump Transferring Gas with Compact Structure. Micromachines 2016, 7, 219. [Google Scholar] [CrossRef] [PubMed]
- Force, M.; Moroi, M.; Wang, S.; Kunselman, A.R.; Ündar, A. In Vitro Hemodynamic Evaluation of ECG-Synchronized Pulsatile Flow Using i-Cor Pump as Short-Term Cardiac Assist Device for Neonatal and Pediatric Population. Artif. Organs 2018, 42, E153–E167. [Google Scholar] [CrossRef]
- Cremers, B.; Link, A.; Werner, C.; Gorhan, H.; Simundic, I.; Matheis, G.; Scheller, B.; Böhm, M.; Laufs, U. Pulsatile Venoarterial Perfusion Using a Novel Synchronized Cardiac Assist Device Augments Coronary Artery Blood Flow During Ventricular Fibrillation. Artif. Organs 2015, 39, 77–82. [Google Scholar] [CrossRef]
- Riveros, A.; Cuellar, M.; Sánchez, P.F.; Muñoz-Camargo, C.; Cruz, J.C.; Sandoval, N.; Mejia, O.D.L.; Briceño, J.C. Design and Characterization of a Fluidic Device for the Evaluation of SIS-Based Vascular Grafts. Processes 2020, 8, 9. [Google Scholar] [CrossRef]









| Reference | Pump Type | Flow Rate | Flow Pattern and Duration | Generated Shear Stress Magnitude | Used Sectors | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|
| Bouhrira [2] | Peristaltic pump with linear actuator | 1–6 mL/min | Pulsatile, physiological, 18 h | Variable shear stress (CFD data) | Blood–brain barrier, endothelial studies | Low-cost, mimics physiological waveform | Limited to low flow rates |
| Helms [4] | Pulsatile peristaltic pump | 64 mL/min | Pulsatile, 10 days | - | Vascular tissue engineering | Promotes smooth muscle differentiation | Requires custom bioreactor setup |
| Eoh [5] | Peristaltic pump | 0.74 mL/min | Pulsatile flow, 30 days | 3–5.5 × 10−4 dyne/cm2 | Vascular tissue engineering | Enhanced elastin and ECM production | Requires bioreactor and scaffold setup |
| Gu Wei [6] | Braille-based peristaltic pump | 74 nL/min | Pulsatile, continuous, 3 weeks | - | Microfluidic cell culture | Portable, programmable, long-term culture | Limited scalability, channel constriction |
| Chong [7] | Reciprocating roller pump | 0.88–4.25 L/min | Pulsatile, physiological, continuous | - | Cardiopulmonary bypass, ECMO | Reproduces realistic blood flow waveforms | Limited to pre-programmed flow profiles |
| Balagaddé [8] | Peristaltic Pump | 250 µm/s | Continuous, semi-continuous circulation, over 200 h | - | Metabolic culture studies | Prevents biofilm formation, long-term monitoring | Requires precise control of microfluidics |
| Gómez-Sjöberg [9] | On-chip peristaltic pump | 6.5 nL/s | Pressure-driven, peristaltic, 7–9 days | 0.05 N/m2 | Stem cell research, differentiation studies | Fully automated, precise media exchange | Bubble formation, slower for flushing tasks |
| Ju [10] | Surface tension-driven micropump | 45 µm/s | Continuous, slow flow | - | Electrofusion, plant cells | Simple, cost-effective, no peripherals required | Limited to slow-flow applications |
| Ju [11] | Surface tension-driven micropump | 45 µm/s | Continuous, reversible | - | Microfluidics, cell transport | Simple, passive, low power requirement | Prone to backward flow at low volume ratios |
| Park [12] | Osmotic pump | 0.15 µL/h | Continuous, laminar, 10 days | Low, minimized | Stem cell differentiation | Long-term stable gradient, minimal handling | Complex initial setup |
| Park [13] | Osmotic pump | 0.15 µL/h | Continuous, gradient, 8–21 days | - | Stem cell differentiation | Stable long-term gradient, minimal media use | Requires careful pressure balance |
| Rhee [14] | Centrifugal force-based system | - | Hydrodynamic, centrifugal, 30–300 s | - | Cell positioning, chemotaxis | Simple setup, adaptable for multiple cell types | Cell viability can be affected at high RCF |
| Ren [15] | Centrifugal microfluidic pump | - | Vortical, spiral toroidal, up to 24 h | - | Cell culture, mixing studies | Short lag phase, efficient mixing | Requires optimized flow patterns |
