Development and Validation of Deep Learning Preoperative Planning Software for Automatic Lumbosacral Screw Selection Using Computed Tomography
Abstract
1. Introduction
2. Materials and Methods
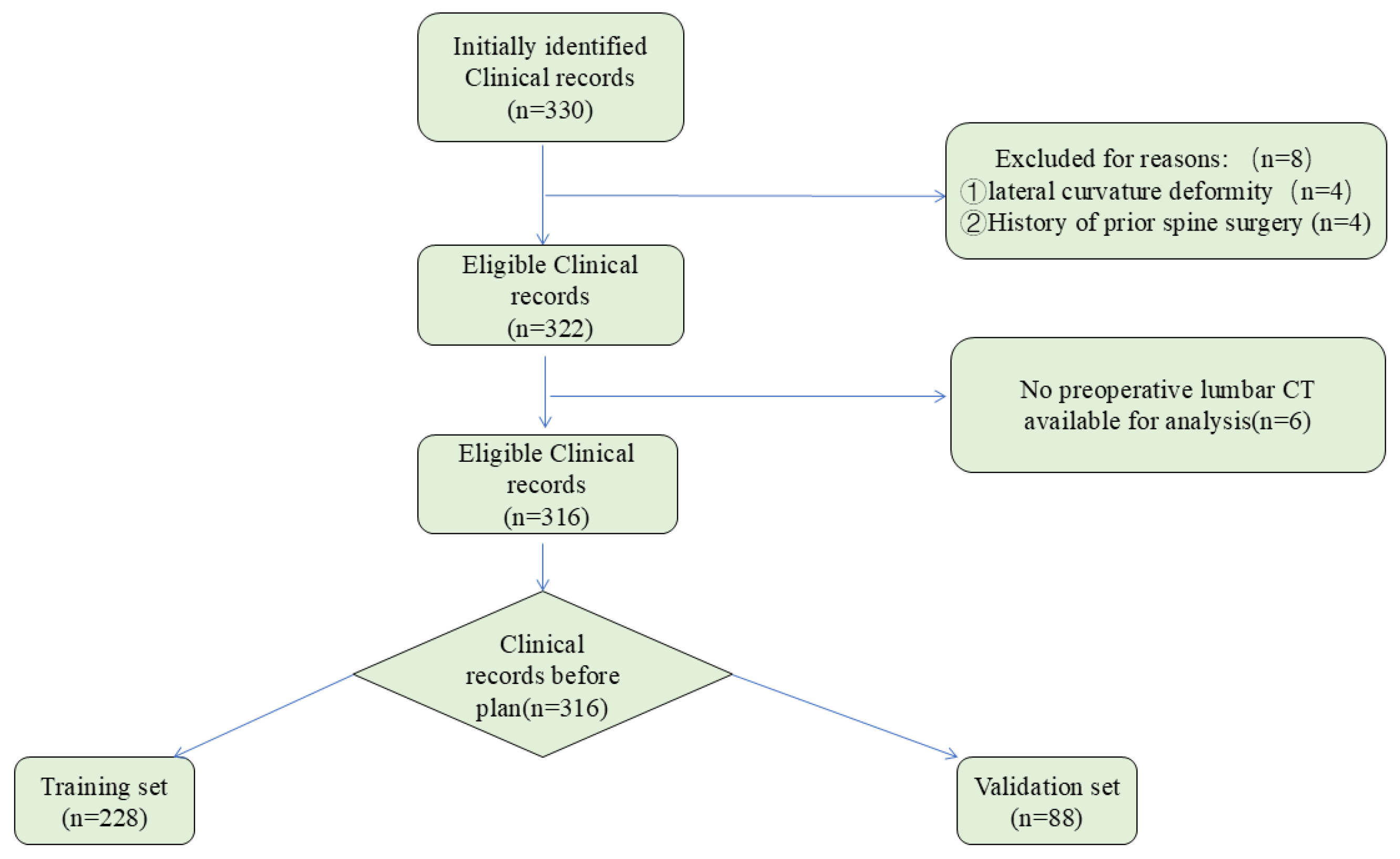
2.1. Patient Selection
2.2. Datasets and Preprocessing
2.3. Mimics Software Manual Pedicle Screws Preoperative Planning
2.4. Development and Construction of the Artificial Intelligence Preoperative Planning for Posterior Lumbar Interbody Fusion (AISPINE)
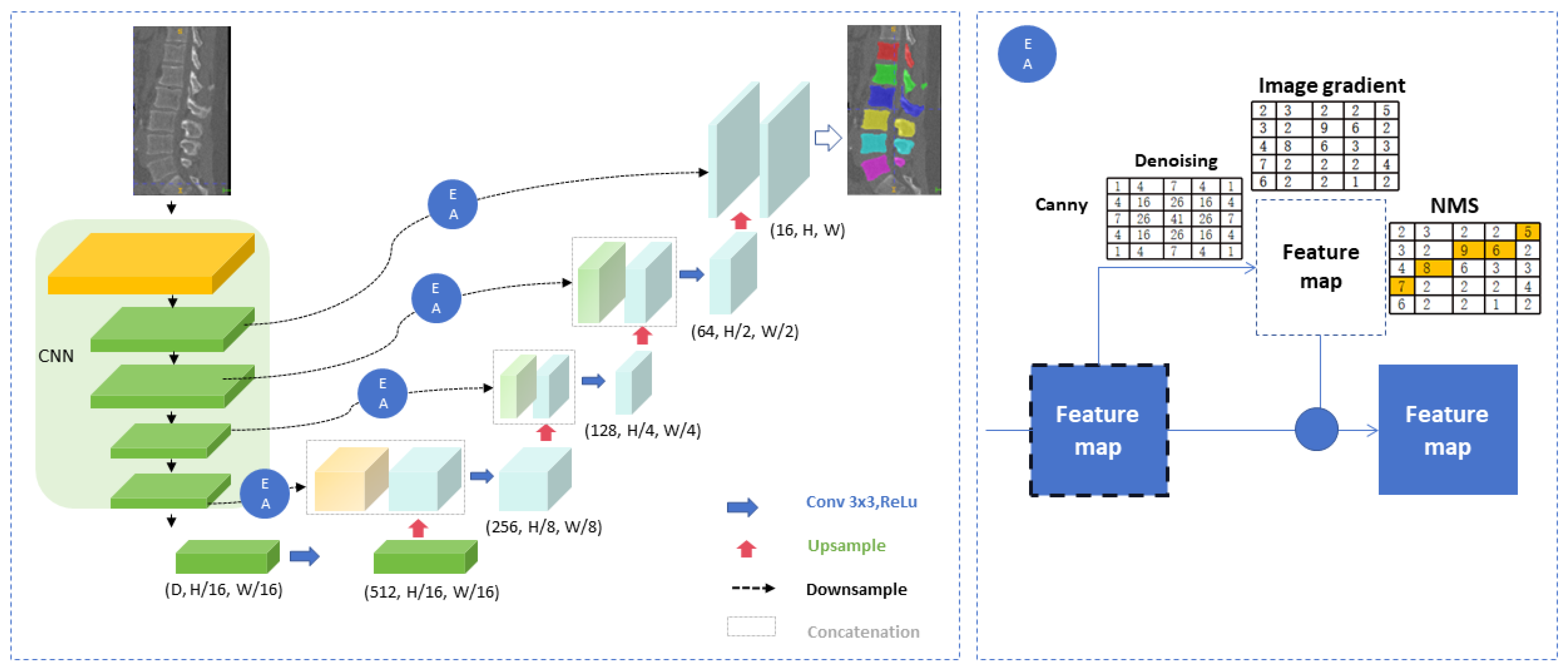
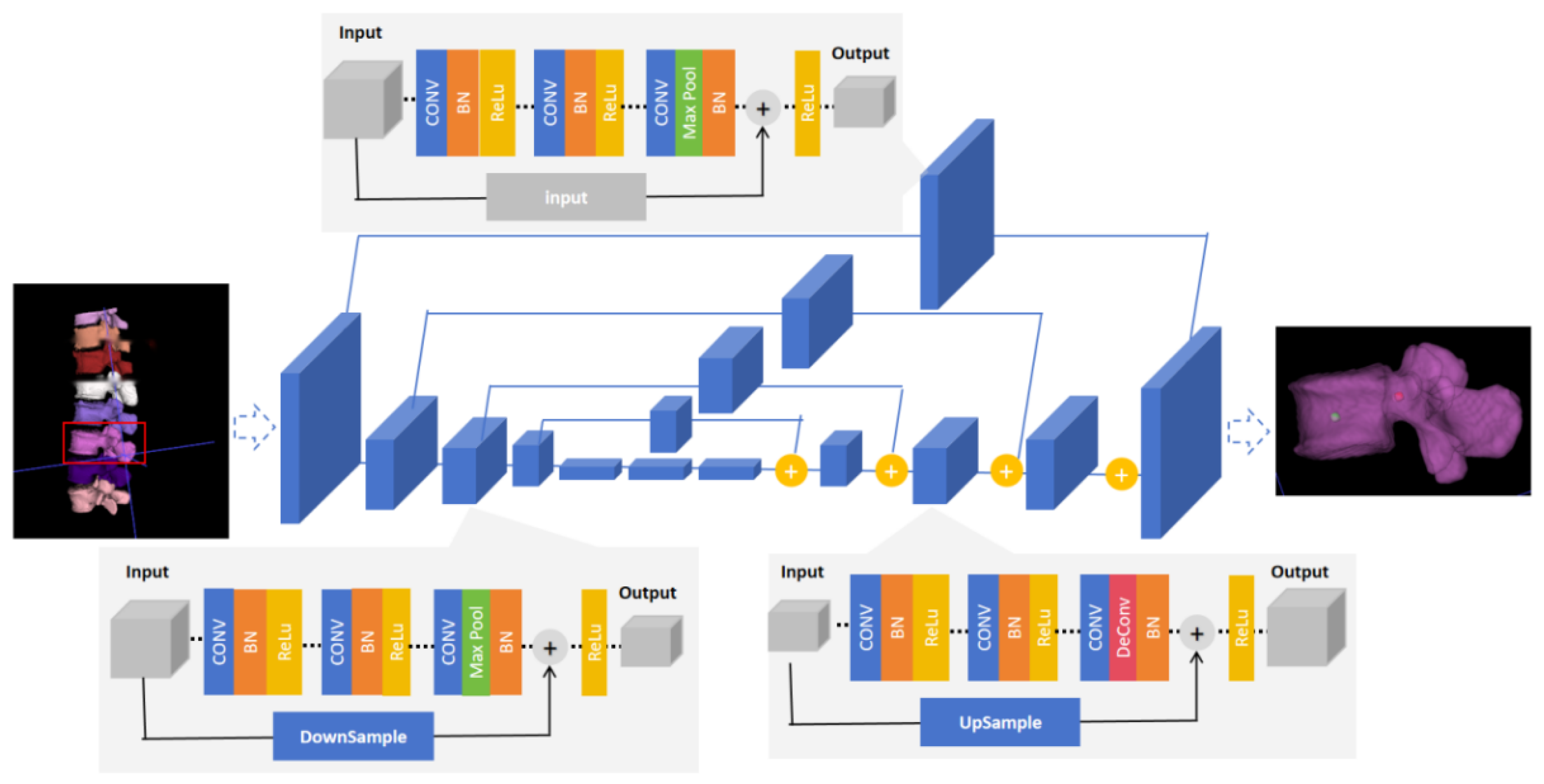
2.4.1. Image Segmentation
2.4.2. The Identification Model of Entry Point and Exit Point
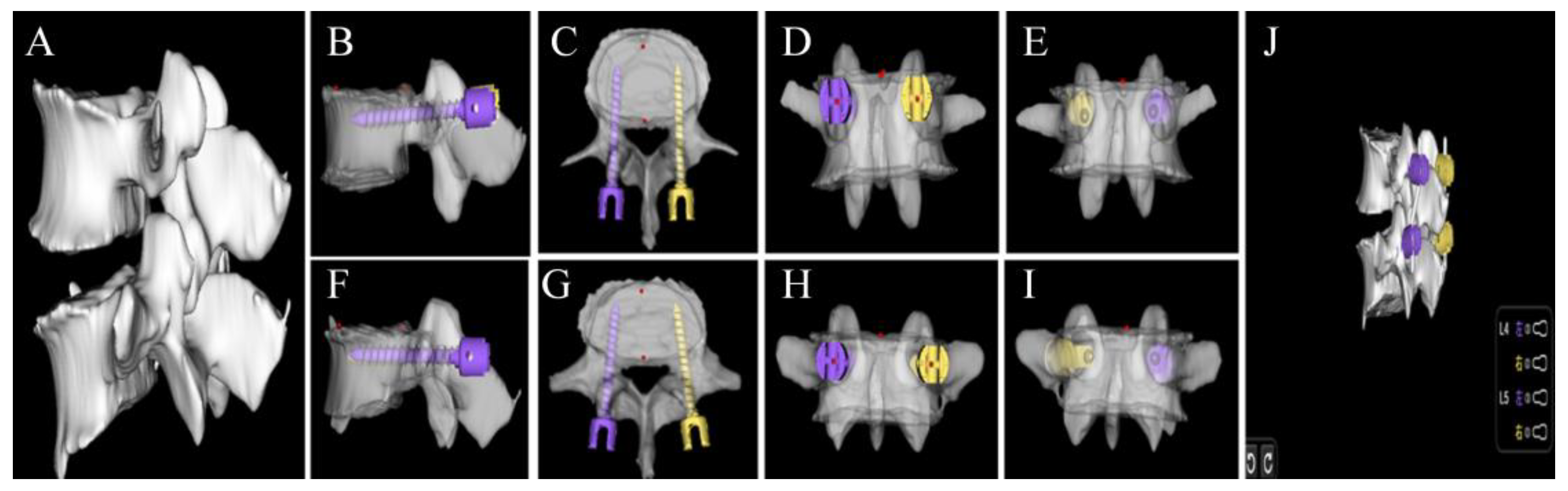
2.4.3. Preoperative Planning Module
2.5. Quantitative Evaluation of Automatic Pedicle Planning
2.6. Qualitative Evaluation of Automatic Pedicle Planning
2.7. Statistics
3. Results
3.1. Descriptive Data
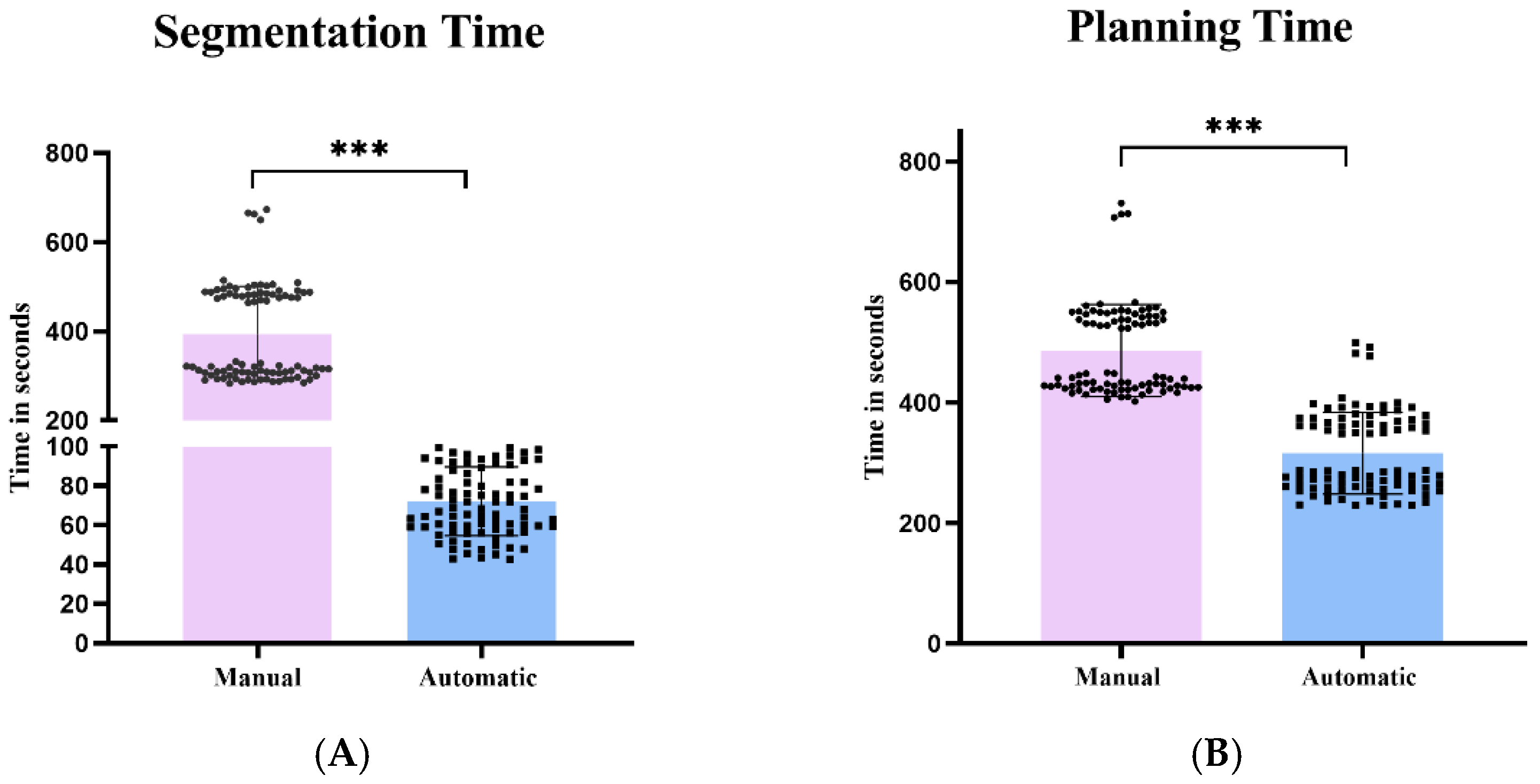
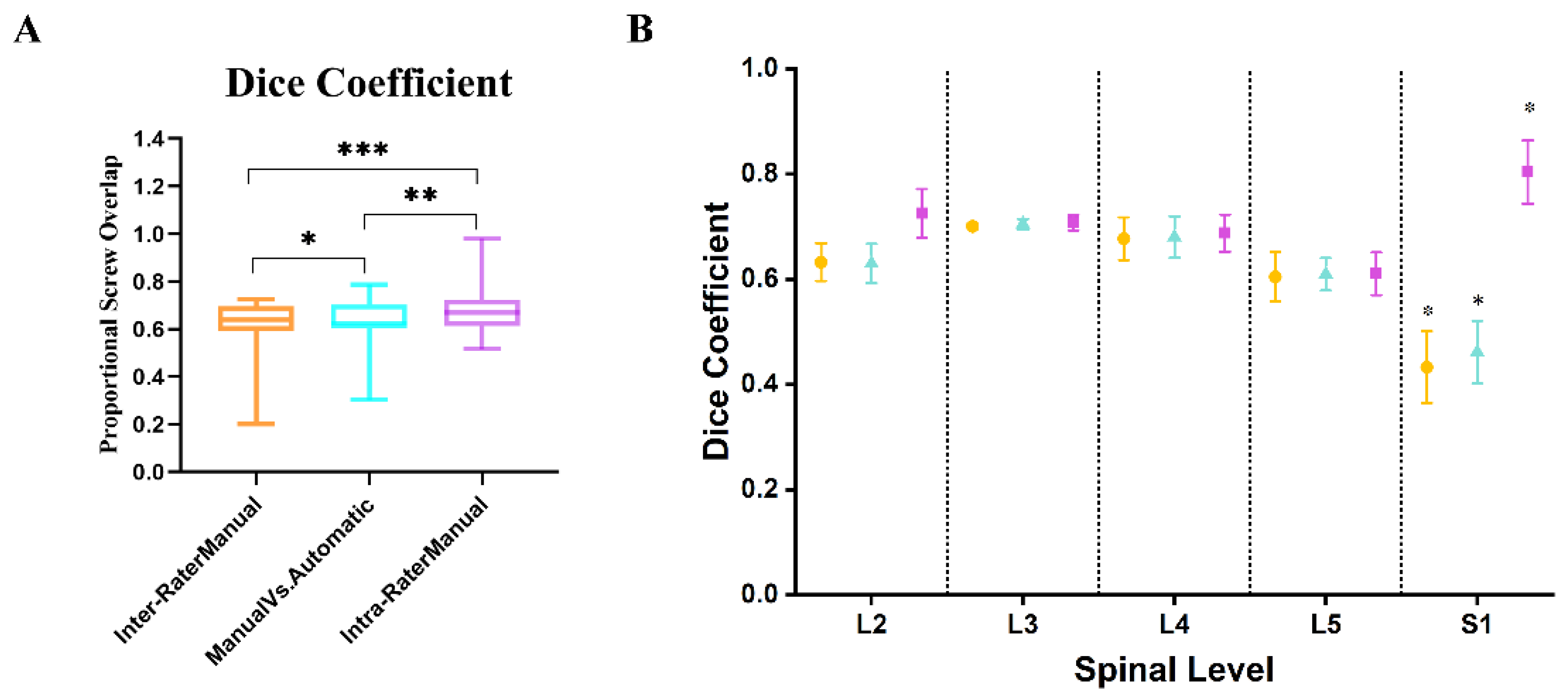
3.2. Quantitative Evaluation of AISPINE Screw Plans
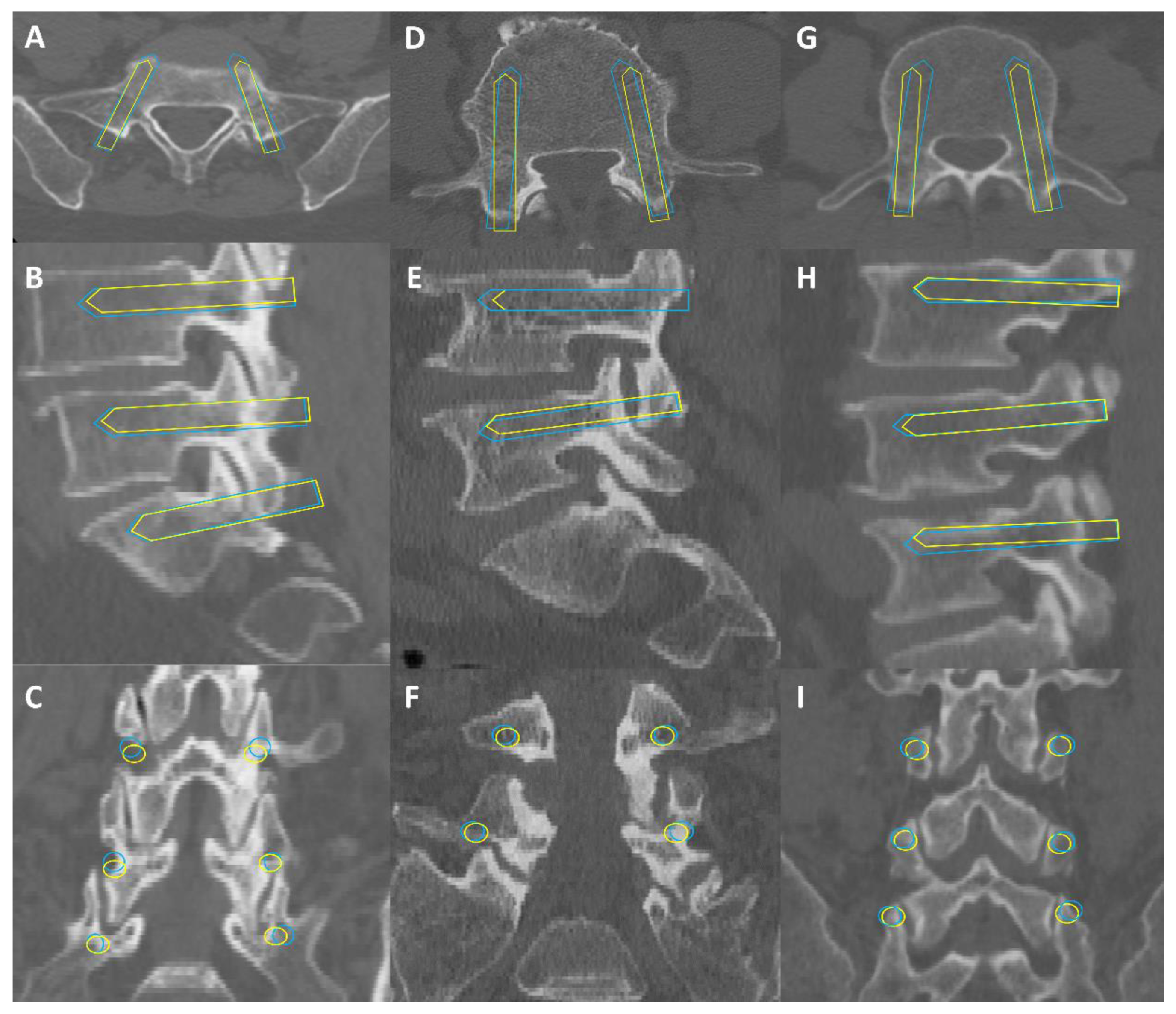
3.3. Qualitative Evaluation of AIPSINE Screw Plans
4. Discussion
4.1. Data Processing
4.2. Quantitative Validation
4.3. Qualitative Validation
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Van Zundert, J.; Cohen, S.P. Low back pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Gill, T.K.; Mittinty, M.M.; March, L.M.; Steinmetz, J.D.; Culbreth, G.T.; Cross, M.; Kopec, J.A.; Woolf, A.D.; Haile, L.M.; Hagins, H.; et al. Global, regional, and national burden of other musculoskeletal disorders, 1990–2020, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e670–e682. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N. Lumbar Disc Disorders and Low-Back Pain: Socioeconomic Factors and Consequences. J. Bone Jt. Surg. 2006, 88, 21–24. [Google Scholar] [CrossRef]
