Instantaneous Generation of Subject-Specific Finite Element Models of the Hip Capsule
Abstract
:1. Introduction
2. Materials and Methods
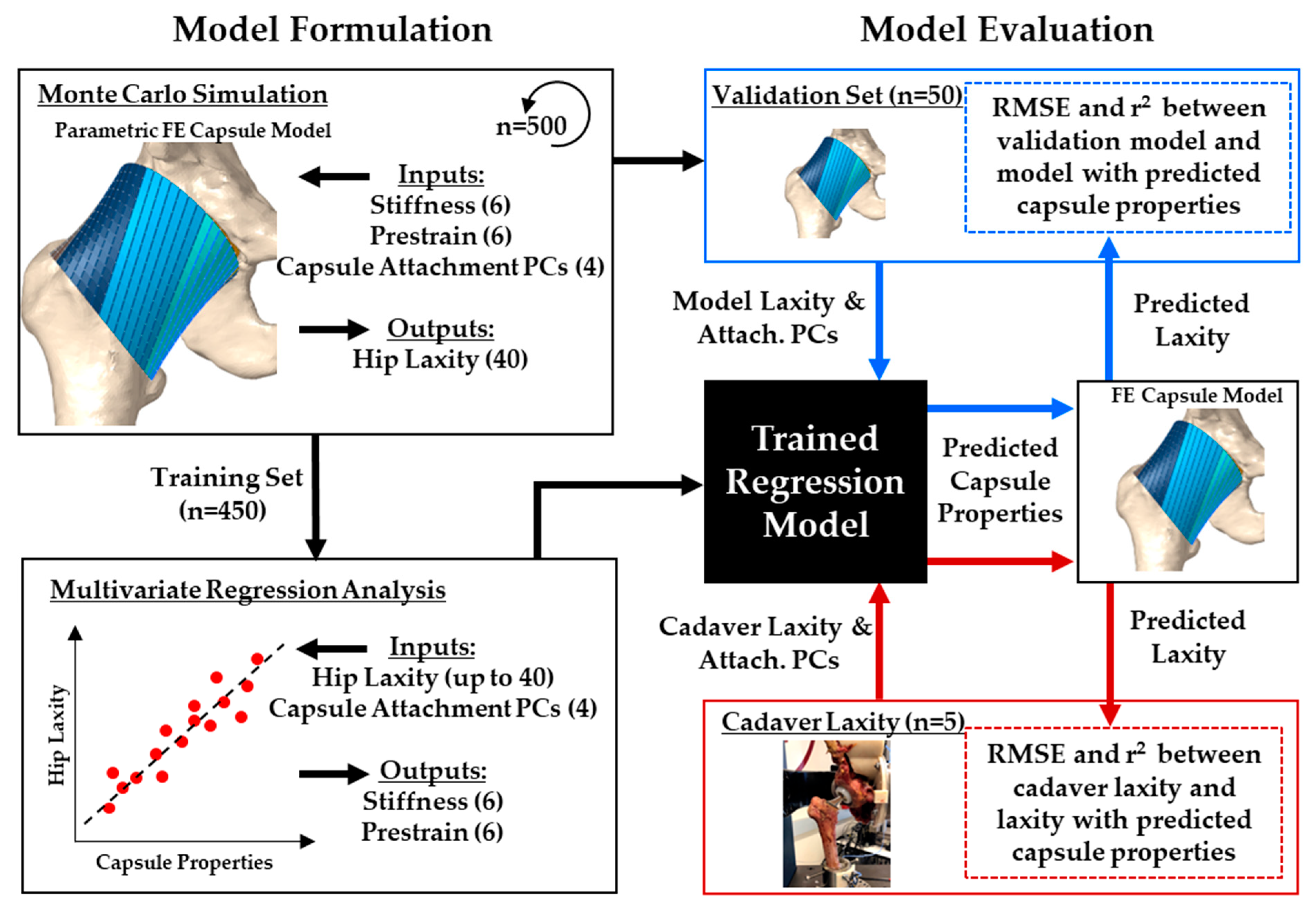
2.1. Overview
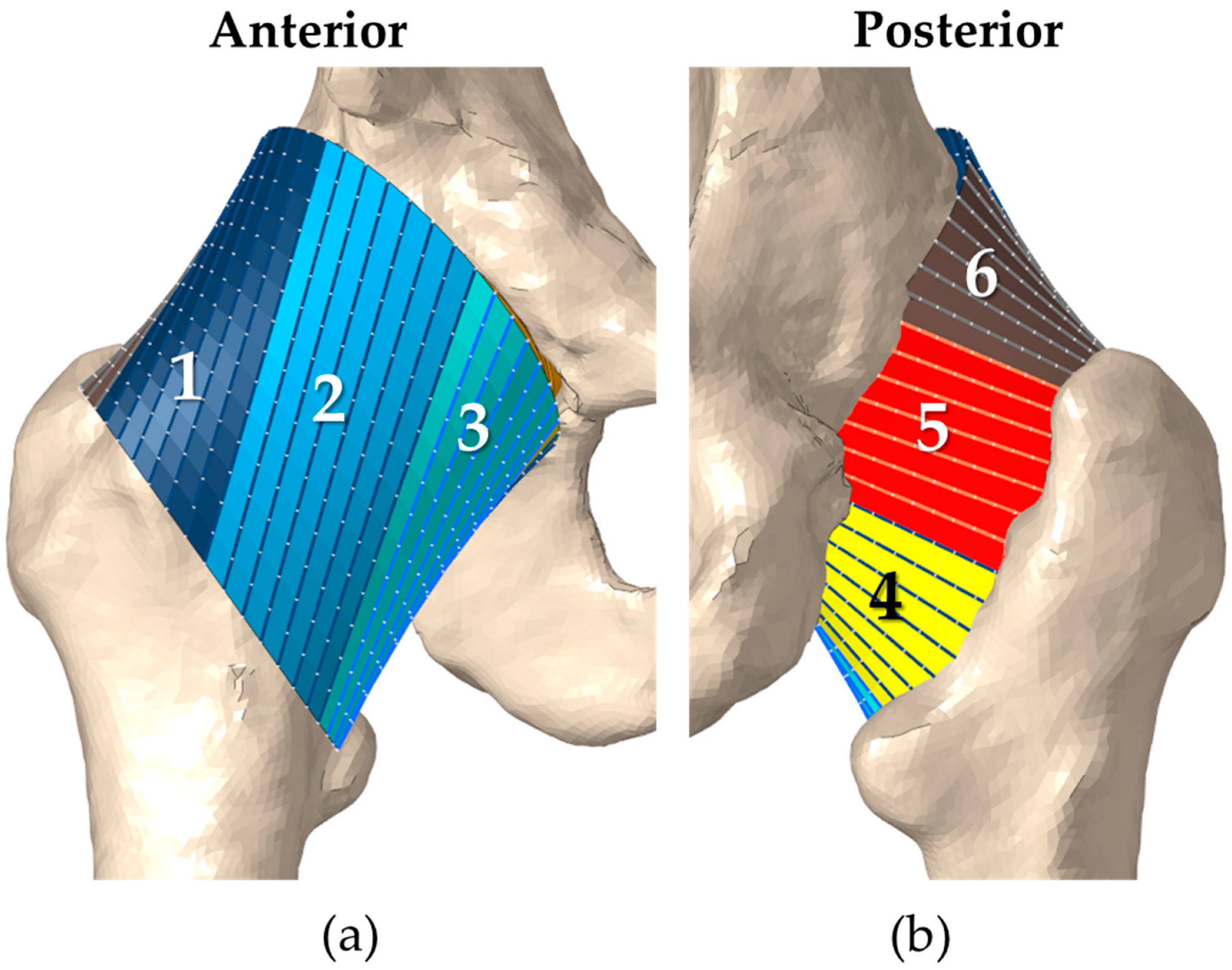
2.2. FE Model of the Implanted Hip Capsule
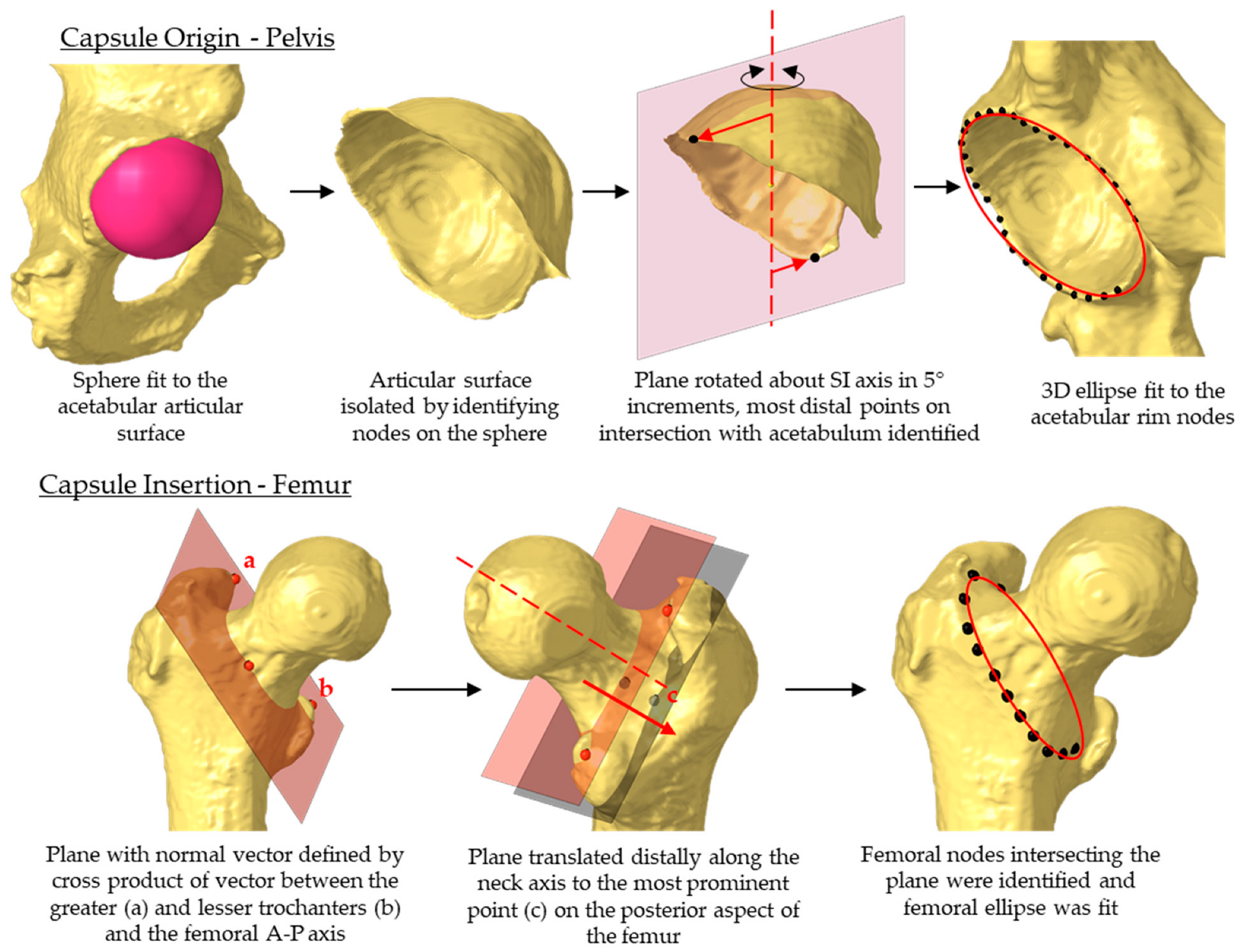
2.3. Statistical Shape Model of the Capsule
2.4. Probabilistic Model Evaluation
2.5. Capsule Predictions Using Multilinear Regression
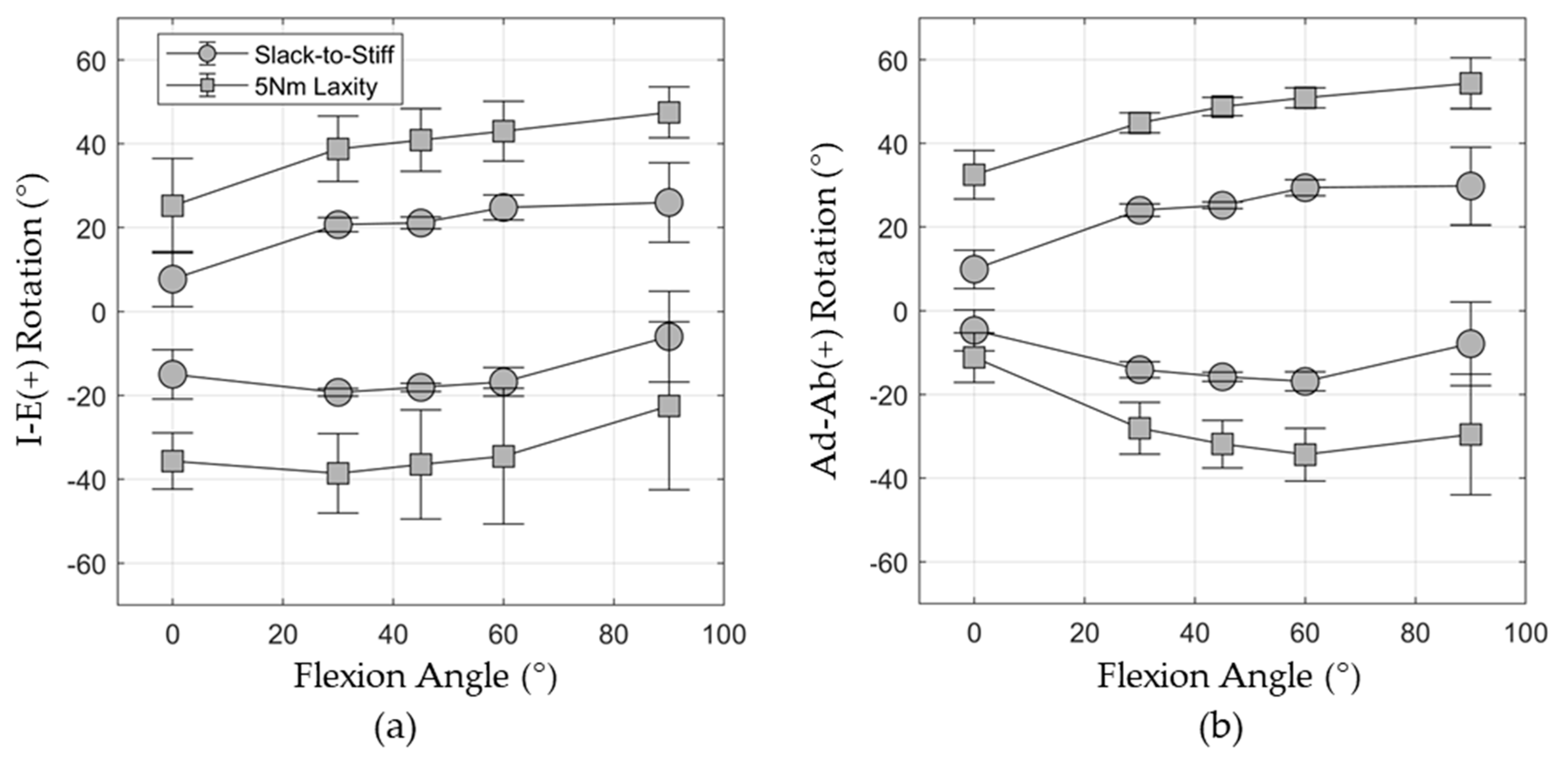
2.6. Capsule Predictions for In Vitro Hip Laxity
3. Results
3.1. Shape Function Model of Hip Capsule and Probabilistic Model Response
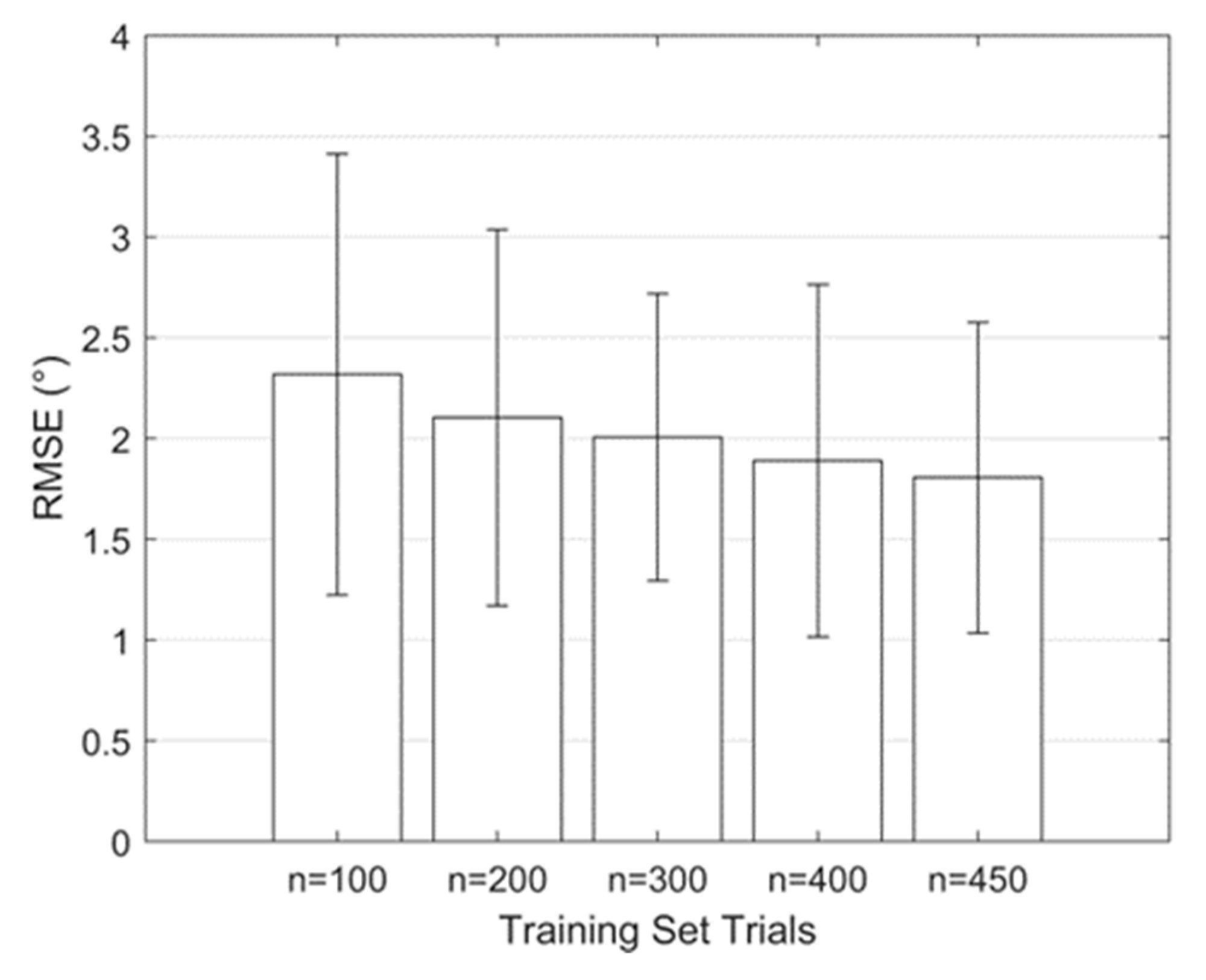
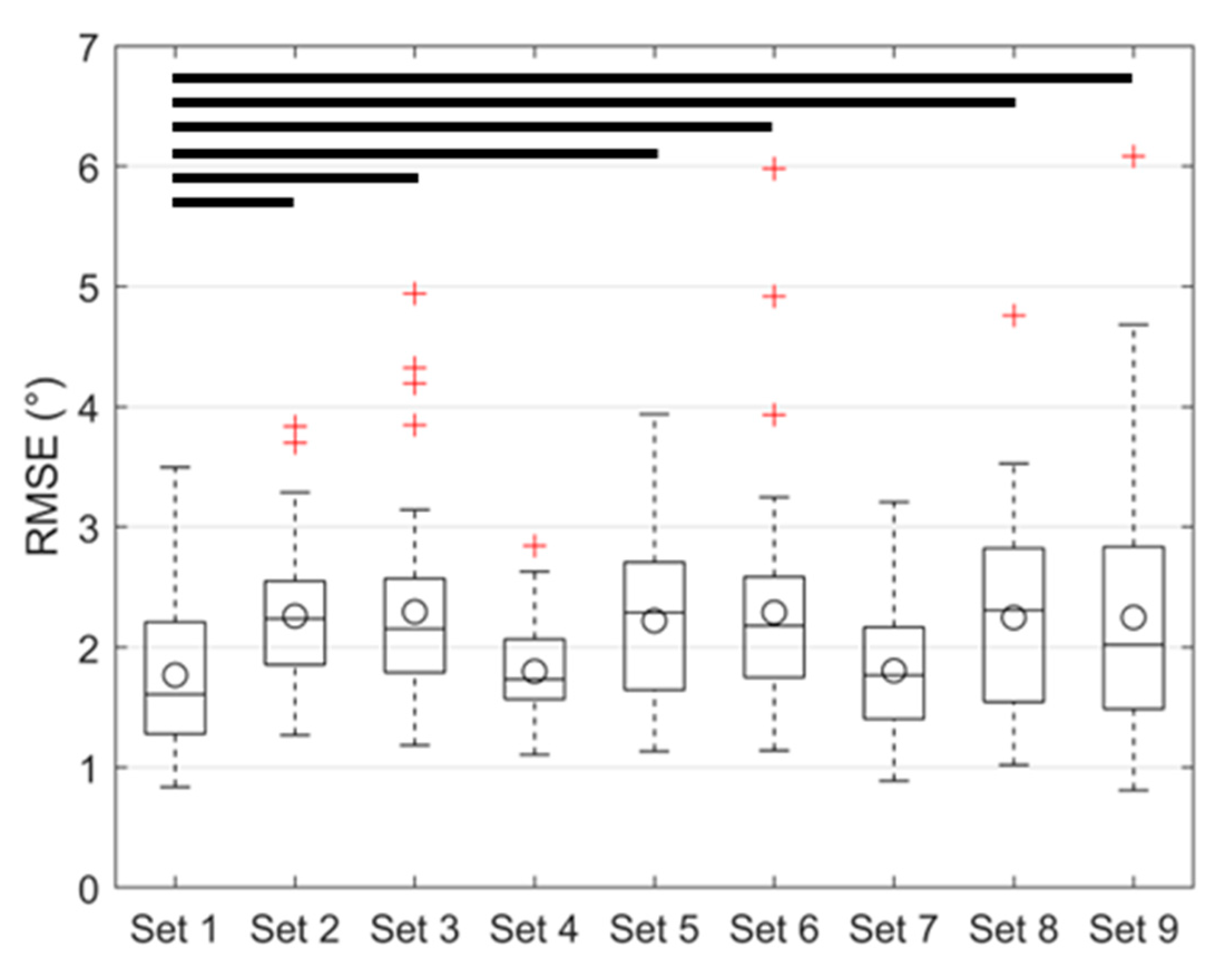
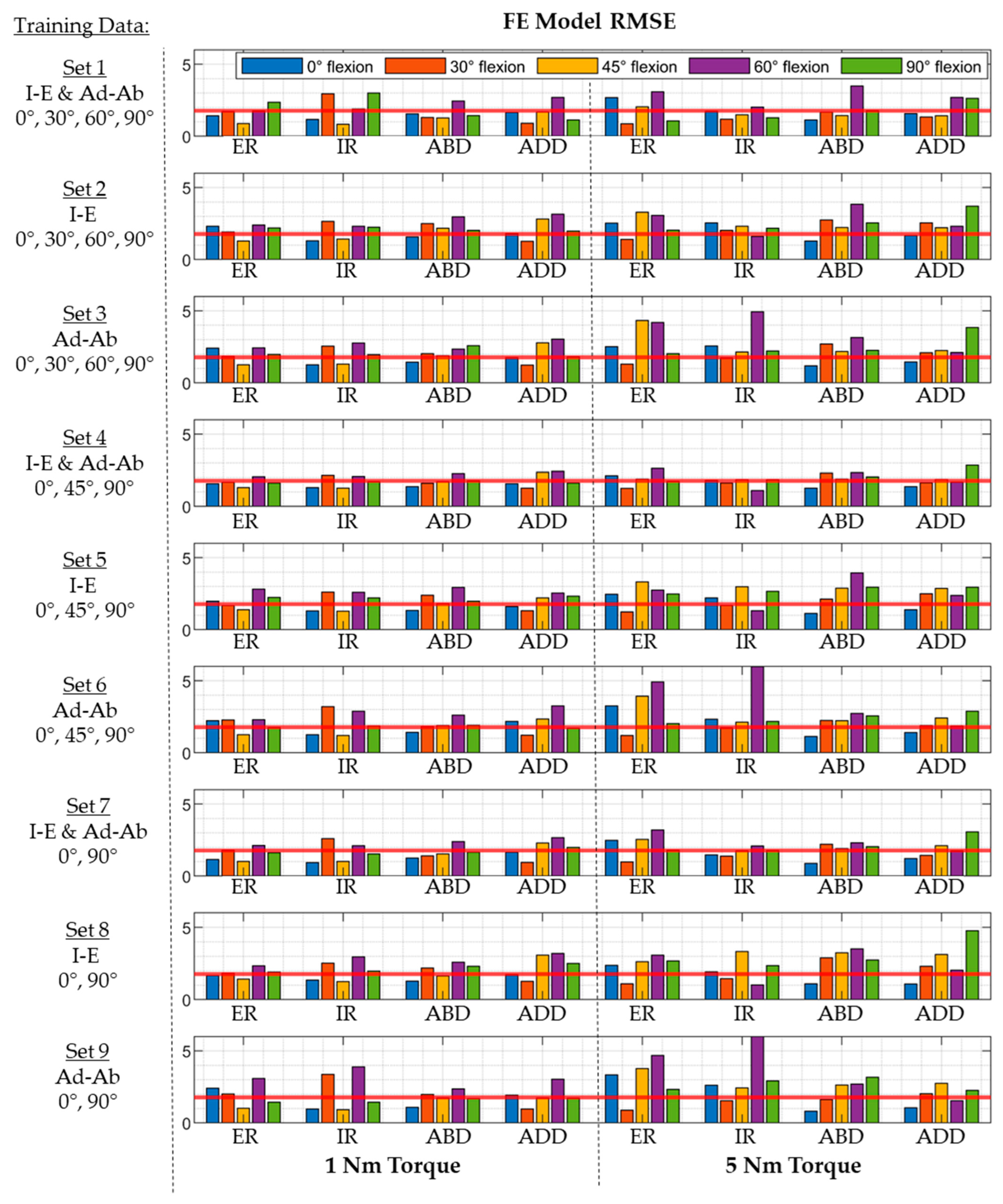
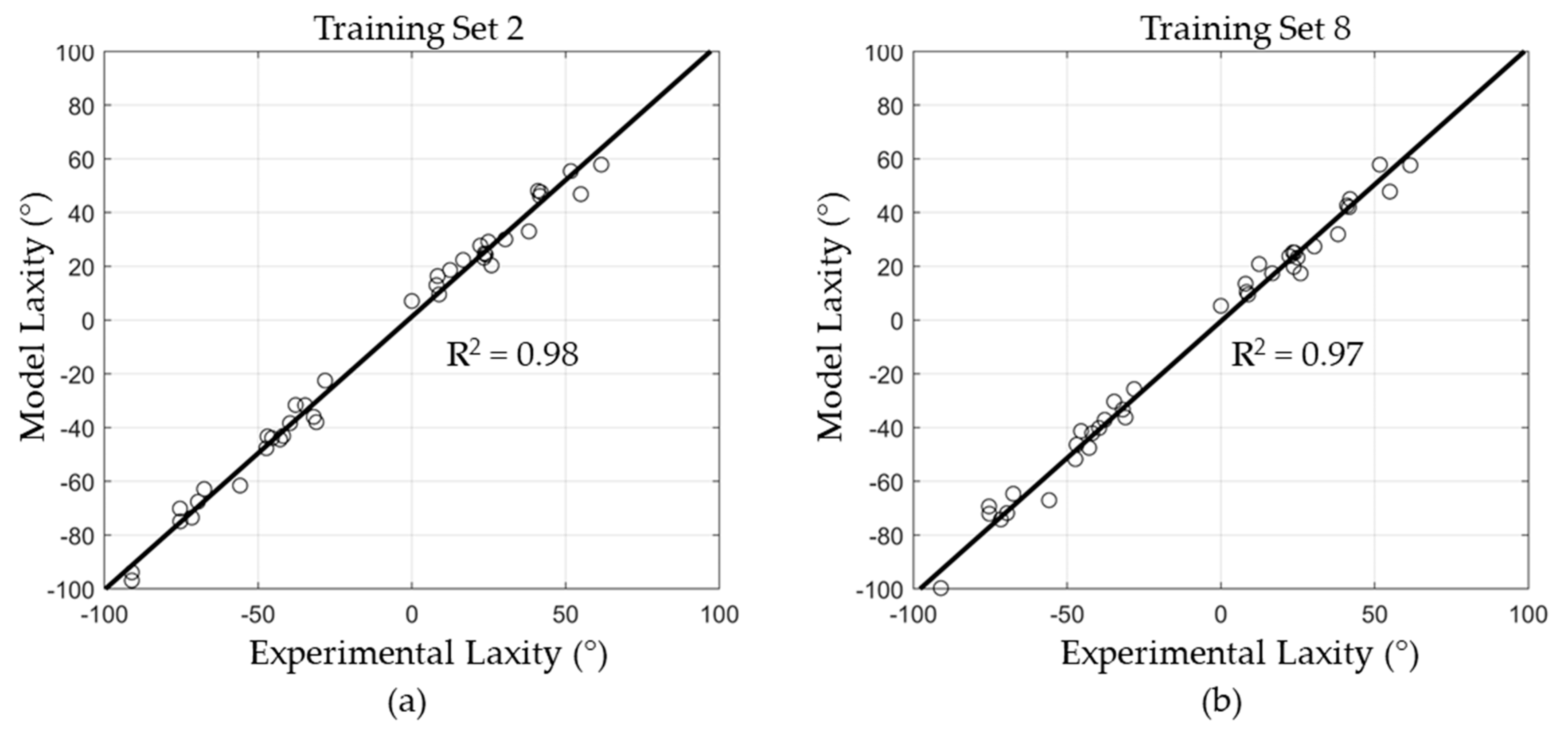
3.2. Regression Model Training, Validation, and Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colombi, A.; Schena, D.; Castelli, C.C. Total hip arthroplasty planning. EFORT Open Rev. 2019, 4, 626. [Google Scholar] [CrossRef]
- Subramanian, P.; Wainwright, T.W.; Bahadori, S.; Middleton, R.G. A review of the evolution of robotic-assisted total hip arthroplasty. Hip Int. 2019, 29, 232–238. [Google Scholar] [CrossRef]
- Ng, N.; Gaston, P.; Simpson, P.M.; Macpherson, G.J.; Patton, J.T.; Clement, N.D. Robotic arm-assisted versus manual total hip arthroplasty: A systematic review and meta-analysis. Bone Jt. J. 2021, 103, 1009–1020. [Google Scholar] [CrossRef]
- Emara, A.K.; Samuel, L.T.; Acuña, A.J.; Kuo, A.; Khlopas, A.; Kamath, A.F. Robotic-arm assisted versus manual total hip arthroplasty: Systematic review and meta-analysis of radiographic accuracy. Int. J. Med. Robot. Comput. Assist. Surg. 2021, 17, e2332. [Google Scholar] [CrossRef]
- Riddick, A.; Smith, A.; Thomas, D.P. Accuracy of preoperative templating in total hip arthroplasty. J. Orthop. Surg. 2014, 22, 173–176. [Google Scholar] [CrossRef]
- Jacofsky, D.J.; Allen, M. Robotics in arthroplasty: A comprehensive review. J. Arthroplast. 2016, 31, 2353–2363. [Google Scholar] [CrossRef]
- Fontalis, A.; Kayani, B.; Thompson, J.W.; Plastow, R.; Haddad, F.S. Robotic total hip arthroplasty: Past, present and future. Orthop. Trauma 2022, 36, 6–13. [Google Scholar] [CrossRef]