| Song [16] | Braille-based peristaltic pump | 4.9 µL/s | Pulsatile, peristaltic, 48–72 h | 1–12 dyne/cm2 | Endothelial cell culture | High control over shear stress, multi-loop setup | Limited to shear stress up to 12 dyne/cm2 |
| Law [17] | Computer-controlled roller pump | - | Pulsatile, physiological, continuous operation | - | Doppler ultrasound studies | Simulates physiological flow, easily cleaned | Limited reverse flow capability |
| Hoenicka [18] | Peristaltic pump | 40–60 mL/min | Continuous, pulsatile, 4–8 days | 0.05–0.22 Pa | Blood vessel metabolism | Maintains endothelial cell viability | Requires precise viscosity control |
| Wang [19] | Peristaltic Pump | 30 µL/min | Continuous, gradient flow, up to 24 h | 0.54–6 dyne/cm2 | Cell culture, force transduction | Real-time observation, spatial shear gradients | Limited scalability |
| Farcas [20] | Low-pulsatility peristaltic pump | 119.5 mL/min | Steady, laminar flow, 8–24 h | 20 dynes/cm2 | Endothelial cell culture | Accurate cell elongation, realistic artery model | Introduces minor pressure fluctuations |
| Ota [21] | Peristaltic pump | 1.1 mL/min | Micro-rotational flow, over 1 day | - | Spheroid formation, cell culture | Precise control of spheroid size, long-term culture | Clogging in channels, requires shredder channels |
| Futai [22] | Braille-based pump | 50 nL/min | Pulsatile, 8–21 days | - | Portable cell culture | Portable, long-term culture, minimal maintenance | Limited scalability |
| Heo [23] | Braille-based peristaltic pump | 500 nL/min | Recirculating, continuous, 10 h | - | Endothelial cell culture | Prevents osmolality shifts, sub-microliter recirculation | Requires parylene coating to prevent evaporation |
| Mehta [24] | Braille-based peristaltic pump | 220 µL/s | Continuous, gradient-driven, up to 12 h | - | Microfluidic bioreactors, cell culture | Precise oxygen control, small volume setup | Requires extensive calibration and control |
| Sasaki [25] | Miniaturized infusion pump | 30 µL/h | Intermittent flowOver 102 h | 0.17 dyne/cm2 | Cell culture, endothelial studies | Small, portable, easy-to-use system | Limited scalability, risk of air bubble formation |
| Unger [26] | Peristaltic elastomeric pump | 2.35 nL/s | Pulsatile, peristaltic | - | Microfluidics, cell culture | Low dead volume, fast actuation, durable | Requires pneumatic control system |
| Wu [27] | Pneumatic peristaltic micro-pump | 185.1 μL/h | Pulsatile, peristaltic, up to 48 h | - | 3D cell culture, micro bioreactors | High-throughput, uniform medium distribution | Complexity in scaling up, time delay due to fluid resistance |
| Reference | Pump Type | Flow Rate | Flow Pattern and Duration | Generated Shear Stress Magnitude | Used Sectors | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|
| Wolf [28] | Micro-centrifugal pump | 10-2000 mL/min | Pulsatile, physiological, up to 25 h | - | Tissue-engineered vascular graft conditioning | Compact, transportable, disposable | Requires batteries for autonomous operation |
| Diamantouros [29] | Pulse-free centrifugal pump with magnetic actuator | 50–1000 mL/min | Pulsatile, supra-physiological, up to 4 weeks | 0.067–13.5 dyne/cm2 | Vascular graft durability testing | Realistic pressure waveforms, compliance monitoring | Complex setup, requires multiple sensors |
| Chaudhury [30] | Piston-based pulsatile pump | 850 mL/s | Pulsatile, physiological, continuous operation | - | Aortic flow experiments | High Reynolds number, MRI-compatible | Requires large-scale setup, limited to high flow rates |
| Shaikh [31] | Pulsatile hydrostatic pressure pump | - | Pulsatile, cyclic, 24–48 h | - | Vascular tissue engineering | Compact, easy sterilization, flexible setup | Limited to pressure-based conditioning only |