- Fenton-White, H.A. Trailblazing: The historical development of the posterior lumbar interbody fusion (PLIF). Spine J. Off. J. N. Am. Spine Soc. 2021, 21, 1528–1541. [Google Scholar] [CrossRef]
- Katonis, P.; Christoforakis, J.; Kontakis, G.; Aligizakis, A.C.; Papadopoulos, C.; Sapkas, G.; Hadjipavlou, A. Complications and problems related to pedicle screw fixation of the spine. Clin. Orthop. Relat. Res. 2003, 411, 86–94. [Google Scholar] [CrossRef]
- Tsahtsarlis, A.; Wood, M. Minimally invasive transforaminal lumber interbody fusion and degenerative lumbar spine disease. Eur. Spine J. Off. Publ. Eur. Spine Soc Eur. Spinal. Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2012, 21, 2300–2305. [Google Scholar] [CrossRef]
- Knez, D.; Mohar, J.; Cirman, R.J.; Likar, B.; Pernuš, F.; Vrtovec, T. Variability Analysis of Manual and Computer-Assisted Preoperative Thoracic Pedicle Screw Placement Planning. Spine 2018, 43, 1487–1495. [Google Scholar] [CrossRef]
- Benito, R.; Bertelsen, Á.; de Ramos, V.; Iribar-Zabala, A.; Innocenti, N.; Castelli, N.; Lopez-Linares, K.; Scorza, D. Fast and versatile platform for pedicle screw insertion planning. Int. J. Comput. Assist. Radiol. Surg. 2023, 18, 1151–1157. [Google Scholar] [CrossRef]
- Aoude, A.A.; Fortin, M.; Figueiredo, R.; Jarzem, P.; Ouellet, J.; Weber, M.H. Methods to determine pedicle screw placement accuracy in spine surgery: A systematic review. Eur. Spine J. 2015, 24, 990–1004. [Google Scholar] [CrossRef]
- Zhang, H.-Q.; Wang, C.-C.; Zhang, R.-J.; Zhou, L.-P.; Jia, C.-Y.; Ge, P.; Shen, C.-L. Predictors of accurate intrapedicular screw placement in single-level lumbar (L4-5) fusion: Robot-assisted pedicle screw, traditional pedicle screw, and cortical bone trajectory screw insertion. BMC Surg. 2022, 22, 284. [Google Scholar] [CrossRef]
