Enzymatically Driven Mineralization of a Calcium–Polyphosphate Bleaching Gel
Abstract
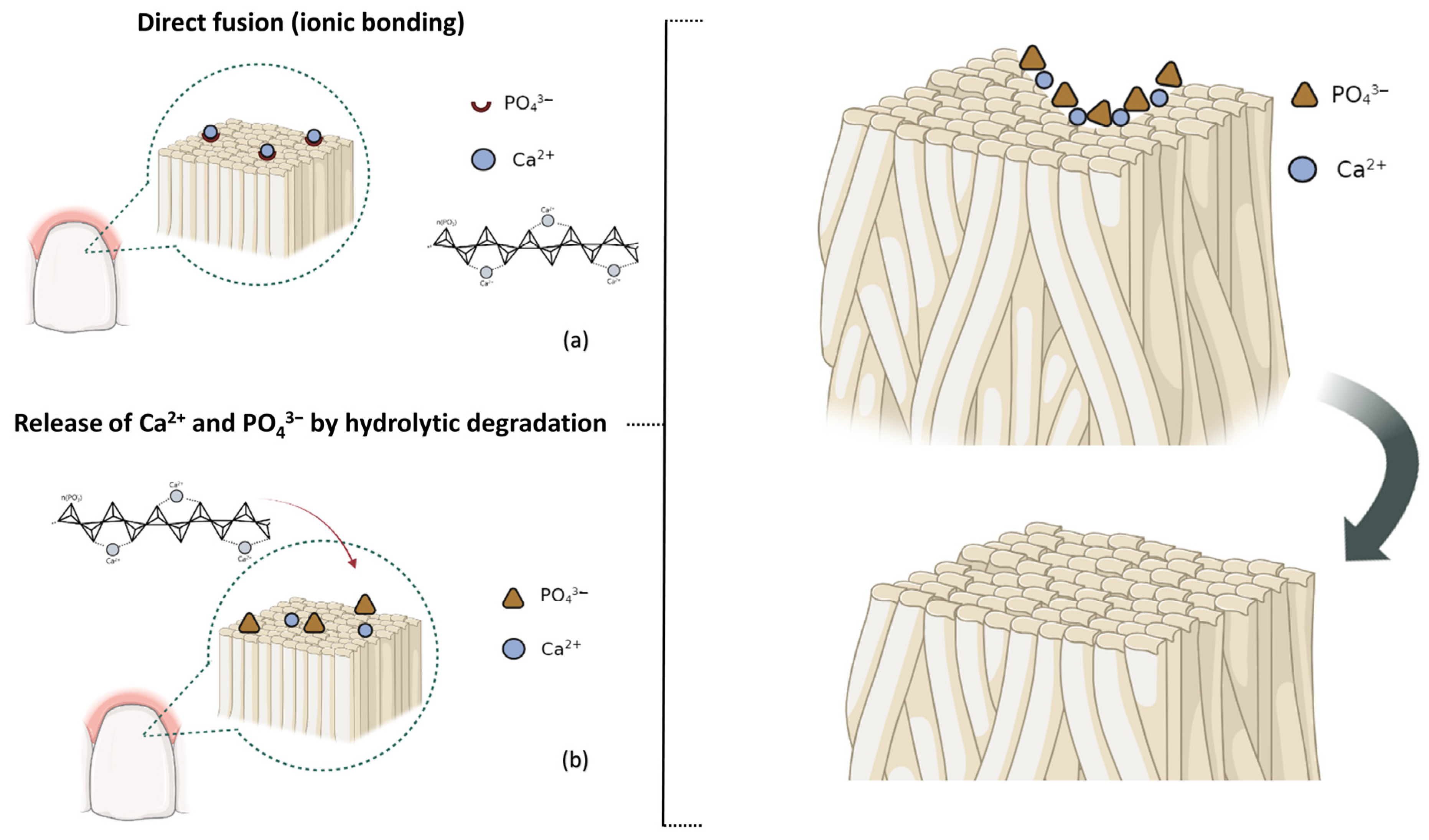
1. Introduction
2. Materials and Methods
2.1. Assessment of ALP Activity and Its Secondary Structure Using Circular Dichroism Spectroscopy (CD)
