Multi-Dimensional Modeling of Cerebral Hemodynamics: A Systematic Review
Abstract
1. Introduction
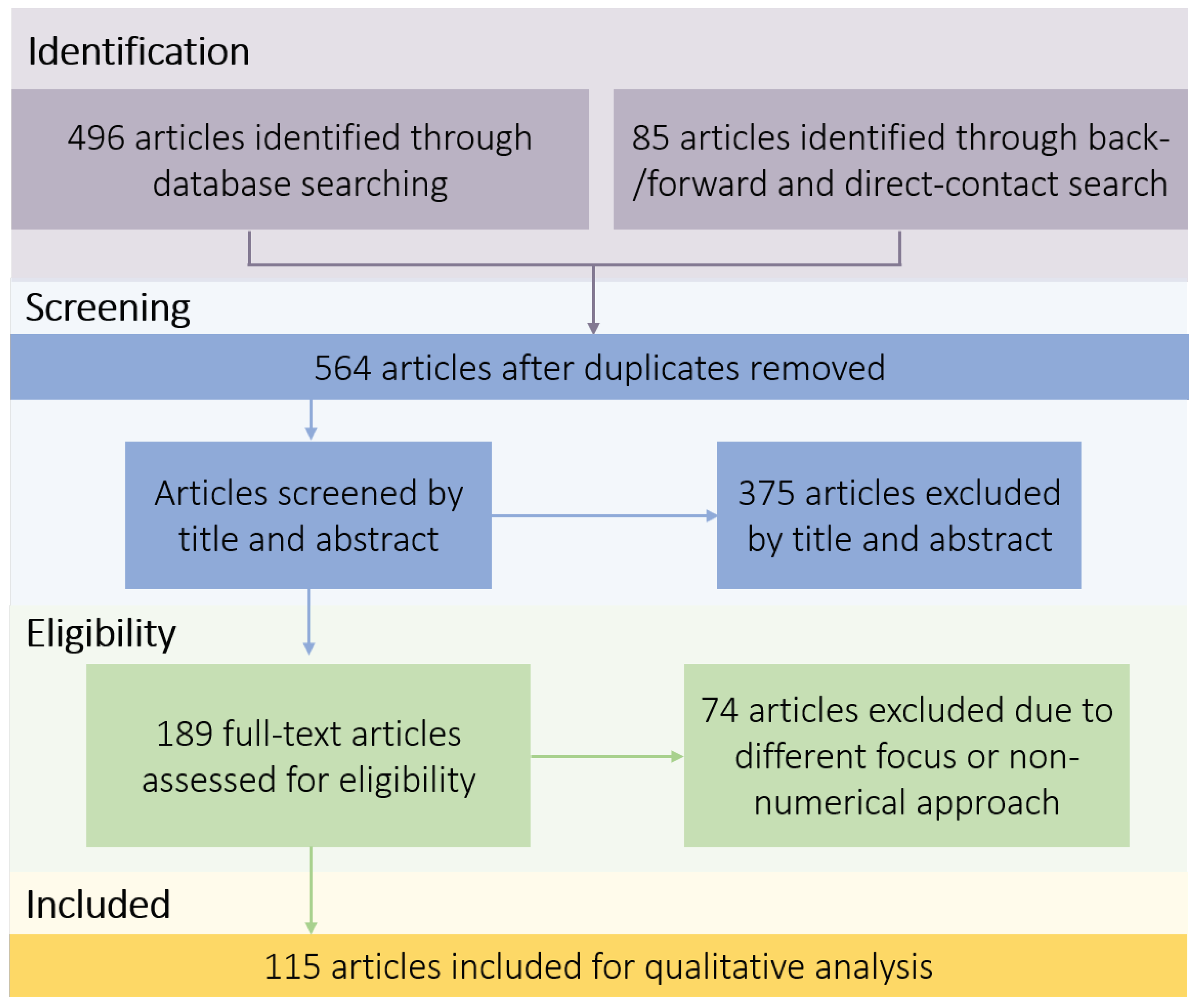
2. Methods
3. Mathematical Models
3.1. Zero-Dimensional Models
- Validation
- Application
3.2. One-Dimensional Models
- Validation
- Application
3.3. Three-Dimensional Models
- Validation
- Application
4. Multi-Scale Models
4.1. Coupling 0D–1D Models
4.2. Coupling 0D–3D Models
4.3. Coupling 1D–3D Models
4.4. Coupling 0D–1D–3D Models
5. Summary and Conclusions
- Multi-scale models as a realistic description of hemodynamic parameters within the circle of Willis
- Advantages derived from multi-scale modeling
- Limitations of the current status regarding multi-scale modeling
- Remaining investigation needed for future improvement
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | Cross-Sectional Area |
| C | Capacitance |
| CFD | Computational Fluid Dynamics |
| CoW | Circle of Willis |
| CT | Computed Tomography |
| D | Dimensional |
| FSI | Fluid Structure Interaction |
| I | Current |
| IA | Intracranial Aneurysm |
| Blood Resistance | |
| L | Inductance |
| LPM | Lumped Parameter Model |
| MRI | Magnetic Resonance Imaging |
| ODE | Ordinary Differential Equation |
| P | Pressure |
| PC | Phase Contrast |
| PDE | Partial Differential Equation |
| PIV | Particle-Image Velocimetry |
| Q | Flowrate |
| R | Resistance |
| SPECT | Single-Photon Emission Computed Tomography |
| t | Time |
| U | Voltage |
| WK | Windkessel |
| z | Vessel Length |
References
- Liu, H.; Wang, D.; Leng, X.; Zheng, D.; Chen, F.; Wong, L.; Shi, L.; Leung, T. State-of-the-art computational models of circle of willis with physiological applications: A Review. IEEE Access 2020, 8, 156261–156273. [Google Scholar] [CrossRef]
- Linn, F.; Rinkel, G.; Algra, A.; Van Gijn, J. Incidence of subarachnoid hemorrhage: Role of region, year, and rate of computed tomography: A meta-analysis. Stroke 1996, 27, 625–629. [Google Scholar] [CrossRef]
- Cebral, J.R.; Mut, F.; Weir, J.; Putman, C.M. Association of hemodynamic characteristics and cerebral aneurysm rupture. AJNR Am. J. Neuroradiol. 2011, 32, 264–270. [Google Scholar] [CrossRef]
- Meng, H.; Tutino, V.; Xiang, J.; Siddiqui, A. High WSS or how WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: Toward a unifying hypothesis. AJNR Am. J. Neuroradiol. 2013, 35, 1254–1262. [Google Scholar] [CrossRef]
- Voß, S.; Beuing, O.; Janiga, G.; Berg, P. Stent-induced vessel deformation after intracranial aneurysm treatment—A hemodynamic pilot study. Comput. Biol. Med. 2019, 111, 103338. [Google Scholar] [CrossRef] [PubMed]
- Markl, M.; Chan, F.P.; Alley, M.T.; Wedding, K.L.; Draney, M.T.; Elkins, C.J.; Parker, D.W.; Wicker, R.; Taylor, C.A.; Herfkens, R.J.; et al. Time-resolved three-dimensional phase-contrast MRI. J. Magn. Reson. Imaging 2003, 17, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Pravdivtseva, M.; Gaidzik, F.; Berg, P.; Ulloa, P.; Larsen, N.; Jansen, O.; Hövener, J.B.; Salehi Ravesh, M. Influence of spatial resolution and compressed sense acceleration factor on flow quantification with 4d flow mri at 3 tesla. Tomography 2022, 8, 457–478. [Google Scholar] [CrossRef]
- Shi, Y.; Lawford, P.; Hose, R. Review of zero-d and 1-D models of blood flow in the cardiovascular system. Biomed. Eng. Online 2011, 10, 1–38. [Google Scholar] [CrossRef]
- Berg, P.; Saalfeld, S.; Voß, S.; Beuing, O.; Janiga, G. A review on the reliability of hemodynamic modeling in intracranial aneurysms: Why computational fluid dynamics alone cannot solve the equation. Neurosurg. Focus 2019, 47, E15. [Google Scholar] [CrossRef]
- Burkhoff, D.; Alexander, J.; Schipke, J. Assessment of windkessel as a model of aortic input impedance. Am. J. Physiol.-Heart Circ. Physiol. 1988, 255, H742–H753. [Google Scholar] [CrossRef]
- Fitchett, D. LV-arterial coupling: Interactive model to predict effect of wave reflections on LV energetics. Am. J. Physiol. 1991, 261, H1026–H1033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Peng, F.; Tong, X.; Feng, X.; Li, Y.; Chen, H.; Niu, H.; Zhang, B.; Song, G.; Li, Y.; et al. Associations between haemodynamics and wall enhancement of intracranial aneurysm. Stroke Vasc. Neurol. 2021, 6, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.M.; Moorhead, K.T.; Chase, J.G.; David, T.; Fink, J. One-dimensional and three-dimensional models of cerebrovascular flow. J. Biomech. Eng. 2005, 127, 440–449. [Google Scholar] [CrossRef]
- Antiga, L.; Piccinelli, M.; Botti, L.; Ene-Iordache, B.; Remuzzi, A.; Steinman, D.A. An image-based modeling framework for patient-specific computational hemodynamics. Med. Biol. Eng. Comput. 2008, 46, 1097–1112. [Google Scholar] [CrossRef]
- Voß, S.; Beuing, O.; Janiga, G.; Berg, P. Multiple Aneurysms AnaTomy CHallenge 2018 (MATCH)-Phase Ib: Effect of morphology on hemodynamics. PLoS ONE 2019, 14, e0216813. [Google Scholar] [CrossRef] [PubMed]
- Korte, J.; Voß, S.; Janiga, G.; Beuing, O.; Behme, D.; Saalfeld, S.; Berg, P. Is accurate lumen segmentation more important than outlet boundary condition in image-based blood flow simulations for intracranial aneurysms? Cardiovasc. Eng. Technol. 2023, 14, 617–630. [Google Scholar] [CrossRef]
- Blanco, P.J.; Urquiza, S.A.; Feijóo, R.A. Assessing the influence of heart rate in local hemodynamics through coupled 3D-1D-0D models. Int. J. Numer. Methods Biomed. Eng. 2010, 26, 890–903. [Google Scholar] [CrossRef]
- Grinberg, L.; Fedosov, D.A.; Karniadakis, G.E. Parallel multiscale simulations of a brain aneurysm. J. Comput. Phys. 2013, 244, 131–147. [Google Scholar] [CrossRef]
- Kim, H.J.; Vignon-Clementel, I.E.; Figueroa, C.A.; LaDisa, J.F.; Jansen, K.E.; Feinstein, J.A.; Taylor, C.A. On coupling a lumped parameter heart model and a three-dimensional finite element aorta model. Ann. Biomed. Eng. 2009, 37, 2153–2169. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Atkinson, K.M.; Koenka, A.C.; Sanchez, C.E.; Moshontz, H.; Cooper, H. Reporting standards for literature searches and report inclusion criteria: Making research syntheses more transparent and easy to replicate. Res. Synth. Methods 2014, 6, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Abdi, K.A. Modeling the circle of willis using electrical analogy method under both normal and pathological circumstances. J. Biomed. Phys. Eng. 2013, 3, 45. [Google Scholar]
- Alastruey, J.; Parker, K.; Peiro, J.; Byrd, S.; Sherwin, S. Modeling the circle of willis to assess the effects of anatomical variations and occlusions on cerebral flows. J. Biomech. 2007, 40, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Olufsen, M.; Nadim, A. On deriving lumped models for blood flow and pressure in the systemic arteries. Math. Biosci. Eng. MBE 2004, 1, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Rideout, V.C. Mathematical and Computer Modeling of Physiological Systems; Prentice Hall: Upper Saddle River, NJ, USA, 1991. [Google Scholar]
