Salivary Gland Bioengineering
Abstract
1. Introduction
2. Learning from Salivary Gland Biology and Pathology
2.1. Salivary Gland Development and Function
2.1.1. Salivary Glands and Their Functional Units
2.1.2. Coordination of Saliva Secretion
2.1.3. Salivary Gland Development
Branching Morphogenesis Creates the Adult Gland Structure
Epithelium, Endothelium, and Neural Crest-Derived Cells Regulate Early Salivary Gland Development
2.2. Fibrosis, Cellular Senescence, and Salivary Gland Pathology
2.2.1. SASP Signaling Drives Neighboring Proliferation-Competent Cells to Senescence (Bystander Effect)
2.2.2. Senescence and Its Impact on Normal Fibroblast Dynamics in Healing and Fibrosis
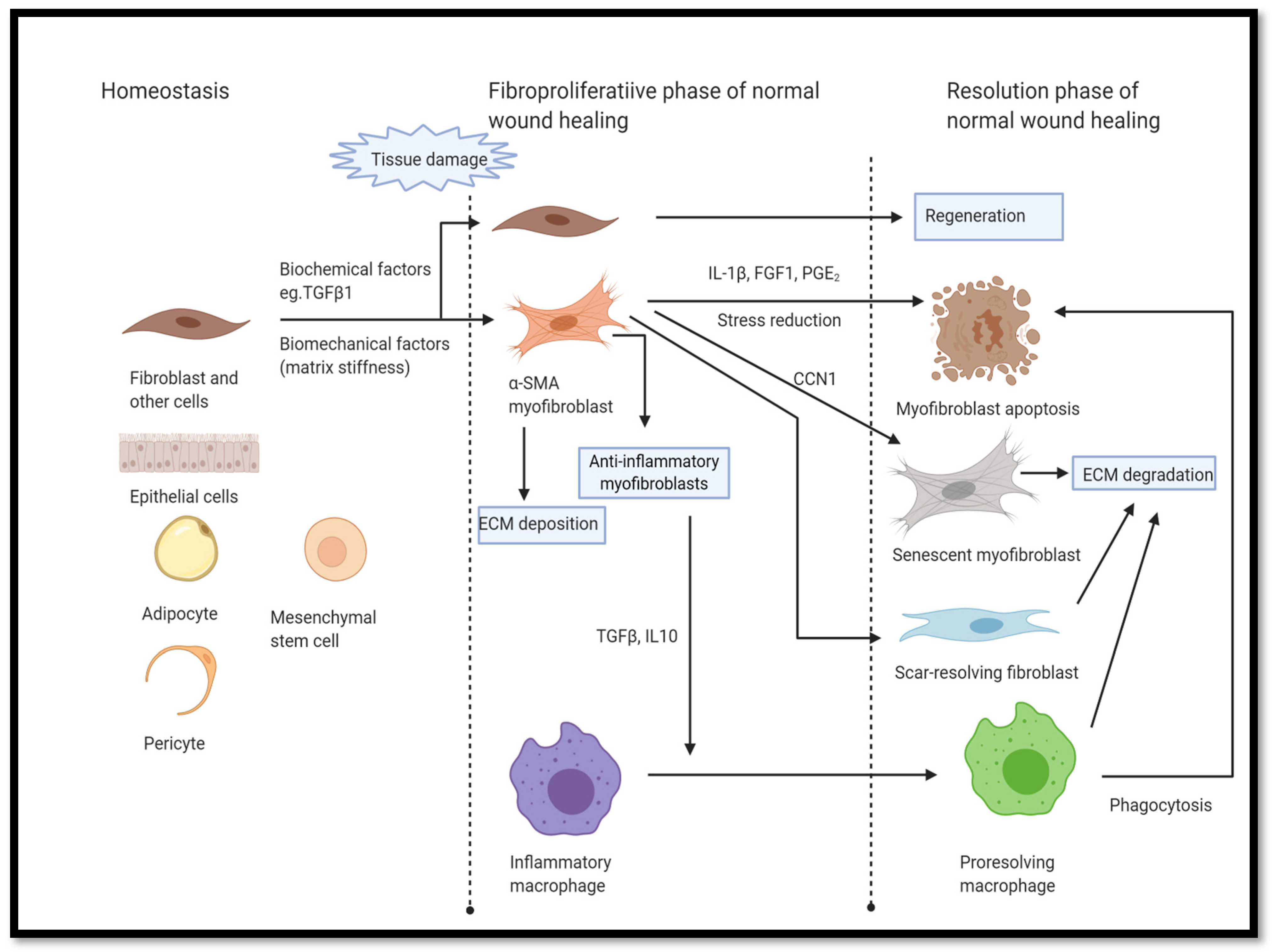
2.2.3. Senolytics and SASP Depressants Support Healing, Reduce Fibrosis, and Improve Transplant Engraftment
3. Cell Selection for Salivary Gland Bioengineering
3.1. Salivary Gland Cell Lines
| Species | Cell Lines | Tissue Sources | Cell Types | Characteristics |
|---|---|---|---|---|
| Human | HSY | Parotid gland adenocarcinoma | Ductal epithelial cells | Form desmosomes and tight junctions (TJs) and exhibit polarization [126,135]; express amylase [136]; respond to muscarinic and β-adrenergic autonomic agonists [137]. |
| HSG | Irradiated SMG | Intercalated duct epithelial cells | Form desmosomes with sporadic TJ and no AQP expression on plastics [138]; differentiate into acinar structures and express amylase on Matrigel; respond to muscarinic and purinergic agonists; express ductal differentiation markers (EGF, NGF, and renin) [125]; express TJs (claudin-1, -2, -3, -4,ccludingn, JAM-A, and ZO-1) and AQP (AQP5) on Matrigel-coated permeable supports [139]. Report to be contaminated with Hela cells [128]. | |
| Mouse | SIMS | A 22-day-old transgenic SMG | Immortalized ductal epithelial cells | Exhibit polarity and express E-cadherin and ZO-1 and duct-specific cytokeratins on Matrigel-coated surfaces; form duct-like structures (cysts) on collagen Type I gels (Col I); When grown on a filter support SIMS cells form a tight monolayer, exhibit vectorial transport function and show exclusive Na+, K(+)-ATPase localization to the basolateral domain [140]; express EGF, NGF, and renin [129]. |
| SIMP | A 12-day-old PyLT transgenic SMG | Immortalized striated ductal epithelial cells | Exhibit polarity and express E-cadherin and ZO-1 and duct-specific cytokeratins on Matrigel-coated surfaces; form duct-like structures on Col I; express duct-specific cytokeratins and differentiation markers (EGF, NGF, and renin) [140] | |
| mSG-DUC1 | SMG | Genetically modified mice, homozygous for floxed alleles of the integrin α3 subunit | mSG-DUC1 cells express the ductal markers, keratin-7 and keratin-19, and form lumenized spheroids [130]. | |
| mSG-PAC1 | SMG | Genetically modified mice, homozygous for floxed alleles of the integrin α3 subunit | Express the ductal markers, keratin-7 and keratin-19, and form lumenized spheroids; express the pro-acinar markers SOX10 and aquaporin-5 [130]. | |
| Rat | SMIE | SMG | Immortalized salivary glandular epithelium-like cells | Form TJs on collagen-coated filters [131]; resemble salivary glandular epithelium with an immature lumen; express ZO-1 and E-cadherin, but low level claudin-3 [141]; have a low level transepithelial resistance (TER) that can be regulated by IGF-1 [142] |
| RSMT-A5 | SMG | Transformed ductal cells | Exhibit a ductal epithelial phenotype and a high density of α1-adrenergic receptors [143]. | |
| SMG-C6 | SMG | Immortalized submandibular acinar epithelial cells | Form TJs and desmosomes, enabling polarization [133]; exhibit secretory features (i.e., domes, granules, and canaliculi) and more cytodifferentiation than SMG-C10 [144]; respond to muscarinic and purinergic agonists (but not to α1 agonists) by increasing [Ca2+]i and respond to β-adrenergic agonists by increasing [cAMP]; lack ductal marker cytokeratin 19 expression and exhibit high TER on collagen-coated polycarbonate filters [145]. | |
| SMG-C10 | SMG | Immortalized submandibular acinar epithelial cells | Form TJs and desmosomes, enabling polarization; respond to β-adrenergic agonists; exhibit high TER on collagen-coated polycarbonate filters; modulate Na+ transport and regulate salivary cell volume [125,133,145,146,147]. | |
| Par-C5 | Rat parotid glands | Immortalized acinar epithelial cells | Form layers of plump cells containing intercellular lumen-like invaginations on their medial surfaces; form secretory granules, TJs, intermediate junctions, desmosomes, and microvilli; respond to α1-adrenergic agonists by increasing [cAMP] [133]; respond to cholinergic, muscarinic, and α1-adrenergic agonists by increasing [Ca2+]i [148,149]; express functional amylase [134]. | |
| Par-C10 | Immortalized acinar epithelial cells | Form monolayers of cuboidal cells with thick ECM at their bases; form secretory granules, TJs, intermediate junctions, desmosomes, and microvilli [133]; respond to α1-adrenergic agonists by increasing [cAMP] [148]; respond to cholinergic, muscarinic, and α1-adrenergic agonists by increasing [Ca2+] [133,148,149]; do not express amylase [134]; exhibit high TER [150,151,152]; express sodium bicarbonate cotransporters and anion exchange proteins on basolateral surfaces, which regulate transepithelial transport. Par-C10 cells achieve transepithelial transport that is sensitive to both intracellular Ca(2+)- and cAMP-dependent stimulation [151]; form 3D differentiated acinar-like spheres on growth-factor-reduced Matrigel, expressing TJs, ion transporters, M3 muscarinic receptors, and AQP3, increasing AQP5 expression under osmotic stress and showing changes in potential difference in response to muscarinic agonist stimulation [152]. |
3.2. Primary Salivary Gland Cells
3.3. Progenitor Cells of the Developing Salivary Gland
3.3.1. Salivary Gland Stem and Progenitor Cells during Development
3.3.2. Stromal-Epithelial Interactions during Development
3.4. Stem Cells for Salivary Gland Tissue Engineering
3.4.1. Salivary Gland Stem Cells (SGSCs)
| Stem Cell Marker | Salivary Gland Location | Method of Identification | Progenitor or Stem | Reference Number |
|---|---|---|---|---|
| c-KIT (CD117) | Ducts | Gene expression | Stem | [177] |
| SCA-1 | Ducts | Gene expression | Stem | [177] |
| Keratin-5 (K5) | Ducts (developing) | Cytoskeletal protein expression, in vivo lineage tracing | Progenitor | [41,178,179] |
| Keratin-14 (K14) | Ducts (developing) | Cytoskeletal protein expression, in vivo lineage tracing | Progenitor | [179] |
| LGR5 | Ducts (human parotid and submandibular) | Gene expression | Stem | [67] |
| CD44 | Not specified | MSC surface antigen | Stem | [67,180,181,182,183] |
| CD49f (integrin) | Not specified | MSC surface antigen | Stem | [67,180,181,182,183] |
| CD90 | Not specified | MSC surface antigen | Stem | [67,180,181,182,183] |
| CD105 | Not specified | MSC surface antigen | Stem | [67,180,181,182,183] |
3.4.2. Mesenchymal Stem Cells (MSCs)
3.4.3. Pluripotent Stem Cells (PSCs): ESCs and iPSCs
4. Biomaterials for Salivary Gland Cell Survival, Differentiation, and Engraftment
4.1. Cell Support System Overview
4.2. Scaffold Fabrication Approaches to Salivary Gland Tissue Engineering
4.2.1. Electrospinning to Synthesize Fibrous Matrices
4.2.2. Phase Separation to Produce Composite Scaffolds
4.2.3. Freeze-Drying to Fabricate Porous Scaffolds
4.2.4. Hydrogel Synthesis to Form Injectable Cell Delivery Vehicles
4.2.5. Self-Assembly to Generate Cellular Clusters and Organoids
5. Potential Engineering Strategies to Improve Salivary Gland Tissue Vascularization, Innervation, and Engraftment
5.1. Prospects for Engineering Vascularized Salivary Gland Tissue
5.2. Prospects for Engineering Innervated Salivary Gland Tissue
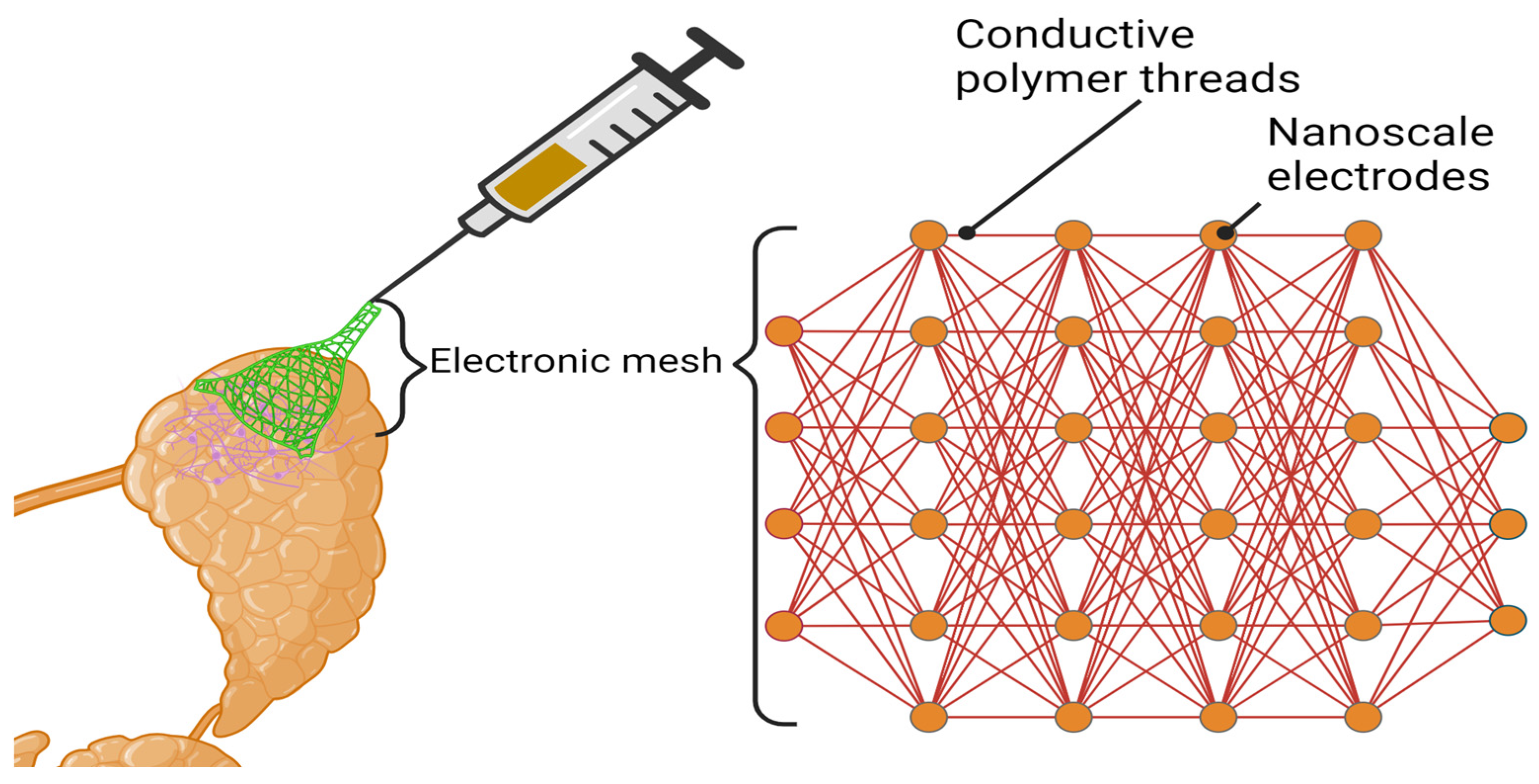
5.2.1. Mesh Electronics and Bio-Hybrid Systems
5.2.2. Biocompatible, Endocytosed Nanotubes in Salivary Gland Tissue
5.2.3. Bioprinting of Neurons and Innervation of Tissues
5.2.4. Nanoparticles to Offer Essential Spatiotemporal Control over Scaffold Development and Engraftment
5.2.5. Injectable Engineered Salivary Gland Transplants
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sasportas, M.; Hosford, A.; Sodini, M.; Waters, J.; Zambricki, A.; Barral, J.; Graves, E.; Brinton, T.; Yock, P.; Quyhh-Thu, L.; et al. Cost-Effectiveness Landscape Analysis of Treatments Addressing Xerostomia in Patients Receiving Head and Neck Radiation Therapy. Oral Surg. Oral Med. Oral Pathol. Oral Raiol. 2013, 116, e37–e51. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.; Ship, J. Dry Mouth and Its Effects on the Oral Health of Elderly People. J. Am. Dent. Assoc. 2007, 138, S15–S20. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.; Pedersen, A.M.L.; Vissink, A.; Andersen, E.; Brown, C.G.; Davies, A.N.; Dutilh, J.; Fulton, J.S.; Jankovic, L.; Lopes, N.N.F.; et al. A Systematic Review of Salivary Gland Hypofunction and Xerostomia Induced by Cancer Therapies: Prevalence, Severity and Impact on Quality of Life. Support. Care Cancer 2010, 18, 1039–1060. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.W.; Wild, C.P. World Cancer Report 2014; World Health Organization: Geneva, Switzerland, 2014.