2.2. Assessment of PO43− Levels
2.3. Enamel Preparation and Bleaching Treatment
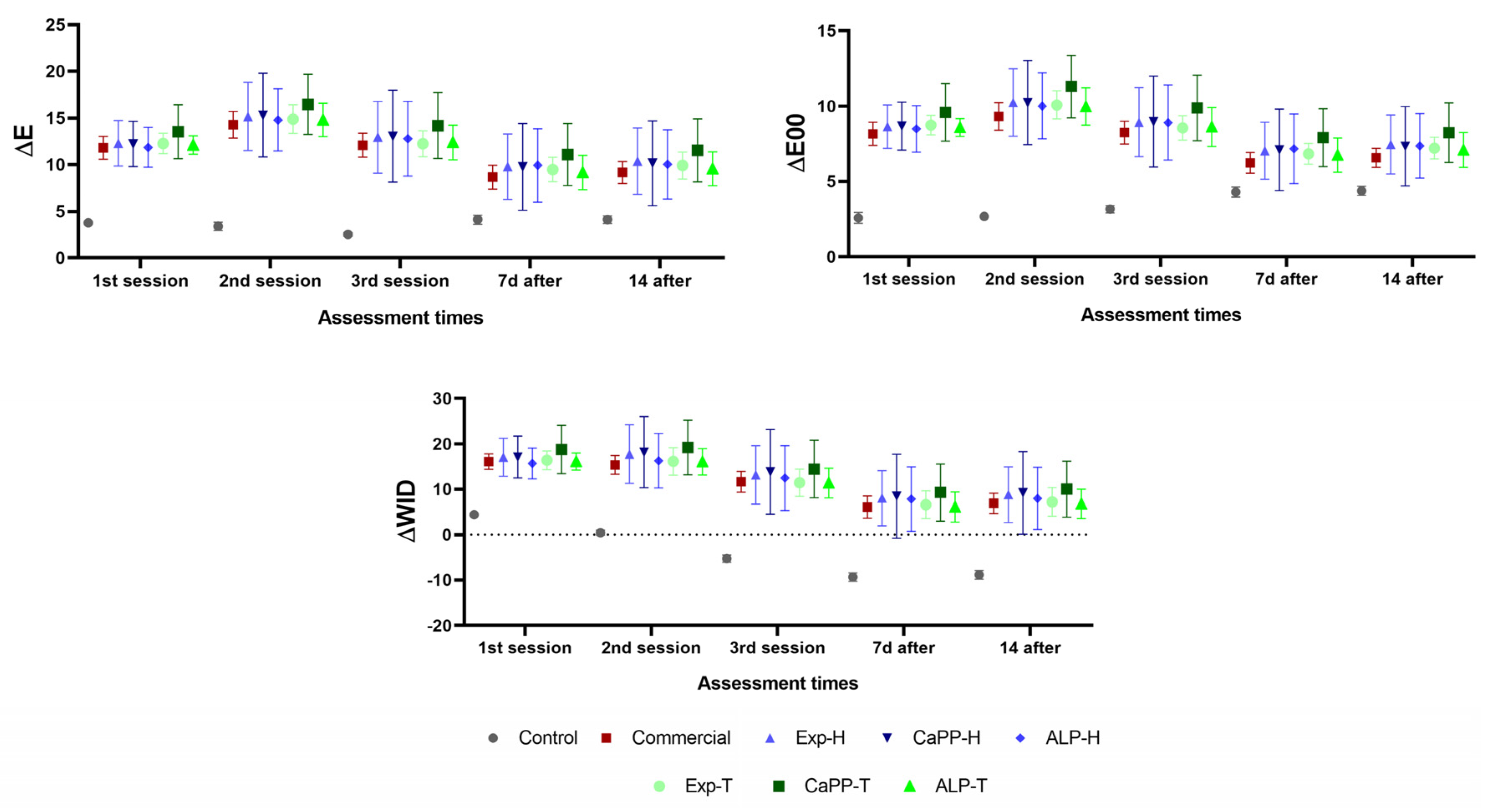
2.4. Color (ΔE, ΔE00, ΔWID) Assessment
2.5. Surface Microhardness (SMH) and Cross-Sectional Microhardness (CSMH) Assessment