- Burattini, R.; Natalucci, S. Complex and frequency-dependent compliance of viscoelastic windkessel resolves contradictions in elastic windkessels. Med. Eng. Phys. 1998, 20, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Chnafa, C.; Brina, O.; Pereira, V.; Steinman, D. Better than nothing: A rational approach for minimizing the impact of outflow strategy on cerebrovascular simulations. Am. J. Neuroradiol. 2017, 39, 337–343. [Google Scholar] [CrossRef]
- Manini, S.; Antiga, L.; Botti, L.; Remuzzi, A. pyNS: An open-source framework for 0d haemodynamic modelling. Ann. Biomed. Eng. 2014, 43, 1461–1473. [Google Scholar] [CrossRef]
- Connolly, M.; He, X.; Gonzalez, N.; Vespa, P.; DiStefano, J.; Hu, X. Reproduction of consistent pulse-waveform changes using a computational model of the cerebral circulatory system. Med. Eng. Phys. 2014, 36, 354–363. [Google Scholar] [CrossRef][Green Version]
- Alastruey, J.; Moore, S.M.; Parker, K.H.; David, T.; Peiró, J.; Sherwin, S.J. Reduced modelling of blood flow in the cerebral circulation: Coupling 1-D, 0-D and cerebral auto-regulation models. Int. J. Numer. Methods Fluids 2008, 56, 1061–1067. [Google Scholar] [CrossRef]
- Ursino, M.; Lodi, C. A simple mathematical model of the interaction between intracranial pressure and cerebral hemodynamics. J. Appl. Physiol. 1997, 82, 1256–1269. [Google Scholar] [CrossRef]
- Lal, R.; Nicoud, F.; Bars, E.L.; Deverdun, J.; Molino, F.; Costalat, V.; Mohammadi, B.; Lal, R.; Nicoud, F.; Bars, E.L.; et al. Non invasive blood flow features estimation in cerebral arteries from uncertain medical data. Biomed. Eng. 2017, 45, 2574–2591. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, J.; Cui, S.; Wang, S. Simulation study of the cardiovascular functional status in hypertensive situation. Comput. Biol. Med. 2002, 32, 345–362. [Google Scholar] [CrossRef] [PubMed]
- McConnell, F.K.; Payne, S. The dual role of cerebral autoregulation and collateral flow in the circle of willis after major vessel occlusion. IEEE Trans. Biomed. Eng. 2017, 64, 1793–1802. [Google Scholar] [CrossRef]
- Stergiopulos, N.; Young, D.; Rogge, T. Computer simulation of arterial flow with applications to arterial and aortic stenoses. J. Biomech. 1993, 25, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Fahrig, R.; Nikolov, H.; Fox, A.; Holdsworth, D. A three-dimensional cerebrovascular flow phantom. Med. Phys. 1999, 26, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Fukasaku, K.; Liu, H.; Takagi, S. A computational model study of the influence of the anatomy of the circle of willis on cerebral hyperperfusion following carotid artery surgery. Biomed. Eng. Online 2011, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Olufsen, M.; Tran, H.; Ottesen, J. Modeling cerebral blood flow control during posture change from sitting to standing. Cardiovasc. Eng. 2004, 4, 47–58. [Google Scholar] [CrossRef]
- Pope, S.R.; Ellwein, L.M.; Zapata, C.L.; Novak, V.; Kelley, C.T.; Olufsen, M.S. Estimation and identification of parameters in a lumped cerebrovascular model. Math. Biosci. Eng. 2009, 6, 93–115. [Google Scholar] [CrossRef]
- Ottesen, J.; Olufsen, M.; Larsen, J. Applied Mathematical Models in Human Physiology; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 2004. [Google Scholar]
- Heldt, T. Computational Models of Cardiovascular Response to Orthostatic Stress. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2005. [Google Scholar]
- Sadraie, S.H.; Abdi, M.; Navidbakhsh, M.; Hassani, K.; Kaka, G.R. Modeling the heart beat, circle of willis and related cerebral stenosis using an equivalent electronic circuit. Biomed. Eng. Appl. Basis Commun. 2014, 26, 1450052. [Google Scholar] [CrossRef]
- MacIntosh, B.; Sideso, E.; Donahue, M.; Chappell, M.; Günther, M.; Handa, A.; Kennedy, J.; Jezzard, P. Intracranial hemodynamics is altered by carotid artery disease and after endarterectomy a dynamic magnetic resonance angiography study. Stroke J. Cereb. Circ. 2011, 42, 979–984. [Google Scholar] [CrossRef]
- O’Rourke, M. Accurate measurement of arterial pressure. J. Hum. Hypertens. 2003, 17, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Hoksbergen, A.W.J.; Fülesdi, B.; Legemate, D.A.; Csiba, L. Collateral configuration of the circle of willis. Stroke 2000, 13, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Westerhof, N.; Bosman, F.; Vries, C.J.D.; Noordergraaf, A. Analog studies of the human systemic arterial tree. J. Biomech. 1969, 2, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Bergel, D. The Visco-Elastic Properties of the Arterial Wall. Ph.D. Thesis, Queen Mary University of London, London, UK, 1960. [Google Scholar]
- Patel, D.J.; Freitas, F.M.D.; Greenfield, J.C.; Fry, D.L. Relationship of radius to pressure along the aorta in living dogs. J. Appl. Physiol. 1963, 18, 1111–1117. [Google Scholar] [CrossRef]
- Noordergraaf, A.; Verdouw, P.D.; Boom, H.B. The use of an analog computer in a circulation model. Prog. Cardiovasc. Dis. 1963, 5, 419–439. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Pu, F.; Fan, Y.; Li, D. The effect of anatomic variations of circle of willis on cerebral blood distribution during posture change from supination to standing: A model study. Bio-Med. Mater. Eng. 2014, 24, 2371–2380. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, L.; Li, X.; Li, S.; Pu, F.; Fan, Y.; Li, D. Modeling the circle of Willis to assess the effect of anatomical variations on the development of unilateral internal carotid artery stenosis. Bio-Med. Mater. Eng. 2014, 24, 491–499. [Google Scholar] [CrossRef]
- Alastruey, J. Numerical Modelling of Pulse Wave Propagation in the Cardiovascular System: Development, Validation and Clinical Applications. Ph.D. Thesis, Departments of Aeronautics and Bioengineering, Imperial College London, London, UK, 2006. [Google Scholar]
- Bessems, D.; Rutten, M.; van de Vosse, F. A wave propagation model of blood flow in large vessels using an approximate velocity profile function. J. Fluid Mech. 2007, 580, 145–168. [Google Scholar] [CrossRef]
- Cassot, F.; Zagzoule, M.; Marc-Vergnes, J.P. Hemodynamic role of the circle of Willis in stenoses of internal carotid arteries. An analytical solution of a linear model. J. Biomech. 2000, 33, 395–405. [Google Scholar] [CrossRef]
- Hillen, B.; Hoogstratent, H.W.; Post, L. A mathematical model of the flow in the circle of willis. J. Biomech. 1986, 19, 187–194. [Google Scholar] [CrossRef]
- Ferrandez, A.; David, T.; Brown, M.D. Numerical models of auto-regulation and blood flow in the cerebral circulation. Comput. Methods Biomech. Biomed. Eng. 2002, 5, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Ferrández, A.; David, T.; Bamford, J.; Scott, J.; Guthrie, A. Computational models of blood flow in the circle of willis. Comput. Methods Biomech. Biomed. Eng. 2001, 4, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Formaggia, L.; Lamponi, D.; Quarteroni, A. One-dimensional models for blood flow in arteries. J. Eng. Math. 2003, 47, 251–276. [Google Scholar] [CrossRef]