- Polaris Market Research. Xerostomia Therapeutics Market Share, Size, Trends, Industry Analysis Report, By Type (OTC, Prescription), By Product, By Region, And Segment Forecasts, 2023–2032, Report Summary. Available online: https://www.polarismarketresearch.com/industry-analysis/xerostomia-therapeutics-market (accessed on 25 August 2023).
- Yoo, C.; Vines, J.B.; Alexander, G.; Murdock, K.; Hwang, P.; Jun, H.W. Adult Stem Cells and Tissue Engineering Strategies for Salivary Gland Regeneration: A Review. Biomater. Res. 2014, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Konkel, J.E.; O’Boyle, C.; Krishnan, S. Distal Consequences of Oral Inflammation. Front. Immunol. 2019, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Weng, P.; Aure, M.; Maruyama, T.; Ovitt, C. Limited Regeneration of Adult Salivary Glands after Severe Injury Involves Cellular Plasticity. Cell Rep. 2018, 24, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Dodds, M.; Roland, S.; Edgar, M.; Thornhill, M. Saliva A Review of Its Role in Maintaining Oral Health and Preventing Dental Disease. BDJ Team 2015, 2, 15123. [Google Scholar] [CrossRef]
- Rose, S. Primary Salivary Glands. Available online: https://app.biorender.com/illustrations/60149f828a786b606b577576 (accessed on 23 August 2023).
- Treuting, P.M.; Dintzis, S.M. Comparative Anatomy and Histology: A Mouse and Human Atlas. In Comparative Anatomy and Histology: A Mouse and Human Atlas; Academic Press: Cambridge, MA, USA, 2012; ISBN 9780123813619. [Google Scholar]
- Proctor, G.B.; Carpenter, G.H. Regulation of Salivary Gland Function by Autonomic Nerves. Auton. Neurosci. Basic Clin. 2007, 133, 3–18. [Google Scholar] [CrossRef]
- de Paula, F.; Teshima, T.H.N.; Hsieh, R.; Souza, M.M.; Nico, M.M.S.; Lourenco, S.V. Overview of Human Salivary Glands: Highlights of Morphology and Developing Processes. Anat. Rec. 2017, 300, 1180–1188. [Google Scholar] [CrossRef]
- Carlson, B.M. The Human Body: Linking Structure and Function; Academic Press: Cambridge, MA, USA, 2018; pp. 1–417. [Google Scholar] [CrossRef]
- Munger, B.L. Histochemical Studies on Seromucous- and Mucoussecreting Cells of Human Salivary Glands. Am. J. Anat. 1964, 115, 411–429. [Google Scholar] [CrossRef]
- Shah, A.; Mulla, A.; Mayank, M. Pathophysiology of Myoepithelial Cells in Salivary Glands. J. Oral Maxillofac. Pathol. 2016, 20, 480. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, T.; Fowler, E.W.; Hao, Y.; Ravikrishnan, A.; Harrington, D.A.; Witt, R.L.; Farach-Carson, M.C.; Pradhan-Bhatt, S.; Jia, X. Biomaterials-Based Strategies for Salivary Gland Tissue Regeneration. Biomater. Sci. 2016, 4, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.S.; Lin, Y.C.; Young, T.H.; Lou, P.J. Effects of Fibroblasts on the Function of Acinar Cells from the Same Human Parotid Gland. Head Neck 2016, 38, E279–E286. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.; Wei, C.; Yamada, K.M. Cell and Fibronectin Dynamics during Branching Morphogenesis. J. Cell Sci. 2006, 119, 3376–3384. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.N.; Likar, K.M.; Zisman-rozen, S.; Cowherd, S.N.; Lassiter, K.S.; Sher, I.; Yates, E.A.; Turnbull, J.E.; Ron, D.; Hoffman, M.P. Specific Heparan Sulfate Structures Modulate FGF10-Mediated Submandibular Gland Epithelial Morphogenesis. J. Biol. Chem. 2008, 283, 9308–9317. [Google Scholar] [CrossRef] [PubMed]
- Jaskoll, T.; Abichaker, G.; Witcher, D.; Sala, F.G.; Bellusci, S.; Hajihosseini, M.K.; Melnick, M. FGF10/FGFR2b Signaling Plays Essential Roles during In Vivo Embryonic Submandibular Salivary Gland Morphogenesis. BMC Dev. Biol. 2005, 5, 11. [Google Scholar] [CrossRef]
- Mattingly, A.; Finley, J.K.; Knox, S.M. Salivary Gland Development and Disease. Wires Dev. Biol. 2015, 4, 573–590. [Google Scholar] [CrossRef]
- Retting, K.N.; Lyons, K.M. BMPs in Development. Handb. Cell Signal. 2010, 2, 1905–1912. [Google Scholar] [CrossRef]
- Jaskoll, T.; Leo, T.; Witcher, D.; Ormestad, M.; Astorga, J.; Bringas, P.; Carlsson, P.; Melnick, M. Sonic Hedgehog Signaling Plays an Essential Role during Embryonic Salivary Gland Epithelial Branching Morphogenesis. Dev. Dyn. 2004, 229, 722–732. [Google Scholar] [CrossRef]
- Sisto, M.; Ribatti, D.; Lisi, S. E-Cadherin Signaling in Salivary Gland Development and Autoimmunity. J. Clin. Med. 2022, 11, 2241. [Google Scholar] [CrossRef]
- Lourenço, S.V.; Kapas, S. Integrin Expression in Developing Human Salivary Glands. Histochem. Cell Biol. 2005, 124, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Chatzeli, L.; Gaete, M.; Tucker, A.S. Fgf10 and Sox9 Are Essential for the Establishment of Distal Progenitor Cells during Mouse Salivary Gland Development. Development 2017, 144, 2294–2305. [Google Scholar] [CrossRef] [PubMed]
- Schöck, F.; Perrimon, N. Molecular Mechanisms of Epithelial Morphogenesis. Annu. Rev. Cell Dev. Biol. 2003, 18, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Kriangkrai, R.; Iseki, S.; Eto, K.; Chareonvit, S. Dual Odontogenic Origins Develop at the Early Stage of Rat Maxillary Incisor Development. Anat. Embryol. 2006, 211, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.; Takamatsu, K.; Yukimori, A.; Kujiraoka, S.; Ishida, S.; Takakura, I.; Yasuhara, R.; Mishima, K. Sox9 Function in Salivary Gland Development. J. Oral Biosci. 2021, 63, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Rothova, M.; Thompson, H.; Lickert, H.; Tucker, A.S. Lineage Tracing of the Endoderm during Oral Development. Dev. Dyn. 2012, 241, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Suzawa, T.; Yamada, A.; Yamaguchi, T.; Mishima, K.; Osumi, N.; Maki, K.; Kamijo, R. Identification of Gene Expression Profile of Neural Crest-Derived Cells Isolated from Submandibular Glands of Adult Mice. Biochem. Biophys. Res. Commun. 2014, 446, 481–486. [Google Scholar] [CrossRef]
- Kwon, H.R.; Nelson, D.A.; Desantis, K.A.; Morrissey, J.M.; Larsen, M. Endothelial Cell Regulation of Salivary Gland Epithelial Patterning. Development 2017, 144, 211–220. [Google Scholar] [CrossRef]
- Emmerson, E.; May, A.J.; Nathan, S.; Cruz-Pacheco, N.; Lizama, C.O.; Maliskova, L.; Zovein, A.C.; Shen, Y.; Muench, M.O.; Knox, S.M. SOX2 Regulates Acinar Cell Development in the Salivary Gland. eLife 2017, 6, e26620. [Google Scholar] [CrossRef]
- Athwal, H.K.; Iii, G.M.; Tibbs, E.; Cornett, A.; Hill, E.; Yeoh, K.; Berenstein, E.; Hoffman, M.P.; Lombaert, I.M.A. Stem Cell Reports Article Sox10 Regulates Plasticity of Epithelial Progenitors toward Secretory Units of Exocrine Glands. Stem Cell Rep. 2019, 12, 366–380. [Google Scholar] [CrossRef]
- Cutler, L.S. The Role of Extracellular Matrix in the Morphogenesis and Differentiation of Salivary Glands. Adv. Dent. Res. 1990, 4, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Aure, M.H.; Symonds, J.M.; Mays, J.W.; Hoffman, M.P. Epithelial Cell Lineage and Signaling in Murine Salivary Glands. J. Dent. Res. 2019, 98, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.N.; Hoffman, M.P. Salivary Gland Development: A Template for Regeneration. Semin. Cell Dev. Biol. 2014, 25–26, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.D. Embryology and Anomalies of the Facial Nerve and Their Surgical Implications. JAMA J. Am. Med. Assoc. 1991, 266, 283. [Google Scholar] [CrossRef]
- Johnson, C.M.; Hoffer, M.E. Facial Nerve, Embryology. In Encyclopedia of Otolaryngology, Head and Neck Surgery; Springer: Berlin/Heidelberg, Germany, 2013; pp. 879–882. [Google Scholar] [CrossRef]
- Knox, S.M.; Lombaert, I.M.A.; Reed, X.; Vitale-Cross, L.; Gutkind, J.S.; Hoffman, M.P. Parasympathetic Innervation Maintains Epithelial Progenitor Cells during Salivary Organogenesis. Science 2010, 329, 1645–1647. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Nakata, H.; Kumchantuek, T.; Sakulsak, N.; Iseki, S. Immunohistochemical Localization of Keratin 5 in the Submandibular Gland in Adult and Postnatal Developing Mice. Histochem. Cell Biol. 2016, 145, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Knosp, W.M.; Knox, S.M.; Lombaert, I.M.A.; Haddox, C.L.; Patel, V.N.; Hoffman, M.P. Submandibular Parasympathetic Gangliogenesis Requires Sprouty-Dependent Wnt Signals from Epithelial Progenitors. Dev. Cell 2015, 32, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Paratcha, G.; Ledda, F. GDNF and GFRα: A Versatile Molecular Complex for Developing Neurons. Trends Neurosci. 2008, 31, 384–391. [Google Scholar] [CrossRef]
- Sharma, M.; Castro-Piedras, I.; Simmons, G.E.; Pruitt, K. Dishevelled: A Masterful Conductor of Complex Wnt Signals. Cell. Signal. 2018, 47, 52. [Google Scholar] [CrossRef]
- Ferreira, J.N.; Hoffman, M.P. Interactions between Developing Nerves and Salivary Glands. Organogenesis 2013, 9, 152–158. [Google Scholar] [CrossRef]
- Ferreira, J.N.A.; Zheng, C.; Lombaert, I.M.A.; Goldsmith, C.M.; Cotrim, A.P.; Symonds, J.M.; Patel, V.N.; Hoffman, M.P. Neurturin Gene Therapy Protects Parasympathetic Function to Prevent Irradiation-Induced Murine Salivary Gland Hypofunction. Mol. Ther. Methods Clin. Dev. 2018, 9, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Heuckeroth, R.O.; Enomoto, H.; Grider, J.R.; Golden, J.P.; Hanke, J.A.; Jackman, A.; Molliver, D.C.; Bardgett, M.E.; Snider, W.D.; Johnson, E.M.; et al. Gene Targeting Reveals a Critical Role for Neurturin in the Development and Maintenance of Enteric, Sensory, and Parasympathetic Neurons. Neuron 1999, 22, 253–263. [Google Scholar] [CrossRef]
- Wang, S.; Sekiguchi, R.; Daley, W.P.; Yamada, K.M. Patterned Cell and Matrix Dynamics in Branching Morphogenesis. J. Cell Biol. 2017, 216, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Sakai, T.; Hsu, J.C.F.; Matsumoto, K.; Chiorini, J.A.; Yamada, K.M. Btbd7 Regulates Epithelial Cell Dynamics and Branching Morphogenesis. Science 2010, 329, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Larsen, M.; Yamada, K.M. Fibronectin Requirement in Branching Morphogenesis. Nature 2003, 423, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, Y.; Yamashina, S. Cellular Dynamics of Epithelial Clefting during Branching Morphogenesis of the Mouse Submandibular Gland. Dev. Dyn. 2010, 239, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Daley, W.P.; Gulfo, K.M.; Sequeira, S.J.; Larsen, M. Identification of a Mechanochemical Checkpoint and Negative Feedback Loop Regulating Branching Morphogenesis. Dev. Biol. 2009, 336, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Daley, W.P.; Gervais, E.M.; Centanni, S.W.; Gulfo, K.M.; Nelson, D.A.; Larsen, M. ROCK1-Directed Basement Membrane Positioning Coordinates Epithelial Tissue Polarity. Development 2012, 139, 411–422. [Google Scholar] [CrossRef]
- Häärä, O.; Koivisto, T.; Miettinen, P.J. EGF-Receptor Regulates Salivary Gland Branching Morphogenesis by Supporting Proliferation and Maturation of Epithelial Cells and Survival of Mesenchymal Cells. Differentiation 2009, 77, 298–306. [Google Scholar] [CrossRef]
- Yamamoto, S.; Fukumoto, E.; Yoshizaki, K.; Iwamoto, T.; Yamada, A.; Tanaka, K.; Suzuki, H.; Aizawa, S.; Arakaki, M.; Yuasa, K.; et al. Platelet-Derived Growth Factor Receptor Regulates Salivary Gland Morphogenesis via Fibroblast Growth Factor Expression. J. Biol. Chem. 2008, 283, 23139–23149. [Google Scholar] [CrossRef]
- Aure, M.H.; Symonds, J.M.; Villapudua, C.U.; Dodge, J.T.; Werner, S.; Knosp, W.M.; Hoffman, M.P. FGFR2 is Essential for Salivary Gland Duct Homeostasis and MAPK-dependent Seromucous Acinar Cell Differentiation. Nat. Commun. 2023, 14, 6485. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, Z.; Myers, C.; Heim, V.M.; Lathrop, C.A.; Rebustini, I.T.; Stewart, J.S.; Larsen, M.; Hoffman, M.P. FGFR2b Signaling Regulates Ex Vivo Submandibular Gland Epithelial Cell Proliferation and Branching Morphogenesis. Development 2005, 132, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, L.; Burke, D.F.; von Delft, F.; Mulloy, B.; Blundell, T.L. Crystal Structure of Fibroblast Growth Factor Receptor Ectodomain Bound to Ligand and Heparin. Nature 2000, 407, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Sumitomo, S.; Shrestha, P.; Tanaka, S.; Takai, Y.; Shikimori, M. Multifunctional Roles of Growth Factors or Biologically Active Peptides in Salivary Glands and Saliva. Oral Med. Pathol. 2008, 12, 115–123. [Google Scholar] [CrossRef][Green Version]
