Prospective Randomized Controlled Clinical Trial to Evaluate the Safety and Efficacy of ACTLINK Plasma Treatment for Promoting Osseointegration and Bone Regeneration in Dental Implants
Abstract
1. Introduction
2. Materials and Methods
2.1. Randomized Controlled Clinical Trial
2.2. Plasma Treatment of the Dental Implant
2.3. Patients and Case Selection
2.4. Treatment Group Allocation
- SLA group: non-plasma-treated SLA implants, n = 14.
- SLA/plasma group: plasma-treated SLA implants, n = 14.
2.5. First-Stage Surgical Procedure and Plasma Treatment on Implants
2.6. Second-Stage Surgical Procedure
2.7. Follow-Up Visit and Clinical Evaluation
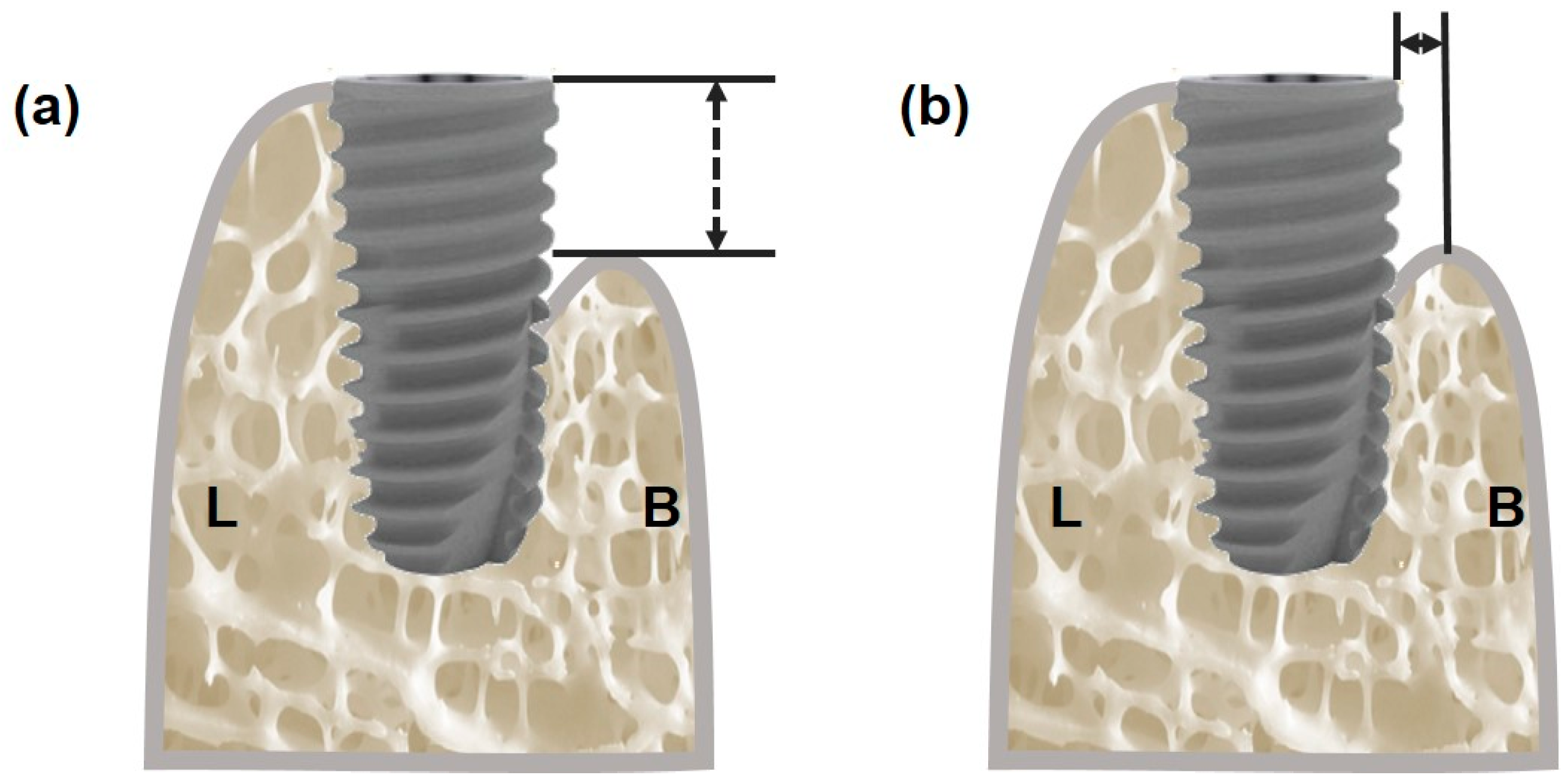
2.8. Implant Stability and Dehiscence Defect Measurement
2.9. Statistical Analysis
3. Results
3.1. Patient Demographic Information
3.2. Implant Placement
3.3. Cumulative Survival Rate
3.4. Clinical Examination of Buccal Bone Defect
3.4.1. Measurement of Vertical Bone Defect
3.4.2. Measurement of Horizontal Bone Defect
3.5. Evaluation of Implant Stability
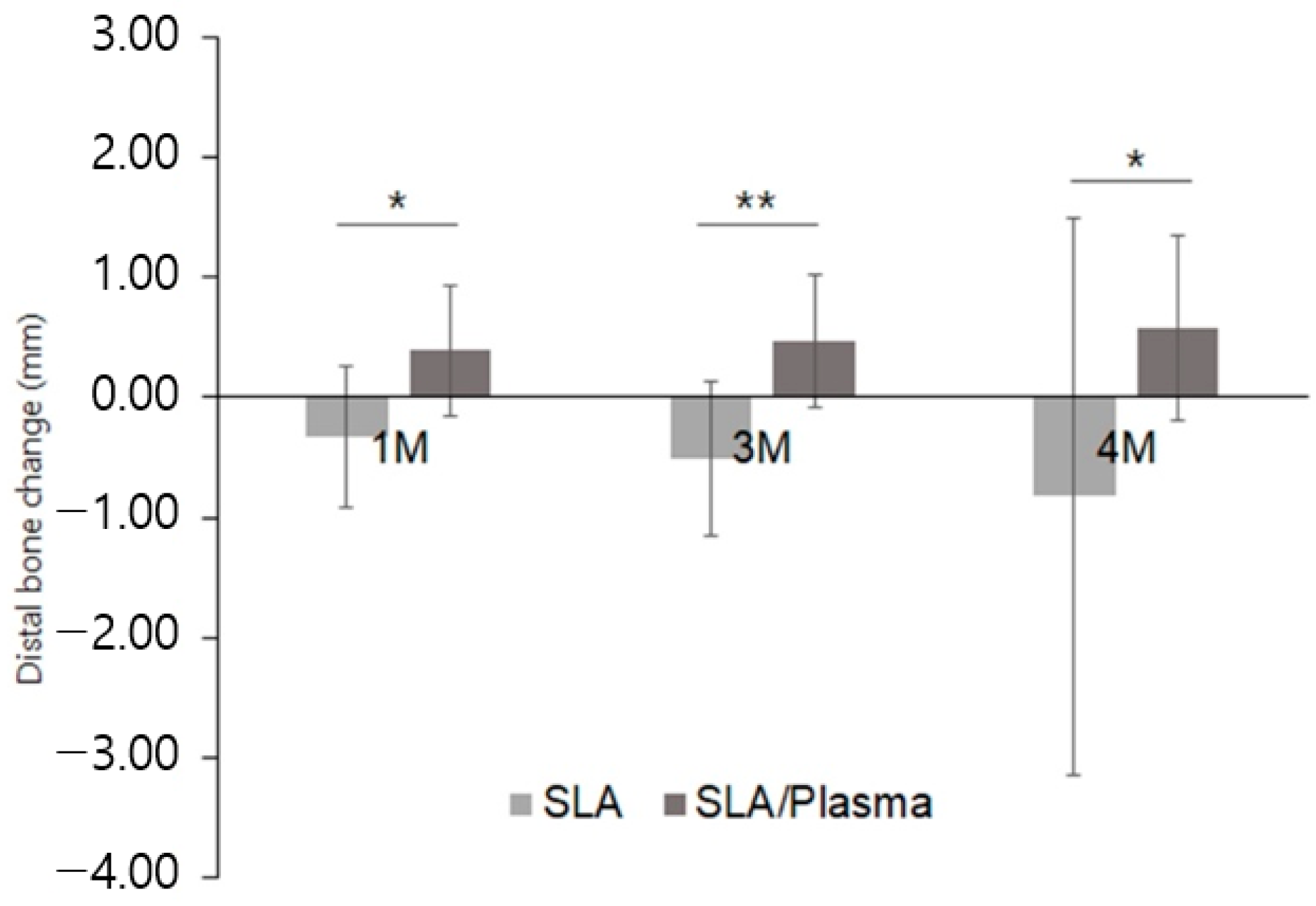
3.6. Evaluation of Marginal Bone Change
3.7. NRS (Numeric Rating Score) Pain Assessment
3.8. Participants’ Satisfaction with the Procedure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brånemark, P.I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Liu, T.T.; Wang, Q.Q.; Zhang, J.; Cao, M.S. Surface modification and functionalities for titanium dental implants. ACS Biomater. Sci. Eng. 2023, 9, 4442–4461. [Google Scholar] [CrossRef] [PubMed]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface modifications and their effects on titanium dental implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [PubMed]
- Att, W.; Hori, N.; Takeuchi, M.; Ouyang, J.; Yang, Y.; Anpo, M.; Ogawa, T. Time-dependent degradation of titanium osteoconductivity: An implication of biological aging of implant materials. Biomaterials 2009, 30, 5352–5363. [Google Scholar] [CrossRef]
- Lee, J.H.; Ogawa, T. The biological aging of titanium implants. Implant Dent. 2012, 21, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Minamikawa, H.; Att, W.; Ikeda, T.; Hirota, M.; Ogawa, T. Longterm progressive degradation of the biological capability of titanium. Materials 2016, 9, 102. [Google Scholar] [CrossRef]
