From Mechanical Machining Technology: A New Solution That Integrates Blades to the Implant to Control the Stress to the Peri-Implant Cortical Bone
Abstract
1. Introduction
2. Founding Paradigms
2.1. Description of the Implant®
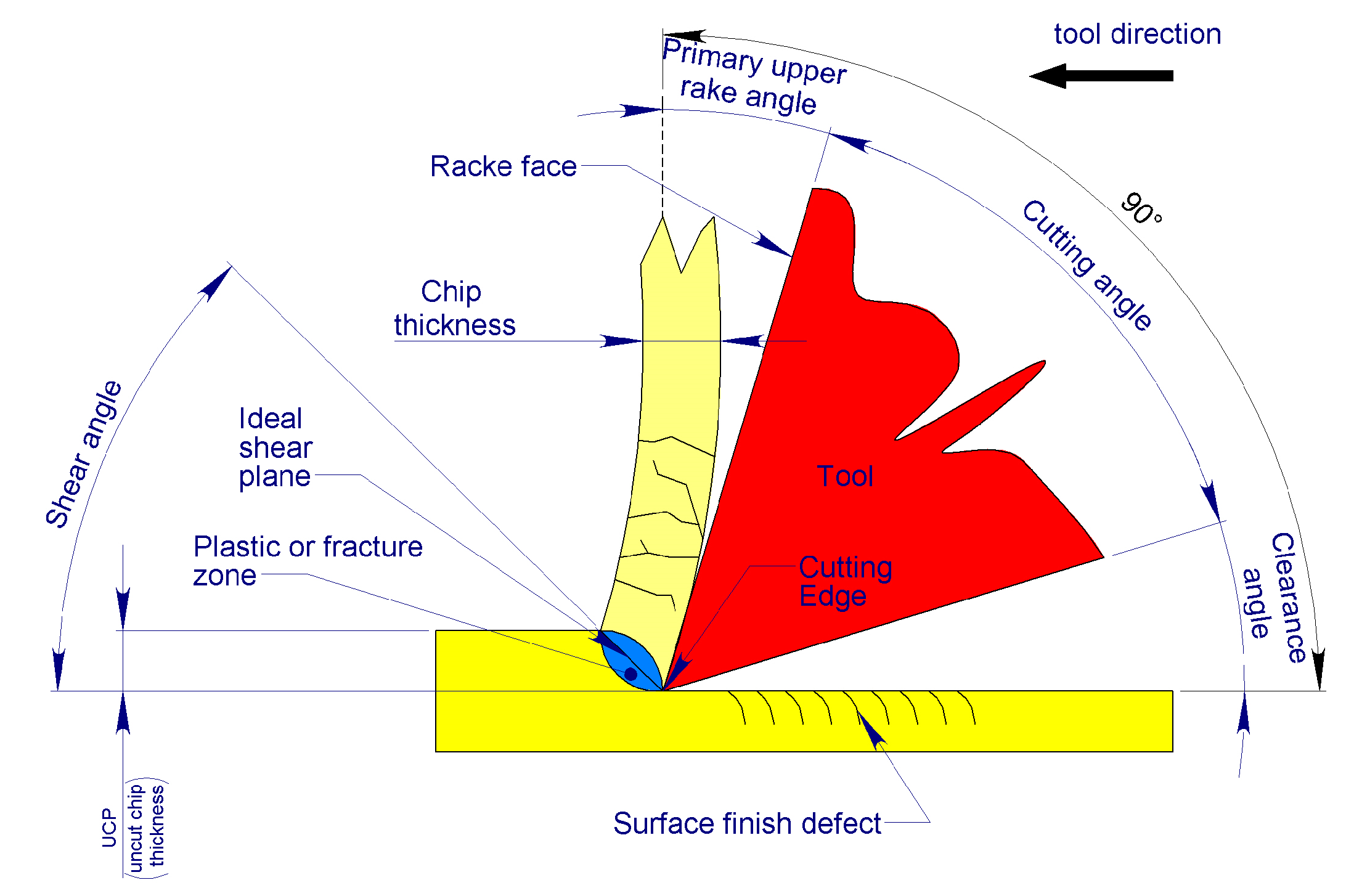
2.2. Mechanics of Bone Chip Formation
2.3. Defining the Type of Swarf to Be Produced by the Blades
3. The Solution® in Practice
3.1. Parameters at the Main Cutting Edge
3.1.1. Definition of Blade Vertex Angle
- Axial penetration thrust: The smaller the angle, the lower the thrust required for the blade to penetrate the bone [34];
- Self-alignment capability: The greater the angle, the lower the alignment capability [42];
- Chip width: The smaller the angle, the larger the normal cross-section of the chip produced [42];
- Chip thickness: The smaller the angle, the smaller the UCT thickness of the chip.