2.6. Statistical Analyses
3. Results
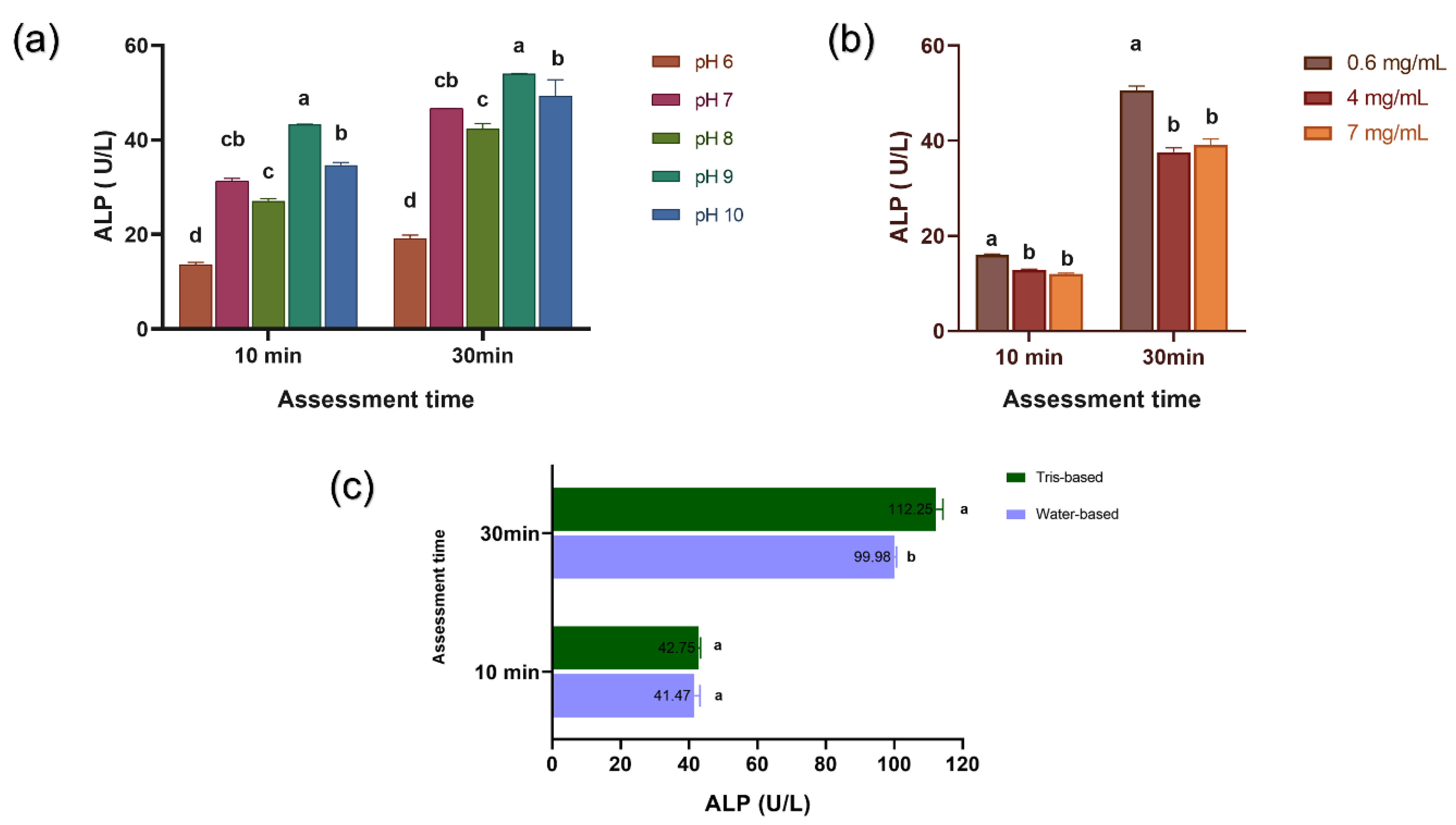
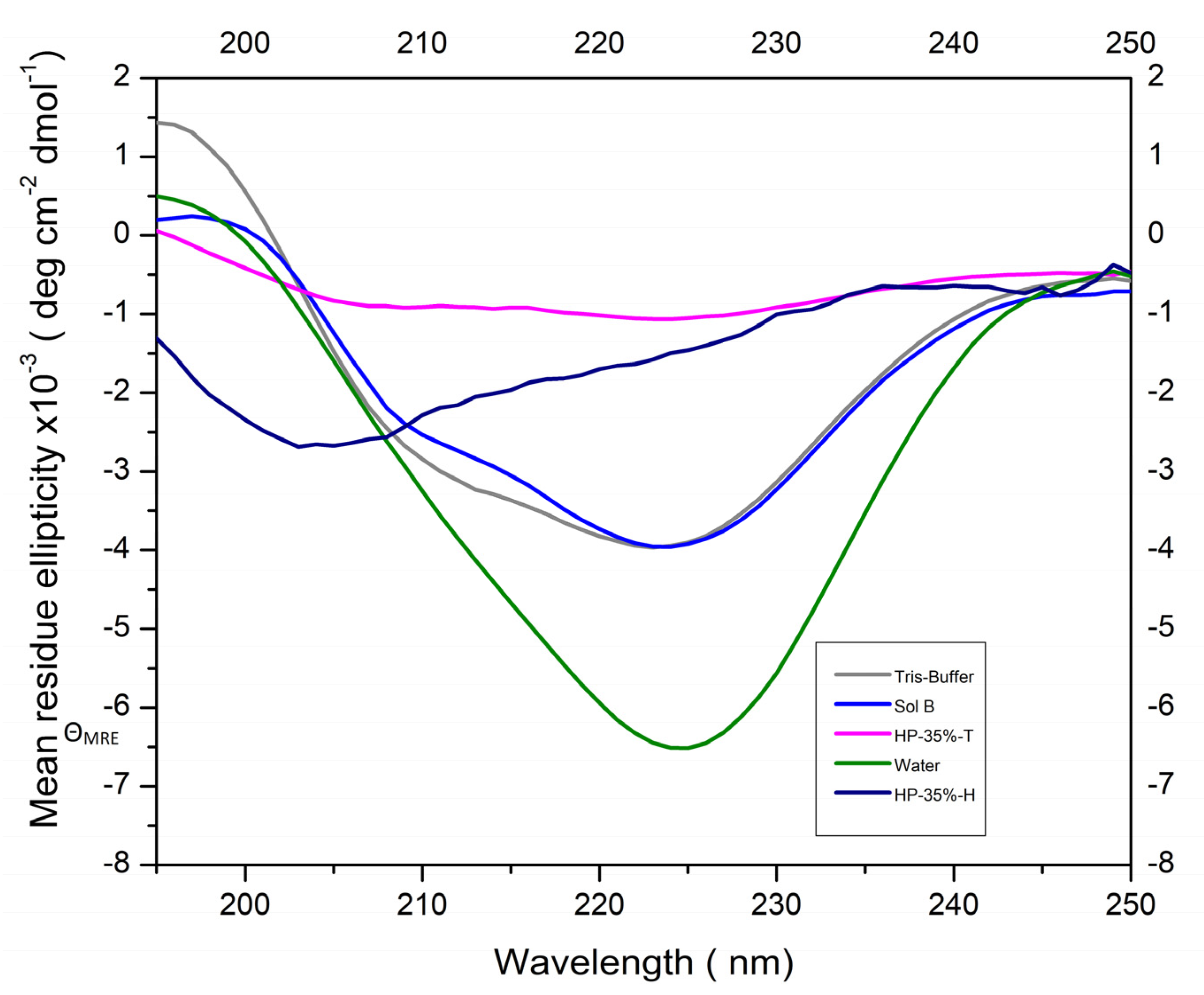
3.1. Activity and Secondary Structure of ALP
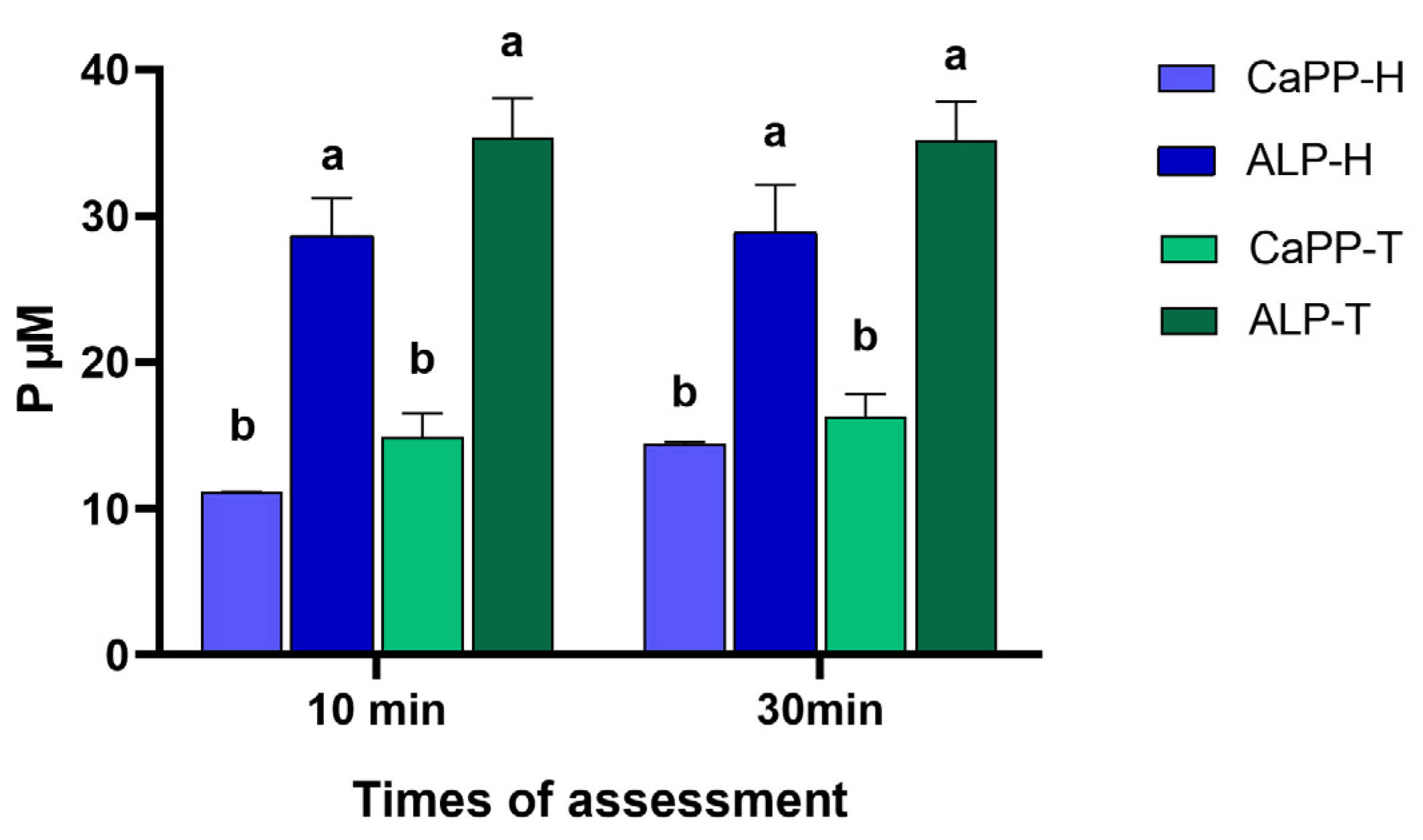
3.2. PO43− Levels
3.3. Color (ΔE, ΔE00, ΔWID)
3.4. Surface Microhardness (SMH)
3.5. Cross-Sectional Microhardness (CSMH)
4. Discussion
5. Conclusions
- -
- ALP activity was higher in mediums with pH 9, reduced in H2O2 mediums, and remained similar in Tris- or water-based thickeners.