| Song [32] | Pulsatile perfusion-based pump | 10 mL per cycle | Pulsatile, physiological, 2 weeks | - | Vascular tissue engineering | Precise control, high patency | Complex setup, requires motor-driven control |
| Ruiz [33] | Linear motor-driven pump | 5 L/min | Pulsatile, physiological | - | Cardiovascular simulation | Simulates normal and pathological conditions | Complex system setup required |
| Vignali [34] | Custom-made piston pump | 5 L/min | Pulsatile, physiological, continuous operation | - | Patient-specific hemodynamics | High versatility generating patient-specific flow | Complex setup, high maintenance |
| Kizilski [35] | Custom-made piston pump | - | Pulsatile, physiological, continuous operation | Pediatric right ventricular outflow tract (RVOT) simulation | Accurate simulation of pediatric heart conditions | Complex setup, requires precise calibration | |
| Fanni [36] | Custom-made piston pump | - | Pulsatile, patient-specific | - | Pulmonary artery simulations | High accuracy in replicating patient-specific flows | Complex setup and calibration required |
| Kaasi [37] | Ventricular assist device (VAD) | - | Pulsatile, physiological, 4 days | - | Heart valve tissue engineering | Mimics ventricular pressures, dynamic conditioning | Complex setup, potential contamination risks |
| Kado [38] | Pneumatic pumps (Abiomed AB5000) | 4.2 L/min | Pulsatile, physiological | - | Mechanical circulatory device testing | Customizable for different heart failure conditions | Requires extensive calibration and monitoring |
| Ruel and Lachance [39] | Custom diaphragm pump (Windkessel model-based) | 160 mL/cycle | Pulsatile, physiological | Tissue-engineered heart valve development | Accurate physiological flow and pressure waveforms | Requires complex setup and calibration | |
| Hung [40] | Syringe pump | 0.2 mL/min | Continuous, uniform flow, 7.5 days | - | High-throughput cell culture | Cost-effective, uniform microenvironment | Requires careful flow rate calibration |
| Yoshino [41] | Hydrostatic pressure system | - | Constant pressure, cyclic, 24 h | - | Endothelial cell research | Controlled positive and negative pressure | Requires careful sealing to maintain pressure |
| Baeckert [42] | Syringe infusion pump | 1 mL/h | Continuous, low flow, variable duration | - | Pediatric critical care | Precise for small-volume infusions | Prone to start-up delays and flow irregularities |
| Huh [43] | Air–liquid two-phase flow pump (vacuum pump) | - | Pulsatile, air–liquid, continuous operation | - | Flow cytometry, microfluidics | Disposable, low-cost, minimal contamination risk | Limited to hydrophobic channel systems |
| King [44] | Microfluidic flow-encoded switching (vacuum pump) | - | Continuous, laminar, long-term culture | <0.1 dyne/cm2 | Dynamic cell culture | High-throughput, scalable, parallel flow control | Requires precise pressure control |
| Tsai and Savaş [45] | Gear-piston pump system | 300 mL/s | Pulsatile, physiological, continuous operation | - | Vascular flow experiments | Accurate waveform replication, easy tuning | Sensitive to flow loop impedance |
| Choi [46] | Gear pump with feedback control | 40.9 L/min | Pulsatile, physiological | - | Cardiovascular simulations | Affordable, precise replication of waveforms | Unable to replicate reverse flow |
| Chodzyński [47] | Custom piston pump with linear motor | 40–700 mL/min | Pulsatile, physiological, continuous operation | - | Cardiovascular simulation | Accurate reproduction of coronary blood flow | Requires complex real-time control system |
| Drost [48] | Arduino-controlled gear pump | 1.5 L/min | Pulsatile, physiological, continuous operation | - | Vascular access hemodynamics | Affordable, customizable | Requires semi-automatic system identification |