- Ligtenberg, A.J.M.; Veerman, E.C.I. Saliva: Secretion and Functions; Karger Medical and Scientific Publishers: Basel, Switzerland, 2014; Volume 24, pp. 1–154. [Google Scholar] [CrossRef]
- Hauser, B.R.; Aure, M.H.; Kelly, M.C.; Hoffman, M.P.; Chibly, A.M. Generation of a Single-Cell RNAseq Atlas of Murine Salivary Gland Development. iScience 2020, 23, 101838. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, L.S.Y.; Lombaert, I.M.A.; Van Der Zwaag, M.; Faber, H.; Brunsting, J.F.; Van Os, R.P.; Coppes, R.P. Salisphere Derived C-Kit+ Cell Transplantation Restores Tissue Homeostasis in Irradiated Salivary Gland. Radiother. Oncol. 2013, 108, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Rugel-Stahl, A.; Elliott, M.E.; Ovitt, C.E. Ascl3 Marks Adult Progenitor Cells of the Mouse Salivary Gland. Stem Cell Res. 2012, 8, 379–387. [Google Scholar] [CrossRef]
- Lombaert, I.M.A.; Brunsting, J.F.; Wierenga, P.K.; Kampinga, H.H.; de Haan, G.; Coppes, R.P. Keratinocyte Growth Factor Prevents Radiation Damage to Salivary Glands by Expansion of the Stem/Progenitor Pool. Stem Cells 2008, 26, 2595–2601. [Google Scholar] [CrossRef]
- Pringle, S.; Maimets, M.; Van Der Zwaag, M.; Stokman, M.A.; Van Gosliga, D.; Zwart, E.; Witjes, M.J.H.; De Haan, G.; Van Os, R.; Coppes, R.P. Human Salivary Gland Stem Cells Functionally Restore Radiation Damaged Salivary Glands. Stem Cells 2016, 34, 640–652. [Google Scholar] [CrossRef]
- Yi, T.G.; Lee, S.; Choi, N.; Shin, H.S.; Kim, J.; Lim, J.Y. Single Cell Clones Purified from Human Parotid Glands Display Features of Multipotent Epitheliomesenchymal Stem Cells. Sci. Rep. 2016, 6, 36303. [Google Scholar] [CrossRef]
- Katsiougiannis, S.; Wong, D.T.W. The Proteomics of Saliva in Sjögren’s Syndrome. Rheum. Dis. Clin. N. Am. 2016, 42, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Marmary, Y.; Adar, R.; Gaska, S.; Wygoda, A.; Maly, A.; Cohen, J.; Eliashar, R.; Mizrachi, L.; Orfaig-geva, C.; Baum, B.J.; et al. Radiation-Induced Loss of Salivary Gland Function Is Driven by Cellular Senescence and Prevented by IL-6 Modulation. Cancer Res. 2016, 76, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Avouac, J.; Sordet, C.; Depinay, C.; Ardizonne, M.; Vacher-Lavenu, M.C.; Sibilia, J.; Kahan, A.; Allanore, Y. Systemic Sclerosis-Associated Sjögren’s Syndrome and Relationship to the Limited Cutaneous Subtype: Results of a Prospective Study of Sicca Syndrome in 133 Consecutive Patients. Arthritis Rheum. 2006, 54, 2243–2249. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.A.; Reynolds, A.B. Blocked Acinar Development, E-Cadherin Reduction, and Intraepithelial Neoplasia upon Ablation of P120-Catenin in the Mouse Salivary Gland. Dev. Cell 2006, 10, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Ellis, G.L.; Auclair, P.L. Salivary Glands, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Sousa-Victor, P.; Gutarra, S.; García-Prat, L.; Rodriguez-Ubreva, J.; Ortet, L.; Ruiz-Bonilla, V.; Jardí, M.; Ballestar, E.; González, S.; Serrano, A.L.; et al. Geriatric Muscle Stem Cells Switch Reversible Quiescence into Senescence. Nature 2014, 506, 316–321. [Google Scholar] [CrossRef]
- Pringle, S.; Wang, X.; Verstappen, G.M.P.J.; Terpstra, J.H.; Zhang, C.K.; He, A.; Patel, V.; Jones, R.E.; Baird, D.M.; Spijkervet, F.K.L.; et al. Salivary Gland Stem Cells Age Prematurely in Primary Sjögren’s Syndrome. Arthritis Rheumatol. 2019, 71, 133–142. [Google Scholar] [CrossRef]
- Wang, X.; Bootsma, H.; Terpstra, J.; Vissink, A.; Van Der Vegt, B.; Spijkervet, F.K.L.; Kroese, F.G.M.; Pringle, S. Progenitor Cell Niche Senescence Reflects Pathology of the Parotid Salivary Gland in Primary Sjögren’s Syndrome. Rheumatology 2020, 59, 3003–3013. [Google Scholar] [CrossRef]
- Hoebers, F.J.P.; Kartachova, M.; De Bois, J.; Van Den Brekel, M.W.M.; Van Tinteren, H.; Van Herk, M.; Rasch, C.R.N.; Valdés Olmos, R.A.; Verheij, M. 99mTc Hynic-Rh-Annexin V Scintigraphy for In Vivo Imaging of Apoptosis in Patients with Head and Neck Cancer Treated with Chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 509–518. [Google Scholar] [CrossRef]
- Robar, J.L.; Day, A.; Clancey, J.; Kelly, R.; Yewondwossen, M.; Hollenhorst, H.; Rajaraman, M.; Wilke, D. Spatial and Dosimetric Variability of Organs at Risk in Head-and-Neck Intensity-Modulated Radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1121–1130. [Google Scholar] [CrossRef]
- May, A.J.; Cruz-Pacheco, N.; Emmerson, E.; Gaylord, E.A.; Seidel, K.; Nathan, S.; Muench, M.O.; Klein, O.D.; Knox, S.M. Diverse Progenitor Cells Preserve Salivary Gland Ductal Architecture after Radiation-Induced Damage. Development 2018, 145, dev166363. [Google Scholar] [CrossRef]
- Hakim, S.G.; Kosmehl, H.; Lauer, I.; Nadrowitz, R.; Wedel, T.; Sieg, P. The Role of Myoepithelial Cells in the Short-Term Radiogenic Impairment of Salivary Glands. An Immunohistochemical, Ultrastructural and Scintigraphic Study. Anticancer Res. 2002, 22, 4121–4128. [Google Scholar] [PubMed]
- Chibly, A.M.; Patel, V.N.; Aure, M.H.; Pasquale, M.C.; Morell, R.J.; Izquierdo, D.M.; Boger, E.; Martin, G.E.; Ghannam, M.; Andrade, J.; et al. Neurotrophin Signaling Is a Central Mechanism of Salivary Dysfunction after Irradiation That Disrupts Myoepithelial Cells. NPJ Regen. Med. 2023, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Borodkina, A.V.; Deryabin, P.I.; Giukova, A.A.; Nikolsky, N.N. “Social Life” of Senescent Cells: What Is SASP and Why Study It? Acta Naturae 2018, 10, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Wordsworth, J.; Wang, C.; Jurk, D.; Lawless, C.; Martin-Ruiz, C.; von Zglinicki, T. A Senescent Cell Bystander Effect: Senescence-Induced Senescence. Aging Cell 2012, 11, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Mikuła-Pietrasik, J.; Sosinska, P.; Janus, J.; Rubis, B.; Brewinska-Olchowik, M.; Piwocka, K.; Ksiãzek, K. Bystander Senescence in Human Peritoneal Mesothelium and Fibroblasts Is Related to Thrombospondin-1-Dependent Activation of Transforming Growth Factor-Β1. Int. J. Biochem. Cell Biol. 2013, 45, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Kucheryavenko, O.; Wordsworth, J.; von Zglinicki, T. The Senescent Bystander Effect Is Caused by ROS-Activated NF-ΚB Signalling. Mech. Ageing Dev. 2018, 170, 30–36. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.F.L.; Ogrodnik, M.; Kucheryavenko, O.; Glibert, J.; Miwa, S.; Cameron, K.; Ishaq, A.; Saretzki, G.; Nagaraja-Grellscheid, S.; Nelson, G.; et al. The Bystander Effect Contributes to the Accumulation of Senescent Cells In Vivo. Aging Cell 2019, 18, e12848. [Google Scholar] [CrossRef]
- Altrieth, A.L.; O’Keefe, K.J.; Gellatly, V.A.; Tavarez, J.R.; Feminella, S.M.; Moskwa, N.L.; Cordi, C.V.; Turrieta, J.C.; Nelson, D.A.; Larsen, M. Identifying Fibrogenic Cells Following Salivary Gland Obstructive Injury. Front. Cell Dev. Biol. 2023, 11, 1190386. [Google Scholar] [CrossRef]
- Fulda, S. Evasion of Apoptosis as a Cellular Stress Response in Cancer. Int. J. Cell Biol. 2010, 2010, 370835. [Google Scholar] [CrossRef]
- Moskwa, N.; Mahmood, A.; Nelson, D.A.; Altrieth, A.L.; Forni, P.E.; Larsen, M. Single-Cell RNA Sequencing Reveals PDFGRα+ Stromal Cell Subpopulations That Promote Proacinar Cell Differentiation in Embryonic Salivary Gland Organoids. Development 2022, 149, dev200167. [Google Scholar] [CrossRef]
- Mack, M.; Yanagita, M. Origin of Myofibroblasts and Cellular Events Triggering Fibrosis. Kidney Int. 2015, 87, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; Ohtani, N.; Youssef, S.A.; Rodier, F.; Toussaint, W.; Mitchell, J.R.; Laberge, R.M.; Vijg, J.; VanSteeg, H.; Dollé, M.E.T.; et al. An Essential Role for Senescent Cells in Optimal Wound Healing through Secretion of PDGF-AA. Dev. Cell 2014, 31, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Sapudom, J.; Wu, X.; Chkolnikov, M.; Ansorge, M.; Anderegg, U.; Pompe, T. Fibroblast Fate Regulation by Time Dependent TGF-Β1 and IL-10 Stimulation in Biomimetic 3D Matrices. Biomater. Sci. 2017, 5, 1858–1867. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Lagares, D. Evasion of Apoptosis by Myofibroblasts: A Hallmark of Fibrotic Diseases. Nat. Rev. Rheumatol. 2020, 16, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Panwar, P.; Lamour, G.; Mackenzie, N.C.W.; Yang, H.; Ko, F.; Li, H.; Brömme, D. Changes in Structural-Mechanical Properties and Degradability of Collagen during Aging-Associated Modifications. J. Biol. Chem. 2015, 290, 23291–23306. [Google Scholar] [CrossRef] [PubMed]
- Fedintsev, A.; Moskalev, A. Stochastic Non-Enzymatic Modification of Long-Lived Macromolecules—A Missing Hallmark of Aging. Ageing Res. Rev. 2020, 62, 101097. [Google Scholar] [CrossRef] [PubMed]
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Olivieri, F.; Rippo, M.R.; Monsurrò, V.; Salvioli, S.; Capri, M.; Procopio, A.D.; Franceschi, C. MicroRNAs Linking Inflamm-Aging, Cellular Senescence and Cancer. Ageing Res. Rev. 2013, 12, 1056–1068. [Google Scholar] [CrossRef]
- Lampi, M.C.; Reinhart-King, C.A. Targeting Extracellular Matrix Stiffness to Attenuate Disease: From Molecular Mechanisms to Clinical Trials. Sci. Transl. Med. 2018, 10, eaao0475. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Rojas, A.; Añazco, C.; González, I.; Araya, P. Extracellular Matrix Glycation and Receptor for Advanced Glycation End-Products Activation: A Missing Piece in the Puzzle of the Association between Diabetes and Cancer. Carcinogenesis 2018, 39, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Gong, X.; Zhu, H.; Wang, C.; Xu, X.; Cui, D.; Qian, W.; Han, X. Inhibition of Wnt/β-catenin signaling promotes engraftment of mesenchymal stem cells to repair lung injury. J. Cell. Physiol. 2014, 229, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, C.; Shi, C.; Sun, F. Activated Wnt Signaling Induces Myofibroblast Differentiation of Mesenchymal Stem Cells, Contributing to Pulmonary Fibrosis. Int. J. Mol. Med. 2014, 33, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, J.M.; Choi, M.E.; Kim, S.K.; Kim, Y.M.; Choi, J.S. Adipose-Derived Mesenchymal Stem Cells Regenerate Radioiodine-Induced Salivary Gland Damage in a Murine Model. Sci. Rep. 2019, 9, 15752. [Google Scholar] [CrossRef] [PubMed]
- Saylam, G.; Baylr, Ö.; Gültekin, S.S.; Plnarll, F.A.; Han, Ü.; Korkmaz, M.H.; Sancaktar, M.E.; Tatar, Ý.; Sargon, M.F.; Tatar, E.C. Protective/Restorative Role of the Adipose Tissue-Derived Mesenchymal Stem Cells on the Radioiodine-Induced Salivary Gland Damage in Rats. Radiol. Oncol. 2017, 51, 307–318. [Google Scholar] [CrossRef]
- Stone, G.W.; Ellis, S.G.; Cox, D.A.; Hermiller, J.; O’Shaughnessy, C.; Mann, J.T.; Turco, M.; Caputo, R.; Bergin, P.; Greenberg, J.; et al. A Polymer-Based, Paclitaxel-Eluting Stent in Patients with Coronary Artery Disease. N. Engl. J. Med. 2004, 350, 221–231. [Google Scholar] [CrossRef]
- Onat, D.; Brillon, D.; Colombo, P.C.; Schmidt, A.M. Human Vascular Endothelial Cells: A Model System for Studying Vascular Inflammation in Diabetes and Atherosclerosis. Curr. Diabetes Rep. 2012, 11, 193–202. [Google Scholar] [CrossRef]
- Rose, S. Myofibroblast Origins and Fate in Normal Wound Healing. Available online: https://app.biorender.com/illustrations/64e6225156a3787ce7161c9e (accessed on 20 July 2023).