- Martin, H.D.; Savage, A.; Braly, B.A.; Palmer, I.J.; Beall, D.P.; Kelly, B. The function of the hip capsular ligaments: A quantitative report. Arthrosc. J. Arthrosc. Relat. Surg. 2008, 24, 188–195. [Google Scholar] [CrossRef]
- Ng, K.G.; Jeffers, J.R.; Beaulé, P.E. Hip joint capsular anatomy, mechanics, and surgical management. J. Bone Jt. Surg. Am. Vol. 2019, 101, 2141. [Google Scholar] [CrossRef]
- van Arkel, R.J.; Ng, K.G.; Muirhead-Allwood, S.K.; Jeffers, J.R. Capsular ligament function after total hip arthroplasty. The Journal of Bone and Joint surgery. Am. Vol. 2018, 100, e94. [Google Scholar]
- Logishetty, K.; Van Arkel, R.J.; Ng, K.C.G.; Muirhead-Allwood, S.K.; Cobb, J.P.; Jeffers, J.R.T. Hip capsule biomechanics after arthroplasty: The effect of implant, approach, and surgical repair. Bone Jt. J. 2019, 101, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Maratt, J.D.; Gagnier, J.J.; Butler, P.D.; Hallstrom, B.R.; Urquhart, A.G.; Roberts, K.C. No difference in dis-location seen in anterior vs posterior approach total hip arthroplasty. J. Arthroplast. 2016, 31, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Mjaaland, K.E.; Svenningsen, S.; Fenstad, A.M.; Havelin, L.I.; Furnes, O.; Nordsletten, L. Implant survival after minimally invasive anterior or anterolateral vs. conventional posterior or direct lateral approach: An analysis of 21,860 total hip arthroplasties from the Norwegian Arthroplasty Register (2008 to 2013). J. Bone Jt. Surg. 2017, 99, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.A.; Hopper, R.H., Jr.; Ho, H.; McDonald III, J.F.; Parks, N.L.; Hamilton, W.G. Direct anterior approach for primary total hip arthroplasty lowers the risk of dislocation compared to the posterior approach: A single institution experience. J. Arthroplast. 2022, 37, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, W.P.; De Hartog, B.; Van Steenbergen, L.N.; Scheurs, B.W.; Nelissen, R.G. Effect of femoral head size and surgical approach on risk of revision for dislocation after total hip arthroplasty: An analysis of 166,231 procedures in the Dutch Arthroplasty Register (LROI). Acta Orthop. 2017, 88, 395–401. [Google Scholar] [CrossRef]