- Huang, P.G.; Muller, L.O. Simulation of one-dimensional blood flow in networks of human vessels using a novel TVD scheme. Int. J. Numer. Methods Biomed. Eng. 2015, 31, e02701. [Google Scholar] [CrossRef]
- Mueller, L.; Toro, E. Well-balanced high-order solver for blood flow in networks of vessels with variable properties. Int. J. Numer. Methods Biomed. Eng. 2013, 29, 1388–1411. [Google Scholar] [CrossRef]
- Huang, G.P.; Yu, H.; Yang, Z.; Schwieterman, R.; Ludwig, B. 1D simulation of blood flow characteristics in the circle of Willis using THINkS. Comput. Methods Biomech. Biomed. Eng. 2018, 21, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Olufsen, M.S.; Peskin, C.S.; Kim, W.Y.; Pedersen, E.M.; Nadim, A.; Larsen, J. Numerical simulation and experimental validation of blood flow in arteries with structured-tree outflow conditions. Ann. Biomed. Eng. 2000, 28, 1281–1299. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Alaraj, A.; Du, X.; Charbel, F.T.; Linninger, A.A. An efficient full space-time discretization method for subject-specific hemodynamic simulations of cerebral arterial blood flow with distensible wall mechanics. J. Biomech. 2019, 87, 37–47. [Google Scholar] [CrossRef]
- Perdikaris, P.; Grinberg, L.; Karniadakis, G.E. An effective fractal-tree closure model for simulating blood flow in large arterial networks. Ann. Biomed. Eng. 2015, 43, 1432–1442. [Google Scholar] [CrossRef]
- Reymond, P.; Merenda, F.; Perren, F.; Rü, D.; Stergiopulos, N. Validation of a one-dimensional model of the systemic arterial tree. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, 208–222. [Google Scholar] [CrossRef]
- Ryu, J.; Hu, X.; Shadden, S.C. A coupled lumped-parameter and distributed network model for cerebral pulse-wave hemodynamics. J. Biomech. Eng. 2015, 137, 101009. [Google Scholar] [CrossRef]
- Moore, S.; David, T.; Chase, J.; Arnold, J.; Fink, J. 3D models of blood flow in the cerebral vasculature. J. Biomech. 2006, 39, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Berg, P.; Stucht, D.; Janiga, G.; Beuing, O.; Speck, O.; Thévenin, D. Cerebral blood flow in a healthy circle of willis and two intracranial aneurysms: Computational fluid dynamics versus four-dimensional phase-contrast magnetic resonance imaging. J. Biomech. Eng. 2014, 136, 041003. [Google Scholar] [CrossRef] [PubMed]
- Cebral, J.R.; Castro, M.A.; Soto, O.; Löhner, R.; Alperin, N. Blood-flow models of the circle of Willis from magnetic resonance data. J. Eng. Math. 2003, 47, 369–386. [Google Scholar] [CrossRef]
- Chaichana, T.; Sun, Z.; Jewkes, J. Computation of hemodynamics in the left coronary artery with variable angulations. J. Biomech. 2011, 44, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; O’Rourke, M.; Nichols, W.W. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles; CRC Press: Boca Raton, FL, USA, 2011; Volume 6. [Google Scholar] [CrossRef]
- Fabbri, D.; Long, Q.; Das, S.; Pinelli, M. Computational modelling of emboli travel trajectories in cerebral arteries: Influence of microembolic particle size and density. Biomech. Model. Mechanobiol. 2014, 13, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Jiang, W.; Zou, Y.; Li, J.; Chen, J.; Deng, X. Numerical simulation of pulsatile non-Newtonian flow in the carotid artery bifurcation. Acta Mech. Sin. Lixue Xuebao 2009, 25, 249–255. [Google Scholar] [CrossRef]
- Bharadvaj, B.K.; Mabon, R.F.; Giddens, D.P. Steady flow in a model of the human carotid bifurcation. Part I—Flow visualization. J. Biomech. 1982, 15, 349–362. [Google Scholar] [CrossRef]
- Ghaffari, M.; Tangen, K.; Alaraj, A.; Du, X.; Charbel, F.T.; Linninger, A.A. Large-scale subject-specific cerebral arterial tree modeling using automated parametric mesh generation for blood flow simulation. Comput. Biol. Med. 2017, 91, 353–365. [Google Scholar] [CrossRef]
- Grinberg, L.; Cheever, E.; Anor, T.; Madsen, J.R.; Karniadakis, G.E. Modeling blood flow circulation in intracranial arterial networks: A comparative 3D/1D simulation study. Ann. Biomed. Eng. 2011, 39, 297–309. [Google Scholar] [CrossRef]
- Mamatyukov, M.; Mikheev, I.; Parshin, D.; Khe, A.; Cherevko, A.; Orlov, K.; Chupakhin, A. Comprehensive research of human brain hemodynamics: Clinical monitoring and computer simulations. AIP Conf. Proc. 2018, 2027, 020009. [Google Scholar] [CrossRef]
- Piskin, S.; Ündar, A.; Pekkan, K. Computational modeling of neonatal cardiopulmonary bypass hemodynamics with full circle of willis anatomy. Artif. Organs 2015, 39, E164–E175. [Google Scholar] [CrossRef] [PubMed]
- Pekkan, K.; Dur, O.; Sundareswaran, K.; Kanter, K.; Fogel, M.; Yoganathan, A.; Undar, A. Neonatal aortic arch hemodynamics and perfusion during cardiopulmonary bypass. J. Biomech. Eng. 2009, 130, 061012. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.E.; Sahebjam, R. Numerical simulation of the blood flow behavior in the circle of Willis. BioImpacts 2014, 4, 89–94. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, Q.; Li, Z.Y. A 3D numerical study of the collateral capacity of the Circle of Willis with anatomical variation in the posterior circulation. Biomed. Eng. Online 2015, 14, S11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Xu, L.J.; Liu, R.; Liu, X.S.; Zhao, B.; Liang, F.Y. Importance of incorporating systemic cerebroarterial hemodynamics into computational modeling of blood flow in intracranial aneurysm. J. Hydrodyn. 2020, 32, 510–522. [Google Scholar] [CrossRef]
- Zhu, F.; Qian, Y.; Xu, B.; Gu, Y.; Karunanithi, K.; Zhu, W.; Chen, L.; Mao, Y.; Morgan, M.K. Quantitative assessment of changes in hemodynamics of the internal carotid artery after bypass surgery for moyamoya disease. J. Neurosurg. 2018, 129, 677–683. [Google Scholar] [CrossRef]
- Devault, K.; Gremaud, P.A.; Olufsen, M.S.; Novak, V.; Zhao, P.; Vernières, G. Blood flow in the circle of willis: Modeling and calibration. Multiscale Model. Simul. 2008, 7, 888–909. [Google Scholar] [CrossRef]
- Formaggia, L.; Lamponi, D.; Tuveri, M.; Veneziani, A. Numerical modeling of 1D arterial networks coupled with a lumped parameters description of the heart. Comput. Methods Biomech. Biomed. Eng. 2006, 9, 273–288. [Google Scholar] [CrossRef]
- Liang, F.; Takagi, S.; Himeno, R.; Liu, H. Multi-scale modeling of the human cardiovascular system with applications to aortic valvular and arterial stenoses. Med. Biol. Eng. Comput. 2009, 47, 743–755. [Google Scholar] [CrossRef]
- Olufsen, M. Modeling the Arterial System with Reference to an Anesthesia Simulator. Ph.D. Thesis, Rotskilde University, Roskilde, Denmark, 1998. [Google Scholar]