- Dietz, N.; Spiessberger, A. Consistent anatomical relationships of pedicle, lamina, and superior articulating process in severe idiopathic scoliosis allow for safe freehand pedicle screw placement: A proof-of-concept technical study. J. Craniovertebral Junction Spine 2024, 15, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Hu, S.; Zeng, W.; Cen, S.; Liu, Y.; Zhang, W.; Shi, B. Effect of L5 spinal canal type on pedicle screw placement based on CT imaging: A retrospective clinical study. Eur. Spine J. 2024, 33, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, X.; Yu, Z.; Zhang, Y.; Lv, J.; Zhang, H.; Wu, Y.; Lin, H.; Dai, J. Accuracy and digital screw path design of TiRobot-assisted pedicle screw placement for lumbar spondylolisthesis. Int. Orthop. 2023, 47, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Sadrameli, S.S.; Boghani, Z.; Steele Iii, W.J.; Holman, P.J. Utility of Patient-Specific Rod Instrumentation in Deformity Correction: Single Institution Experience. Spine Surg. Relat. Res. 2020, 4, 256–260. [Google Scholar] [CrossRef]
- Atici, Y.; Akman, Y.E.; Balioglu, M.B.; Kargin, D.; Kaygusuz, M.A. Two level pedicle substraction osteotomies for the treatment of severe fixed sagittal plane deformity: Computer software-assisted preoperative planning and assessing. Eur. Spine J. Off. Publ. Eur. Spine Soc Eur. Spinal. Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2016, 25, 2461–2470. [Google Scholar] [CrossRef]
- Siddiqui, M.I.; Wallace, D.J.; Salazar, L.M.; Vardiman, A.B. Robot-Assisted Pedicle Screw Placement: Learning Curve Experience. World Neurosurg. 2019, 130, e417–e422. [Google Scholar] [CrossRef]
- Goerres, J.; Uneri, A.; De Silva, T.; Ketcha, M.; Reaungamornrat, S.; Jacobson, M.; Vogt, S.; Kleinszig, G.; Osgood, G.; Wolinsky, J.-P.; et al. Spinal pedicle screw planning using deformable atlas registration. Phys. Med. Biol. 2017, 62, 2871–2891. [Google Scholar] [CrossRef]
- Vijayan, R.; De Silva, T.; Han, R.; Zhang, X.; Uneri, A.; Doerr, S.; Ketcha, M.D.; Perdomo-Pantoja, A.; Theodore, N.; Siewerdsen, J.H. Automatic pedicle screw planning using atlas-based registration of anatomy and reference trajectories. Phys. Med. Biol. 2019, 64, 165020. [Google Scholar] [CrossRef]