- -
- The PO43− levels were higher following the incorporation of ALP into gel thickener solutions, indicating high polymer scission.
- -
- Both HP-CaPP-ALP (ALP-H and ALP-T) solutions demonstrated adequate bleaching effectiveness.
- -
- The HP-CaPP-ALP gels exhibited increased enamel surface microhardness after treatment when compared to the commercial or experimental gels without CaPP. Moreover, the ALP-T group exhibited the highest microhardness values after treatment.
- -
- The higher PO43− levels in the ALP-T group increased microhardness without decreasing bleaching effectiveness, which suggests a bioinspired remineralization potential.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barot, T.; Rawtani, D.; Kulkarni, P. Nanotechnology-based materials as emerging trends for dental applications. Rev. Adv. Mater. Sci. 2021, 60, 173–189. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Degli Esposti, L.; Iafisco, M.; Brambilla, E. Dental tissue remineralization by bioactive calcium phosphate nanoparticles formulations. Sci. Rep. 2022, 12, 5994. [Google Scholar] [CrossRef]
- Guanipa Ortiz, M.I.; Santos, J.J.; dos Burga, J.S.; Rodrigues-Filho, U.P.; Aguiar, F.H.B.; Rischka, K.; Lima, D.A.N.L. Calcium-Polyphosphate Submicroparticles (CaPP) Improvement Effect of the Experimental Bleaching Gels’ Chemical and Cellular-Viability Properties. Gels 2023, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G.; Ackermann, M.; Neufurth, M.; Tolba, E.; Wang, S.; Feng, Q.; Schröder, H.C.; Wang, X. A Novel Biomimetic Approach to Repair Enamel Cracks/Carious Damages and to Reseal Dentinal Tubules by Amorphous Polyphosphate. Polymers 2017, 9, 120. [Google Scholar] [CrossRef]
- Ackermann, M.; Tolba, E.; Neufurth, M.; Wang, S.; Schröder, H.C.; Wang, X.; Müller, W.E. Biomimetic transformation of polyphosphate microparticles during restoration of damaged teeth. Dent. Mater. 2019, 35, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Omelon, S.; Georgiou, J.; Henneman, Z.J.; Wise, L.M.; Sukhu, B.; Hunt, T.; Wynnyckyj, C.; Holmyard, D.; Bielecki, R.; Grynpas, M.D. Control of Vertebrate Skeletal Mineralization by Polyphosphates. PLoS ONE 2009, 4, e5634. [Google Scholar] [CrossRef] [PubMed]
- Omelon, S.; Georgiou, J.; Variola, F.; Dean, M.N. Colocation and role of polyphosphates and alkaline phosphatase in apatite biomineralization of elasmobranch tesserae. Acta Biomater. 2014, 10, 3899–3910. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, J.; Chen, H.; Ding, Z.; Ouyang, Y.; Zhang, Q.; Yan, Y. A novel degradable tricalcium silicate/calcium polyphosphate/polyvinyl alcohol organic-inorganic composite cement for bone filling. J. Biomater. Appl. 2021, 36, 772–788. [Google Scholar] [CrossRef]
- Lorenz, B.; Schröder, H.C. Mammalian intestinal alkaline phosphatase acts as highly active exopolyphosphatase. Biochim. Biophys. Acta—Protein Struct. Mol. Enzymol. 2001, 1547, 254–261. [Google Scholar] [CrossRef]
- Balladares, L.; Alegría-Acevedo, L.F.; Montenegro-Arana, A.; Arana-Gordillo, L.A.; Pulido, C.; Salazar-Gracez, M.T.; Reis, A.; Loguercio, A. Effects of pH and Application Technique of In-office Bleaching Gels on Hydrogen Peroxide Penetration into the Pulp Chamber. Oper. Dent. 2019, 44, 659–667. [Google Scholar] [CrossRef]
- Crastechini, E.; Bühler Borges, A.; Gomes Torres, C.R. Effect of remineralizing gels on microhardness, color and wear susceptibility of bleached enamel. Oper. Dent. 2019, 44, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.R.G.; Zanatta, R.F.; Silva, T.J.; Borges, A.B. Effect of Calcium and Fluoride Addition to Hydrogen Peroxide Bleaching Gel On Tooth Diffusion, Color, and Microhardness. Oper. Dent. 2019, 44, 424–432. [Google Scholar] [CrossRef]