- Al Qahtani, M.S.; Wu, Y.; Spintzyk, S.; Krieg, P.; Killinger, A.; Schweizer, E.; Stephan, I.; Scheideler, L.; Geis-Gerstorfer, J.; Rupp, F. UV-A and UV-C light induced hydrophilization of dental implants. Dent. Mater. 2015, 31, e157–e167. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.B.; Cohen, D.J.; Levit, M.M.; Puetzer, J.L.; Boyan, B.D.; Schwartz, Z. Hydrophilic implants generated using a lowcost dielectric barrier discharge plasma device at the time of placement exhibit increased osseointegration in an animal pre-clinical study: An effect that is sex-dependent. Dent. Mater. 2022, 38, 632–645. [Google Scholar] [CrossRef]
- Guo, L.; Zou, Z.; Smeets, R.; Kluwe, L.; Hartjen, P.; Cacaci, C.; Gosau, M.; Henningsen, A. time dependency of non-thermal oxygen plasma and ultraviolet irradiation on cellular attachment and mRNA expression of growth factors in osteoblasts on titanium and zirconia surfaces. Int. J. Mol. Sci. 2020, 21, 8598. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, H.; Chen, Z.; Wang, Y.; Yang, X.; Yu, H. Improvement in osseointegration of titanium dental implants after exposure to ultraviolet-C light for varied durations: An experimental study in beagle dogs. J. Oral Maxillofac. Surg. 2022, 80, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Dobslaw, D.; Ortlinghaus, O.; Dobslaw, C. A combined process of non-thermal plasma and a low-cost mineral adsorber for VOC removal and odor abatement in emissions of organic waste treatment plants. J. Environ. Chem. Eng. 2018, 6, 2281–2289. [Google Scholar] [CrossRef]
- Kyere-Yeboah, K.; Bique, I.K.; Qiao, X. Advances of non-thermal plasma discharge technology in degrading recalcitrant wastewater pollutants. A comprehensive review. Chemosphere 2023, 320, 138061. [Google Scholar] [CrossRef] [PubMed]
- Dobslaw, D.; Ortlinghaus, O. Biological waste air and waste gas treatment: Overview, challenges, operational efficiency, and current trends. Sustainability 2020, 12, 8577. [Google Scholar] [CrossRef]
- Attri, P.; Koga, K.; Okumura, T.; Chawarambwa, F.L.; Putri, T.E.; Tsukada, Y.; Kamataki, K.; Itagaki, N.; Shiratani, M. Treatment of organic wastewater by a combination of non-thermal plasma and catalyst: A review. Rev. Mod. Plasma Phys. 2022, 6, 17. [Google Scholar] [CrossRef]