- Korez, R.; Ibragimov, B.; Likar, B.; Pernus, F.; Vrtovec, T. A Framework for Automated Spine and Vertebrae Interpolation-Based Detection and Model-Based Segmentation. IEEE Trans. Med. Imaging 2015, 34, 1649–1662. [Google Scholar] [CrossRef]
- Pereanez, M.; Lekadir, K.; Castro-Mateos, I.; Pozo, J.M.; Lazary, A.; Frangi, A.F. Accurate Segmentation of Vertebral Bodies and Processes Using Statistical Shape Decomposition and Conditional Models. IEEE Trans. Med. Imaging 2015, 34, 1627–1639. [Google Scholar] [CrossRef]
- Martín-Noguerol, T.O.; Miranda, M.O.; Amrhein, T.J.; Paulano-Godino, F.; Xiberta, P.; Vilanova, J.C.; Luna, A. The role of Artificial intelligence in the assessment of the spine and spinal cord. Eur. J. Radiol. 2023, 161, 110726. [Google Scholar] [CrossRef] [PubMed]
- Hallinan, J.T.P.D.; Zhu, L.; Yang, K.; Makmur, A.; Algazwi, D.A.R.; Thian, Y.L.; Lau, S.; Choo, Y.S.; Eide, S.E.; Yap, Q.V.; et al. Deep Learning Model for Automated Detection and Classification of Central Canal, Lateral Recess, and Neural Foraminal Stenosis at Lumbar Spine MRI. Radiology 2021, 300, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.-D.; Sun, Y.-L.; Kong, D.-W.; Yin, M.-C.; Chen, J.; Lin, Y.-P.; Ma, X.-F.; Wang, H.-S.; Yuan, G.-J.; Yao, M.; et al. Deep learning-based high-accuracy quantitation for lumbar intervertebral disc degeneration from MRI. Nat. Commun. 2022, 13, 841. [Google Scholar] [CrossRef] [PubMed]
- Doerr, S.A.; Weber-Levine, C.; Hersh, A.M.; Awosika, T.; Judy, B.; Jin, Y.; Raj, D.; Liu, A.; Lubelski, D.; Jones, C.K.; et al. Automated prediction of the Thoracolumbar Injury Classification and Severity Score from CT using a novel deep learning algorithm. Neurosurg. Focus 2022, 52, E5. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Nagahama, K.; Abe, Y.; Hyugaji, Y.; Takahata, M.; Iwasaki, N. Morphological analysis of Kambin’s triangle using 3D CT/MRI fusion imaging of lumbar nerve root created automatically with artificial intelligence. Eur. Spine J. 2021, 30, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.; Kausch, L.; Ishak, B.; Norajitra, T.; Vollmuth, P.; Kiening, K.; Unterberg, A.; Maier-Hein, K.; Neumann, J.-O. Development and validation of an automated planning tool for navigated lumbosacral pedicle screws using a convolutional neural network. Spine J. 2022, 22, 1666–1676. [Google Scholar] [CrossRef]
- Esfandiari, H.; Newell, R.; Anglin, C.; Street, J.; Hodgson, A.J. A deep learning framework for segmentation and pose estimation of pedicle screw implants based on C-arm fluoroscopy. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 1269–1282. [Google Scholar] [CrossRef]
- Magliano, A.; Naddeo, F.; Naddeo, A. A user-friendly system for identifying the optimal insertion direction and to choose the best pedicle screws for patient-specific spine surgery. Heliyon 2024, 10, e26334. [Google Scholar] [CrossRef]
- Zou, K.H.; Warfield, S.K.; Bharatha, A.; Tempany, C.M.; Kaus, M.R.; Haker, S.J.; Wells, W.M.; Jolesz, F.A.; Kikinis, R. Statistical Validation of Image Segmentation Quality Based on a Spatial Overlap Index1. Acad. Radiol. 2006, 11, 178–189. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Hsu, W.-L.; Hsu, R.-J.; Liu, D.-W. Fully Convolutional Network for the Semantic Segmentation of Medical Images: A Survey. Diagnostics 2022, 12, 2765. [Google Scholar] [CrossRef]
- Xiong, X.; Graves, S.A.; Gross, B.A.; Buatti, J.M.; Beichel, R.R. Lumbar and Thoracic Vertebrae Segmentation in CT Scans Using a 3D Multi-Object Localization and Segmentation CNN. Tomography 2024, 10, 738–760. [Google Scholar] [CrossRef] [PubMed]