- Oliveira Duque, C.C.; Soares, D.G.; Basso, F.G.; Hebling, J.; de Souza Costa, C.A. Bleaching effectiveness, hydrogen peroxide diffusion, and cytotoxicity of a chemically activated bleaching gel. Clin. Oral. Investig. 2014, 18, 1631–1637. [Google Scholar] [CrossRef]
- Soares, D.G.; Marcomini, N.; Duque, C.C.d.O.; Bordini, E.A.F.; Zuta, U.O.; Basso, F.G.; Hebling, J.; Costa, C.A.D.S. Increased whitening efficacy and reduced cytotoxicity are achieved by the chemical activation of a highly concentrated hydrogen peroxide bleaching gel. J. Appl. Oral. Sci. 2019, 27, e20180453. [Google Scholar] [CrossRef]
- Monteiro, N.R.; Basting, R.T.; Do Amaral, F.L.B.; França, F.M.G.; Turssi, C.P.; Gomes, O.P.; Filho, P.N.L.; Kantovitz, K.R.; Basting, R.T. Titanium dioxide nanotubes incorporated into bleaching agents: Physicochemical characterization and enamel color change. J. Appl. Oral. Sci. 2020, 28, e20190771. [Google Scholar] [CrossRef] [PubMed]
- Akabane, S.T.; Danelon, M.; Nunes, G.P.; Gruba, A.S.; Alberto de Souza-Costa, C.; Oliveira Duque, C.; Gallinari, M.d.O.; Briso, A.L.F.; Delbem, A.C.B. Evaluation of the aesthetic effect, enamel microhardness and trans-amelodentinal cytotoxicity of a new bleaching agent for professional use containing trimetaphosphate and fluoride. J. Mech. Behav. Biomed. Mater. 2021, 114, 104225. [Google Scholar] [CrossRef]
- Yang, S.Y.; Han, A.R.; Kim, K.M.; Kwon, J.S. Effects of incorporating 45S5 bioactive glass into 30% hydrogen peroxide solution on whitening efficacy and enamel surface properties. Clin. Oral. Investig. 2022, 26, 5301–5312. [Google Scholar] [CrossRef]
- Ortiz, M.I.G.; Santos, J.; Rodrigues-Filho, U.; Aguiar, F.; Rischka, K.; Lima, D. Maintenance of enamel properties after bleaching with high-concentrated hydrogen-peroxide gel containing calcium polyphosphate sub-microparticles. Clin. Oral. Investig. 2023, 27, 5275–5285. [Google Scholar] [CrossRef] [PubMed]
- Rauen, C.A.; Filho, J.C.C.; Bittencourt, B.F.; Gomes, G.M.; Gomes, J.C.; Gomes, O.M.M. Effect of bleaching agents containing fluoride or calcium on enamel microhardness, roughness and permeability. Brazilian J. Oral. Sci. 2015, 14, 262–266. [Google Scholar] [CrossRef]
- Pinto, A.V.D.; Bridi, E.C.; Amaral, F.L.B.; Franca, F.M.G.; Turssi, C.P.; Pérez, C.A.; Martinez, E.; Flório, F.; Basting, R. Enamel Mineral Content Changes After Bleaching with High and Low Hydrogen Peroxide Concentrations: Colorimetric Spectrophotometry and Total Reflection X-ray Fluorescence Analyses. Oper. Dent. 2017, 42, 308–318. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Neufurth, M.; Tolba, E.; Wang, S.; Geurtsen, W.; Feng, Q.; Schröder, H.C.; Wang, X. A biomimetic approach to ameliorate dental hypersensitivity by amorphous polyphosphate microparticles. Dent. Mater. 2016, 32, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Hasselgren, G. Alkaline phosphatase in developing teeth and bone of man and macaque monkey. Acta Odontol. Scand. 1978, 36, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline phosphatase: An overview. Indian. J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef]
- Haarhaus, M.; Brandenburg, V.; Kalantar-Zadeh, K.; Stenvinkel, P.; Magnusson, P. Alkaline phosphatase: A novel treatment target for cardiovascular disease in CKD. Nat. Rev. Nephrol. 2017, 13, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.E.; Boesze-Battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Rahutomo, S.; Kovar, J.L.; Thompson, M.L. Malachite Green Method for Determining Phosphorus Concentration in Diverse Matrices. Commun. Soil. Sci. Plant Anal. 2019, 50, 1743–1752. [Google Scholar] [CrossRef]