- Swanson, T.V.; Kukreja, M.M.; Ballard, J.C.; Calleja, H.G.; Brown, J.M. The “capsular noose”: A new capsular repair technique to diminish dislocation risk after the posterior approach total hip arthroplasty. Int. J. Surg. Open 2019, 17, 8–14. [Google Scholar] [CrossRef]
- Pedneault, C.; Tanzer, D.; Nooh, A.; Smith, K.; Tanzer, M. Capsular closure outweighs head size in preventing dislocation following revision total hip arthroplasty. HIP Int. 2020, 30, 141–146. [Google Scholar] [CrossRef]
- Vandeputte, F.J.; Vanbiervliet, J.; Sarac, C.; Driesen, R.; Corten, K. Capsular resection versus capsular repair in direct anterior approach for total hip arthroplasty: A randomized controlled trial. Bone Jt. J. 2021, 103, 321–328. [Google Scholar] [CrossRef]
- Schwartz, A.M.; Goel, R.K.; Sweeney, A.P.; Bradbury, T.L., Jr. Capsular management in direct anterior total hip arthroplasty: A randomized, single-blind, controlled trial. J. Arthroplast. 2021, 36, 2836–2842. [Google Scholar] [CrossRef]
- Elkins, J.M.; Stroud, N.J.; Rudert, M.J.; Tochigi, Y.; Pedersen, D.R.; Ellis, B.J.; Brown, T.D. The capsule’s contribution to total hip construct stability–a finite element analysis. J. Orthop. Res. 2011, 29, 1642–1648. [Google Scholar] [CrossRef]
- Myers, C.A.; Fitzpatrick, C.K.; Huff, D.N.; Laz, P.J.; Rullkoetter, P.J. Development and calibration of a probabilistic finite element hip capsule representation. Comput. Methods Biomech. Biomed. Eng. 2020, 23, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Anantha Krishnan, A.; Myers, C.A.; Scinto, M.; Marshall, B.N.; Clary, C.W. Specimen-specific finite element representations of implanted hip capsules. Comput. Methods Biomech. Biomed. Eng. 2023, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ezquerro, F.; Vacas, F.G.; Postigo, S.; Prado, M.; Simón, A. Calibration of the finite element model of a lumbar functional spinal unit using an optimization technique based on differential evolution. Med. Eng. Phys. 2011, 33, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Ewing, J.A.; Kaufman, M.K.; Hutter, E.E.; Granger, J.F.; Beal, M.D.; Piazza, S.J.; Siston, R.A. Estimating subject-specific soft-tissue properties in a TKA knee. J. Orthop. Res. 2016, 34, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, J.E.; Dai, Y.; Goodlett, C.; Davis, B.; Bandi, M. Incorporating population-level variability in orthopedic biomechanical analysis: A review. J. Biomech. Eng. 2014, 136, 021004. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Kulkarni, K.; Xie, S.; Bull, A.M.; Jones, G.G. The accuracy of statistical shape models in predicting bone shape: A systematic review. Int. J. Med. Robot. Comput. Assist. Surg. 2023, 19, e2503. [Google Scholar] [CrossRef] [PubMed]
- Sarkalkan, N.; Weinans, H.; Zadpoor, A.A. Statistical shape and appearance models of bones. Bone 2014, 60, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Smoger, L.M.; Fitzpatrick, C.K.; Clary, C.W.; Cyr, A.J.; Maletsky, L.P.; Rullkoetter, P.J.; Laz, P.J. Statistical modeling to characterize relationships between knee anatomy and kinematics. J. Orthop. Res. 2015, 33, 1620–1630. [Google Scholar] [CrossRef]
- Gibbons, K.D.; Clary, C.W.; Rullkoetter, P.J.; Fitzpatrick, C.K. Development of a statistical shape-function model of the implanted knee for real-time prediction of joint mechanics. J. Biomech. 2019, 88, 55–63. [Google Scholar] [CrossRef]
- Shalhoub, S.; Cyr, A.; Maletsky, L.P. Correlation between knee anatomy and joint laxity using principal component analysis. J. Orthop. Res. 2022, 40, 2502–2509. [Google Scholar] [CrossRef]
- Baldwin, M.A.; Clary, C.W.; Fitzpatrick, C.K.; Deacy, J.S.; Maletsky, L.P.; Rullkoetter, P.J. Dynamic finite element knee simulation for evaluation of knee replacement mechanics. J. Biomech. 2012, 45, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, C.; Baldwin, M.; Rullkoetter, P. Computationally Efficient Finite Element Evaluation of Natural Patellofemoral Mechanics. J. Biomech. Eng. 2010, 132, 121013. [Google Scholar] [CrossRef] [PubMed]
- Brockett, C.; Williams, S.; Jin, Z.; Isaac, G.; Fisher, J. Friction of total hip replacements with different bearings and loading conditions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 81, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Guezmil, M.; Bensalah, W.; Mezlini, S. Tribological behavior of UHMWPE against TiAl6V4 and CoCr28Mo alloys under dry and lubricated conditions. J. Mech. Behav. Biomed. Mater. 