- Wang, J.; Parker, K. Wave propagation in a model of the arterial circulation. J. Biomech. 2004, 37, 457–470. [Google Scholar] [CrossRef] [PubMed]
- McEniery, C.M.; Yasmin, N.; Hall, I.R.; Qasem, A.; Wilkinson, I.B.; Cockcroft, J.R.; ACCT Investigators. Normal vascular aging: Differential effects on wave reflection and aortic pulse wave velocity. J. Am. Coll. Cardiol. 2005, 46, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.Y.; Takagi, S.; Himeno, R.; Liu, H. Biomechanical characterization of ventricular-arterial coupling during aging: A multi-scale model study. J. Biomech. 2009, 42, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Sarwal, S.; Watts, K.; Marble, A. Computational simulation of blood flow in human systemic circulation incorporating an external force field. Med. Biol. Eng. Comput. 1995, 33, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Davatgar, M. Numerical Simulation of Blood flow in the Systemic Vasculature Incorporatin Gravitational Force with Application to Cerebral Circulation. Ph.D. Thesis, Graduate School of Biomedical Engineering, University of New South Wales, Kensington, Australia, 2006. [Google Scholar]
- Werner, J.; Siskin, G.; Mandato, K.; Englander, M.; Herr, A. Review of venous anatomy for venographic interpretation in chronic cerebrospinal venous insufficiency. J. Vasc. Interv. Radiol. 2011, 22, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Fortune, J.; Feustel, P. Effect of patient position on size and location of the subclavian vein for percutaneous puncture. Arch. Surg. 2003, 138, 996–1000. [Google Scholar] [CrossRef]
- Stringer, M.; Restieaux, M.; Fisher, A.; Crosado, B. The vertebral venous plexuses: The internal veins are muscular and external veins have valves. Clin. Anat. 2012, 25, 609–618. [Google Scholar] [CrossRef]
- Liu, W.; Wu, S.; Lin, X. Anatomical observation of the retromandibular vein by mandibular angle ostectomy. Chin. J. Tissue Eng. Res. 2010, 14, 9113–9116. [Google Scholar] [CrossRef]
- Tanoue, S.; Kiyosue, H.; Okahara, M.; Sagara, Y.; Hori, Y.; Kashiwagi, J.; Mori, H. Para-cavernous sinus venous structures: Anatomic variations and pathologic conditions evaluated on fat-suppressed 3D fast gradient-echo MR images. AJNR Am. J. Neuroradiol. 2006, 27, 1083–1089. [Google Scholar]
- Caruso, R.; Rosenbaum, A.; Chang, J.; Joy, S. Craniocervical junction venous anatomy on enhanced mr images: The suboccipital cavernous sinus. AJNR Am. J. Neuroradiol. 1999, 20, 1127–1131. [Google Scholar]
- Balak, N.; Ersoy, G.; Uslu, U.; Tanriover, N.; Tapul, L.; Cetin, G.; Isik, N.; Elmaci, I. Microsurgical and histomorphometric study of the occipital sinus: Quantitative measurements using a novel approach of stereology. Clin. Anat. 2010, 23, 386–393. [Google Scholar] [CrossRef]
- Louis, R.G.; Loukas, M.; Wartmann, C.T.; Tubbs, R.S.; Apaydin, N.; Gupta, A.A.; Spentzouris, G.; Ysique, J.R. Clinical anatomy of the mastoid and occipital emissary veins in a large series. Surg. Radiol. Anat. 2008, 31, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Kutoglu, T.; Turut, M.; Kocabiyik, N.; Ozan, H.; Yildirim, M. Anatomical analysis of azygos vein system in human cadavers. Rom. J. Morphol. Embryol. = Rev. Roum. Morphol. Embryol. 2012, 53, 1051–1056. [Google Scholar]
- Arnautovic, K.; Al-Mefty, O.; Pait, T.; Krisht, A.; Husain, M. The suboccipital cavernous sinus. Neurosurg. Focus 1996, 1, E2. [Google Scholar] [CrossRef]
- Zachrisson, H.; Lindenberger, M.; Lanne, T. Diameter and compliance of the greater saphenous vein-the effect of age and glyceryl trinitrates. Faseb J. 2008, 22, 1211.10. [Google Scholar] [CrossRef]
- Kölegård, R.; Mekjavic, I.; Eiken, O. Increased distensibility in dependent veins following prolonged bedrest. Eur. J. Appl. Physiol. 2009, 106, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Hertzberg, B.; Kliewer, M.; DeLong, D.; Lalouche, K.; Paulson, E.; Frederick, M.; Carroll, B. Sonographic assessment of lower limb vein diameters: Implications for the diagnosis and characterization of deep venous thrombosis. AJR Am. J. Roentgenol. 1997, 168, 1253–1257. [Google Scholar] [CrossRef]
- Oguzkurt, L.; Tercan, F.; Pourbagher, M.A.; Kizilkilic, O.; Turkoz, R.; Boyvat, F. Computed tomography findings in 10 cases of iliac vein compression (May–Thurner) syndrome. Eur. J. Radiol. 2005, 55, 421–425. [Google Scholar] [CrossRef]
- Das, K.; Begum, S.; Dey, S.; Quddus, M.; Mohiuddin, A. Sonographic Measurement Of Inferior Vena Cava Diameter—A Noninvasive Tool To Detect Acute Blood Loss. Ibrahim Med. Coll. J. 2012, 5, 45–50. [Google Scholar] [CrossRef]
- Desser, T.S.; Sze, D.Y.; Jeffrey, R.B. Imaging and Intervention in the Hepatic Veins. Am. J. Roentgenol. 2003, 180, 1583–1591. [Google Scholar] [CrossRef]
- Wafae, N.; Hirose, K.; Franco, C.; Wafae, G.; Ruiz, C.; Daher, L.; Person, O. The anatomy of the human thyroid veins and its surgical application. Folia Morphol. 2008, 67, 221–225. [Google Scholar]
- Wéber, R.; Gyürki, D.; Paál, G. First blood: An efficient, hybrid one- and zero-dimensional, modular hemodynamic solver. Int. J. Numer. Methods Biomed. Eng. 2023, 39, e3701. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Chen, S.; Simaan, M.; Boston, J.; Antaki, J. A nonlinear state-space model of a combined cardiovascular system and a rotary pump. In Proceedings of the 44th IEEE Conference on Decision and Control, Seville, Spain, 12–15 December 2005; Volume 2005, pp. 897–902. [Google Scholar] [CrossRef]
- Kim, H.; Vignon-Clementel, I.; Coogan, J.; Figueroa, C.; Jansen, K.; Taylor, C. Patient-specific modeling of blood flow and pressure in human coronary arteries. Ann. Biomed. Eng. 2010, 38, 3195–3209. [Google Scholar] [CrossRef] [PubMed]
- Charlton, P.H.; Harana, J.M.; Vennin, S.; Li, Y.; Chowienczyk, P.; Alastruey, J. Modeling arterial pulse waves in healthy aging: A database for in silico evaluation of hemodynamics and pulse wave indexes. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H1062–H1085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fujiwara, N.; Kobayashi, M.; Yamada, S.; Liang, F.; Takagi, S.; Oshima, M. Development of a numerical method for patient-specific cerebral circulation using 1D–0D simulation of the entire cardiovascular system with SPECT data. Ann. Biomed. Eng. 2016, 44, 2351–2363. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Hoshina, K.; Yamamoto, S.; Nemoto, Y.; Akai, T.; Shigematsu, K.; Watanabe, T.; Ohshima, M. Development of an image-based modeling system to investigate evolutional geometric changes of a stent graft in an abdominal aortic aneurysm. Circ. J. Off. J. Jpn. Circ. Soc. 2015, 79, 1534–1541. [Google Scholar] [CrossRef]
- Oliver, J.; Webb, D. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 554–566. [Google Scholar] [CrossRef] [PubMed]