- Akdoğan, E.; Şirin, H.T. Plasma surface modification strategies for the prep-aration of antibacterial biomaterials: A review of the recent literature. Mater. Sci. Eng. C 2021, 131, 112474. [Google Scholar] [CrossRef]
- Jaffer, Z.J.; Abdalameer, N.K.; Noori, A.S. Plasma surface treatment of metals: A comprehensive review of recent developments and future prospects. Int. J. Nanosci. 2024, 23, 2330008. [Google Scholar] [CrossRef]
- Minati, L.; Migliaresi, L.; Lunelli, L.; Viero, G.; Serra, M.D.; Speranza, G. Plasma assisted surface treatments of biomaterials. Biophys. Chem. 2017, 229, 151–164. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Desmet, T.; Dubruel, P.; Leys, C. Plasma surface modification of biodegradable polymers: A review. Plasma Process. Polym. 2011, 8, 171–190. [Google Scholar] [CrossRef]
- Duske, K.; Koban, I.; Kindel, E.; Schroder, K.; Nebe, B.; Holtfreter, B.; Jablonowski, L.; Weltmann, K.D.; Kocher, T. Atmospheric plasma enhances wettability and cell spreading on dental implant metals. J. Clin. Periodontol. 2012, 39, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Moriguchi, Y.; Okada, K.; Myoui, A.; Yoshikawa, H.; Hamaguchi, S. Improvement of hydrophilicity of interconnected porous hydroxyapatite by dielectric barrier discharge plasma treatment. IEEE Trans. Plasma Sci. 2011, 39, 2166–2167. [Google Scholar] [CrossRef]
- Tsujita, H.; Nishizaki, H.; Miyake, A.; Takao, S.; Komasa, S. Effect of plasma treatment on titanium surface on the tissue surrounding implant material. Int. J. Mol. Sci. 2021, 22, 6931. [Google Scholar] [CrossRef] [PubMed]
- Guastaldi, F.P.; Yoo, D.; Marin, C.; Jimbo, R.; Tovar, N.; Zanetta-Barbosa, D.; Coelho, P.G. Plasma treatment maintains surface energy of the implant surface and enhances osseointegration. Int. J. Biomater. 2013, 2013, 354125. [Google Scholar] [CrossRef] [PubMed]