- Serrador, L.; Villani, F.P.; Moccia, S.; Santos, C.P. Knowledge distillation on individual vertebrae segmentation exploiting 3D U-Net. Comput. Med. Imaging Graph. 2024, 113, 102350. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Hong, H.; Choi, Y.R.; Kim, D.H.; Kim, J.Y.; Yoon, J.-H.; Yoon, S.H. CT analysis of thoracolumbar body composition for estimating whole-body composition. Insights Imaging 2023, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- Canny, J. A Computational Approach to Edge Detection. IEEE Trans. Pattern Anal. Mach.Intell. 1986, 8, 679–698. [Google Scholar] [CrossRef] [PubMed]
- Ackermans, L.L.; Volmer, L.; Timmermans, Q.M.; Brecheisen, R.; Damink, S.M.O.; Dekker, A.; Loeffen, D.; Poeze, M.; Blokhuis, T.J.; Wee, L.; et al. Clinical evaluation of automated segmentation for body composition analysis on abdominal L3 CT slices in polytrauma patients. Injury 2022, 53, S30–S41. [Google Scholar] [CrossRef]
- Jarvers, J.-S.; Schleifenbaum, S.; Pfeifle, C.; Oefner, C.; Edel, M.; Von Der Höh, N.; Heyde, C.-E. Comparison of three different screw trajectories in osteoporotic vertebrae: A biomechanical investigation. BMC Musculoskelet. Disord. 2021, 22, 418. [Google Scholar] [CrossRef]
- Kim, H.-J.; Chun, H.-J.; Kang, K.-T.; Moon, S.-H.; Kim, H.-S.; Park, J.-O.; Moon, E.-S.; Kim, B.-R.; Sohn, J.-S.; Ko, Y.-N.; et al. The Biomechanical Effect of Pedicle Screws’ Insertion Angle and Position on the Superior Adjacent Segment in 1 Segment Lumbar Fusion. Spine 2012, 37, 1637–1644. [Google Scholar] [CrossRef]
- Caprara, S.; Fasser, M.R.; Spirig, J.M.; Widmer, J.; Snedeker, J.G.; Farshad, M.; Senteler, M. Bone density optimized pedicle screw instrumentation improves screw pull-out force in lumbar vertebr. Comput. Methods Biomech. Biomed. Eng. 2022, 25, 464–474. [Google Scholar] [CrossRef]
- Yang, J.; Deng, H.; Zhang, Y.; Zhou, Y.; Miao, T. Application of amodal segmentation for shape reconstruction and occlusion recovery in occluded tomatoes. Front. Plant Sci. 2024, 15, 1376138. [Google Scholar] [CrossRef]