- Kashefi, A.; Mahdinia, M.; Firoozabadi, B.; Amirkhosravi, M.; Ahmadi, G.; Saidi, M. Multidimensional modeling of the stenosed carotid artery: A novel CAD approach accompanied by an extensive lumped model. Acta Mech. Sin. 2014, 30, 259–273. [Google Scholar] [CrossRef]
- Gijsen, F.; van de Vosse, F.; Janssen, J. The influence of the non-Newtonian properties of blood on the flow in large arteries: Steady flow in a carotid bifurcation model. J. Biomech. 1999, 32, 601–608. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Z.; Xiong, H.; Ghista, D.; Ren, L.; Zhang, H.; Wu, W.; Huang, W.; Hau, W.K. Three-dimensional hemodynamics analysis of the circle of Willis in the patient-specific nonintegral arterial structures. Biomech. Model. Mechanobiol. 2016, 15, 1439–1456. [Google Scholar] [CrossRef]
- Morbiducci, U.; Gallo, D.; Massai, D.; Consolo, F.; Ponzini, R.; Antiga, L.; Bignardi, C.; Deriu, M.A.; Redaelli, A. Outflow conditions for image-based hemodynamic models of the carotid bifurcation: Implications for indicators of abnormal flow. J. Biomech. Eng. 2010, 132, 091005. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, B.; Zhang, L.; Zhang, Y.; Liu, J.; Huang, S.; Xi, X.; Liu, Y. Numerical study of hemodynamic changes in the Circle of Willis after stenosis of the internal carotid artery. Comput. Methods Programs Biomed. 2024, 243, 107881. [Google Scholar] [CrossRef]
- Ho, H.; Sands, G.; Schmid, H.; Mithraratne, K.; Mallinson, G.; Hunter, P. A hybrid 1D and 3D approach to hemodynamics modelling for a patient-specific cerebral vasculature and aneurysm. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention–MICCAI 2009: 12th International Conference, London, UK, 20–24 September 2009; Volume 12, pp. 323–330. [Google Scholar] [CrossRef]
- Liang, F.; Liu, X.; Yamaguchi, R.; Liu, H. Sensitivity of flow patterns in aneurysms on the anterior communicating artery to anatomic variations of the cerebral arterial network. J. Biomech. 2016, 49, 3731–3740. [Google Scholar] [CrossRef] [PubMed]
- Marzo, A.; Singh, P.; Larrabide, I.; Radaelli, A.; Coley, S.; Gwilliam, M.; Wilkinson, I.D.; Lawford, P.; Reymond, P.; Patel, U.; et al. Computational hemodynamics in cerebral aneurysms: The effects of modeled versus measured boundary conditions. Ann. Biomed. Eng. 2011, 39, 884–896. [Google Scholar] [CrossRef] [PubMed]
- Neidlin, M.; Büsen, M.; Brockmann, C.; Wiesmann, M.; Sonntag, S.J.; Steinseifer, U.; Kaufmann, T.A. A numerical framework to investigate hemodynamics during endovascular mechanical recanalization in acute stroke. Int. J. Numer. Methods Biomed. Eng. 2016, 32, e02748. [Google Scholar] [CrossRef] [PubMed]
- Enzmann, D.; Ross, M.; Marks, M.; Pelc, N. Blood flow in major cerebral arteries measured by phase-contrast cine MR. AJNR Am. J. Neuroradiol. 1994, 15, 123–129. [Google Scholar]
- Wåhlin, A.; Ambarki, K.; Birgander, R.; Wieben, O.; Johnson, K.; Malm, J.; Eklund, A. Measuring pulsatile flow in cerebral arteries using 4d phase-contrast mr imaging. AJNR Am. J. Neuroradiol. 2013, 34, 1740–1745. [Google Scholar] [CrossRef]
- Ooij, P.; Zwanenburg, J.; Visser, F.; Majoie, C.; Vanbavel, E.; Hendrikse, J.; Nederveen, A. Quantification and visualization of flow in the circle of willis: Time-resolved three-dimensional phase contrast mri at 7 T compared with 3 T. Magn. Reson. Med. 2013, 69, 868–876. [Google Scholar] [CrossRef]
- Passerini, T.; de Luca, M.; Formaggia, L.; Quarteroni, A.; Veneziani, A. A 3D/1D geometrical multiscale model of cerebral vasculature. J. Eng. Math. 2009, 64, 319–330. [Google Scholar] [CrossRef]
- Stergiopulos, N.; Westerhof, B.; Westerhof, N. Total arterial inertance as the fourth element of the Windkessel model. Am. J. Physiol. 1999, 276, H81–H88. [Google Scholar] [CrossRef]
- Šutalo, I.; Bui, A.; Ahmed, S.; Liffman, K.; Manasseh, R. Modeling of Flow Through The Circle of Willis and Cerebral Vasculature to Assess The Effects of Changes In The Peripheral Small Cerebral Vasculature on The Inflows. Eng. Appl. Comput. Fluid Mech. 2015, 8, 609–622. [Google Scholar] [CrossRef][Green Version]
- Urquiza, S.A.; Blanco, P.J.; Vénere, M.J.; Feijóo, R.A. Multidimensional modelling for the carotid artery blood flow. Comput. Methods Appl. Mech. Eng. 2006, 195, 4002–4017. [Google Scholar] [CrossRef]
- Hughes, T.J.R.; Brooks, A.N. Streamline upwind/Petrov-Galerkin formulations for convection dominated flows with particular emphasis on the incompressible Navier-Stokes equations. Comput. Methods Appl. Mech. Eng. 1982, 32, 199–259. [Google Scholar] [CrossRef]
- Liang, F.; Oshima, M.; Huang, H.; Liu, H.; Takagi, S. Numerical study of cerebroarterial hemodynamic changes following carotid artery operation: A comparison between multiscale modeling and stand-alone three-dimensional modeling. J. Biomech. Eng. 2015, 137, 101011. [Google Scholar] [CrossRef]
- Oshima, M.; Torii, R.; Tokuda, S.; Yamada, S.; Koizumi, A. Patient-specific modeling and multi-scale blood simulation for computational hemodynamic study on the human cerebrovascular system. Curr. Pharm. Biotechnol. 2012, 13, 2153–2165. [Google Scholar] [CrossRef] [PubMed]
- Kokalari, I.; Karaja, T.; Guerrisi, M. Review on lumped parameter method for modeling the blood flow in systemic arteries. J. Biomed. Sci. Eng. 2013, 6, 92–99. [Google Scholar] [CrossRef]
- Avolio, A.P. Multi-branched model of the human arterial system. Biol. Eng. Comput. 1980, 18, 709–718. [Google Scholar] [CrossRef]
- van de Vosse, F.; Stergiopulos, N. Pulse wave propagation in the arterial tree. Annu. Rev. Fluid Mech. 2011, 43, 467–499. [Google Scholar] [CrossRef]
- Sethaput, T. Mathematical Model for Hemodynamic and Intracranial Windkessel Mechanism. Ph.D. Thesis, Case Western Reserve University, Cleveland, OH, USA, 2013. [Google Scholar]
- Quarteroni, A.; Ragni, S.; Veneziani, A. Coupling between lumped and distributed models for blood flow problems. Comput. Vis. Sci. 2001, 4, 111–124. [Google Scholar] [CrossRef]
- Schaaf, B.; Abbrecht, P. Digital computer simulation of human systemic arterial pulse wave transmission: A nonlinear model. J. Biomech. 1972, 5, 345–364. [Google Scholar] [CrossRef][Green Version]
- Stettler, J.C.; Niederer, P.; Anliker, M. Theoretical analysis of arterial hemodynamics including the influence of bifurcations-Part I: Mathematical model and prediction of normal pulse patterns. Ann. Biomed. Eng. 1981, 9, 145–164. [Google Scholar] [CrossRef]
- Alastruey, J.; Parker, K.H.; Sherwin, S.J. Arterial pulse wave haemodynamics. In Proceedings of the 11th International Conference on Pressure, Lisbon, Portugal, 24–26 October 2012; pp. 401–443. [Google Scholar]
- Pullan, A.; Smith, N.; Hunter, P. An Anatomically Based Model of Transient Coronary Blood Flow in the Heart. SIAM J. Appl. Math. 2002, 62, 990–1018. [Google Scholar] [CrossRef]