- Nevins, M.; Chen, C.Y.; Parma-Benfenati, S.; Kim, D.M. Gas plasma treatment improves titanium dental implant osseointegration-a preclinical in vivo experimental study. Bioengineering 2023, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Hälg, G.A.; Thoma, D.S.; Hämmerle, C.H. A randomized, controlled clinical trial to evaluate a new membrane for guided bone regeneration around dental implants. Clin. Oral Implant. Res. 2009, 20, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, C.H.; Lang, N.P. Single stage surgery combining transmucosal implant placement with guided bone regeneration and bioresorbable materials. Clin. Oral Implant. Res. 2001, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 20, S286–S291. [Google Scholar]
- Sadrkhah, M.; Faraji, G.; Khorasani, S.; Mesbah, M. Excellent mechanical properties, wettability and biological response of ultrafine-grained pure Ti dental implant surface modified by SLActive. J. Mater. Eng. Perform. 2023, 32, 11408–11421. [Google Scholar] [CrossRef]
- Sonntag, R.; Reinders, J.; Gibmeier, J.; Kretzer, J.P. Fatigue performance of medical Ti6Al4V alloy after mechanical surface treatments. PLoS ONE 2015, 10, e0121963. [Google Scholar] [CrossRef]
- Ku, C.H.; Pioletti, D.P.; Browne, M.; Gregson, P.J. Effect of different Ti-6Al-4V surface treatments on osteoblasts behaviour. Biomaterials 2002, 23, 1447–1454. [Google Scholar] [CrossRef]
- Santiago-Medina, P.; Sundaram, P.A.; Diffoot-Carlo, N. The effects of micro arc oxidation of gamma titanium aluminide surfaces on osteoblast adhesion and differentiation. J. Mater. Sci. Mater. Med. 2014, 25, 1577–1587. [Google Scholar] [CrossRef][Green Version]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Gasik, M.; Braem, A.; Chaudhari, A.; Duyck, J.; Vleugels, J. Titanium implants with modified surfaces: Meta-analysis of in vivo osteointegration. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Minamikawa, H.; Ikeda, T.; Att, W.; Hagiwara, Y.; Hirota, M.; Tabuchi, M.; Aita, H.; Park, W.; Ogawa, T. Photofunctionalization increases the bioactivity and osteoconductivity of the titanium alloy Ti6Al4V. J. Biomed. Mater. Res. A 2014, 102, 3618–3630. [Google Scholar] [CrossRef]
- Pesce, P.; Menini, M.; Santori, G.; Giovanni, E.; Bagnasco, F.; Canullo, L. Photo and plasma activation of dental implant titanium surfaces. A systematic review with meta-analysis of pre-clinical studies. J. Clin. Med. 2020, 9, 2817. [Google Scholar] [CrossRef]
- Atsumi, M.; Park, S.H.; Wang, H.L. Methods used to assess implant stability: Current status. Int. J. Oral Maxillofac. Implant. 2007, 22, 743–754. [Google Scholar]