- Deng, J.; Carlson, N.; Takeyama, K.; Dal Cin, P.; Shipp, M.; Letai, A. BH3 Profiling Identifies Three Distinct Classes of Apoptotic Blocks to Predict Response to ABT-737 and Conventional Chemotherapeutic Agents. Cancer Cell 2007, 12, 171–185. [Google Scholar] [CrossRef]
- Green, D.R.; Llambi, F. Cell Death Signaling. Cold Spring Harb. Perspect. Biol. 2015, 7, a006080. [Google Scholar] [CrossRef]
- Ambudkar, I. Calcium Signaling Defects Underlying Salivary Gland Dysfunction. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 1771–1777. [Google Scholar] [CrossRef] [PubMed]
- Mccarthy, D.A.; Clark, R.R.; Toni, R.; Trebak, M.; Andres, J.; Mccarthy, D.A.; Clark, R.R.; Bartling, T.R.; Trebak, M.; Melendez, J.A. Redox Control of the Senescence Regulator Interleukin-1 Alpha and the Secretory Phenotype*. J. Biol. Chem. 2013, 288, 32149–32159. [Google Scholar] [CrossRef] [PubMed]
- Orjalo, A.V.; Bhaumik, D.; Gengler, B.K.; Scott, G.K.; Campisi, J. Cell Surface-Bound IL-1α Is an Upstream Regulator of the Senescence-Associated IL-6/IL-8 Cytokine Network. Proc. Natl. Acad. Sci. USA 2009, 106, 17031–17036. [Google Scholar] [CrossRef] [PubMed]
- Hai, B.; Zhao, Q.; Deveau, M.A.; Liu, F. Delivery of Sonic Hedgehog Gene Repressed Irradiation-Induced Cellular Senescence in Salivary Glands by Promoting DNA Repair and Reducing Oxidative Stress. Theranostics 2018, 8, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Korfei, M.; Mutze, K.; Klee, S.; Skronska-Wasek, W.; Alsafadi, H.N.; Ota, C.; Costa, R.; Schiller, H.B.; Lindner, M.; et al. Senolytic Drugs Target Alveolar Epithelial Cell Function and Attenuate Experimental Lung Fibrosis Ex Vivo. Eur. Respir. J. 2017, 50, 1602367. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Deguan, L.; Zhang, J.; Wang, Y.; Chen, M.; Lin, S.; Huang, L.; Chung, J.E.; Citrin, D.; Wang, Y.; et al. Inhibition of Bcl-2/Xl with ABT-263 Selectively Kills Senescent Type II Pneumocytes and Reverses Persistent Pumonary Fibrosis Induced by Ionizing Radiation in Mice. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J.; Oberg, A.L.; Birch, J.; Salmonowicz, H.; Zhu, Y.; et al. Cellular Senescence Mediates Fibrotic Pulmonary Disease. Nat. Commun. 2017, 8, 14532. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; Lebrasseur, N.K.; Childs, B.G.; Van De Sluis, B.; Kirkland, J.L.; Van Deursen, J.M. Clearance of P16 Ink4a-Positive Senescent Cells Delays Ageing-Associated Disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Humphreys, B.D. Mechanisms of Renal Fibrosis. Annu. Rev. Physiol. 2018, 80, 309–326. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, Y.; Ding, Q.; Wang, S.S.; Rastogi, P.; Dai, D.-F.; Lu, D.; Purvis, M.; Cao, C.; Wang, A.; et al. Epithelial Innate Immunity Mediates Tubular Cell Senescence after Kidney Injury. JCI Insight 2019, 4, e125490. [Google Scholar] [CrossRef]
- Luo, C.; Zhou, S.; Zhou, Z.; Liu, Y.; Yang, L.; Liu, J.; Zhang, Y.; Li, H.; Liu, Y.; Hou, F.F.; et al. Wnt9a Promotes Renal Fibrosis by Accelerating Cellular Senescence in Tubular Epithelial Cells. J. Am. Soc. Nephrol. 2018, 29, 1238–1256. [Google Scholar] [CrossRef] [PubMed]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in Idiopathic Pulmonary Fibrosis: Results from a First-in-Human, Open-Label, Pilot Study. eBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.; Rodrigues, C.E.; Gomes, S.A.; Noronha, I.L. Acute Kidney Injury as a Condition of Renal Senescence. Cell Transplant 2018, 27, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Lagoumtzi, S.M.; Chondrogianni, N. Senolytics and Senomorphics: Natural and Synthetic Therapeutics in the Treatment of Aging and Chronic Diseases. Free Radic. Biol. Med. 2021, 171, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Gasek, N.S.; Kuchel, G.A.; Kirkland, J.L.; Xu, M. Strategies for Targeting Senescent Cells in Human Disease. Nat. Aging 2021, 1, 870–879. [Google Scholar] [CrossRef]
- Nelson, J.; Manzella, K.; Baker, O.J. Current Cell Models for Bioengineering a Salivary Gland: A Mini-Review of Emerging Technologies. Oral Dis. 2013, 19, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, T.; Hayashi, Y.; Nagamine, S.; Yoshida, H.; Yura, Y.; Sato, M. Generation of Cells with Phenotypes of Both Intercalated Duct-Type and Myoepithelial Cells in Human Parotid Gland Adenocarcinoma Clonal Cells Grown in Athymic Nude Mice. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1986, 51, 187–195. [Google Scholar] [CrossRef]
- Sato, M.; Hayashi, Y.; Yoshida, H.; Yanagawa, T.; Yura, Y.; Nitta, T. Search for Specific Markers of Neoplastic Epithelial Duct and Myoepithelial Cell Lines Established from Human Salivary Gland and Characterization of Their Growth In Vitro. Cancer 1984, 54, 2959. [Google Scholar] [CrossRef]
- Lin, L.C.; Elkashty, O.; Ramamoorthi, M.; Trinh, N.; Liu, Y.; Sunavala-Dossabhoy, G.; Pranzatelli, T.; Michael, D.G.; Chivasso, C.; Perret, J.; et al. Cross-Contamination of the Human Salivary Gland HSG Cell Line with HeLa Cells: A STR Analysis Study. Oral Dis. 2018, 24, 1477–1483. [Google Scholar] [CrossRef]
- Athwal, H.K.; Lombaert, I.M.A. 3D Organoid Formation from the Murine Salivary Gland Cell Line SIMS. Bio-protocol 2019, 9, e3386. [Google Scholar] [CrossRef]
- Thiemann, R.F.; Nelson, D.A.; Michael DiPersio, C.; Larsen, M.; LaFlamme, S.E. Establishment of a Murine Pro-Acinar Cell Line to Characterize Roles for FGF2 and A3β1 Integrins in Regulating Pro-Acinar Characteristics. Sci. Rep. 2019, 9, 10984. [Google Scholar] [CrossRef]
- He, X.J.; Frank, D.P.; Tabak, L.A. Establishment Immortalized and Characterization of 12S Adenoviral E1A Rat Submandibular Gland Epithelial Cells. Biochem. Biophys. Res. Commun. 1990, 170, 336–343. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wu, X.; Brown, A.M.; Wellner, R.B.; Baum, B.J. Characteristics of Alpha 1-Adrenergic Receptors in a Rat Salivary Cell Line, RSMT-A5. Gen. Pharmacol. 1989, 20, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Quissell, D.O.; Barzen, K.A.; Redman, R.S.; Camden, J.M.; Turner, J.T. Development and Characterization of SV40 Immortalized Rat Submandibular Acinar Cell Lines. Vitr. Cell. Dev. Biol.-Anim. 1997, 33, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Aletta, J.M.; Wen, J.; Zhang, X.; Higgins, D.; Rubin, R.P. Rat Serum Induces a Differentiated Phenotype in a Rat Parotid Acinar Cell Line. Am. J. Physiol. Gastrointest. Liver Physiol. 1998, 275, G259–G268. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Futagi, M.; Fukumoto, E.; Saito, K.; Yoshizaki, K.; Ishikawa, M.; Arakaki, M.; Hino, R.; Sugawara, Y.; Ishikawa, M.; et al. Connexin 43 Is Necessary for Salivary Gland Branching Morphogenesis and FGF10-induced ERK1/2 Phosphorylation. J. Biol. Chem. 2016, 291, 904–912. [Google Scholar] [CrossRef]
- Oishi, Y.; Arakawa, T.; Tanimura, A.; Itakura, M.; Takahashi, M.; Tajima, Y.; Mizoguchi, I.; Takuma, T. Role of VAMP-2, VAMP-7, and VAMP-8 in constitutive exocytosis from HSY cells. Histochem. Cell Biol. 2016, 125, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Patton, L.L.; Pollack, S.; Wellner, R.B. Responsiveness of a Human Parotid Epithelial Cell Line (HSY) to Autonomic Stimulation: Muscarinic Control of K+ Transport. Vitr. Cell Dev. Biol. Anim. 1991, 27, 779–785. [Google Scholar] [CrossRef]
- Shirasuna, K.; Sato, M.; Miyazaki, T. A Neoplastic Epithelial Duct Cell Line Established from an Irradiated Human Salivary Gland. Cancer 1981, 48, 745–752. [Google Scholar] [CrossRef]
- Maria, O.M.; Maria, O.; Liu, Y.; Komarova, S.V.; Tran, S.D. Matrigel Improves Functional Properties of Human Submandibular Salivary Gland Cell Line. Int. J. Biochem. Cell Biol. 2011, 43, 622–631. [Google Scholar] [CrossRef]
- Laoide, B.M.; Courty, Y.; Gastinne, I.; Thibaut, C.; Kellermann, O.; Rougeon, F. Immortalised Mouse Submandibular Epithelial Cell Lines Retain Polarised Structural and Functional Properties. J. Cell Sci. 1996, 109, 2789–2800. [Google Scholar] [CrossRef]
- He, X.; Kuijpers, G.A.J.; Goping, G.; Kulakusky, J.A.; Zheng, C.; Delporte, C.; Tse, C.M.; Redman, R.S.; Donowitz, M.; Pollard, H.B.; et al. A Polarized Salivary Cell Monolayer Useful for Studying Transepithelial Fluid Movement In Vitro. Pflügers Arch. 1998, 435, 375–381. [Google Scholar] [CrossRef]
- Mitsui, R.; Fujita-Yoshigaki, J.; Narita, T.; Matsuki-Fukushima, M.; Satoh, K.; Qi, B.; Guo, M.Y.; Katsumata-Kato, O.; Sugiya, H. Maintenance of Paracellular Barrier Function by Insulin-like Growth Factor-I in Submandibular Gland Cells. Arch. Oral Biol. 2010, 55, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M. In Vitro Transformation of Submandibular Gland Epithelial Cells and Fibroblasts of Adult Rats by Methylcholanthrene. Cancer Res. 1973, 33, 2779–2789. [Google Scholar] [PubMed]
- Liu, X.B.; Sun, X.; Mörk, A.C.; Dodds, M.W.; Martinez, J.R.; Zhang, G.H. Characterization of the Calcium Signaling System in the Submandibular Cell Line SMG-C6. Proc. Soc. Exp. Biol. Med. 2000, 225, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Barlow-Walden, L.; Woodson, T.; Kerecman, J.D.; Zhang, G.H.; Martinez, J.R. Ion Transport in an Immortalized Rat Submandibular Cell Line SMG-C6 (44550). Exp. Biol. Med. 2000, 225, 39–48. [Google Scholar] [CrossRef]
- Aure, M.H.; Røed, A.; Galtung, H.K. Intracellular Ca2+ Responses and Cell Volume Regulation upon Cholinergic and Purinergic Stimulation in an Immortalized Salivary Cell Line. Eur. J. Oral Sci. 2010, 118, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Bruchas, M.R.; Toews, M.L.; Bockman, C.S.; Abel, P.W. Characterization of the A1-Adrenoceptor Subtype Activating Extracellular Signal-Regulated Kinase in Submandibular Gland Acinar Cells. Eur. J. Pharmacol. 2008, 578, 349–358. [Google Scholar] [CrossRef]
- Liu, X.B.; Mörk, A.C.; Sun, X.; Castro, R.; Martinez, J.R.; Zhang, G.H. Regulation of Ca2+ Signals in a Parotid Cell Line Par-C5. Arch. Oral Biol. 2001, 46, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Bockman, C.S.; Bradley, M.E.; Dang, H.K.; Zeng, W.; Scofield, M.A.; Dowd, F.J. Molecular and Pharmacological Characterization of Muscarinic Receptor Subtypes in a Rat Parotid Gland Cell Line: Comparison with Native Parotid Gland. J. Pharmacol. Exp. Ther. 2001, 297, 718–726. [Google Scholar]
- Turner, J.T.; Redman, R.S.; Camden, J.M.; Landon, L.A.; Quissell, D.O. A Rat Parotid Gland Cell Line, Par-C10, Exhibits Neurotransmitter-Regulated Transepithelial Anion Secretion. Am. J. Physiol. 1998, 275, C367–C374. [Google Scholar] [CrossRef]
- Demeter, I.; Szucs, A.; Hegyesi, O.; Foldes, A.; Racz, G.Z.; Burghardt, B.; Steward, M.C.; Varga, G. Vectorial Bicarbonate Transport by Par-C10 Salivary Cells. J. Physiol. Pharmacol. 2009, 60, 197–204. [Google Scholar]
- Baker, O.J. Tight Junctions in Salivary Epithelium. J. Biomed. Biotechnol. 2010, 2010, 278948. [Google Scholar] [CrossRef] [PubMed]
- Szlávik, V.; Szabó, B.; Vicsek, T.; Barabás, J.; Bogdán, S.; Gresz, V.; Varga, G.; O’Connell, B.; Vág, J. Differentiation of Primary Human Submandibular Gland Cells Cultured on Basement Membrane Extract. Tissue Eng. Part A 2008, 14, 1915–1926. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G.M.; Dewson, G.; Wardlaw, A.J.; Levi-Schaffer, F.; Moqbel, R. A Comparative Study of Different Methods for the Assessment of Apoptosis and Necrosis in Human Eosinophils. J. Immunol. Methods 1998, 217, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Joraku, A.; Sullivan, C.A.; Yoo, J.; Atala, A. In-Vitro Reconstitution of Three-Dimensional Human Salivary Gland Tissue Structures. Differentiation 2007, 75, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Chopra, D.P.; Xue-Hu, I.C. Secretion of α-Amylase in Human Parotid Gland Epithelial Cell Culture. J. Cell. Physiol 1993, 155, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Joraku, A.; Sullivan, C.A.; Yoo, J.J.; Atala, A. Tissue Engineering of Functional Salivary Gland Tissue. Laryngoscope 2005, 115, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Sugito, T.; Kagami, H.; Hata, K.; Nishiguchi, H.; Ueda, M. Transplantation of Cultured Salivary Gland Cells into an Atrophic Salivary Gland. Cell Transplant. 2004, 13, 691–699. [Google Scholar] [CrossRef]