- Sherwin, S.J.; Franke, V.; Peiró, J.; Parker, K. One-dimensional modelling of a vascular network in space-time variables. J. Eng. Math. 2003, 47, 217–250. [Google Scholar] [CrossRef]
- Smith, F.; Ovenden, N.; Franke, P.; Doorly, D. What happens to pressure when a flow enters a side branch? J. Fluid Mech. 2003, 479, 231–258. [Google Scholar] [CrossRef]
- Moorhead, K.T.; Doran, C.V.; Chase, J.G.; David, T. Lumped parameter and feedback control models of the auto-regulatory response in the circle of willis. Comput. Methods Biomech. Biomed. Eng. 2004, 7, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xinmiao, Z.; Yawei, W.; Wentao, F.; Jing, J.; Zhunjun, S.; Wang, B.; Yongjun, W.; Yubo, F. Effects of abnormal vertebral arteries and the circle of Willis on vertebrobasilar dolichoectasia: A multi-scale simulation study. Clin. Biomech. 2022, 101, 105853. [Google Scholar] [CrossRef] [PubMed]
- Blanco, P.J.; Müller, L.O.; Watanabe, S.M.; Feijóo, R.A. On the anatomical definition of arterial networks in blood flow simulations: Comparison of detailed and simplified models. Biomech. Model. Mechanobiol. 2020, 19, 1663–1678. [Google Scholar] [CrossRef]
- Majka, M.; Gadda, G.; Taibi, A.; Gałazka, M.; Zieliński, P. Earliest effects of sudden occlusions on pressure profiles in selected locations of the human systemic arterial system. Phys. Rev. E 2017, 95, 032414. [Google Scholar] [CrossRef]
- Azer, K.; Peskin, C.S. A one-dimensional model of blood flow in arteries with friction and convection based on the Womersley velocity profile. Cardiovasc. Eng. 2007, 7, 51–73. [Google Scholar] [CrossRef]
- Xie, X.; Yuanyuan, W.; Zhu, H.; Zhou, J. Computation of hemodynamics in tortuous left coronary artery: A morphological parametric study. J. Biomech. Eng. 2014, 136, 101006. [Google Scholar] [CrossRef]
- Perron, S.; Boivin, S.; Hérard, J.M. A finite volume method to solve the 3D Navier–Stokes equations on unstructured collocated meshes. Comput. Fluids 2004, 33, 1305–1333. [Google Scholar] [CrossRef]
- Rezaie, H.; Ashrafizadeh, A.; Mojra, A. A patient-specific three-dimensional hemodynamic model of the circle of willis. Cardiovasc. Eng. Technol. 2017, 8, 495–504. [Google Scholar] [CrossRef]
- Guangyu, Z.; Yuan, Q.; Yang, J.; Yeo, J. The role of the circle of Willis in internal carotid artery stenosis and anatomical variations: A computational study based on a patient-specific three-dimensional model. Biomed. Eng. Online 2015, 14, 107. [Google Scholar] [CrossRef]
- Valen-Sendstad, K.; Bergersen, A.W.; Shimogonya, Y.; Goubergrits, L.; Bruening, J.; Pallares, J.; Cito, S.; Piskin, S.; Pekkan, K.; Geers, A.J.; et al. Real-world variability in the prediction of intracranial aneurysm wall shear stress: The 2015 international aneurysm cfd challenge. Cardiovasc. Eng. Technol. 2018, 9, 544–564. [Google Scholar] [CrossRef]
- Jahed, M.; Ghalichi, F.; Farhoudi, M. Fluid-structure interaction of patient-specific circle of willis with aneurysm: Investigation of hemodynamic parameters. Bio-Med. Mater. Eng. 2018, 29, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Razaghi, R.; Biglari, H.; Karimi, A. Risk of rupture of the cerebral aneurysm in relation to traumatic brain injury using a patient-specific fluid-structure interaction model. Comput. Methods Programs Biomed. 2019, 176, 9–16. [Google Scholar] [CrossRef]
- Rahma, A.G.; Yousef, K.; Abdelhamid, T. Blood flow CFD simulation on a cerebral artery of a stroke patient. SN Appl. Sci. 2022, 4, 261. [Google Scholar] [CrossRef]
- Xiang, J.; Siddiqui, A.; Meng, H. The effect of inlet waveforms on computational hemodynamics of patient-specific intracranial aneurysms. J. Biomech. 2014, 47, 3882–3890. [Google Scholar] [CrossRef]
- Mut, F.; Ruijters, D.; Babic, D.; Bleise, C.; Lylyk, P.; Cebral, J. Effects of changing physiologic conditions on the in vivo quantification of hemodynamic variables in cerebral aneurysms treated with flow diverting devices. Int. J. Numer. Methods Biomed. Eng. 2014, 30, 135–142. [Google Scholar] [CrossRef]
- Saalfeld, S.; Voß, S.; Beuing, O.; Preim, B.; Berg, P. Flow-splitting-based computation of outlet boundary conditions for improved cerebrovascular simulation in multiple intracranial aneurysms. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1805–1813. [Google Scholar] [CrossRef]
- Berg, P.; Saalfeld, S.; Janiga, G.; Brina, O.; Cancelliere, N.; Machi, P.; Pereira, V. Virtual stenting of intracranial aneurysms: A pilot study for the prediction of treatment success based on hemodynamic simulations. Int. J. Artif. Organs 2018, 41, 698–705. [Google Scholar] [CrossRef]
- Sekhane, D. Image-based computational fluid dynamics (CFD) modeling cerebral blood flow in the circle of willis. J. Adv. Res. Phys. 2016, 6, 1–5. [Google Scholar]
- Piechna, A.; Cieslicki, K. Reversed robin hood syndrome in the light of nonlinear model of cerebral circulation. Int. J. Appl. Mech. Eng. 2017, 22, 459–464. [Google Scholar] [CrossRef]
- Alnæs, M.S.; Isaksen, J.; Mardal, K.A.; Romner, B.; Morgan, M.K.; Ingebrigtsen, T. Computation of hemodynamics in the circle of Willis. Stroke 2007, 38, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.W.; Choi, S.; Cheong, Y.; Kim, Y.H.; Park, H.K. Evaluation of aneurysm-associated wall shear stress related to morphological variations of circle of Willis using a microfluidic device. J. Biomech. 2015, 48, 348–353. [Google Scholar] [CrossRef]
- Šutalo, I.D.; Bui, A.; Ahmed, S.; Liffman, K.; Manasseh, R. Modelling of flow through the circle of willis and cerebral vasculature. Model. Med. Biol. VIII 2009, 13, 83–92. [Google Scholar] [CrossRef]
- Roloff, C.; Stucht, D.; Beuing, O.; Berg, P. Comparison of intracranial aneurysm flow quantification techniques: Standard PIV vs stereoscopic PIV vs tomographic PIV vs phase-contrast MRI vs CFD. J. Neurointerventional Surg. 2018, 11, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Brindise, M.; Rothenberger, S.; Dickerhoff, B.; Schnell, S.; Markl, M.; Saloner, D.; Rayz, V.; Vlachos, P. Multi-modality cerebral aneurysm haemodynamic analysis: In vivo 4D flow MRI, in vitro volumetric particle velocimetry and in silico computational fluid dynamics. J. R. Soc. Interface 2019, 16, 20190465. [Google Scholar] [CrossRef]
- Korte, J.; Gaidzik, F.; Larsen, N.; Schütz, E.; Damm, T.; Wodarg, F.; Hövener, J.B.; Jansen, O.; Janiga, G.; Berg, P.; et al. In vitro and in silico assessment of flow modulation after deploying the Contour Neurovascular System in intracranial aneurysm models. J. Neurointerventional Surg. 2023. [Google Scholar] [CrossRef]
- Steinman, D.; Milner, J.; Norley, C.; Lownie, S.; Holdsworth, D. Image-Based Computational Simulation of Flow Dynamics in a Giant Intracranial Aneurysm. AJNR Am. J. Neuroradiol. 2003, 24, 559–566. [Google Scholar]
- Perktold, K.; Resch, M.; Florian, H. Pulsatile non-newtonian flow characteristics in a three-dimensional human carotid bifurcation model, ASME. J. Biomech. Eng. 1991, 113, 464–475. [Google Scholar] [CrossRef]