- Rodrigo, D.; Aracil, L.; Martin, C.; Sanz, M. Diagnosis of implant stability and its impact on implant survival: A prospective case series study. Clin. Oral. Implant. Res. 2010, 21, 255–261. [Google Scholar] [CrossRef]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta. Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef]
- Jo, W.L.; Lim, Y.W.; Kwon, S.Y.; Bahk, J.H.; Kim, J.; Shin, T.; Kim, Y. Non-thermal atmospheric pressure plasma treatment increases hydrophilicity and promotes cell growth on titanium alloys in vitro. Sci. Rep. 2023, 13, 14792. [Google Scholar] [CrossRef]
- Rabel, K.; Kohal, R.J.; Steinberg, T.; Rolauffs, B.; Adolfsson, E.; Altmann, B. Human osteoblast and fibroblast response to oral implant biomaterials functionalized with non-thermal oxygen plasma. Sci. Rep. 2021, 11, 17302. [Google Scholar] [CrossRef]
- Seon, G.M.; Seo, H.J.; Kwon, S.Y.; Lee, M.H.; Kwon, B.J.; Kim, M.S.; Koo, M.A.; Park, B.J.; Park, J.C. Titanium surface modification by using microwave-induced argon plasma in various conditions to enhance osteoblast biocompatibility. Biomater. Res. 2015, 19, 13. [Google Scholar] [CrossRef]
- Lee, H.; Jeon, H.J.; Jung, A.; Kim, J.; Kim, J.Y.; Lee, S.H.; Kim, H.; Yeom, M.S.; Choe, W.; Gweon, B.; et al. Improvement of osseointegration efficacy of titanium implant through plasma surface treatment. Biomed. Eng. Lett. 2022, 12, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Danna, N.R.; Beutel, B.G.; Tovar, N.; Witek, L.; Marin, C.; Bonfante, E.A.; Granato, R.; Suzuki, M.; Coelho, P.G. Assessment of atmospheric pressure plasma treatment for implant osseointegration. Biomed. Res. Int. 2015, 2015, 761718. [Google Scholar] [CrossRef]
- Naujokat, H.; Harder, S.; Schulz, L.Y.; Wiltfang, J.; Flörke, C.; Açil, Y. Surface conditioning with cold argon plasma and its effect on the osseointegration of dental implants in miniature pigs. J. Craniomaxillofac. Surg. 2019, 47, 484–490. [Google Scholar] [CrossRef]