2016, 63, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Grood, E.S.; Suntay, W.J. A joint coordinate system for the clinical description of three-dimensional motions: Application to the knee. J. Biomech. Eng. 1983, 105, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Telleria, J.J.; Lindsey, D.P.; Giori, N.J.; Safran, M.R. A quantitative assessment of the insertional footprints of the hip joint capsular ligaments and their spanning fibers for reconstruction. Clin. Anat. 2014, 27, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, M.; Nimura, A.; Honda, E.; Utsunomiya, H.; Uchida, S.; Akita, K. An anatomical study of the ante-rosuperior capsular attachment site on the acetabulum. J. Bone Jt. Surgery. Am. Vol. 2019, 101, 1554. [Google Scholar] [CrossRef]
- Wingstrand, H.; Wingstrand, A. Biomechanics of the hip joint capsule—A mathematical model and clinical implications. Clin. Biomech. (Bristol Avon) 1997, 12, 273–280. [Google Scholar] [CrossRef]
- Zhang, J.; Malcolm, D.; Hislop-Jambrich, J.; Thomas, C.D.L.; Nielsen, P.M. An anatomical region-based sta-tistical shape model of the human femur. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2014, 2, 176–185. [Google Scholar] [CrossRef]
- van Arkel, R.J.; Amis, A.A.; Jeffers, J.R. The envelope of passive motion allowed by the capsular ligaments of the hip. J. Biomech. 2015, 48, 3803–3809. [Google Scholar] [CrossRef]
- Karunaseelan, K.J.; Dandridge, O.; Muirhead-Allwood, S.K.; van Arkel, R.J.; Jeffers, J.R. Capsular ligaments provide a passive stabilizing force to protect the hip against edge loading. Bone Jt. Res. 2021, 10, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhu, X.; Zeng, Y.; Zhang, H.; Zeng, J.; Feng, W.; Zeng, Y. The effect of posterior capsule repair in total hip arthroplasty: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2020, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.S.; Jimenez, A.E.; Shapira, J.; Saks, B.R.; Glein, R.M.; Maldonado, D.R.; Domb, B.G. Capsular repair may improve outcomes in subjects undergoing hip arthroscopy for femoroacetabular impingement: A systematic review of comparative outcome studies. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 37, 2975–2990. [Google Scholar] [CrossRef] [PubMed]