| Lee [49] | Moving-actuator type pump | 8 L/min | Pulsatile, physiological, continuous operation | - | Ventricular assist device (VAD), TAH | Single device for LVAD (Left Ventricular Assist Device), RVAD (Right Ventricular Assist Device), BVAD (Biventricular Assist Device), or TAH (Total Artificial Heart) | Requires complex compliance and control systems |
| Mechoor [50] | Real-time programmable pulsatile pump | 500 mL/min | Pulsatile, physiological | - | Cardiovascular experimentation | Accurate, adjustable flow patterns | Requires feedback control for waveform accuracy |
| Kurniawan [51] | Syringe Pump | 300 µL/min | Continuous, programmable. Continuous operation | - | Microfluidics, drug delivery | High precision, customizable flow rates | Limited to small-scale application |
| Reference | Pump Type | Flow Rate | Flow Pattern and Duration | Generated Shear Stress Magnitude | Use Sectors | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|
| Kassis [52] | Piezoelectric pump (PiFlow system) | 1–3000 mL/min | Pulsatile, programmable, continuous operation | - | Microfluidics, cell culture | Low-cost, scalable, biocompatible | Limited to moderate flow resistance systems |
| Chen [53] | Low-frequency-driven piezoelectric pump with flexible valve | 18.1 mL/min | Pulsatile, low frequency, continuous operation | - | Microfluidics, drug delivery | Precise flow control, high particle tolerance | Reverse leakage needs control |
| Wang [54] | Resonant piezoelectric diaphragm pump | 186.8 mL/min | Pulsatile, resonant, continuous operation | - | Gas transfer, microfluidics | High output pressure, compact design | Complex structure, working noise |
| Force [55] | ECG-synchronized i-cor diagonal pump | 200–1800 mL/min | Pulsatile, physiological, continuous operation | - | Pediatric cardiac assist | Higher hemodynamic energy, reduces afterload | FDA (Food and Drug Administration) approval pending, off-label use |
| Cremers [56] | Diagonal pulsatile pump (i-cor system) | 0–8 L/min | Pulsatile, ECG-synchronized, continuous operation | - | Cardiogenic shock, ECMO (Extracorporeal Membrane Oxygenation) | Increased coronary perfusion, precise control | Requires synchronization with ECG (Electrocardiogram), complex setup |
| Riveros [57] | Baxter infusion pump | 1200 mL/h | Pulsatile, physiological, continuous operation | 12 dynes/cm2 | Vascular graft testing | Cost-effective, easy assembly | Requires calibration for flow consistency |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazhar, N.; Islam, M.S.; Raza, M.Z.; Mahin, S.K.H.; Islam, M.R.; Chowdhury, M.E.H.; Al-Ali, A.; Agouni, A.; Yalcin, H.C. Comparative Analysis of In Vitro Pumps Used in Cardiovascular Investigations: Focus on Flow Generation Principles and Characteristics of Generated Flows. Bioengineering 2024, 11, 1116. https://doi.org/10.3390/bioengineering11111116
Mazhar N, Islam MS, Raza MZ, Mahin SKH, Islam MR, Chowdhury MEH, Al-Ali A, Agouni A, Yalcin HC. Comparative Analysis of In Vitro Pumps Used in Cardiovascular Investigations: Focus on Flow Generation Principles and Characteristics of Generated Flows. Bioengineering. 2024; 11(11):1116. https://doi.org/10.3390/bioengineering11111116
Chicago/Turabian StyleMazhar, Noaman, Munshi Sajidul Islam, Muhammad Zohaib Raza, SM. Khaled Hossain Mahin, Mohammed Riazul Islam, Muhammad E. H. Chowdhury, Abdulla Al-Ali, Abdelali Agouni, and Huseyin C. Yalcin. 2024. "Comparative Analysis of In Vitro Pumps Used in Cardiovascular Investigations: Focus on Flow Generation Principles and Characteristics of Generated Flows" Bioengineering 11, no. 11: 1116. https://doi.org/10.3390/bioengineering11111116
APA StyleMazhar, N., Islam, M. S., Raza, M. Z., Mahin, S. K. H., Islam, M. R., Chowdhury, M. E. H., Al-Ali, A., Agouni, A., & Yalcin, H. C. (2024). Comparative Analysis of In Vitro Pumps Used in Cardiovascular Investigations: Focus on Flow Generation Principles and Characteristics of Generated Flows. Bioengineering, 11(11), 1116. https://doi.org/10.3390/bioengineering11111116