- Banu, R.F.; Kumar, V.A. Early implant bone loss in the preprosthetic phase: A retrospective study. J. Oral Implantol. 2023, 49, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; Catena, A.; Pérez-Sayáns, M.; Fernández-Barbero, J.E.; O’Valle, F.; Padi-al-Molina, M. Early marginal bone loss around dental implants to define success in implant dentistry: A retrospective study. Clin. Implant Dent. Relat. Res. 2022, 24, 630–642. [Google Scholar] [CrossRef]




| (a) Inclusion Criteria |
|
| (b) Exclusion Criteria |
|
| Number of Patients (%) | p-Value | |||
|---|---|---|---|---|
| SLA Group (n = 14) | SLA/Plasma Group (n = 14) | Total (n = 28) | ||
| Age | ||||
| Mean ± SD | 64.21 ± 8.51 | 61.43 ± 15.58 | 62.82 ± 12.40 | 0.839 a |
| Median [Min–Max] | 66.50 [51.00–82.00] | 67.50 [23.00–78.00] | 67.00 [23.00–82.00] | |
| Gender | ||||
| Male | 5 (35.71) | 9 (64.29) | 14 (50.00) | 0.131 b |
| Female | 9 (64.29) | 5 (35.71) | 14 (50.00) | |
| Smoking | ||||
| Smoker | 2 (14.29) | 1 (7.14) | 3 (10.71) | 0.541 b |
| Non-smoker | 12 (85.71) | 13 (92.86) | 25 (89.29) | |
| Number of Patients (%) | |||
|---|---|---|---|
| SLA Group (n = 14) | SLA/Plasma Group (n = 14) | Total (n = 28) | |
| Location | |||
| Maxilla | 4 (28.57) | 7 (50.00) | 11 (39.29) |
| Mandible | 10 (71.43) | 7 (50.00) | 17 (60.71) |
| Position | |||
| Anterior | 5 (35.71) | 5 (35.71) | 10 (35.71) |
| Posterior | 9 (64.29) | 9 (64.29) | 18 (64.29) |
| SLA Group (n = 14) | SLA/Plasma Group (n = 14) | p-Value a | |
|---|---|---|---|
| First stage | |||
| Mean ± SD | 1.37 ± 1.39 | 1.18 ± 1.09 | 0.839 |
| Median [Min–Max] | 1.09 [0.00–4.76] | 1.00 [0.00–4.22] | |
| Second stage | |||
| Mean ± SD | 0.21 ± 0.44 | 0.29 ± 0.51 | 0.667 |
| Median [Min–Max] | 0.00 [0.00–1.53] | 0.00 [0.00–1.72] | |
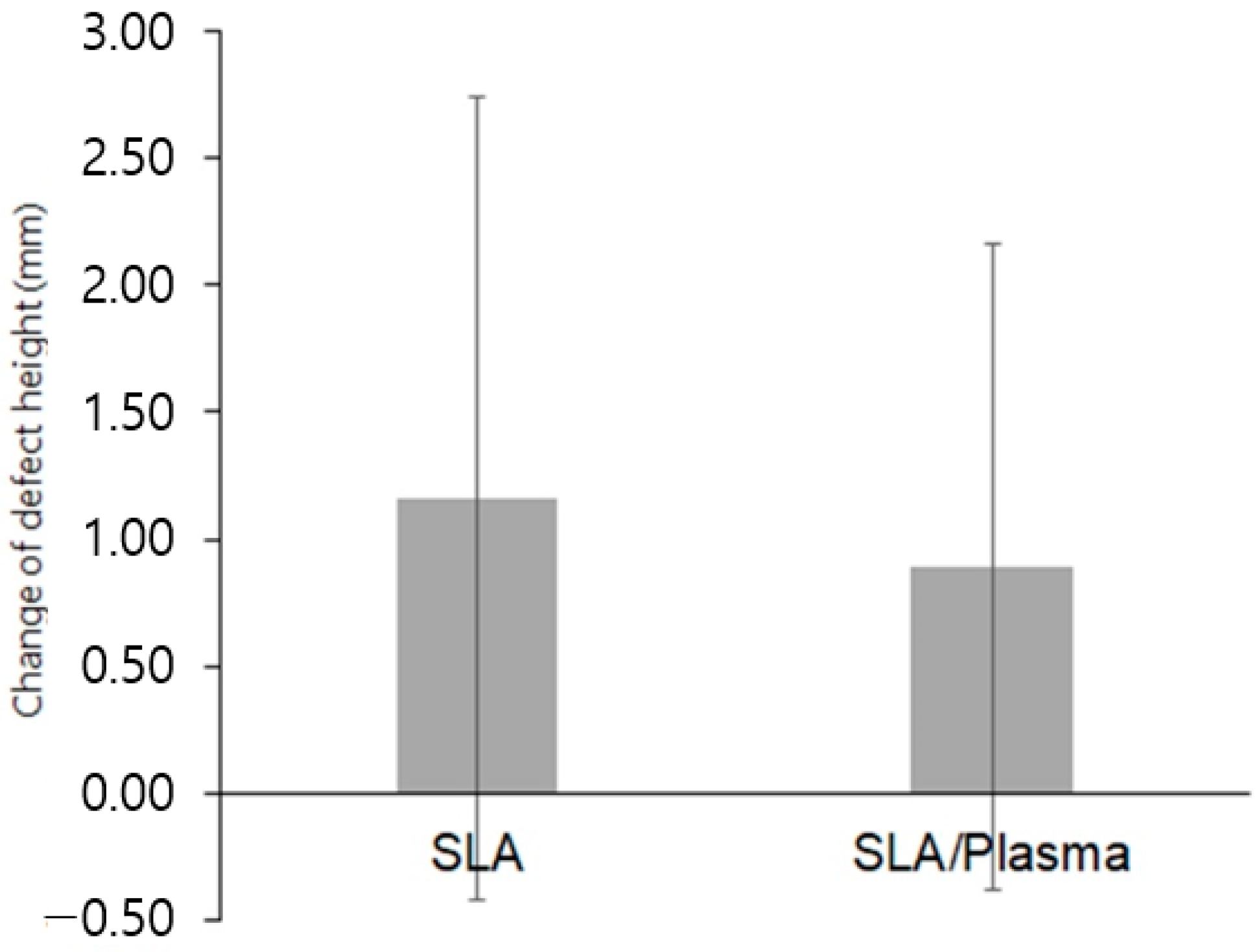
| Change in defect height b | |||
| Mean ± SD | 1.16 ± 1.58 | 0.89 ± 1.27 | 0.541 |
| Median [Min–Max] | 1.89 [−1.21–4.76] | 0.67 [−1.02–4.22] |
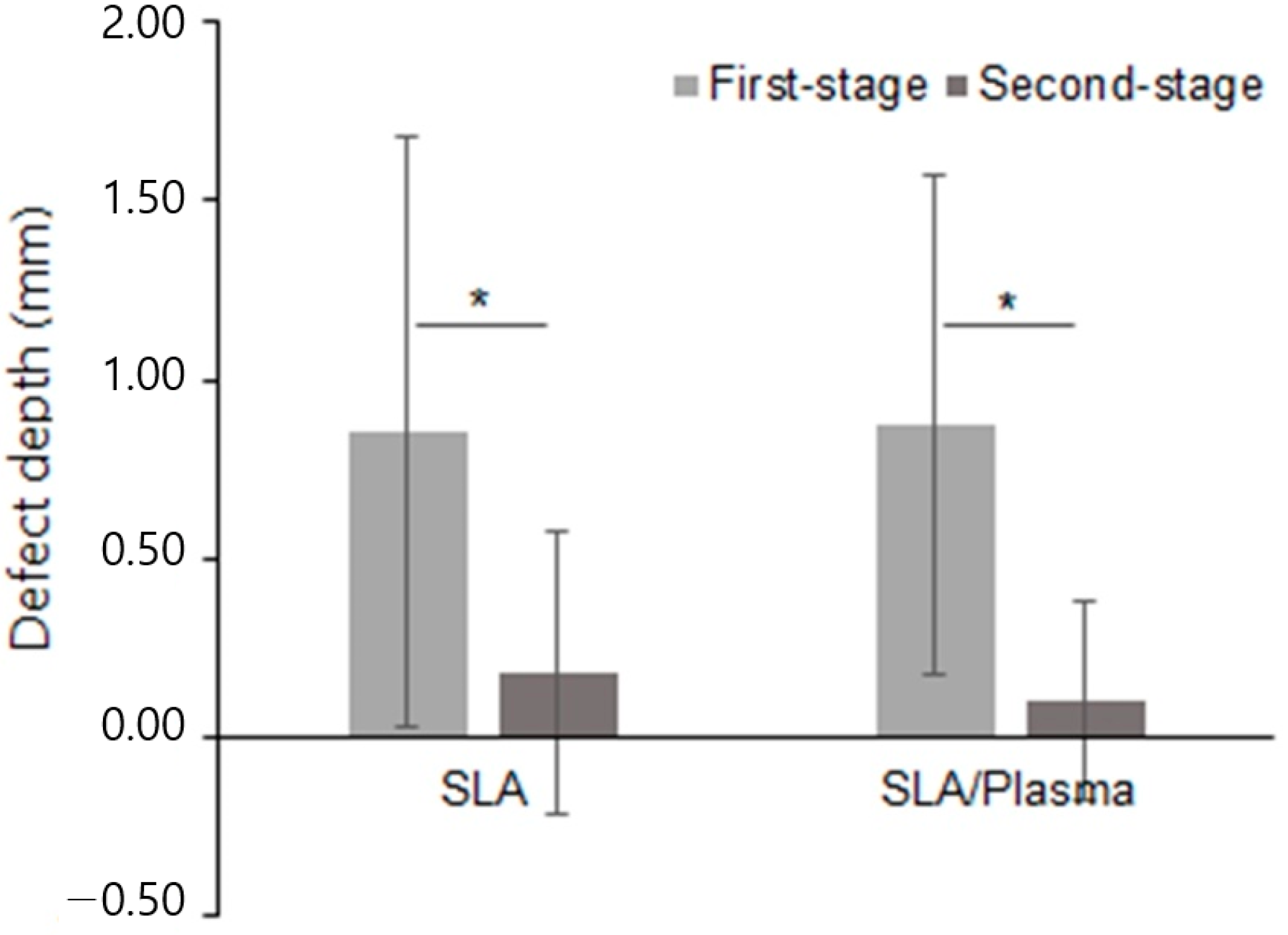
| SLA Group (n = 14) | SLA/Plasma Group (n = 14) | p-Value a | |
|---|---|---|---|
| First-stage | |||
| Mean ± SD | 0.85 ± 0.83 | 0.87 ± 0.70 | 0.734 |
| Median [Min–Max] | 0.61 [0.00–3.33] | 0.58 [0.00–2.50] | |
| Second-stage | |||
| Mean ± SD | 0.18 ± 0.39 | 0.10 ± 0.28 | 0.571 |
| Median [Min–Max] | 0.00 [0.00–1.39] | 0.00 [0.00–0.96] | |
| Change in defect depth b | |||
| Mean ± SD | 0.67 ± 1.02 | 0.77 ± 0.80 | 0.734 |