- Batailler, C.; Fary, C.; Verdier, R.; Aslanian, T.; Caton, J.; Lustig, S. The evolution of outcomes and indications for the dual-mobility cup: A systematic review. Int. Orthop. 2017, 41, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Grace, T.R.; Goh, G.S.; Lee, G.C.; Kamath, A.F.; Kurtz, S.M.; Courtney, P.M. Dual Mobility Reduces Dislocations—Why I Use It in All Revisions. J. Arthroplast. 2021, 36, S63–S69. [Google Scholar] [CrossRef] [PubMed]
- Zajc, J.; Fokter, S.K. Bimodular femoral stems in primary total hip arthroplasty. Expert Rev. Med. Devices 2023, 20, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Vajapey, S.P.; Fideler, K.L.; Lynch, D.; Li, M. Use of dual mobility components in total hip arthroplasty: Indications and outcomes. J. Clin. Orthop. Trauma 2020, 11, S760–S765. [Google Scholar] [CrossRef]
- Wyles, C.C.; Maradit-Kremers, H.; Larson, D.R.; Lewallen, D.G.; Taunton, M.J.; Trousdale, R.T.; Sierra, R.J. Creation of a total hip arthroplasty subject-specific dislocation risk calculator. J. Bone Jt. Surg. 2022, 104, 1068–1080. [Google Scholar] [CrossRef]
- Myers, C.A.; Huff, D.N.; Mason, J.B.; Rullkoetter, P.J. Effect of intraoperative treatment options on hip joint stability following total hip arthroplasty. J. Orthop. Res. Off. Publ. Orthop. Re-Search Soc. 2022, 40, 604–613. [Google Scholar] [CrossRef]
- Fitzpatrick, C.K.; Baldwin, M.A.; Rullkoetter, P.J.; Laz, P.J. Combined probabilistic and principal component analysis approach for multivariate sensitivity evaluation and application to implanted patellofemoral mechanics. J. Biomech. 2011, 44, 13–21. [Google Scholar] [CrossRef]
- Bah, M.T.; Nair, P.B.; Taylor, M.; Browne, M. Efficient computational method for assessing the effects of implant positioning in cementless total hip replacements. J. Biomech. 2011, 44, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Perilli, E.; Martelli, S. Development of a surrogate model based on subject weight, bone mass and geometry to predict femoral neck strains and fracture loads. J. Biomech. 2017, 55, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, F.E.; Nyman, E., Jr.; Coburn, J.C. Prediction of contact mechanics in metal-on-metal Total Hip Re-placement for parametrically comprehensive designs and loads. J. Biomech. 2015, 48, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, C.K.; Hemelaar, P.; Taylor, M. Computationally efficient prediction of bone–implant interface micromotion of a cementless tibial tray during gait. J. Biomech. 