- Ogawa, M.; Oshima, M.; Imamura, A.; Sekine, Y.; Ishida, K.; Yamashita, K.; Nakajima, K.; Hirayama, M.; Tachikawa, T.; Tsuji, T. Functional Salivary Gland Regeneration by Transplantation of a Bioengineered Organ Germ. Nat. Commun. 2013, 4, 2498. [Google Scholar] [CrossRef]
- Coughlin, M.D. Target Organ Stimulation of Parasympathetic Nerve Growth in the Developing Mouse Submandibular Gland. Dev. Biol. 1975, 43, 140–158. [Google Scholar] [CrossRef]
- Chibly, A.M.; Aure, M.H.; Patel, V.N.; Hoffman, M.P. Salivary Gland Function, Development, and Regeneration. Physiol. Rev. 2022, 102, 1495–1552. [Google Scholar] [CrossRef]
- Lombaert, I.M.; Knox, S.M.; Hoffman, M.P. Salivary Gland Progenitor Cell Biology Provides a Rationale For Therapeutic Salivary Gland Regeneration. Oral Dis. 2011, 17, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Lombaert, I.M.A.; Hoffman, M.P. Epithelial Stem/Progenitor Cells in the Embryonic Mouse Submandibular Gland. Salivary Gland. 2010, 14, 90–106. [Google Scholar] [CrossRef]
- Koslow, M.; O’Keefe, K.J.; Hosseini, Z.F.; Nelson, D.A.; Larsen, M. ROCK Inhibitor Increases Proacinar Cells in Adult Salivary Gland Organoids. Stem Cell Res. 2019, 41, 101608. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Nogawa, H. Branching Morphogenesis of Mouse Salivary Epithelium in Basement Membrane-like Substratum Separated from Mesenchyme by the Membrane Filter. Development 1991, 111, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Okumura, K.; Shinohara, M.; Endo, F. Capability of Tissue Stem Cells to Organize into Salivary Rudiments. Stem Cells Int. 2012, 2012, 502136. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hosseini, Z.F.; Nelson, D.A.; Moskwa, N.; Sfakis, L.M.; Castracane, J.; Larsen, M. FGF2-Dependent Mesenchyme and Laminin-111 Are Niche Factors in Salivary Gland Organoids. J. Cell Sci. 2018, 131, jcs208728. [Google Scholar] [CrossRef]
- Patel, V.N.; Rebustini, I.T.; Hoffman, M.P. Salivary Gland Branching Morphogenesis. Differentiation 2006, 74, 349–364. [Google Scholar] [CrossRef]
- Feng, J.; van der Zwaag, M.; Stokman, M.A.; van Os, R.; Coppes, R.P. Isolation and Characterization of Human Salivary Gland Cells for Stem Cell Transplantation to Reduce Radiation-Induced Hyposalivation. Radiother. Oncol. 2009, 92, 466–471. [Google Scholar] [CrossRef]
- Rocchi, C.; Barazzuol, L.; Coppes, R.P. The Evolving Definition of Salivary Gland Stem Cells. NPJ Regen. Med. 2021, 6, 4. [Google Scholar] [CrossRef]
- Srinivasan, P.P.; Patel, V.N.; Liu, S.; Harrington, D.A.; Hoffman, M.P.; Jia, X.; Witt, R.L.; Farach-Carson, M.C.; Pradhan-Bhatt, S. Primary Salivary Human Stem/Progenitor Cells Undergo Microenvironment-Driven Acinar-Like Differentiation in Hyaluronate Hydrogel Culture. Stem Cells Transl. Med. 2017, 6, 110–120. [Google Scholar] [CrossRef]
- Sui, Y.; Zhang, S.; Li, Y.; Zhang, X.; Hu, W.; Feng, Y.; Xiong, J.; Zhang, Y.; Wei, S. Generation of Functional Salivary Gland Tissue from Human Submandibular Gland Stem/Progenitor Cells. Stem Cell Res. Ther. 2020, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, L.S.Y.; Maimets, M.; Pringle, S.A.; Van Der Zwaag, M.; Van Os, R.P.; Coppes, R.P. Regeneration of Irradiated Salivary Glands with Stem Cell Marker Expressing Cells. Radiother. Oncol. 2011, 99, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Serrano Martinez, P.; Cinat, D.; van Luijk, P.; Baanstra, M.; de Haan, G.; Pringle, S.; Coppes, R.P. Mouse Parotid Salivary Gland Organoids for the in Vitro Study of Stem Cell Radiation Response. Oral Dis. 2021, 27, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Tubbesing, K.; Moskwa, N.; Khoo, T.C.; Nelson, D.A.; Sharikova, A.; Feng, Y.; Larsen, M.; Khmaladze, A. Raman Microspectroscopy Fingerprinting of Organoid Differentiation State. Cell. Mol. Biol. Lett. 2022, 27, 53. [Google Scholar] [CrossRef] [PubMed]
- Pringle, S.; Van Os, R.; Coppes, R.P. Concise Review: Adult Salivary Gland Stem Cells and a Potential Therapy for Xerostomia. Stem Cells 2013, 31, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Hisatomi, Y.; Okumura, K.; Nakamura, K.; Matsumoto, S.; Satoh, A.; Nagano, K.; Yamamoto, T.; Endo, F. Flow Cytometric Isolation of Endodermal Progenitors from Mouse Salivary Gland Differentiate into Hepatic and Pancreatic Lineages. Hepatology 2004, 39, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, A.R.; Vitale-Cross, L.; Amornphimoltham, P.; Gutkind, J.S.; Molinolo, A. Rapid Development of Salivary Gland Carcinomas Upon Conditional Expression of K-ras Driven by the Cytokeratin 5 Promoter. Am. J. Pathol. 2006, 168, 1654–1665. [Google Scholar] [CrossRef] [PubMed]
- Lombaert, I.M.; Abrams, S.R.; Li, L.; Eswarakumar, V.P.; Sethi, A.J.; Witt, R.L.; Hoffman, M.P. Combined KIT and FGFR2b Signaling Regulates Epithelial Progenitor Expansion during Organogenesis. Stem Cell Rep. 2013, 1, 604–619. [Google Scholar] [CrossRef]
- Schwarz, S.; Rotter, N. Human Salivary Gland Stem Cells: Isolation, Propagation, and Characterization. Methods Mol. Biol. 2012, 879, 403–442. [Google Scholar] [CrossRef]
- Sato, A.; Okumura, K.; Matsumoto, S.; Hattori, K.; Hattori, S.; Shinohara, M.; Endo, F. Isolation, Tissue Localization, and Cellular Characterization of Progenitors Derived from Adult Human Salivary Glands. Cloning Stem Cells 2007, 9, 191–205. [Google Scholar] [CrossRef]
- Jeong, J.; Baek, H.; Kim, Y.J.; Choi, Y.; Lee, H.; Lee, E.; Kim, E.S.; Hah, J.H.; Kwon, T.-K.; Choi, I.J.; et al. Human Salivary Gland Stem Cells Ameliorate Hyposalivation of Radiation-Damaged Rat Salivary Glands. Exp. Mol. Med. 2013, 45, e58. [Google Scholar] [CrossRef] [PubMed]
- Rotter, N.; Oder, J.; Schlenke, P.; Lindner, U.; Böhrnsen, F.; Kramer, J.; Rohwedel, J.; Huss, R.; Brandau, S.; Wollenberg, B.; et al. Isolation and Characterization of Adult Stem Cells from Human Salivary Glands. Stem Cells Dev. 2008, 17, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Mackay, A.M.; Beck, S.C.; Murphy, J.M.; Barry, F.P.; Chichester, C.O.; Pittenger, M.F. Chondrogenic Differentiation of Cultured Human Mesenchymal Stem Cells from Marrow. Tissue Eng. 1998, 4, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Dezawa, M.; Ishikawa, H.; Itokazu, Y.; Yoshihara, T.; Hoshino, M.; Takeda, S.I.; Ide, C.; Nabeshima, Y.I. Developmental Biology: Bone Marrow Stromal Cells Generate Muscle Cells and Repair Muscle Degeneration. Science 2005, 309, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Makino, S.; Fukuda, K.; Miyoshi, S.; Konishi, F.; Kodama, H.; Pan, J.; Sano, M.; Takahashi, T.; Hori, S.; Abe, H.; et al. Cardiomyocytes Can Be Generated from Marrow Stromal Cells In Vitro. J. Clin. Investig. 1999, 103, 697. [Google Scholar] [CrossRef]
- Snykers, S.; Vanhaecke, T.; Papeleu, P.; Luttun, A.; Jiang, Y.; Vander Heyden, Y.; Verfaillie, C.; Rogiers, V. Sequential Exposure to Cytokines Reflecting Embryogenesis: The Key for In Vitro Differentiation of Adult Bone Marrow Stem Cells into Functional Hepatocyte-like Cells. Toxicol. Sci. 2006, 94, 330–341. [Google Scholar] [CrossRef]
- Scintu, F.; Reali, C.; Pillai, R.; Badiali, M.; Sanna, M.A.; Argiolu, F.; Ristaldi, M.S.; Sogos, V. Differentiation of Human Bone Marrow Stem Cells into Cells with a Neural Phenotype: Diverse Effects of Two Specific Treatments. BMC Neurosci. 2006, 7, 14. [Google Scholar] [CrossRef]
- Marinkovic, M.; Tran, O.N.; Wang, H.; Abdul-Azees, P.; Dean, D.D.; Chen, X.D.; Yeh, C.K. Autologous Mesenchymal Stem Cells Offer a New Paradigm for Salivary Gland Regeneration. Int. J. Oral Sci. 2023, 15, 18. [Google Scholar] [CrossRef]
- Caplan, A.I. Adult Mesenchymal Stem Cells for Tissue Engineering versus Regenerative Medicine. J. Cell. Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.M.; Motan, D.A.L.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal Stem Cells and Immunomodulation: Current Status and Future Prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef]
- Lim, J.Y.; Yi, T.; Choi, J.S.; Jang, Y.H.; Lee, S.; Kim, H.J.; Song, S.U.; Kim, Y.M. Intraglandular Transplantation of Bone Marrow-Derived Clonal Mesenchymal Stem Cells for Amelioration of Post-Irradiation Salivary Gland Damage. Oral Oncol. 2013, 49, 136–143. [Google Scholar] [CrossRef]
- Kojima, T.; Kanemaru, S.I.; Hirano, S.; Tateya, I.; Ohno, S.; Nakamura, T.; Ito, J. Regeneration of Radiation Damaged Salivary Glands with Adipose-Derived Stromal Cells. Laryngoscope 2011, 121, 1864–1869. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Roh, S. Transdifferentiation of Mouse Adipose-Derived Stromal Cells into Acinar Cells of the Submandibular Gland Using a Co-Culture System. Exp. Cell Res. 2015, 334, 160–172. [Google Scholar] [CrossRef]
- Lim, J.Y.; Ra, J.C.; Shin, I.S.; Jang, Y.H.; An, H.Y.; Choi, J.S.; Kim, W.C.; Kim, Y.M. Systemic Transplantation of Human Adipose Tissue-Derived Mesenchymal Stem Cells for the Regeneration of Irradiation-Induced Salivary Gland Damage. PLoS ONE 2013, 8, e71167. [Google Scholar] [CrossRef]
- Xiong, X.; Shi, X.; Chen, F. Human Adipose Tissue-Derived Stem Cells Alleviate Radiation-Induced Xerostomia. Int. J. Mol. Med. 2014, 34, 749–755. [Google Scholar] [CrossRef]
- Lim, J.Y.; Yi, T.; Lee, S.; Kim, J.; Kim, S.N.; Song, S.U.; Kim, Y.M. Establishment and Characterization of Mesenchymal Stem Cell-like Clonal Stem Cells from Mouse Salivary Glands. Tissue Eng. Part C Methods 2015, 21, 447–457. [Google Scholar] [CrossRef]
- McCoy, S.S.; Giri, J.; Das, R.; Paul, P.K.; Pennati, A.; Parker, M.; Liang, Y.; Galipeau, J. Minor Salivary Gland Mesenchymal Stromal Cells Derived from Patients with Sjӧgren’s Syndrome Deploy Intact Immune Plasticity. Cytotherapy 2021, 23, 301–310. [Google Scholar] [CrossRef]
- Denewar, M.; Amin, L.E. Role of Bone Marrow-Derived Mesenchymal Stem Cells on the Parotid Glands of Streptozotocin Induced Diabetes Rats. J. Oral Biol. Craniofac. Res. 2020, 10, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Lindroos, B.; Suuronen, R.; Miettinen, S. The Potential of Adipose Stem Cells in Regenerative Medicine. Stem Cell Rev. Rep. 2011, 7, 269–291. [Google Scholar] [CrossRef] [PubMed]
- Gaebel, R.; Furlani, D.; Sorg, H.; Polchow, B.; Frank, J.; Bieback, K.; Wang, W.; Klopsch, C.; Ong, L.L.; Li, W.; et al. Cell Origin of Human Mesenchymal Stem Cells Determines a Different Healing Performance in Cardiac Regeneration. PLoS ONE 2011, 6, e15652. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Mack, D.; Kapetanovic, E.; Atala, A.; Jackson, J.D.; Yoo, J.; Soker, S. Bioprinted Amniotic Fluid-Derived Stem Cells Accelerate Healing of Large Skin Wounds. Stem Cells Transl. Med. 2012, 1, 792–802. [Google Scholar] [CrossRef] [PubMed]
- De Coppi, P.; Bartsch, G.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J.; et al. Isolation of Amniotic Stem Cell Lines with Potential for Therapy. Nat. Biotechnol. 2007, 25, 100–106. [Google Scholar] [CrossRef] [PubMed]