- Barletta, E.A.; Ricci, R.L.; Di Silva, R.G.; Gaspar, R.H.M.L.; Araújo, J.F.M.; Neves, M.W.F.; de Aquino, J.L.B.; Barba Belsuzarri, T.A. Fusiform aneurysms: A review from its pathogenesis to treatment options. Surg. Neurol. Int. 2018, 9, 189. [Google Scholar] [CrossRef]
- Stahl, J.; Marsh, L.; Thormann, M.; Ding, A.; Saalfeld, S.; Behme, D.; Berg, P. Assessment of the flow-diverter efficacy for intracranial aneurysm treatment considering pre- and post-interventional hemodynamics. Comput. Biol. Med. 2023, 156, 106720. [Google Scholar] [CrossRef] [PubMed]
- Formaggia, L.; Nobile, F.; Quarteroni, A.; Veneziani, A. Computing and visualization in science multiscale modelling of the circulatory system: A preliminary analysis. Comput. Visual Sci. 1999, 2, 75–83. [Google Scholar] [CrossRef]
- Pontrelli, G. A multiscale approach for modelling wave propagation in an arterial segment. Comput. Methods Biomech. Biomed. Eng. 2004, 7, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.O.; Toro, E.F. A global multiscale mathematical model for the human circulation with emphasis on the venous system. Int. J. Numer. Methods Biomed. Eng. 2014, 30, 681–725. [Google Scholar] [CrossRef]
- Lee, K.E.; Ryu, A.J.; Shin, E.S.; Shim, E.B. Physiome approach for the analysis of vascular flow reserve in the heart and brain. Pflugers Arch. Eur. J. Physiol. 2017, 469, 613–628. [Google Scholar] [CrossRef]
- Voß, S.; Glaßer, S.; Hoffmann, T.; Beuing, O.; Weigand, S.; Jachau, K.; Preim, B.; Thévenin, D.; Janiga, G.; Berg, P. Fluid-structure simulations of a ruptured intracranial aneurysm: Constant versus patient-specific wall thickness. Comput. Math. Methods Med. 2016, 2016, 9854539. [Google Scholar] [CrossRef]
- Tanaka, H.; Fujita, N.; Enoki, T.; Matsumoto, K.; Watanabe, Y.; Murase, K.; Nakamura, H. Relationship between variations in the circle of Willis and flow rates in internal carotid and basilar arteries determined by means of magnetic resonance imaging with semiautomated lumen segmentation: Reference data from 125 healthy volunteers. AJNR Am. J. Neuroradiol. 2006, 27, 1770–1775. [Google Scholar]
- Gobin, Y.; Cloughesy, T.; Chow, K.; Duckwiler, G.; Sayre, J.; Milanese, K.; Viñuela, F. Intraarterial chemotherapy for brain tumors by using a spatial dose fractionation algorithm and pulsatile delivery. Radiology 2001, 218, 724–732. [Google Scholar] [CrossRef]
- Blanco, P.; Leiva, J.; Buscaglia, G. A black-box decomposition approach for coupling heterogeneous components in hemodynamics simulations. Int. J. Numer. Methods Biomed. Eng. 2013, 29, 408–427. [Google Scholar] [CrossRef] [PubMed]
- Chourdakis, G.; Davis, K.; Rodenberg, B.; Schulte, M.; Simonis, F.; Uekermann, B.; Abrams, G.; Bungartz, H.; Cheung Yau, L.; Desai, I.; et al. preCICE v2: A sustainable and user-friendly coupling library. Open Res. Eur. 2022, 2, 51. [Google Scholar] [CrossRef] [PubMed]

| Article | D | Anatomy | Loop | Wall Model | Source of Inputdata | Software/Solver | Derived Results | Comparison |
|---|---|---|---|---|---|---|---|---|
| ABDI et al. (2013) [22] | 0D | CoW, Heart, | closed | neglected | Literature [23] | MATLAB | Pressure–time curves | Experiments [24,25] |
| PS | ||||||||
| BURATTINI et al. (1998) [26] | 0D | Heart | open | viscoelastic | Animal study | Computer simulation | Compliance | WK2, WK3, VW, FPM models |
| BURKHOFF et al. (1988) [10] | 0D | Aorta | open | neglected | Animal study | Computer simulation | Pressure, Impedance | Animal study |
| CHNAFA et al. (2017) [27] | 0D | ICA, MCA, | open | neglected | 3D angiograms | pyNS [28] | Flow | 3D CFD results |
| ACA | ||||||||
| CONNOLLY et al. (2014) [29] | 0D | CoW | open | elastic | Literature [23,30,31] | MATLAB CVX | Flow, Pulse waveforms | Vasoconstriction to vasodilation |
| LAL et al. (2017) [32] | 0D | CoW, Aorta | open | elastic | TOF MRA, Kalman filter | In-house | Resisistance, Compliance | Data assimilation |
| LI et al. (2002) [33] | 0D | CoW, Heart | closed | neglected | Doppler measurements | Delphi 5.0 | Pressure | Doppler measurements |
| MCCONNELL et al. (2017) [34] | 0D | CoW | open | rigid | Literature [23,35,36] | In-house | Flow | Literature [37] |
| OLUFSEN et al. (2004) [38] | 0D | CoW, Heart, | closed | neglected | Doppler measurements | MATLAB | Pressure, Velocity | Doppler measurements |
| Full body | ||||||||
| POPE et al. (2009) [39] | 0D | CoW, Heart | closed | neglected | Doppler measurements, | MATLAB | Pressure, Velocity | Doppler measurements |
| Literature [40,41] | ||||||||
| SADRAIE et al. (2014) [42] | 0D | CoW, Heart | open | neglected | Literature [23,43,44] | MATLAB Simulink | Pressure wave propagation | Literature [45] |
| WESTERHOF et al. (1969) [46] | 0D | Full body | open | neglected | Literature [47,48,49] | n/a | Impedance, Pressure | Doppler measurements |
| ZHANG et al. (2014) [50] | 0D | CoW | open | rigid | Literature [23] | n/a | Flow | Doppler measurements |
| ZHANG et al. (2014) [51] | 0D | CoW | open | rigid | Literature [36] | Fourth-order Runge–Kutta algorithm | Flow | Doppler measurements |
| ALASTRUEY et al. (2007) [23] | 1D | CoW, Aorta | open | elastic | Literature [35,36] | discontinuous Galerkin scheme | Flow/pressure wave propagation | Literature [52] |
| BESSEMS et al. (2007) [53] | 1D | None | open | elastic | Galerkin | Galerkin weighted-residual method | Flow/pressure wave propagation | Womersley profiles |
| weighted-residuals method | ||||||||
| CASSOT et al. (2000) [54] | 1D | CoW | open | viscoelastic | Literature [55] | In-house | Resistance, Pressure | Non-linear numerical model |
| FERRANDEZ et al. (2002) [56] | 1D | CoW | open | rigid | Simulated pressure | Fluent, ANSIC | Flow | Literature [57] |
| FITCHETT et al. (1991) [11] | 1D | CoW, Heart, | closed | viscoelastic | Numerically assessed | n/a | Pressure, Stroke work, | n/a |
| Full body | Impulse reponse | |||||||
| FORMAGGIA et al. (2003) [58] | 1D | Endograft | open | elastic | Pressure waveforms | Finite element Taylor–Galerkin scheme | Flow, Pulse waveforms | 2D fluid–structure interaction |
| HUANG et al. (2015) [59] | 1D | CoW, Full body | closed | elastic | n/a | TVD scheme | Velocity, Pressure, Flow | Literature [60] |
| HUANG et al. (2018) [61] | 1D | CoW | open | elastic | MRI/CT measurements | THINkS | Flow, Velocity | Literature [60] |
| OLUFSEN et al. (2000) [62] | 1D | Full body | open | elastic | MRI measurements | Two-step Lax–Wendroff method | Algorithms, Pressure | MRI measurements |
| PARK et al. (2019) [63] | 1D | CoW | open | elastic | MRI measurements | Method of weighted residuals | Pressure, Flow, Area | MRI measurements |
| Fourier series | ||||||||
| PERDIKARIS et al. (2015) [64] | 1D | CoW, Arm | closed | viscoelastic | MRI/PCMRI measurements | Discontinuous Galerkin solver | Pressure, Flow | n/a |