2014, 47, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Ziaeipoor, H.; Martelli, S.; Pandy, M.; Taylor, M. Efficacy and efficiency of multivariate linear regression for rapid prediction of femoral strain fields during activity. Med. Eng. Phys. 2019, 63, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Bartsoen, L.; Faes, M.G.; Andersen, M.S.; Wirix-Speetjens, R.; Moens, D.; Jonkers, I.; Vander Sloten, J. Bayesian parameter estimation of ligament properties based on tibio-femoral kinematics during squatting. Mech. Syst. Signal Process. 2023, 182, 109525. [Google Scholar] [CrossRef]
- Bartsoen, L.; Faes, M.G.R.; Wirix-Speetjens, R.; Moens, D.; Jonkers, I.; Sloten, J.V. Probabilistic planning for ligament-balanced TKA-Identification of critical ligament properties. Front. Bioeng. Biotechnol. 2022, 10, 930724. [Google Scholar] [CrossRef] [PubMed]
- Brynskog, E.; Iraeus, J.; Reed, M.P.; Davidsson, J. Predicting pelvis geometry using a morphometric model with overall anthropometric variables. J. Biomech. 2021, 126, 110633. [Google Scholar] [CrossRef]
- van Veldhuizen, W.A.; van der Wel, H.; Kuipers, H.Y.; Kraeima, J.; Ten Duis, K.; Wolterink, J.M.; IJpma, F.F. Development of a Statistical Shape Model and Assessment of Anatomical Shape Variations in the Hemipelvis. J. Clin. Med. 2023, 12, 3767. [Google Scholar] [CrossRef]
- van Arkel, R.J.; Amis, A.A.; Cobb, J.P.; Jeffers, J.R.T. The capsular ligaments provide more hip rotational re-straint than the acetabular labrum and the ligamentum teres: An experimental study. Bone Jt. J. 2015, 97, 484–491. [Google Scholar] [CrossRef]
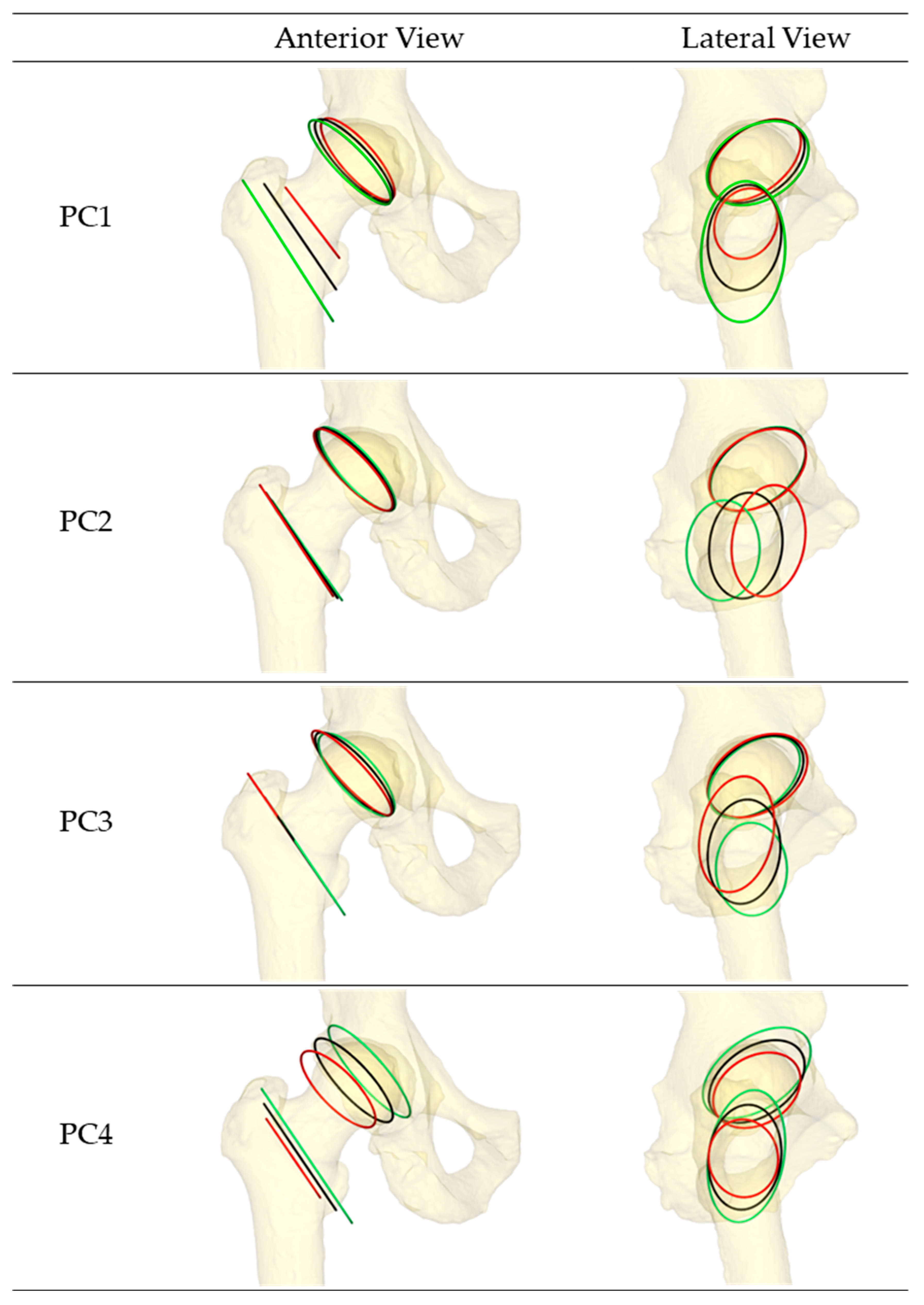
| PC (Variance Explained) | Acetabulum (Origin) | Femur (Insertion) | |||
|---|---|---|---|---|---|
| Inclination (°) | Version (°) | Inclination (°) | Version (°) | ||
| Mean | 137.9 | 18.5 | 124.1 | 0.0 | |
| PC1 (36.8%) | +2 SD | 138.5 | 20.7 | 127.2 | 0.0 |
| −2 SD | 137.5 | 16.6 | 122.5 | 0.0 | |
| PC2 (18.0%) | +2 SD | 138.0 | 17.7 | 123.3 | 0.0 |
| −2 SD | 137.8 | 19.4 | 125.0 | 0.0 | |
| PC3 (13.0%) | +2 SD | 136.6 | 14.4 | 124.4 | 0.0 |
| −2 SD | 138.9 | 22.7 | 123.9 | 0.0 | |
| PC4 (9.1%) | +2 SD | 137.5 | 21.7 | 124.4 | 0.0 |
| −2 SD | 137.4 | 15.6 | 124.0 | 0.0 | |
| Parameter ID | Parameter | Mean ± Standard Deviation |