- The quantity of heat generated is as follows: the wider the angle, the greater the creep deformation of the materials, and thus, the greater the heat produced [42].
3.1.2. Definition of the Upper Rake Angle of the Main Cutting Edge, α
3.1.3. Defining the Clearance Angle Below the Main Cutting Edge
3.2. Parameters at the Secondary Cutting Edge
3.3. Defining the Number of Blades
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bassi, F.; Cicciù, M.; Di Lenarda, R.; Galindo Moreno, P.; Galli, F.; Herford, A.S.; Jokstad, A.; Lombardi, T.; Nevins, M.; Sennerby, L.; et al. Piezoelectric bone surgery compared with conventional rotary instruments in oral surgery and implantology: Summary and consensus statements of the International Piezoelectric Surgery Academy Consensus Conference 2019. Int. J. Oral Implants 2020, 13, 235–239. [Google Scholar] [PubMed]
- Stacchi, C.; Bassi, F.; Troiano, G.; Rapani, A.; Lombardi, T.; Jokstad, A.; Sennerby, L.; Schierano, G. Piezoelectric bone surgery for implant site preparation compared with conventional drilling techniques: A systematic review, meta-analysis and trial sequential analysis. Int. J. Oral Implants 2020, 13, 141–158. [Google Scholar] [PubMed]
- Li, X.; Lin, X.; Guo, J.; Wang, Y. The Stability and Survival Rate of Dental Implants After Preparation of the Site by Piezosurgery vs. Conventional Drilling: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implants 2020, 30, e51–e56. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Kato, S.; Bengazi, F.; Urbizo Velez, J.; Tumedei, M.; Kotsu, M.; Botticelli, D. Healing at implants installed in osteotomies prepared either with a piezoelectric device or drills: An experimental study in dogs. Oral Maxillofac. Surg. 2021, 25, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Bengazi, F.; Lang, N.P.; Canciani, E.; Viganò, P.; Velez, J.U.; Botticelli, D. Osseointegration of implants with dendrimers surface characteristics installed conventionally or with Piezosurgery®. A comparative study in the dog. Clin. Oral Implants Res. 2014, 25, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Arakji, H.; Osman, E.; Aboelsaad, N.; Shokry, M. Evaluation of implant site preparation with piezosurgery versus conventional drills in terms of operation time, implant stability and bone density (randomized controlled clinical trial-split mouth design). BMC Oral Health 2022, 22, 567. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mamidi, A.R.; Gottumukkala, S.N.V.S.; Mantena, S.R.; Penmetsa, G.S.; Ramesh, K.S.V.; Pasupuleti, M.K. Comparison of clinical and radiological outcomes using crestal approach sinus kit and piezoelectric surgery for sinus membrane elevation: A prospective clinical trial. Dent. Res. J. 2022, 19, 88. [Google Scholar] [PubMed] [PubMed Central]
- Cesaretti, G.; Lang, N.P.; Salata, L.A.; Schweikert, M.T.; Gutierrez Hernandez, M.E.; Botticelli, D. Sub-crestal positioning of implants results in higher bony crest resorption: An experimental study in dogs. Clin. Oral Implants Res. 2015, 26, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Viganò, P.; Botticelli, D.; Salata, L.A.; Schweikert, M.T.; Urbizo Velez, J.; Lang, N.P. Healing at implant sites prepared conventionally or by means of Sonosurgery®. An experimental study in dogs. Clin. Oral Implants Res. 2015, 26, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Agabiti, I.; Botticelli, D. Two-Stage Ridge Split at Narrow Alveolar Mandibular Bone Ridges. J. Oral Maxillofac. Surg. 2017, 75, e1–e2115. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, S.; Piattelli, A.; Baldi, N.; Ferri, M.; Iezzi, G.; Botticelli, D. Bone Healing at Implants Placed in Sites Prepared Either with a Sonic Device or Drills: A Split-Mouth Histomorphometric Randomized Controlled Trial. Int. J. Oral Maxillofac. Implants 2020, 35, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, C.A.; Fonseca, E.M.M.; Jorge, R.N. 3D Printing as an Efficient Way to Prototype and Develop Dental Implants. Biomedinformatics 2022, 2, 671–679. [Google Scholar] [CrossRef]