| REYMOND et al. (2009) [65] | 1D | CoW, Heart, | open | viscoelastic | MRI/Doppler measurements, | Witzig–Womersley Theory | Pressure, Flow, Area | MRI/Doppler measurements, |
| Full body | Tonometry | Tonometry | ||||||
| RYU et al. (2015) [66] | 1D | CoW, Heart | open | elastic | Literature [23,35,36,67] | Second-order spatial scheme | Flow velocity, Activation factor, | Simulations |
| Two-step Adams–Bashforth temporal scheme | Active tension, Flow | |||||||
| BERG et al. (2014) [68] | 3D | CoW | open | rigid | MRI measurements | ANSYS Fluent | Velocity fields, Angular similarity index, | n/a |
| Magnitude similarity index | ||||||||
| CEBRAL et al. (2003) [69] | 3D | CoW | open | rigid | MRI/PCMRI measurements | In-house simulation | Flow, Resistances, | n/a |
| Shear parameters (OSI, WSS) | ||||||||
| CHAICHANA et al. (2011) [70] | 3D | Coronary arteries | open | rigid | Literature [71] | ANSYS CFX, MATLAB | WSS, WSS gradient, Velocity | n/a |
| FABBRI et al. (2014) [72] | 3D | CoW | open | rigid | MRI measurements | ANSYS CFX, Particle–fluid interaction | Particle tracking | n/a |
| FAN et al. (2009) [73] | 3D | Carotid | open | rigid | Literature [74] | FLUENT, GAMBIT | Velocity, WSS | n/a |
| GHAFFARI et al. (2017) [75] | 3D | CoW | open | rigid | MRI measurements, | ANSY Fluent | Morphology, Wall shear, | Statistical analysis |
| NOVA scans | Vorticity, Pressure, Flow | |||||||
| GRINBERG et al. (2011) [76] | 3D | CoW | open | rigid | PC-MRI measurements | High-order spectral/hp element solver | Pressure, Flow distribution | n/a |
| MAMATYUKOV et al. (2018) [77] | 3D | CoW | open | elastic | Doppler measurements | ANSYS CFX | Velocity | n/a |
| PISKIN et al. (2015) [78] | 3D | CoW | open | rigid | Literature [79] | ANSYS Fluent | Velocity, WSS, Pressure | Literature [79] |
| RAZAVI et al. (2014) [80] | 3D | CoW | open | rigid | MRI measurements | COMSOL | Velocity, Flow, Shear rate | n/a |
| REN et al. (2015) [81] | 3D | CoW | open | rigid | MRI/PCMRI measurements | ANSYS Fluent | Velocity, Flow | n/a |
| ZHANG et al. (2020) [82] | 3D | CoW | open | rigid | MRI measurements | ANSYS CFX | Velocity, Shear parameters | n/a |
| ZHU et al. (2018) [83] | 3D | CoW | open | rigid | MRI/PCMRI measurements, | ANSYS CFX | Pressure drop, WSS, Flow | NOVA data |
| DEVAULT et al. (2008) [84] | 0D-1D | CoW | open | viscoelastic | MRI measurements | Chebyshev collocation methods | Velocity, Pressure, Flow | Transcranial Doppler |
| FORMAGGIA et al. (2006) [85] | 0D-1D | Heart and arteries | open | viscoelastic | Blood pressure measurements | Second-order Taylor–Galerkin FE scheme | Flow, Pressure | n/a |
| LIANG et al. (2009) [86] | 0D-1D | CoW, Full body | closed | elastic | Literature [35,87,88] | Two-step Lax–Wendroff method | Blood flow, Pressure | Literature [89] |
| Fourth-order Runge–Kutta method | Stroke volume | |||||||
| LIANG et al. (2009) [90] | 0D-1D | CoW, Full body | closed | elastic | Literature [35,87,88] | Two-step Lax–Wendroff method | Blood flow, Pressure | n/a |
| ‘Ghost- point’ method | Stroke volume | |||||||
| LIANG et al. (2011) [37] | 0D-1D | CoW, Heart | closed | elastic | In vivo data, Literature [23,65,84] | Two-step Lax–Wendroff method | Flow, Hyperfusion | In vivo data, |
| Fourth-order Runge–Kutta method | Literature [23,65,84] | |||||||
| MÜLLER et al. (2014) [60] | 0D-1D | Total | closed | elastic | Literature [37] | Dumbser–Enaux–Toro (DET) solver | Pressure, Flow | MRI measurements, |
| Literature [91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109] | ||||||||
| WÉBER et al. (2023) [110] | 0D-1D | CoW, Heart, | open | Poynting– | Literature [65,111,112] | C++ | Pressure, Flow | Literature [113] |
| Full body | Thomson | |||||||
| ZHANG et al. (2016) [114] | 0D-1D | CoW, Heart, | closed | elastic | In-house software „V-modeler“ [115], | In-house simulation | Flow | Literature [23,35,65,116] |
| Full body | MRI/PC-MRI measurements, | |||||||
| SPECT data | ||||||||
| KASHEFI et al. (2014) [117] | 0D-3D | Carotid, Heart, | open | elastic | Literature [74] | Matlab Simulink, ANSYS Fluent | Velocity distribution, | Steady simulation [118] |
| Full body | Pressure, Flow | |||||||
| LIU et al. (2016) [119] | 0D-3D | CoW, Aorta | open | rigid | MRI/Doppler measurements | Comsol Multiphysics | Velocity, Flow | Doppler measurements |
| MORBIDUCCI et al. (2010) [120] | 0D-3D | Carotid | open | rigid | CTA (angiographic images) | ANSYS Fluent | Wall shear parameters | n/a |
| SUN et al. (2023) [121] | 0D-3D | CoW | closed | rigid | CTA | ANSYS CFX | ||
| HO et al. (2009) [122] | 1D-3D | CoW | open | rigid | CTA | ANSYS CFX | Velocity | Doppler measurements |
| LIANG et al. (2016) [123] | 1D-3D | CoW, IA | open | rigid | MRI measurements | In-house code, Two-Step Lax-Wendroff method | Velocity, Flow, OSI, WSS | PIV measurements |
| MARZO et al. (2011) [124] | 1D-3D | CoW | open | elastic | 3DRA/PC-MRI measurements | ANSYS CFX | Flow, Shear Parameters | n/a |
| NEIDLIN et al. (2016) [125] | 1D-3D | CoW | open | rigid | CTA | ANSYS CFX | Flow, Velocity | Literature [126,127,128] |
| PASSERINI et al. (2009) [129] | 1D-3D | CoW, Carotid | open | rigid | Literature [23,30,130] | LifeV | Flow, Velocity | n/a |
| ŠUTALO et al. (2014) [131] | 1D-3D | CoW | open | elastic | CT | ANSYS CFX | Flow, Pressure, Velocity | n/a |
| URQUIZA et al. (2006) [132] | 1D-3D | Full body | open | elastic | Literature [74] | SUPG [133] | Flow, Velocity, Shear stress | Literature |
| BLANCO et al. (2010) [17] | 0D-1D-3D | Full body, Aorta | closed | viscoelastic | MRI measurements | Gauss–Seidel method | Flow, OSI, Particle tracking | n/a |
| LIANG et al. (2015) [134] | 0D-1D-3D | CoW, Full body | open | rigid | Literature [86,90] | Two-step Lax–Wendroff method | WSS, Flow, Velocity | 3D simulation |
| Fourth-order Runge–Kutta | ||||||||
| OSHIMA et al. (2012) [135] | 0D-1D-3D | CoW | open | rigid | MRI measurements | In-house | WSS, Pressure, Flow | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korte, J.; Klopp, E.S.; Berg, P. Multi-Dimensional Modeling of Cerebral Hemodynamics: A Systematic Review. Bioengineering 2024, 11, 72. https://doi.org/10.3390/bioengineering11010072
Korte J, Klopp ES, Berg P. Multi-Dimensional Modeling of Cerebral Hemodynamics: A Systematic Review. Bioengineering. 2024; 11(1):72. https://doi.org/10.3390/bioengineering11010072
Chicago/Turabian StyleKorte, Jana, Ehlar Sophie Klopp, and Philipp Berg. 2024. "Multi-Dimensional Modeling of Cerebral Hemodynamics: A Systematic Review" Bioengineering 11, no. 1: 72. https://doi.org/10.3390/bioengineering11010072
APA StyleKorte, J., Klopp, E. S., & Berg, P. (2024). Multi-Dimensional Modeling of Cerebral Hemodynamics: A Systematic Review. Bioengineering, 11(1), 72. https://doi.org/10.3390/bioengineering11010072