- English, K.; Wood, K.J. Mesenchymal Stromal Cells in Transplantation Rejection and Tolerance. Cold Spring Harb. Perspect. Med. 2013, 3, a015560. [Google Scholar] [CrossRef] [PubMed]
- Khalili, S.; Liu, Y.; Kornete, M.; Roescher, N.; Kodama, S.; Peterson, A.; Piccirillo, C.A.; Tran, S.D. Mesenchymal Stromal Cells Improve Salivary Function and Reduce Lymphocytic Infiltrates in Mice with Sjögren’s-like Disease. PLoS ONE 2012, 7, e38615. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.Q.; Zhang, L.L.; An, Y.; Xu, F.F.; An, R.; Xu, H.Y.; Liu, Y.P.; Liu, B. In Vitro Transdifferentiation of Adipose Tissue-Derived Stem Cells into Salivary Gland Acinar-like Cells. Am. J. Transl. Res. 2019, 11, 2908. [Google Scholar]
- Almansoori, A.A.; Khentii, N.; Kim, B.; Kim, S.M.; Lee, J.H. Mesenchymal Stem Cell Therapy in Submandibular Salivary Gland Allotransplantation: Experimental Study. Transplantation 2019, 103, 1111–1120. [Google Scholar] [CrossRef]
- Grønhøj, C.; Jensen, D.H.; Glovinski, P.V.; Jensen, S.B.; Bardow, A.; Oliveri, R.S.; Specht, L.; Thomsen, C.; Darkner, S.; Kiss, K.; et al. First-in-Man Mesenchymal Stem Cells for Radiation-Induced Xerostomia (MESRIX): Study Protocol for a Randomized Controlled Trial. Trials 2017, 18, 108. [Google Scholar] [CrossRef]
- Martin, G.R. Isolation of a Pluripotent Cell Line from Early Mouse Embryos Cultured in Medium Conditioned by Teratocarcinoma Stem Cells. Proc. Natl. Acad. Sci. USA 1981, 78, 7634–7638. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Kaufman, M.H. Establishment in Culture of Pluripotential Cells from Mouse Embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Kalishman, J.; Golos, T.G.; Durning, M.; Harris, C.P.; Becker, R.A.; Hearn, J.P. Isolation of a Primate Embryonic Stem Cell Line. Proc. Natl. Acad. Sci. USA 1995, 92, 7844–7848. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Yamanaka, S. Induced Pluripotent Stem Cells: Past, Present, and Future. Cell Stem Cell 2012, 10, 678–684. [Google Scholar] [CrossRef]
- Park, A.; Hong, P.; Won, S.T.; Thibault, P.A.; Vigant, F.; Oguntuyo, K.Y.; Taft, J.D.; Lee, B. Sendai Virus, an RNA Virus with No Risk of Genomic Integration, Delivers CRISPR/Cas9 for Efficient Gene Editing. Mol. Ther. Methods Clin. Dev. 2016, 3, 16057. [Google Scholar] [CrossRef]
- Cunningham, J.J.; Ulbright, T.M.; Pera, M.F.; Looijenga, L.H.J. Lessons from Human Teratomas to Guide Development of Safe Stem Cell Therapies. Nat. Biotechnol. 2012, 30, 849–857. [Google Scholar] [CrossRef]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef]
- Singh, V.K.; Kalsan, M.; Kumar, N.; Saini, A.; Chandra, R. Induced Pluripotent Stem Cells: Applications in Regenerative Medicine, Disease Modeling, and Drug Discovery. Front. Cell Dev. Biol. 2015, 3, 2. [Google Scholar] [CrossRef]
- Miura, K.; Okada, Y.; Aoi, T.; Okada, A.; Takahashi, K.; Okita, K.; Nakagawa, M.; Koyanagi, M.; Tanabe, K.; Ohnuki, M.; et al. Variation in the Safety of Induced Pluripotent Stem Cell Lines. Nat. Biotechnol. 2009, 27, 743–745. [Google Scholar] [CrossRef]
- Sato, Y.; Bando, H.; Di Piazza, M.; Gowing, G.; Herberts, C.; Jackman, S.; Leoni, G.; Libertini, S.; MacLachlan, T.; McBlane, J.W.; et al. Tumorigenicity Assessment of Cell Therapy Products: The Need for Global Consensus and Points to Consider. Cytotherapy 2019, 21, 1095–1111. [Google Scholar] [CrossRef]
- Kunitomi, A.; Yuasa, S.; Sugiyama, F.; Saito, Y.; Seki, T.; Kusumoto, D.; Kashimura, S.; Takei, M.; Tohyama, S.; Hashimoto, H.; et al. H1foo Has a Pivotal Role in Qualifying Induced Pluripotent Stem Cells. Stem Cell Rep. 2016, 6, 825–833. [Google Scholar] [CrossRef]
- Kawakami, M.; Ishikawa, H.; Tachibana, T.; Tanaka, A.; Mataga, I. Functional Transplantation of Salivary Gland Cells Differentiated from Mouse Early ES Cells In Vitro. Hum. Cell 2013, 26, 80–90. [Google Scholar] [CrossRef]
- Ono, H.; Obana, A.; Usami, Y.; Sakai, M.; Nohara, K.; Egusa, H.; Sakai, T. Regenerating Salivary Glands in the Microenvironment of Induced Pluripotent Stem Cells. BioMed Res. Int. 2015, 2015, 293570. [Google Scholar] [CrossRef]
- Tanaka, J.; Ogawa, M.; Hojo, H.; Kawashima, Y.; Mabuchi, Y.; Hata, K.; Nakamura, S.; Yasuhara, R.; Takamatsu, K.; Irié, T.; et al. Generation of Orthotopically Functional Salivary Gland from Embryonic Stem Cells. Nat. Commun. 2018, 9, 4216. [Google Scholar] [CrossRef]
- Ramesh, P.; Moskwa, N.; Hanchon, Z.; Koplas, A.; Nelson, D.A.; Mills, K.L.; Castracane, J.; Larsen, M.; Sharfstein, S.T.; Xie, Y. Engineering Cryoelectrospun Elastin-Alginate Scaffolds to Serve as Stromal Extracellular Matrices. Biofabrication 2022, 14, 035010. [Google Scholar] [CrossRef]
- Mosier, A.P.; Peters, S.B.; Larsen, M.; Cady, N.C. Microfluidic Platform for the Elastic Characterization of Mouse Submandibular Glands by Atomic Force Microscopy. Biosensors 2014, 4, 18–27. [Google Scholar] [CrossRef]
- Aframian, D.J.; Cukierman, E.; Nikolovski, J.; Mooney, D.J.; Yamada, K.M.; Baum, B.J. The Growth and Morphological Behavior of Salivary Epithelial Cells on Matrix Protein-Coated Biodegradable Substrata. Tissue Eng. 2000, 6, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cukierman, E.; Swaim, W.D.; Yamada, K.M.; Baum, B.J. Extracellular Matrix Protein-Induced Changes in Human Salivary Epithelial Cell Organization and Proliferation on a Model Biological Substratum. Biomaterials 1999, 20, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Soscia, D.; Sequeira, S.; Schramm, R.; Jayarathanam, K.; Cantara, S.; Larsen, M.; Castracane, J. Salivary Gland Cell Differentiation and Organization on Micropatterned PLGA Nanofiber Craters. Biomaterials 2013, 34, 6773–6784. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, S.; Soscia, D.; Oztan, B.; Mosier, A.; Jean-Gilles, R.; Castracane, J.; Larsen, M. The Regulation of Focal Adhesion Complex Formation and Salivary Gland Epithelial Cell Organization by Nanofibrous PLGA Scaffolds. Biomaterials 2012, 33, 3175–3186. [Google Scholar] [CrossRef] [PubMed]
- Teimouri, A.; Azadi, M.; Emadi, R.; Lari, J.; Chermahini, A.N. Preparation, Characterization, Degradation and Biocompatibility of Different Silk Fibroin Based Composite Scaffolds Prepared by Freeze-Drying Method for Tissue Engineering Application. Polym. Degrad. Stab. 2015, 121, 18–29. [Google Scholar] [CrossRef]
- Zhang, B.X.; Zhang, Z.L.; Lin, A.L.; Wang, H.; Pilia, M.; Ong, J.L.; Dean, D.D.; Chen, X.D.; Yeh, C.K. Silk Fibroin Scaffolds Promote Formation of the Ex Vivo Niche for Salivary Gland Epithelial Cell Growth, Matrix Formation, and Retention of Differentiated Function. Tissue Eng. Part A 2015, 21, 1611–1620. [Google Scholar] [CrossRef]
- Pradhan, S.; Liu, C.; Zhang, C.; Jia, X.; Farach-Carson, M.C.; Witt, R.L. Lumen Formation in Three-Dimensional Cultures of Salivary Acinar Cells. Otolaryngol. Head Neck Surg. 2010, 142, 191–195. [Google Scholar] [CrossRef]
- Pradhan-Bhatt, S.; Harrington, D.A.; Duncan, R.L.; Jia, X.; Witt, R.L.; Farach-Carson, M.C. Implantable Three-Dimensional Salivary Spheroid Assemblies Demonstrate Fluid and Protein Secretory Responses to Neurotransmitters. Tissue Eng. Part A 2013, 19, 1610. [Google Scholar] [CrossRef]
- Pradhan-Bhatt, S.; Harrington, D.A.; Duncan, R.L.; Farach-Carson, M.C.; Jia, X.; Witt, R.L. A Novel In Vivo Model for Evaluating Functional Restoration of a Tissue-Engineered Salivary Gland. Laryngoscope 2014, 124, 456–461. [Google Scholar] [CrossRef]
- Shubin, A.D.; Felong, T.J.; Graunke, D.; Ovitt, C.E.; Benoit, D.S.W. Development of Poly(Ethylene Glycol) Hydrogels for Salivary Gland Tissue Engineering Applications. Tissue Eng. Part A 2015, 21, 1733–1751. [Google Scholar] [CrossRef]
- Peters, S.B.; Naim, N.; Nelson, D.A.; Mosier, A.P.; Cady, N.C.; Larsen, M. Biocompatible Tissue Scaffold Compliance Promotes Salivary Gland Morphogenesis and Differentiation. Tissue Eng. Part A 2014, 20, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, H.; Matsumoto, T.; Sakai, T.; Yamaguchi, S.; An, S.H.; Abe, M.; Wakisaka, S.; Lee, K.Y.; Egusa, H.; Imazato, S. Hydrogel-Based Biomimetic Environment for In Vitro Modulation of Branching Morphogenesis. Biomaterials 2011, 32, 6754–6763. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, M.; Ramesh, P.; Toro, M.; Evans, E.; Moskwa, N.; Zhang, X.; Sharfstein, S.T.; Larsen, M.; Xie, Y. Alginate Hydrogel Microtubes for Salivary Gland Cell Organization and Cavitation. Bioengineering 2022, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.; Jones, J.P.; Lei, P.; Andreadis, S.T.; Baker, O.J. Laminin-111 Peptides Conjugated to Fibrin Hydrogels Promote Formation of Lumen Containing Parotid Gland Cell Clusters. Biomacromolecules 2016, 17, 2293–2301. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.; Dos Santos, H.T.; Maslow, F.; Small, T.; Samuel, R.Z.; Lei, P.; Andreadis, S.T.; Baker, O.J. Fibrin Hydrogels Fortified with FGF-7/10 and Laminin-1 Peptides Promote Regeneration of Irradiated Salivary Glands. Acta Biomater. 2023, 172, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Shimizu, O.; Shiratsuchi, H.; Tamagawa, T.; Tonogi, M. Wound Healing after Applying a Gelatin-Based Hydrogel Sheet to Resected Rat Submandibular Gland. J. Oral Sci. 2020, 62, 222–225. [Google Scholar] [CrossRef]
- Adine, C.; Ng, K.K.; Rungarunlert, S.; Souza, G.R.; Ferreira, J.N. Engineering Innervated Secretory Epithelial Organoids by Magnetic Three-Dimensional Bioprinting for Stimulating Epithelial Growth in Salivary Glands. Biomaterials 2018, 180, 52–66. [Google Scholar] [CrossRef] [PubMed]
- MacLaren, R.E.; Pearson, R.A.; MacNeil, A.; Douglas, R.H.; Salt, T.E.; Akimoto, M.; Swaroop, A.; Sowden, J.C.; Ali, R.R. Retinal Repair by Transplantation of Photoreceptor Precursors. Nature 2006, 444, 203–207. [Google Scholar] [CrossRef]
- Payne, S.L.; Anandakumaran, P.N.; Varga, B.V.; Morshead, C.M.; Nagy, A.; Shoichet, M.S. In Vitro Maturation of Human IPSC-Derived Neuroepithelial Cells Influences Transplant Survival in the Stroke-Injured Rat Brain. Tissue Eng. Part A 2018, 24, 351–360. [Google Scholar] [CrossRef]
- Hatzimichael, E.; Tuthill, M. Hematopoietic Stem Cell Transplantation. Stem Cells Cloning 2010, 3, 105. [Google Scholar]
- Cavazzana, M.; Six, E.; Lagresle-Peyrou, C.; André-Schmutz, I.; Hacein-Bey-Abina, S. Gene Therapy for X-Linked Severe Combined Immunodeficiency: Where Do We Stand? Hum. Gene Ther. 2016, 27, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Voermans, C.; Kooi, M.L.K.; Rodenhuis, S.; Van Der Lelie, H.; Van Der Schoot, C.E.; Gerritsen, W.R. In Vitro Migratory Capacity of CD34+ Cells Is Related to Hematopoietic Recovery after Autologous Stem Cell Transplantation. Blood 2001, 97, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.M.; McGuinness, U.M.; Aguayo, A.J. Axons from CNS Neurones Regenerate into PNS Grafts. Nature 1980, 284, 264–265. [Google Scholar] [CrossRef] [PubMed]
- Peled, A.; Petit, I.; Kollet, O.; Magid, M.; Ponomaryov, T.; Byk, T.; Nagler, A.; Ben-Hur, H.; Many, A.; Shultz, L.; et al. Dependence of Human Stem Cell Engraftment and Repopulation of NOD/SCID Mice on CXCR4. Science 1999, 283, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Chakrapani, V.Y.; Gnanamani, A.; Giridev, V.R.; Madhusoothanan, M.; Sekaran, G. Electrospinning of Type i Collagen and PCL Nanofibers Using Acetic Acid. J. Appl. Polym. Sci. 2012, 125, 3221–3227. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, N.; Li, S.; Battig, M.R.; Wang, Y. Programmable Hydrogels for Controlled Cell Catch and Release Using Hybridized Aptamers and Complementary Sequences. J. Am. Chem. Soc. 2012, 134, 15716–15719. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Choi, J.B.; Jo, S.Y.; Lim, Y.M.; Gwon, H.J.; Khil, M.S.; Nho, Y.C. Characterization and Structure Analysis of PLGA/Collagen Nanofibrous Membranes by Electrospinning. J. Appl. Polym. Sci. 2012, 125, E595–E603. [Google Scholar] [CrossRef]