- Andreucci, C.A.; Fonseca, E.M.M.; Jorge, R.N. Increased Material Density within a New Biomechanism. Math. Comput. Appl. 2022, 27, 90. [Google Scholar] [CrossRef]
- Velikov, S.; Susin, C.; Heuberger, P.; Irastorza-Landa, A. A New Site Preparation Protocol That Supports Bone Quality Evaluation and Provides Predictable Implant Insertion Torque. J. Clin. Med. 2020, 9, 494. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elias, C.N.; Rocha, F.A.; Nascimento, A.L.; Coelho, P.G. Influence of implant shape, surface morphology, surgical technique and bone quality on the primary stability of dental implants. J. Mech. Behav. Biomed. Mater. 2012, 16, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Möhlhenrich, S.C.; Heussen, N.; Modabber, A.; Bock, A.; Hölzle, F.; Wilmes, B.; Danesh, G.; Szalma, J. Influence of bone density, screw size and surgical procedure on orthodontic mini-implant placement—Part B: Implant stability. Int. J. Oral Maxillofac. Surg. 2021, 50, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Pantani, F.; Botticelli, D.; Garcia IRJr Salata, L.A.; Borges, G.J.; Lang, N.P. Influence of lateral pressure to the implant bed on osseointegration: An experimental study in dogs. Clin. Oral Implants Res. 2010, 21, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Al-Tarawneh, S.K.; Thalji, G.; Cooper, L.F. Macrogeometric Differentiation of Dental Implant Primary Stability: An In Vitro Study. Int. J. Oral Maxillofac. Implants 2022, 37, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Benalcázar-Jalkh, E.B.; Nayak, V.V.; Gory, C.; Marquez-Guzman, A.; Bergamo, E.T.; Tovar, N.; Coelho, P.G.; Bonfante, E.A.; Witek, L. Impact of implant thread design on insertion torque and osseointegration: A preclinical model. Med. Oral Patol. Oral Cir. Bucal 2023, 28, e48–e55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romanos, G.E.; Kuyunov, O.; Sacks, D.; Calvo-Guirado, J.L.; Delgado-Ruiz, R. Apical stability of implants with progressive thread design in vitro, based on clinicians with different levels of experience. J. Periodontol. 2019, 90, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, A.S.; Walid, M.A.; Alkhodary, M.A. The effect of osseodensification and different thread designs on the dental implant primary stability. F1000Research 2018, 7, 1898. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Irastorza-Landa, A.; Heuberger, P.; Ploeg, H.L. Effect of insertion factors on dental implant insertion torque/energy-experimental results. J. Mech. Behav. Biomed. Mater. 2020, 112, 103995. [Google Scholar] [CrossRef] [PubMed]
- Eom, T.G.; Kim, H.W.; Jeon, G.R.; Yun, M.J.; Huh, J.B.; Jeong, C.M. Effects of Different Implant Osteotomy Preparation Sizes on Implant Stability and Bone Response in the Minipig Mandible. Int. J. Oral Maxillofac. Implants. 2016, 31, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Kotsu, M.; Urbizo Velez, J.; Bengazi, F.; Tumedei, M.; Fujiwara, S.; Kato, S.; Botticelli, D. Healing at implants installed from ~70- to <10-Ncm insertion torques: An experimental study in dogs. Oral Maxillofac. Surg. 2021, 25, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Duyck, J.; Corpas, L.; Vermeiren, S.; Ogawa, T.; Quirynen, M.; Vandamme, K.; Jacobs, R.; Naert, I. Histological, histomorphometrical, and radiological evaluation of an experimental implant design with a high insertion torque. Clin. Oral Implants Res. 2010, 21, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Júnior, J.A.; Treichel, T.L.E.; do Prado, T.D.; Dedavid, B.A.; de Aza, P.N. Effects of insertion torque values on the marginal bone loss of dental implants installed in sheep mandibles. Sci. Rep. 2022, 12, 538. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Santis, D.; Cucchi, A.; Rigoni, G.; Longhi, C.; Nocini, P.F. Relationship Between Primary Stability and Crestal Bone Loss of Implants Placed with High Insertion Torque: A 3-Year Prospective Study. Int. J. Oral Maxillofac. Implants 2016, 31, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Amari, Y.; Piattelli, A.; Apaza Alccayhuaman, K.A.; Mesa, N.F.; Ferri, M.; Iezzi, G.; Botticelli, D. Bone healing at non-submerged implants installed with different insertion torques: A split-mouth histomorphometric randomized controlled trial. Int. J. Implants Dent. 2019, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Stocchero, M.; Toia, M.; Cecchinato, D.; Becktor, J.P.; Coelho, P.G.; Jimbo, R. Biomechanical, Biologic, and Clinical Outcomes of Undersized Implant Surgical Preparation: A Systematic Review. Int. J. Oral Maxillofac. Implants 2016, 31, 1247–1263. [Google Scholar] [CrossRef] [PubMed]
- Roca-Millan, E.; González-Navarro, B.; Domínguez-Mínger, J.; Marí-Roig, A.; Jané-Salas, E.; López-López, J. Implant insertion torque and marginal bone loss: A systematic review and meta-analysis. Int. J. Oral Implants 2020, 13, 345–353. [Google Scholar] [PubMed]
- Nimbalkar, S.; Dhatrak, P.; Gherde, C.; Joshi, S. A review article on factors affecting bone loss in dental implants. Mater. Today Proc. 2021, 43, 970–976. [Google Scholar] [CrossRef]
- Weiner, S.; Wagner, H.D. THE MATERIAL BONE: Structure-Mechanical Function Relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Jacobs, C.H.; Pope, M.H.; Berry, J.T.; Hoaglund, F. A study of the bone machining process—Orthogonal cutting. J. Biomech. 1974, 7, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Peterlik, H.; Roshger, P.; Klaushofer, K.; Fratzl, P. Orientation dependent fracture toughness of lamellar bone. Int. J. Fract. 2006, 139, 395–405. [Google Scholar] [CrossRef]
- Peterlik, H.; Roshger, P.; Klaushofer, K.; Fratzl, P. From brittle to ductile fracture of bone. Nat. Mater. 2006, 5, 52–55. [Google Scholar] [CrossRef]
- Merchant, M.E. Mechanics of the metal cutting process. 1. Orthogonal Cutting and a Type 2 chip. J. Appl. Phys. 1945, 16, 267–275. [Google Scholar] [CrossRef]
- Nomura, T.; Gold, E.; Powers, M.P.; Shingaki, S.; Katz, J.L. Micromechanics—Structure relationship in the human mandible. Dent. Mater. 2003, 19, 167–173. [Google Scholar] [CrossRef]
- Zhang, Y.; Robles-Linares, J.A.; Chen, L.; Liao, Z.; Shih, A.J. Advanced in machining of hard tissue—From material removal mechanism to tooling solution. Int. J. Mach. Tools Manuf. 2022, 172, 103838. [Google Scholar] [CrossRef]
- Liao, Z.; Axinte, D.A. On chip formation mechanism in orthogonal cutting of bone. Int. J. Mach. Tools Manuf. 2016, 102, 41–55. [Google Scholar] [CrossRef]
- Robles-Linares, J.A.; Liao, Z.; Axinte, D.; Gameros, A. The effect of interstitial fluid on the machining behaviour of cortical bone. J. Mater. Process. Technol. 2022, 307, 117697. [Google Scholar] [CrossRef]
- Kalpakjian, S.; Schmitdt, S.R. Tecnologia Meccanica; Pearson Italia: Milano, Italy, 2014. [Google Scholar]
- Lee, J.; Gozen, B.A.; Ozdoganlar, O.B. Modeling and experimentation of bone drilling force. J. Biomech. 2012, 45, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Basiaga, M.; Szewczenko, J. Numerical and experimental analyses of drills used in osteosynthesis. Acta Bioeng. Biomech. 2011, 13, 29–36. [Google Scholar] [PubMed]