- Fu, W.; Tong, J.; Liu, G.; Zheng, Y.; Wang, S.; Abdelrahim, M.E.A.; Gong, S. Robot-assisted technique vs conventional freehand technique in spine surgery: A meta-analysis. Int. J. Clin. Pract. 2020, 75, e13964. [Google Scholar] [CrossRef]
- Gubian, A.; Kausch, L.; Neumann, J.-O.; Kiening, K.; Ishak, B.; Maier-Hein, K.; Unterberg, A.; Scherer, M. CT-Navigated Spinal Instrumentations–Three-Dimensional Evaluation of Screw Placement Accuracy in Relation to a Screw Trajectory Plan. Medicina 2022, 58, 1200. [Google Scholar] [CrossRef] [PubMed]
- Scarone, P.; Chatterjea, A.; Jenniskens, I.; Klüter, T.; Weuster, M.; Lippross, S.; Presilla, S.; Distefano, D.; Chianca, V.; Sedaghat, S.; et al. Percutaneous thoraco-lumbar-sacral pedicle screw placement accuracy results from a multi-center, prospective clinical study using a skin marker-based optical navigation system. Eur. Spine J. 2022, 31, 3098–3108. [Google Scholar] [CrossRef] [PubMed]
- Proietti, L.; Scaramuzzo, L.; Schirò, G.; Sessa, S.; Tamburrelli, F.; Cerulli, G. Degenerative facet joint changes in lumbar percutaneous pedicle screw fixation without fusion. Orthop. Traumatol. Surg. Res. 2015, 101, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, Q.; Yu, H.; Xie, X.; Shi, Z.; Li, S.; Qiu, H.; Li, C.; Qin, J. Automated segmentation of vertebral cortex with 3D U-Net-based deep convolutional neural network. Front. Bioeng. Biotechnol. 2022, 10, 996723. [Google Scholar] [CrossRef]
- Varoquaux, G.; Cheplygina, V. Machine learning for medical imaging: Methodological failures and recommendations for the future. NPJ Digit. Med. 2022, 5, 48. [Google Scholar] [CrossRef]

| Variable | Patients in Test Set (n = 88) | All Patients (n = 316) |
|---|---|---|
| Age, years | 62.6 ± 10.3 | 63.5 ± 9.3 |
| Gender, n | ||
| Male | 40 (45.5%) | 151 (47.9%) |
| Female | 48 (54.5%) | 165 (52.1%) |
| BMI, Kg/m2 | 26.5 ± 4.2 | 26.5 ± 4.0 |
| Diagnosis, n | ||
| Lumbar disc herniation | 31 (35.2%) | 80 (25.3%) |
| Lumbar spinal stenosis | 38 (43.2%) | 153 (48.4%) |