- Bhattarai, N.; Zhang, M. Controlled Synthesis and Structural Stability of Alginate-Based Nanofibers. Nanotechnology 2007, 18, 455601. [Google Scholar] [CrossRef]
- Sfakis, L.; Sharikova, A.; Tuschel, D.; Costa, F.X.; Larsen, M.; Khmaladze, A.; Castracane, J. Core/Shell Nanofiber Characterization by Raman Scanning Microscopy. Biomed. Opt. Express 2017, 8, 1025. [Google Scholar] [CrossRef]
- Jean-Gilles, R.; Soscia, D.; Sequeira, S.; Melfi, M.; Gadre, A.; Castracane, J.; Larsen, M. Novel Modeling Approach to Generate a Polymeric Nanofiber Scaffold for Salivary Gland Cells. J. Nanotechnol. Eng. Med. 2010, 1, 031008. [Google Scholar] [CrossRef]
- Hong, H.J.; Cho, J.M.; Yoon, Y.J.; Choi, D.; Lee, S.; Lee, H.; Ahn, S.; Koh, W.G.; Lim, J.Y. Thermoresponsive Fiber-Based Microwells Capable of Formation and Retrieval of Salivary Gland Stem Cell Spheroids for the Regeneration of Irradiation-damaged Salivary Glands. J. Tissue Eng. 2022, 13, 20417314221085645. [Google Scholar] [CrossRef] [PubMed]
- Cantara, S.; Soscia, D.; Sequeira, S.; Jean-Gilles, R.; Castracane, J.; Larsen, M. Selective Functionalization of Nanofiber Scaffolds to Regulate Salivary Gland Epithelial Cell Proliferation and Polarity. Biomaterials 2012, 33, 8372–8382. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, A.S.; Lim, S.A.; Martin, D.; Cooper-White, J.J. Polyurethane/Poly(Lactic-Co-Glycolic) Acid Composite Scaffolds Fabricated by Thermally Induced Phase Separation. Biomaterials 2007, 28, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.D.; Chan, P.K.; Feng, X. Morphology Development and Characterization of the Phase-Separated Structure Resulting from the Thermal-Induced Phase Separation Phenomenon in Polymer Solutions under a Temperature Gradient. Chem. Eng. Sci. 2004, 59, 1491–1504. [Google Scholar] [CrossRef]
- Bischof, J.C.; He, X. Thermal Stability of Proteins. Ann. N. Y. Acad. Sci. 2006, 1066, 12–33. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhang, H. Controlled Freezing and Freeze Drying: A Versatile Route for Porous and Micro-/Nano-Structured Materials. J. Chem. Technol. Biotechnol. 2011, 86, 172–184. [Google Scholar] [CrossRef]
- Ikeda, A.; Taketa, H.; Sathi, G.A.; Hirano, Y.; Iida, S.; Matsumoto, T. Functional Peptide KP24 Enhances Submandibular Gland Tissue Growth in vitro. Regen. Ther. 2016, 3, 108–113. [Google Scholar] [CrossRef]
- Taketa, H.; Sathi, G.A.; Farahat, M.; Rahman, K.A.; Sakai, T.; Hirano, Y.; Kuboki, T.; Torii, Y.; Matsumoto, T. Peptide-Modified Substrate for Modulating Gland Tissue Growth and Morphology In Vitro. Sci. Rep. 2015, 5, 11468. [Google Scholar] [CrossRef]
- Kazi, G.A.S.; Yamanaka, T.; Osamu, Y. Chitosan Coating an Efficient Approach to Improve the Substrate Surface for In Vitro Culture System. J. Electrochem. Soc. 2019, 166, B3025–B3030. [Google Scholar] [CrossRef]
- Hsiao, Y.C.; Chen, C.N.; Chen, Y.T.; Yang, T.L. Controlling Branching Structure Formation of the Salivary Gland by the Degree of Chitosan Deacetylation. Acta Biomater. 2013, 9, 8214–8223. [Google Scholar] [CrossRef]
- Yang, T.L.; Young, T.H. The Enhancement of Submandibular Gland Branch Formation on Chitosan Membranes. Biomaterials 2008, 29, 2501–2508. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.L.; Young, T.H. Chitosan Cooperates with Mesenchyme-Derived Factors Inregulating Salivary Gland Epithelial Morphogenesis. J. Cell. Mol. Med. 2009, 13, 2853–2863. [Google Scholar] [CrossRef]
- Yang, T.L.; Hsiao, Y.C. Chitosan Facilitates Structure Formation of the Salivary Gland by Regulating the Basement Membrane Components. Biomaterials 2015, 66, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.M.; Zhang, Y.; Munguia-Lopez, J.G.; Tran, S.D. Egg White Alginate as a Novel Scaffold Biomaterial for 3D Salivary Cell Culturing. Biomimetics 2021, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.; dos Santos, H.T.; Maslow, F.; Trump, B.G.; Lei, P.; Andreadis, S.T.; Baker, O.J. Laminin-1 Peptides Conjugated to Fibrin Hydrogels Promote Salivary Gland Regeneration in Irradiated Mouse Submandibular Glands. Front. Bioeng. Biotechnol. 2021, 9, 729180. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Link, J.M.; Hu, J.C.Y.; Athanasiou, K.A. The Self-Assembling Process and Applications in Tissue Engineering. Cold Spring Harb. Perspect. Med. 2017, 7, a025668. [Google Scholar] [CrossRef]
- Wei, C.; Larsen, M.; Ph, D.; Hoffman, M.P.; Ph, D.; Yamada, K.M.; Ph, D. Self-Organization and Branching Morphogenesis of Primary Salivary Epithelial Cells. Tissue Eng. 2007, 13, 721–735. [Google Scholar] [CrossRef]
- Dost, P. Ultrasonographic Biometry in Normal Salivary Glands. Eur. Arch. Oto-Rhino-Laryngol. 1997, 254, 18–19. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D Bioprinting for Engineering Complex Tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef]
- Shpichka, A.I.; Koroleva, A.V.; Deiwick, A.; Timashev, P.S.; Semenova, E.F.; Moiseeva, I.Y.; Konoplyannikov, M.A.; Chichkov, B.N. Evaluation of Vasculogenic Potential of Modified Fibrin Hydrogel. Tsitologiia 2016, 58, 785–791. [Google Scholar] [CrossRef]
- Nedvetsky, P.I.; Emmerson, E.; Finley, J.K.; Ettinger, A.; Cruz-Pacheco, N.; Prochazka, J.; Haddox, C.L.; Northrup, E.; Hodges, C.; Mostov, K.E.; et al. Parasympathetic Innervation Regulates Tubulogenesis in the Developing Salivary Gland. Dev. Cell 2014, 30, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.M.; Marga, F.; Forgacs, G.; Heesch, C.M. Biofabrication and Testing of a Fully Cellular Nerve Graft. Biofabrication 2013, 5, 045007. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, K.V.; Hoffman, M.P. Anatomy, Biogenesis and Regeneration of Salivary Glands. Monogr. Oral Sci. 2014, 24, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Yang, X.; Zhou, T.; Lieber, C.M. Mesh Electronics: A New Paradigm for Tissue-like Brain Probes. Curr. Opin. Neurobiol. 2018, 50, 33–41. [Google Scholar] [CrossRef]
- Viveros, R.D.; Zhou, T.; Hong, G.; Fu, T.M.; Lin, H.Y.G.; Lieber, C.M. Advanced One- and Two-Dimensional Mesh Designs for Injectable Electronics. Nano Lett. 2019, 19, 4180–4187. [Google Scholar] [CrossRef]
- Goldman, E.B.; Zak, A.; Tenne, R.; Kartvelishvily, E.; Levin-Zaidman, S.; Neumann, Y.; Stiubea-Cohen, R.; Palmon, A.; Hovav, A.H.; Aframian, D.J. Biocompatibility of Tungsten Disulfide Inorganic Nanotubes and Fullerene-like Nanoparticles with Salivary Gland Cells. Tissue Eng. Part A 2015, 21, 1013–1023. [Google Scholar] [CrossRef]
- Chen, X.; Wu, P.; Rousseas, M.; Okawa, D.; Gartner, Z.; Zettl, A.; Bertozzi, C.R. Boron Nitride Nanotubes Are Noncytotoxic and Can Be Functionalized for Interaction with Proteins and Cells. J. Am. Chem. Soc. 2009, 131, 890–891. [Google Scholar] [CrossRef]
- Oprych, K.M.; Whitby, R.L.D.; Mikhalovsky, S.V.; Tomlins, P.; Adu, J. Repairing Peripheral Nerves: Is There a Role for Carbon Nanotubes? Adv. Healthc. Mater. 2016, 5, 1253–1271. [Google Scholar] [CrossRef]
- Lorber, B.; Hsiao, W.K.; Hutchings, I.M.; Martin, K.R. Adult Rat Retinal Ganglion Cells and Glia Can Be Printed by Piezoelectric Inkjet Printing. Biofabrication 2014, 6, 015001. [Google Scholar] [CrossRef]
- Pateman, C. Development of Microstereolithography and Photopolymerisable Polymers for Peripheral Nerve Repair . Available online: https://etheses.whiterose.ac.uk/11795/2014 (accessed on 14 November 2023).
- Varghese, J.J.; Schmale, I.L.; Wang, Y.; Hansen, M.E.; Newlands, S.D.; Ovitt, C.E.; Benoit, D.S.W. Retroductal Nanoparticle Injection to the Murine Submandibular Gland. J. Vis. Exp. 2018, 135, 57521. [Google Scholar] [CrossRef]
- Arany, S.; Benoit, D.S.W.; Dewhurst, S.; Ovitt, C.E. Nanoparticle-Mediated Gene Silencing Confers Radioprotection to Salivary Glands In Vivo. Mol. Ther. 2013, 21, 1182–1194. [Google Scholar] [CrossRef]
- Aguado, B.A.; Mulyasasmita, W.; Su, J.; Lampe, K.J.; Heilshorn, S.C. Improving Viability of Stem Cells during Syringe Needle Flow through the Design of Hydrogel Cell Carriers. Tissue Eng. Part A 2012, 18, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.H.; Rose, F.R.A.J.; Shakesheff, K.M.; Modo, M.; White, L.J. Translational Considerations in Injectable Cell-Based Therapeutics for Neurological Applications: Concepts, Progress and Challenges. NPJ Regen. Med. 2017, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Rodell, C.B.; Kaminski, A.L.; Burdick, J.A. Rational Design of Network Properties in Guest-Host Assembled and Shear-Thinning Hyaluronic Acid Hydrogels. Biomacromolecules 2013, 14, 4125–4134. [Google Scholar] [CrossRef] [PubMed]
- Shuai, F.; Zhang, Y.; Yin, Y.; Zhao, H.; Han, X. Fabrication of an Injectable Iron (III) Crosslinked Alginate-Hyaluronic Acid Hydrogel with Shear-Thinning and Antimicrobial Activities. Carbohydr. Polym. 2021, 260, 117777. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.S.; Murphy, K.C.; Binder, B.Y.K.; Vissers, C.B.; Leach, J.K. Increased Survival and Function of Mesenchymal Stem Cell Spheroids Entrapped in Instructive Alginate Hydrogels. Stem Cells Transl. Med. 2016, 5, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Frisch, S.M.; Francis, H. Disruption of Epithelial Cell-Matrix Interactions Induces Apoptosis. J. Cell Biol. 1994, 124, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Juliano, R. Mitogenic Signal Transduction by Integrin- and Growth Factor Receptor-Mediated Pathways. Mol. Cells 2004, 17, 188–202. [Google Scholar] [PubMed]
- Plow, E.F.; Haas, T.A.; Zhang, L.; Loftus, J.; Smith, J.W. Ligand Binding to Integrins. J. Biol. Chem. 2000, 275, 21785–21788. [Google Scholar] [CrossRef]
- Pierschbacher, M.D.; Ruoslahti, E. Cell Attachment Activity of Fibronectin Can Be Duplicated by Small Synthetic Fragments of the Molecule. Nature 1984, 309, 30–33. [Google Scholar] [CrossRef]
- Somaa, F.A.; Wang, T.Y.; Niclis, J.C.; Bruggeman, K.F.; Kauhausen, J.A.; Guo, H.; McDougall, S.; Williams, R.J.; Nisbet, D.R.; Thompson, L.H.; et al. Peptide-Based Scaffolds Support Human Cortical Progenitor Graft Integration to Reduce Atrophy and Promote Functional Repair in a Model of Stroke. Cell Rep. 2017, 20, 1964–1977. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD Modified Polymers: Biomaterials for Stimulated Cell Adhesion and Beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef] [PubMed]
- Tam, R.Y.; Fuehrmann, T.; Mitrousis, N.; Shoichet, M.S. Regenerative Therapies for Central Nervous System Diseases: A Biomaterials Approach. Neuropsychopharmacology 2014, 39, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Fan, Y.; Li, X. The Use of Bioactive Peptides to Modify Materials for Bone Tissue Repair. Regen. Biomater. 2017, 4, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.F.; Singh, G.; Lesani, P.; Zreiqat, H. Promise and Perspective of Nanomaterials in Antisenescence Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2022, 8, 3133–3141. [Google Scholar] [CrossRef]
- Wissler Gerdes, E.O.; Zhu, Y.; Tchkonia, T.; Kirkland, J.L. Discovery, Development, and Future Application of Senolytics: Theories and Predictions. FEBS J. 2020, 287, 2418–2427. [Google Scholar] [CrossRef]
- Rose, S. Electronic Mesh. Available online: https://app.biorender.com/illustrations/6512ced1cee7246df2c27068 (accessed on 14 November 2023).