- Bosshardt, D.D.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Lang, N.P. The role of bone debris in early healing adjacent to hydrophilic and hydrophobic implant surfaces in man. Clin. Oral Implants Res. 2011, 22, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implants Res. 2011, 22, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Lang, N.P.; De Santis, E.; Morelli, F.; Favero, G.; Botticelli, D. Bone-healing pattern at the surface of titanium implants: An experimental study in the dog. Clin. Oral Implants Res. 2014, 25, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Karmani, S.; Lam, F. The design and function of surgical drills and K-wires. Biomechanics 2004, 18, 484–490. [Google Scholar] [CrossRef]
- Saha, S.; Pal, S.; Albright, J. Surgical Drilling: Design and performance of an improved drill. J. Biomech. Eng. 1982, 104, 245. [Google Scholar] [CrossRef]
- Canullo, L.; Iacono, R.; Pires Godoy, E.; Punzo, A.; Cavicchia, A.; Gianfreda, F.; Bollero, P. Hybrid Funnel Technique: A Novel Approach for Implant Site Preparation: A Pilot Study. Dent. J. 2022, 10, 157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ikar, M.; Grobecker-Karl, T.; Karl, M.; Steiner, C. Mechanical stress during implant surgery and its effects on marginal bone: A literature review. Quintessence Int. 2020, 51, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Khayat, P.G.; Arnal, H.M.; Tourbah, B.I.; Sennerby, L. Clinical outcome of dental implants placed with high insertion torques (up to 176 Ncm). Clin. Implants Dent. Relat. Res. 2013, 15, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, E.; Stegaroiu, R.; Nomura, S.; Miyakawa, O. Biomechanical aspects of marginal bone resorption around osseointegrated implants: Considerations based on a three-dimensional finite element analysis. Clin. Oral Implants Res. 2004, 15, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, M.; Ferri, M.; Guzon, F.M.M.; Asano, A.; Alccayhuaman, K.A.A.; Rossi, E.F.; Botticelli, D. Influence on marginal bone levels at implants equipped with blades aiming to control the lateral pressure on the cortical bone. An experimental study in dogs. Oral Maxillofac. Surg. 2024, 28, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Balan, V.; Ferri, M.; Pires Godoy, E.; Artioli, L.G.; Botticelli, D.; Silva, E.R.; Xavier, S.P. Controlled Lateral Pressure on Cortical Bone Using Blade-Equipped Implants: An Experimental Study in Rabbits. Bioengineering 2024, 11, 835. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferri, M.; Guzzo, M.; Omori, H.; Hazama, Y.; Masotta, N.V.; Botticelli, D. From Mechanical Machining Technology: A New Solution That Integrates Blades to the Implant to Control the Stress to the Peri-Implant Cortical Bone. Bioengineering 2024, 11, 1077. https://doi.org/10.3390/bioengineering11111077
Ferri M, Guzzo M, Omori H, Hazama Y, Masotta NV, Botticelli D. From Mechanical Machining Technology: A New Solution That Integrates Blades to the Implant to Control the Stress to the Peri-Implant Cortical Bone. Bioengineering. 2024; 11(11):1077. https://doi.org/10.3390/bioengineering11111077
Chicago/Turabian StyleFerri, Mauro, Marco Guzzo, Hiroyuki Omori, Yuma Hazama, Nicodemo Vittorio Masotta, and Daniele Botticelli. 2024. "From Mechanical Machining Technology: A New Solution That Integrates Blades to the Implant to Control the Stress to the Peri-Implant Cortical Bone" Bioengineering 11, no. 11: 1077. https://doi.org/10.3390/bioengineering11111077
APA StyleFerri, M., Guzzo, M., Omori, H., Hazama, Y., Masotta, N. V., & Botticelli, D. (2024). From Mechanical Machining Technology: A New Solution That Integrates Blades to the Implant to Control the Stress to the Peri-Implant Cortical Bone. Bioengineering, 11(11), 1077. https://doi.org/10.3390/bioengineering11111077