| Median [Min–Max] | 0.54 [−1.03–3.33] | 0.55 [−0.36–2.50] |
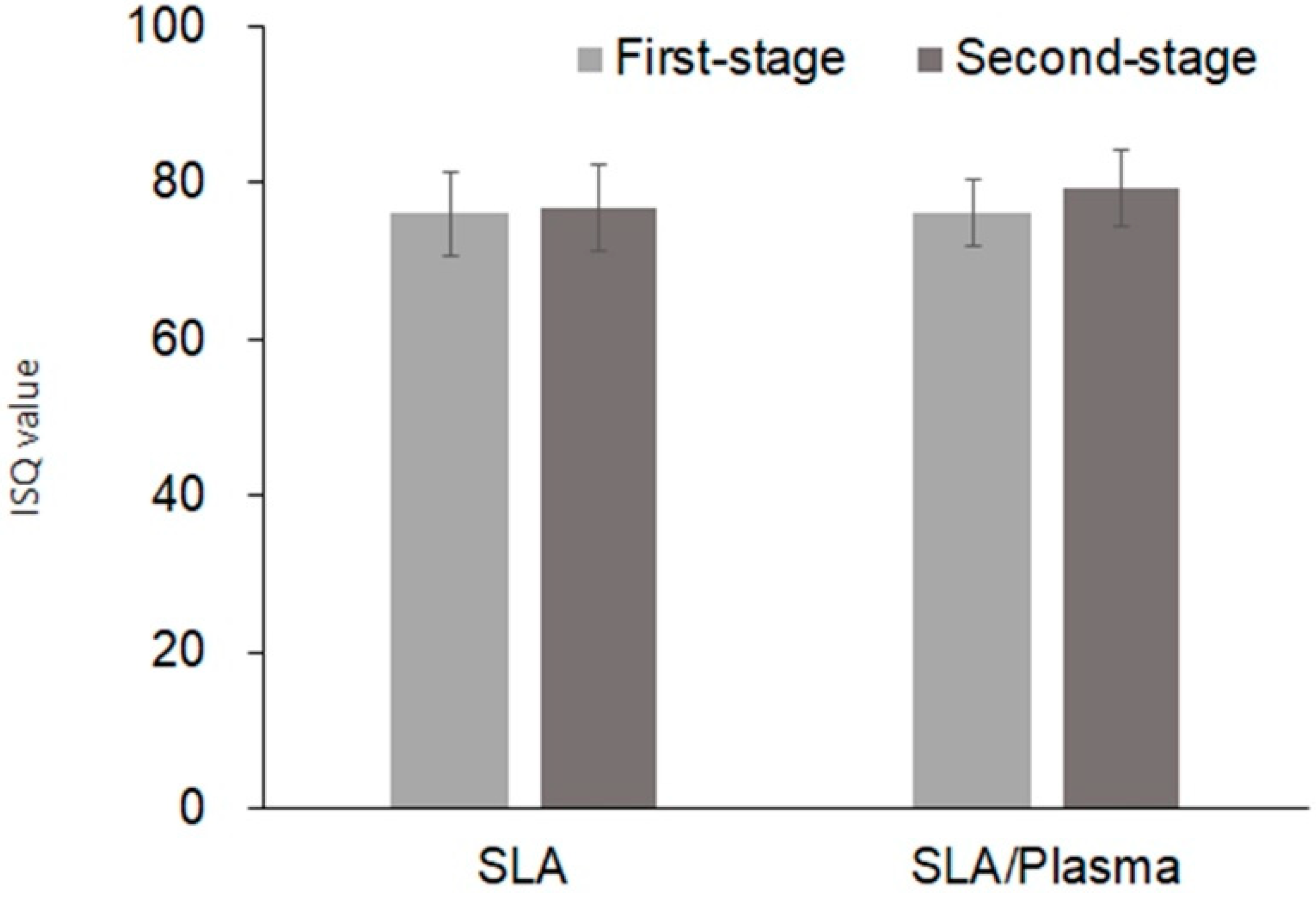
| SLA Group (n = 14) | SLA/Plasma Group (n = 14) | p-Value a | |
|---|---|---|---|
| First stage | |||
| Mean ± SD | 76.07 ± 4.22 | 76.04 ± 5.44 | 0.874 |
| Median [Min–Max] | 75.25 [68.50–85.00] | 76.50 [64.50–84.00] | |
| Second stage | |||
| Mean ± SD | 79.18 ± 4.87 | 76.73 ± 5.51 | 0.402 |
| Median [Min–Max] | 79.00 [70.00–88.50] | 78.50 [65.00–82.50] | |
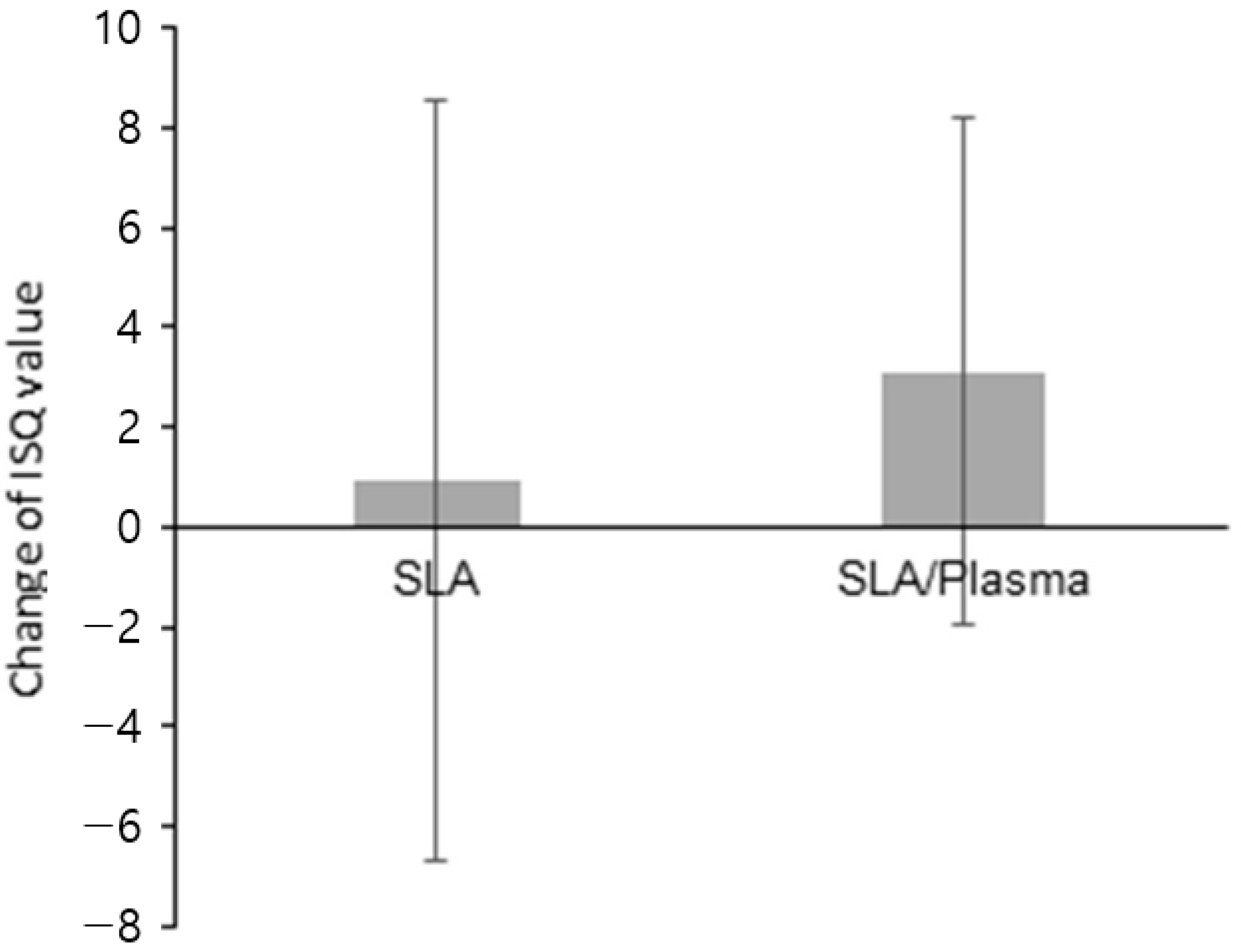
| Change of ISQ value b | |||
| Mean ± SD | 3.11 ± 5.08 | 0.92 ± 7.61 | 0.375 |
| Median [Min–Max] | 3.75 [−5.50–10.50] | 1.50 [−10.00–16.50] |
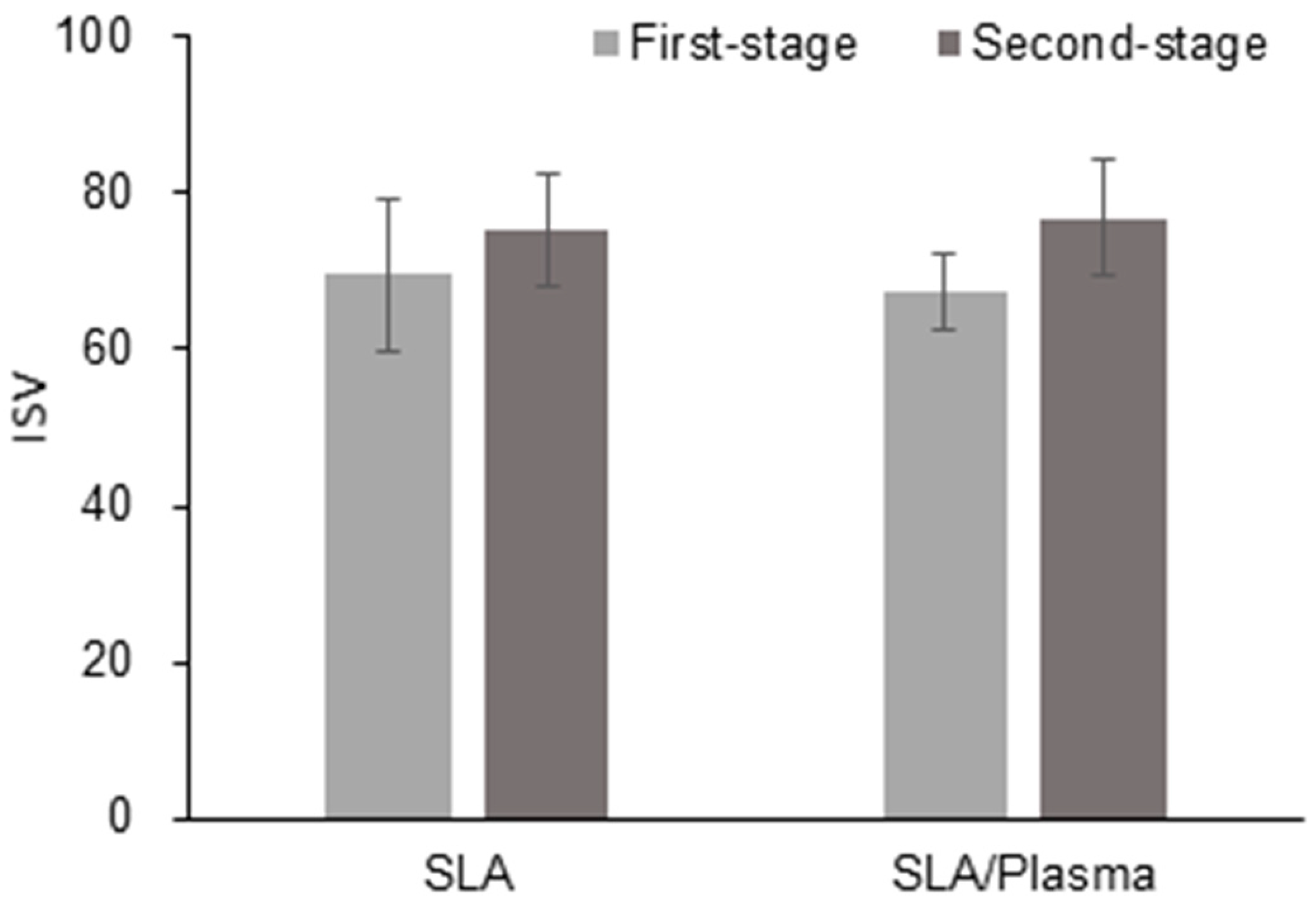
| SLA Group (n = 14) | SLA/Plasma Group (n = 14) | p-Value a | |
|---|---|---|---|
| First stage | |||
| Mean ± SD | 67.25 ± 4.81 | 69.61 ± 9.66 | 0.352 |
| Median [Min–Max] | 67.75 [59.00–77.50] | 69.50 [49.50–85.00] | |
| Second stage | |||
| Mean ± SD | 76.89 ± 7.27 | 75.29 ± 7.19 | 0.603 |
| Median [Min–Max] | 79.00 [60.00–85.50] | 75.00 [64.50–86.50] | |
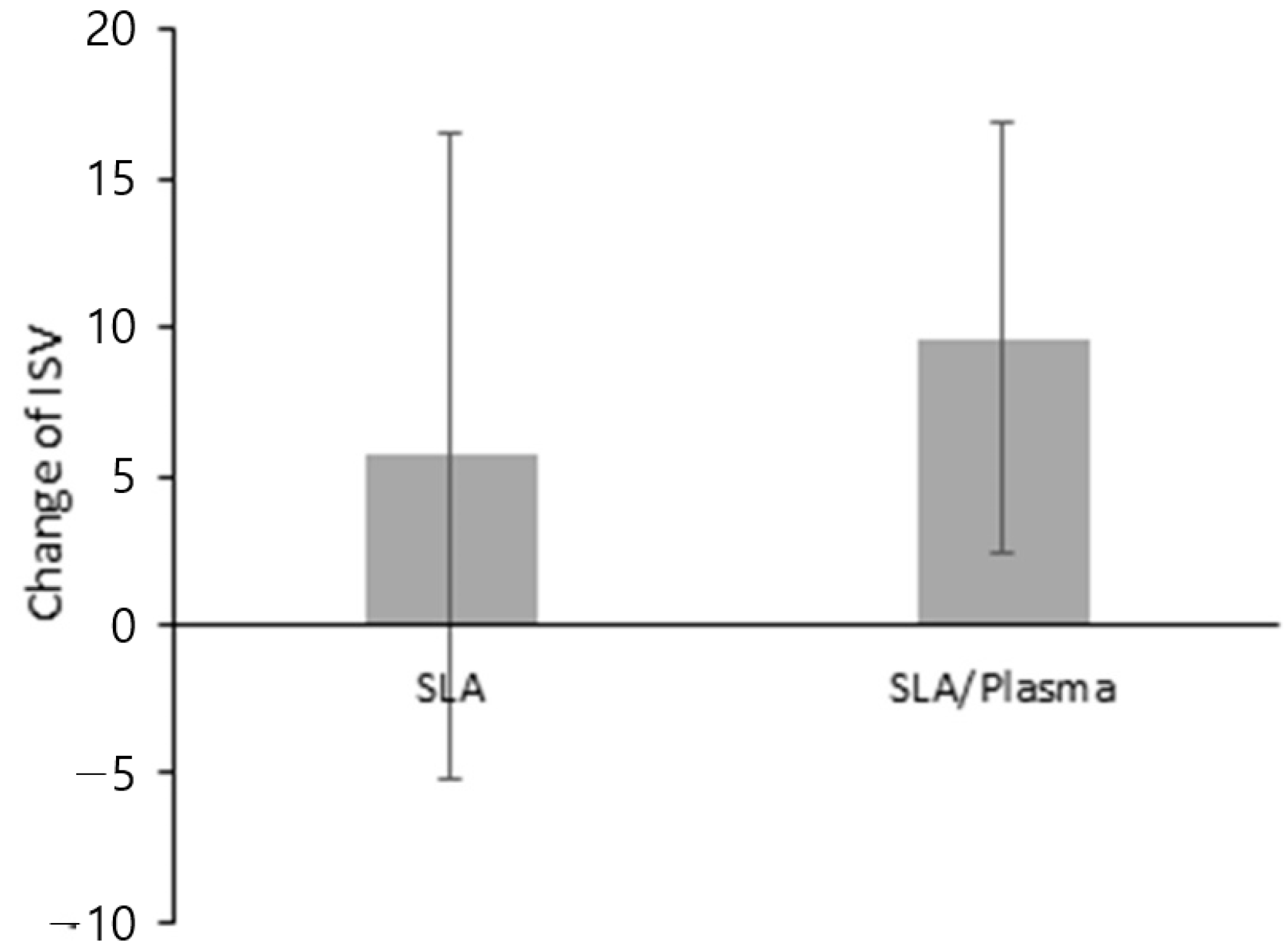
| Change in ISV b | |||
| Mean ± SD | 9.64 ± 7.28 | 5.68 ± 10.84 | 0.454 |
| Median [Min–Max] | 12.00 [−3.00–22.50] | 6.25 [−10.50–20.00] |
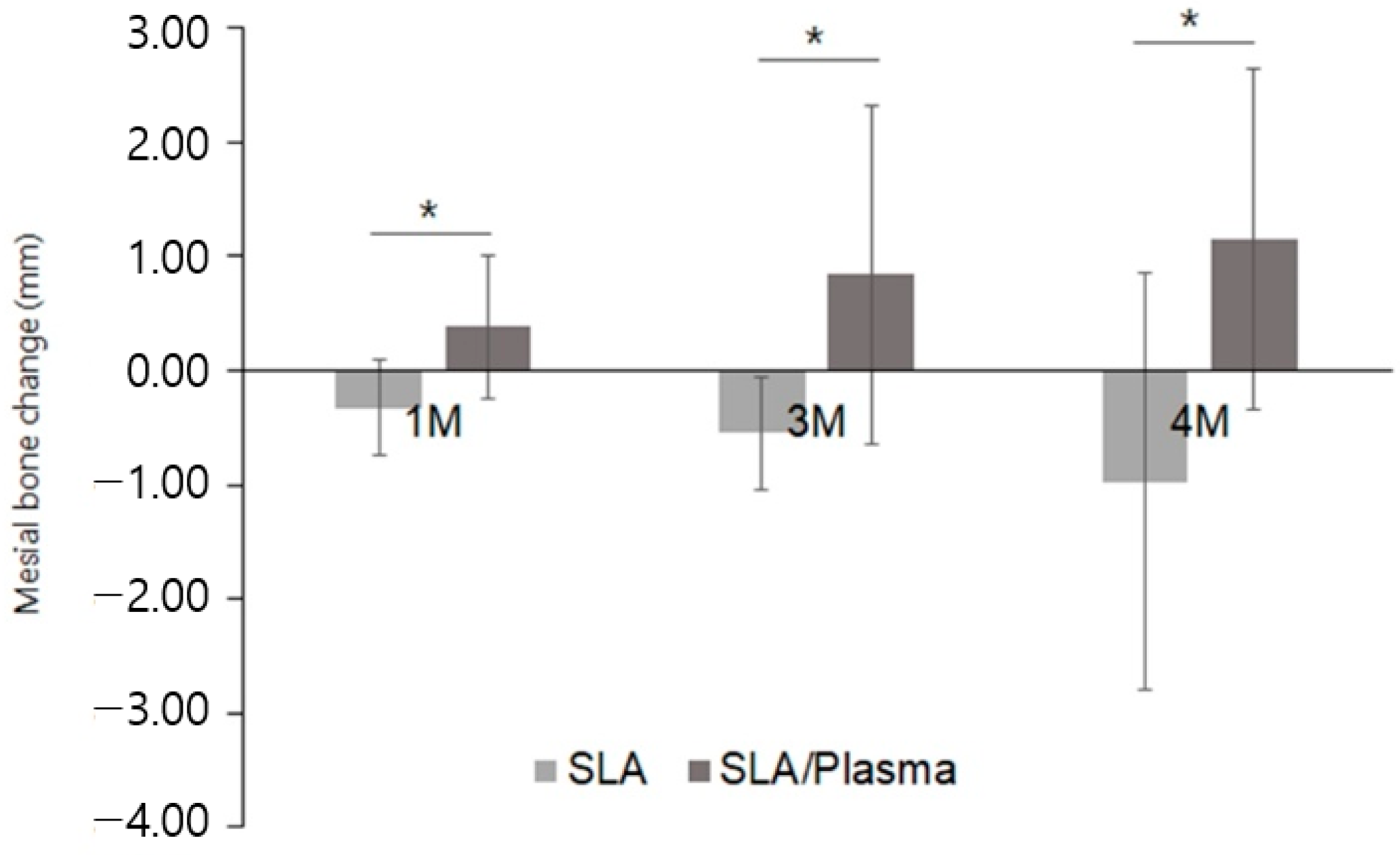
| SLA Group (n = 14) | SLA/Plasma Group (n = 14) | p-Value d | |
|---|---|---|---|
| One month a | |||
| Mean ± SD | −0.32 ± 0.42 | 0.38 ± 0.63 | 0.001 |
| Median [Min–Max] | −0.29 [−1.20–0.24] | 0.28 [−0.39–2.14] | |
| Three months b | |||
| Mean ± SD | −0.54 ± 0.49 | 0.84 ± 1.48 | 0.001 |
| Median [Min–Max] | −0.48 [−4.54–0.25] | 0.51 [−0.54–5.20] | |
| Second-stage surgery (four months) c | |||
| Mean ± SD | −0.97 ± 1.82 | 1.15 ± 1.50 | 0.001 |
| Median [Min–Max] | −0.55 [−6.90–0.05] | 0.65 [0.00–5.60] |
| SLA Group (n = 14) | SLA/Plasma Group (n = 14) | p-Value d | |
|---|---|---|---|
| One month a | |||
| Mean ± SD | −0.33 ± 0.59 | 0.39 ± 0.54 | 0.002 |
| Median [Min–Max] | −0.45 [−1.34–0.80] | 0.27 [−0.41–1.23] | |
| Three months b | |||
| Mean ± SD | −0.50 ± 0.64 | 0.46 ± 0.55 | <0.001 |
| Median [Min–Max] | −0.50 [−1.21–0.96] | 0.39 [−0.30–1.36] | |
| Second-stage surgery (four months) c | |||
| Mean ± SD | −0.82 ± 2.32 | 0.58 ± 0.77 | 0.005 |