|---|---|---|
| 1 | Sector 1—Stiffness (N/mm) | 61.0 ± 15.0 |
| 2 | Sector 2—Stiffness (N/mm) | 62.3 ± 15.0 |
| 3 | Sector 3—Stiffness (N/mm) | 58.5 ± 15.0 |
| 4 | Sector 4—Stiffness (N/mm) | 53.9 ± 15.0 |
| 5 | Sector 5—Stiffness (N/mm) | 70.5 ± 15.0 |
| 6 | Sector 6—Stiffness (N/mm) | 50.4 ± 15.0 |
| 7 | Sector 1—Pre-strain | 0.99 ± 0.05 |
| 8 | Sector 2—Pre-strain | 0.71 ± 0.05 |
| 9 | Sector 3—Pre-strain | 0.62 ± 0.05 |
| 10 | Sector 4—Pre-strain | 0.57 ± 0.05 |
| 11 | Sector 5—Pre-strain | 0.48 ± 0.05 |
| 12 | Sector 6—Pre-strain | 0.57 ± 0.05 |
| 13 | Attachment Region PC1 | ±2.0 |
| 14 | Attachment Region PC2 | ±2.0 |
| 15 | Attachment Region PC3 | ±2.0 |
| 16 | Attachment Region PC4 | ±2.0 |
| Training Set | Laxity the Training Set | Hip Flexion (Deg) | # Input Parameters (N) |
|---|---|---|---|
| Set 1 | I-E laxity and Ad-Ab laxity | 0, 30, 60, 90 | 32 |
| Set 2 | I-E laxity | 0, 30, 60, 90 | 16 |
| Set 3 | Ad-Ab laxity | 0, 30, 60, 90 | 16 |
| Set 4 | I-E Laxity and Ad-Ab laxity | 0, 45, 90 | 24 |
| Set 5 | I-E laxity | 0, 45, 90 | 12 |
| Set 6 | Ad-Ab laxity | 0, 45, 90 | 12 |
| Set 7 | I-E laxity and Ad-Ab laxity | 0, 90 | 16 |
| Set 8 | I-E laxity | 0, 90 | 8 |
| Set 9 | Ad-Ab laxity | 0, 90 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anantha-Krishnan, A.; Myers, C.A.; Fitzpatrick, C.K.; Clary, C.W. Instantaneous Generation of Subject-Specific Finite Element Models of the Hip Capsule. Bioengineering 2024, 11, 37. https://doi.org/10.3390/bioengineering11010037
Anantha-Krishnan A, Myers CA, Fitzpatrick CK, Clary CW. Instantaneous Generation of Subject-Specific Finite Element Models of the Hip Capsule. Bioengineering. 2024; 11(1):37. https://doi.org/10.3390/bioengineering11010037
Chicago/Turabian StyleAnantha-Krishnan, Ahilan, Casey A. Myers, Clare K. Fitzpatrick, and Chadd W. Clary. 2024. "Instantaneous Generation of Subject-Specific Finite Element Models of the Hip Capsule" Bioengineering 11, no. 1: 37. https://doi.org/10.3390/bioengineering11010037
APA StyleAnantha-Krishnan, A., Myers, C. A., Fitzpatrick, C. K., & Clary, C. W. (2024). Instantaneous Generation of Subject-Specific Finite Element Models of the Hip Capsule. Bioengineering, 11(1), 37. https://doi.org/10.3390/bioengineering11010037