- Huang, R.; Wan, B.; Hultz, M.; Diaz, J.M.; Tang, Y. Phosphatase-Mediated Hydrolysis of Linear Polyphosphates. Environ. Sci. Technol. 2018, 52, 1183–1190. [Google Scholar] [CrossRef]
- Freiria, A.C.B.; Ortiz, M.I.G.; de Sobral, D.F.S.; Aguiar, F.H.B.; Lima, D.A.N.L. Nano-hydroxyapatite-induced remineralization of artificial white spot lesions after bleaching treatment with 10% carbamide peroxide. J. Esthet. Restor. Dent. 2022, 34, 1290–1299. [Google Scholar] [CrossRef]
- Pérez, M.D.M.; Ghinea, R.; Rivas, M.J.; Yebra, A.; Ionescu, A.M.; Paravina, R.D.; Herrera, L.J. Development of a customized whiteness index for dentistry based on CIELAB color space. Dent. Mater. 2016, 32, 461–467. [Google Scholar] [CrossRef]
- Paravina, R.D.; Pérez, M.M.; Ghinea, R. Acceptability and perceptibility thresholds in dentistry: A comprehensive review of clinical and research applications. J. Esthet. Restor. Dent. 2019, 31, 103–112. [Google Scholar] [CrossRef]
- Pini, N.I.P.; Piccelli, M.R.; Vieira-Junior, W.F.; Ferraz, L.N.; Aguiar, F.H.B.; Lima, D.A.N.L. In-office tooth bleaching with chitosan-enriched hydrogen peroxide gels: In vitro results. Clin. Oral. Investig. 2022, 26, 471–479. [Google Scholar] [CrossRef]
- Vieira, W.F.; Ferraz, L.N.; Giorgi, M.C.C.; Ambrosano, G.M.B.; Aguiar, F.H.B.; Lima, D.A.N.L. Effect of Mouth Rinse Treatments on Bleached Enamel Properties, Surface Morphology, and Tooth Color. Oper. Dent. 2019, 44, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; He, P.; Yao, J.; Li, M.; Bai, J.; Xue, F.; Chu, C.; Cong, Y.; Chu, P.K. Self-Assembled Multilayered Coatings with Multiple Cyclic Self-Healing Capability, Bacteria Killing, Osteogenesis, and Angiogenesis Properties on Magnesium Alloys. Adv. Healthcare Mater. 2023, 11, e2302519. [Google Scholar] [CrossRef]
- Zhao, Y.; He, P.; Wang, B.; Bai, J.; Xue, F.; Chu, C. Incorporating pH/NIR responsive nanocontainers into a smart self-healing coating for a magnesium alloy with controlled drug release, bacteria killing and osteogenesis properties. Acta Biomater. 2024, 174, 463–481. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Duque, C.C.; Soares, D.G.; Basso, F.G.; Hebling, J.; de Souza Costa, C.A. Influence of enamel/dentin thickness on the toxic and esthetic effects of experimental in-office bleaching protocols. Clin. Oral. Investig. 2017, 21, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Zhu, Y.; Zhou, H.M. Conformational changes and inactivation of calf intestinal alkaline phosphatase in trifluoroethanol solutions. Int. J. Biochem. Cell Biol. 2000, 32, 887–894. [Google Scholar] [CrossRef]
- Hethey, J.; Lai, J.; Loutet, S.; Martin, M.; Tang, V. Effects of Tricine, Glycine and Tris Buffers on Alkaline Phosphatase Activity. J. Exp. Micorbiology Immunol. 2002, 2, 33–38. [Google Scholar]
- Júnior, N.A.N.; Nunes, G.P.; Gruba, A.S.; Danelon, M.; da Silva, L.M.A.V.; de Farias Batista, G.; Briso, A.L.F.; Delbem, A.C.B. Evaluation of bleaching efficacy, microhardness, and trans-amelodentinal diffusion of a novel bleaching agent for an in-office technique containing hexametaphosphate and fluoride. Clin. Oral. Investig. 2022, 26, 5071–5078. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Schröder, H.C.; Wang, X. Inorganic Polyphosphates as Storage for and Generator of Metabolic Energy in the Extracellular Matrix. Chem. Rev. 2019, 119, 12337–12374. [Google Scholar] [CrossRef]
- Zuta, U.O.; Duque, C.C.; De Alencar ESilva Leite, M.L.; Bordini, E.A.F.; Basso, F.G.; Hebling, J.; Costa, C.d.S.; Soares, D. Effects of enzymatic activation of bleaching gels on hydrogen peroxide degradation rates, bleaching effectiveness, and cytotoxicity. Oper. Dent. 2019, 44, 414–423. [Google Scholar] [CrossRef]