| Lumbar spondylolisthesis | 19 (21.6%) | 83 (26.3%) |
| Construct length in spinal segments, n | ||
| 1 | 49 | 199 (63.0%) |
| 2 | 35 | 102 (32.3%) |
| 3 | 4 | 15 (4.7%) |
| Screws evaluated per spinal segment, n | ||
| L2 | 4 (0.9%) | 30 (2.0%) |
| L3 | 48 (11.0%) | 176 (11.5%) |
| L4 | 164 (37.4%) | 534 (35.0%) |
| L5 | 168 (38.4%) | 604 (39.5%) |
| S1 | 54 (12.3%) | 184 (12%) |
| L2 | L3 | L4 | L5 | S1 | Total | |
|---|---|---|---|---|---|---|
| Dice | 0.9533 | 0.9518 | 0.9506 | 0.9481 | 0.9438 | 0.9459 |
| N = 438 Screws | GR-Grade A | Non GR-Grade A | Badu 0 | Non Badu 0 |
|---|---|---|---|---|
| Manual planning | * 438 (100%) | 0 | 438 (100%) | 0 |
| AISPINE planning | 428 (97.7%) | 10 (2.3%) | 426 (97.3%) | 12 (2.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Zou, C.; Liu, X.; Liu, D.; Zhang, Y.; Zang, L. Development and Validation of Deep Learning Preoperative Planning Software for Automatic Lumbosacral Screw Selection Using Computed Tomography. Bioengineering 2024, 11, 1094. https://doi.org/10.3390/bioengineering11111094
Wang B, Zou C, Liu X, Liu D, Zhang Y, Zang L. Development and Validation of Deep Learning Preoperative Planning Software for Automatic Lumbosacral Screw Selection Using Computed Tomography. Bioengineering. 2024; 11(11):1094. https://doi.org/10.3390/bioengineering11111094
Chicago/Turabian StyleWang, Baodong, Congying Zou, Xingyu Liu, Dong Liu, Yiling Zhang, and Lei Zang. 2024. "Development and Validation of Deep Learning Preoperative Planning Software for Automatic Lumbosacral Screw Selection Using Computed Tomography" Bioengineering 11, no. 11: 1094. https://doi.org/10.3390/bioengineering11111094
APA StyleWang, B., Zou, C., Liu, X., Liu, D., Zhang, Y., & Zang, L. (2024). Development and Validation of Deep Learning Preoperative Planning Software for Automatic Lumbosacral Screw Selection Using Computed Tomography. Bioengineering, 11(11), 1094. https://doi.org/10.3390/bioengineering11111094