| Median [Min–Max] | −0.48 [−8.10–1.36] | 0.37 [−0.53–1.90] |
| SLA Group (n = 14) | SLA/Plasma Group (n = 14) | p-Value a | |
|---|---|---|---|
| One month | |||
| Mean ± SD | 0.57 ± 0.85 | 0.29 ± 1.07 | 0.246 |
| Median [Min–Max] | 0.00 [0.00–2.00] | 0.00 [0.00–4.00] | |
| Three months | |||
| Mean ± SD | 0.14 ± 0.53 | 0.14 ± 0.36 | 0.804 |
| Median [Min–Max] | 0.00 [0.00–1.00] | 0.00 [0.00–1.00] | |
| Second-stage surgery | |||
| Mean ± SD | 0.21 ± 0.43 | 0.21 ± 0.80 | 0.571 |
| Median [Min–Max] | 0.00 [0.00–1.00] | 0.00 [0.00–3.00] |
| SLA Group (n = 14) | SLA/Plasma Group (n = 14) | p-Value a | |
|---|---|---|---|
| Mean ± SD | 4.21 ± 0.89 | 4.57 ± 0.51 | 0.376 |
| Median [Min–Max] | 4.00 [2.00–5.00] | 5.00 [4.00–5.00] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, J.-S.; Cho, W.-T.; Lee, J.-H.; Joo, J.-Y.; Lee, J.-Y.; Lim, Y.; Jeon, H.-J.; Huh, J.-B. Prospective Randomized Controlled Clinical Trial to Evaluate the Safety and Efficacy of ACTLINK Plasma Treatment for Promoting Osseointegration and Bone Regeneration in Dental Implants. Bioengineering 2024, 11, 980. https://doi.org/10.3390/bioengineering11100980
Kwon J-S, Cho W-T, Lee J-H, Joo J-Y, Lee J-Y, Lim Y, Jeon H-J, Huh J-B. Prospective Randomized Controlled Clinical Trial to Evaluate the Safety and Efficacy of ACTLINK Plasma Treatment for Promoting Osseointegration and Bone Regeneration in Dental Implants. Bioengineering. 2024; 11(10):980. https://doi.org/10.3390/bioengineering11100980
Chicago/Turabian StyleKwon, Jin-Seon, Won-Tak Cho, Jong-Ho Lee, Ji-Young Joo, Jae-Yeol Lee, Youbong Lim, Hyun-Jeong Jeon, and Jung-Bo Huh. 2024. "Prospective Randomized Controlled Clinical Trial to Evaluate the Safety and Efficacy of ACTLINK Plasma Treatment for Promoting Osseointegration and Bone Regeneration in Dental Implants" Bioengineering 11, no. 10: 980. https://doi.org/10.3390/bioengineering11100980
APA StyleKwon, J.-S., Cho, W.-T., Lee, J.-H., Joo, J.-Y., Lee, J.-Y., Lim, Y., Jeon, H.-J., & Huh, J.-B. (2024). Prospective Randomized Controlled Clinical Trial to Evaluate the Safety and Efficacy of ACTLINK Plasma Treatment for Promoting Osseointegration and Bone Regeneration in Dental Implants. Bioengineering, 11(10), 980. https://doi.org/10.3390/bioengineering11100980