| Treatment Groups | Composition | Batch | Manipulation |
|---|---|---|---|
| Control | No bleaching gel | - | - |
| Commercial | Glycol, inorganic fillers, H2O2-30–35 wt%, mixture of pigments, deionized water, thickener. | 061222 | Mix the components in a 3:1 proportion for 30 s. |
| Exp-H/Exp-T | Glycerol, propylene glycol, H2O2-35 wt%, deionized water or Tris buffer solution, acrylic acid thickener. | 23/16101 | |
| CaPP-H/CaPP-T | Glycerol, propylene glycol, H2O2-35 wt%, deionized water or Tris buffer solution, acrylic acid thickener, CaPP 0.5 wt%. | 23/16102 | |
| ALP-H/ALP-T | Glycerol, propylene glycol, H2O2-35 wt%, deionized water or Tris buffer solution, acrylic acid thickener, CaPP 0.5 wt%, ALP (thickener). | 23/16103 |
| Distance (µm)/Multiple Comparisons (Distances) | 1 Group | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Commercial | Exp-H | CaPP-H | ALP-H | Exp-T | CaPP-T | ALP-T | |
| Mean (Standard Deviation) | ||||||||
| 10/d | 253.80 (46.09) | 229.27 (48.36) | 225.79 (33.32) | 261.52 (44.47) | 257.23 (63.62) | 252.63 (41.45) | 268.00 (41.14) | 282.98 (44.07) |
| 20/c | 315.00 (38.43) | 299.19 (42.05) | 290.14 (39.99) | 327.24 (29.84) | 325.07 (56.57) | 308.15 (46.48) | 338.72 (44.76) | 354.99 (18.63) |
| 40/b | 357.19 (29.88) | 343.43 (25.72) | 309.19 (35.97) | 352.00 (26.87) | 354.71 (47.32) | 346.58 (28.99) | 354.93 (36.11) | 381.47 (20.81) |
| 60/b | 356.66 (26.13) | 352.95 (26.42) | 317.96 (47.54) | 359.04 (23.27) | 358.61 (58.31) | 340.80 (42.03) | 359.27 (37.05) | 382.85 (19.23) |
| 80/b | 363.74 (28.75) | 345.53 (29.77) | 322.34 (41.43) | 352.46 (18.62) | 352.47 (57.49) | 339.12 (31.32) | 362.22 (36.78) | 386.80 (19.36) |
| 100/a | 370.85 (39.25) | 346.59 (30.10) | 329.56 (42.04) | 359.77 (18.33) | 360.73 (62.74) | 349.64 (29.56) | 367.34 (40.88) | 390.13 (15.44) |
| 120/a | 370.64 (32.70) | 354.94 (25.09) | 329.87 (42.09) | 369.51 (19.73) | 357.56 (58.48) | 348.79 (30.21) | 367.02 (39.38) | 384.07 (15.88) |
| 140/a | 371.80 (36.63) | 342.79 (31.93) | 330.85 (45.03) | 370.35 (15.59) | 360.98 (61.72) | 343.10 (29.79) | 368.86 (48.97) | 394.55 (18.15) |
| 160/a | 374.00 (36.79) | 350.06 (39.45) | 335.78 (46.73) | 364.65 (18.90) | 360.43 (63.80) | 344.84 (27.62) | 370.25 (36.30) | 393.73 (12.76) |
| 180/a | 371.32 (36.31) | 348.36 (38.10) | 330.31 (44.89) | 369.91 (28.38) | 365.69 (61.55) | 348.78 (26.32) | 365.95 (36.70) | 402.54 (16.18) |
| Multiple comparisons (groups) | B | C | C | B | BC | BC | B | A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz, M.I.G.; Corrales Ureña, Y.R.; Aguiar, F.H.B.; Lima, D.A.N.L.; Rischka, K. Enzymatically Driven Mineralization of a Calcium–Polyphosphate Bleaching Gel. Bioengineering 2024, 11, 83. https://doi.org/10.3390/bioengineering11010083
Ortiz MIG, Corrales Ureña YR, Aguiar FHB, Lima DANL, Rischka K. Enzymatically Driven Mineralization of a Calcium–Polyphosphate Bleaching Gel. Bioengineering. 2024; 11(1):83. https://doi.org/10.3390/bioengineering11010083
Chicago/Turabian StyleOrtiz, Mariangela Ivette Guanipa, Yendry Regina Corrales Ureña, Flávio Henrique Baggio Aguiar, Débora Alves Nunes Leite Lima, and Klaus Rischka. 2024. "Enzymatically Driven Mineralization of a Calcium–Polyphosphate Bleaching Gel" Bioengineering 11, no. 1: 83. https://doi.org/10.3390/bioengineering11010083
APA StyleOrtiz, M. I. G., Corrales Ureña, Y. R., Aguiar, F. H. B., Lima, D. A. N. L., & Rischka, K. (2024). Enzymatically Driven Mineralization of a Calcium–Polyphosphate Bleaching Gel. Bioengineering, 11(1), 83. https://doi.org/10.3390/bioengineering11010083