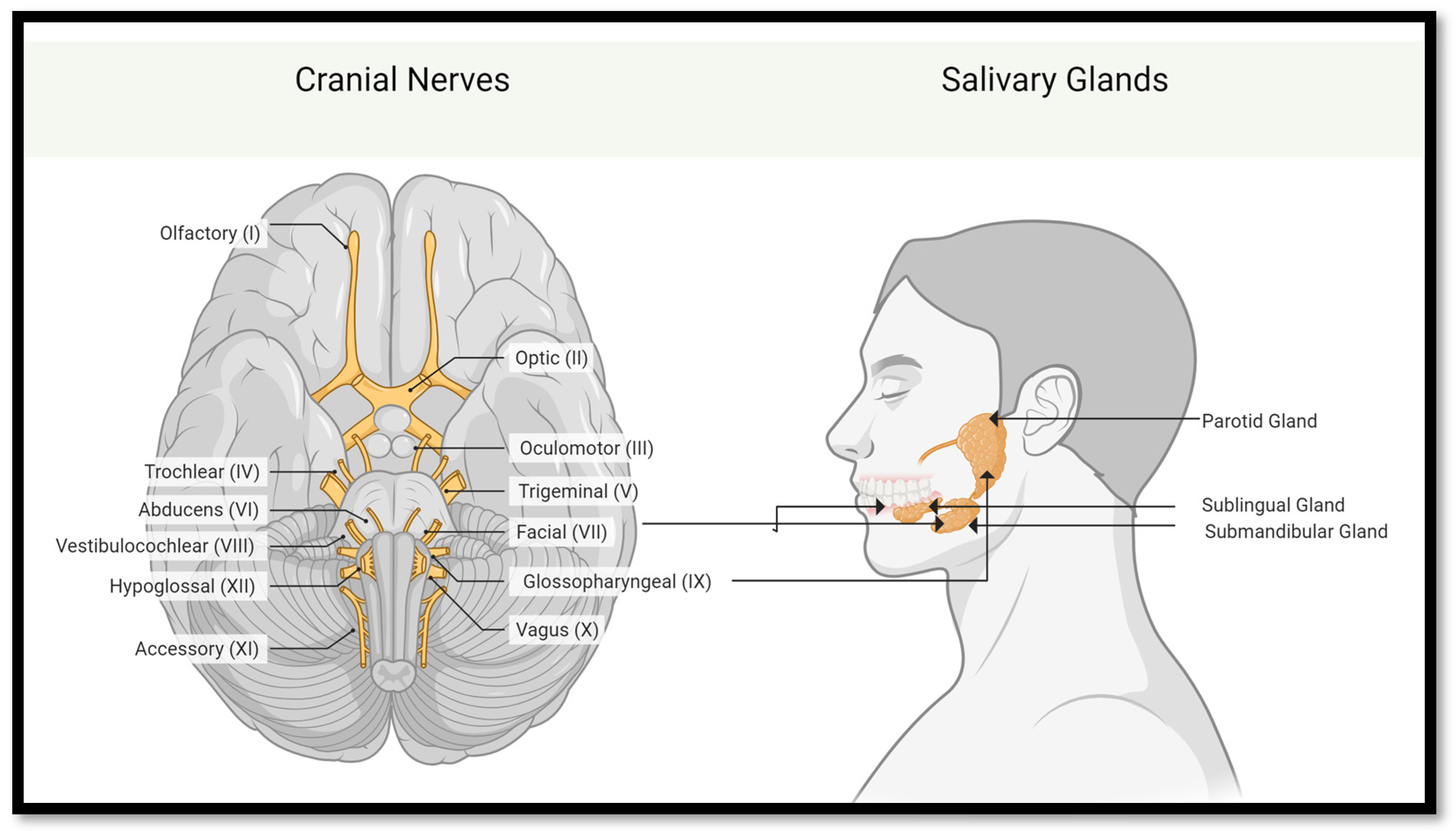
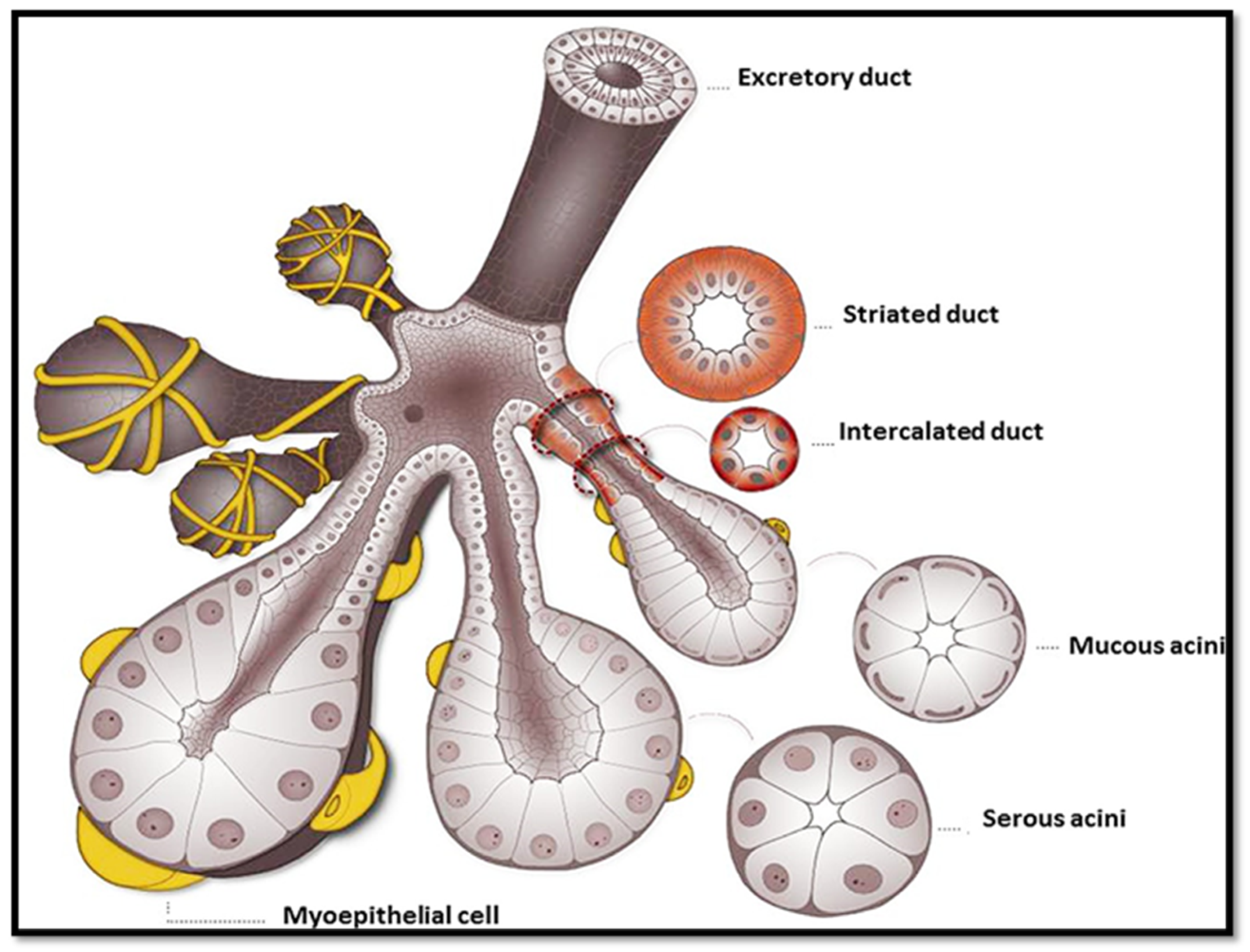
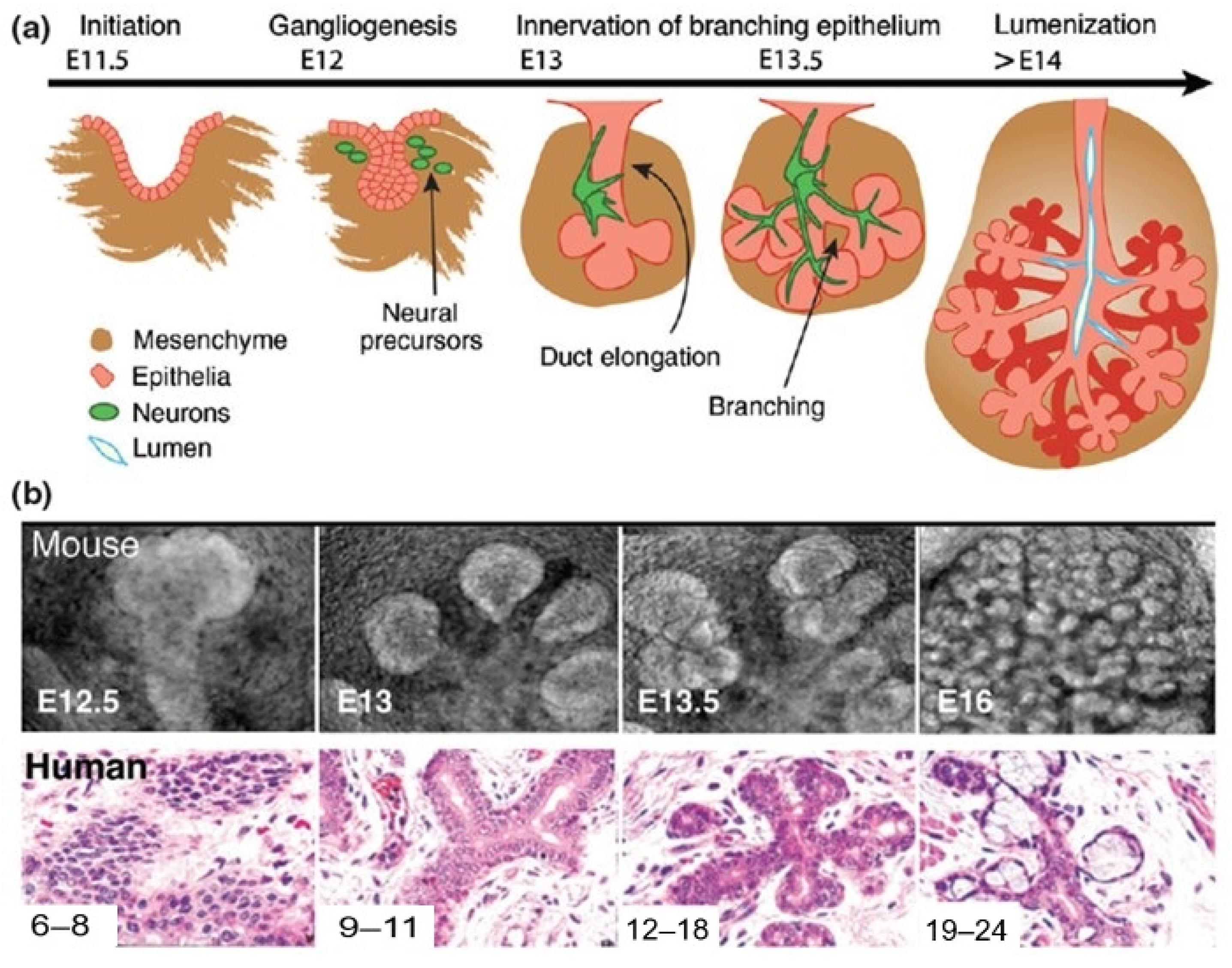
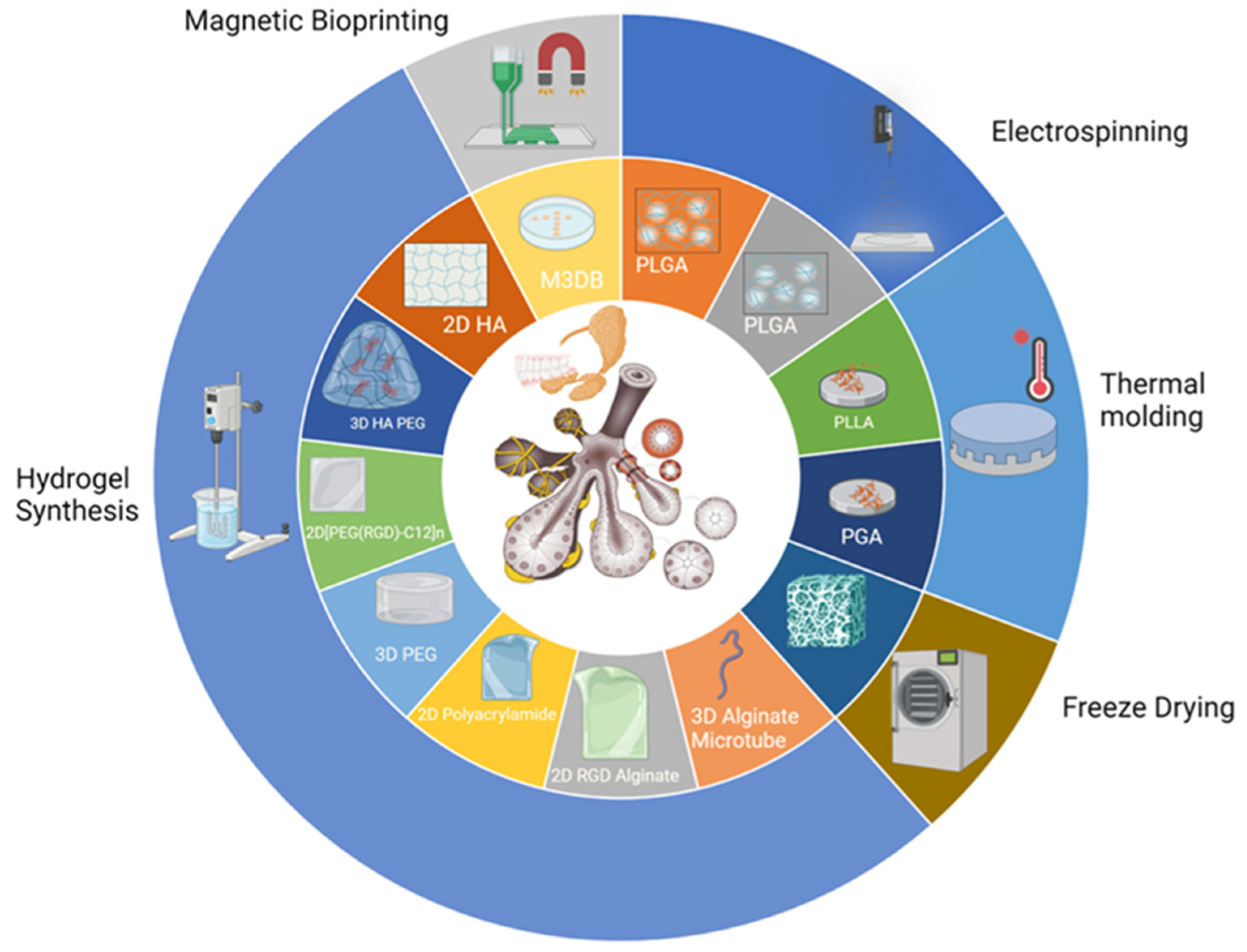
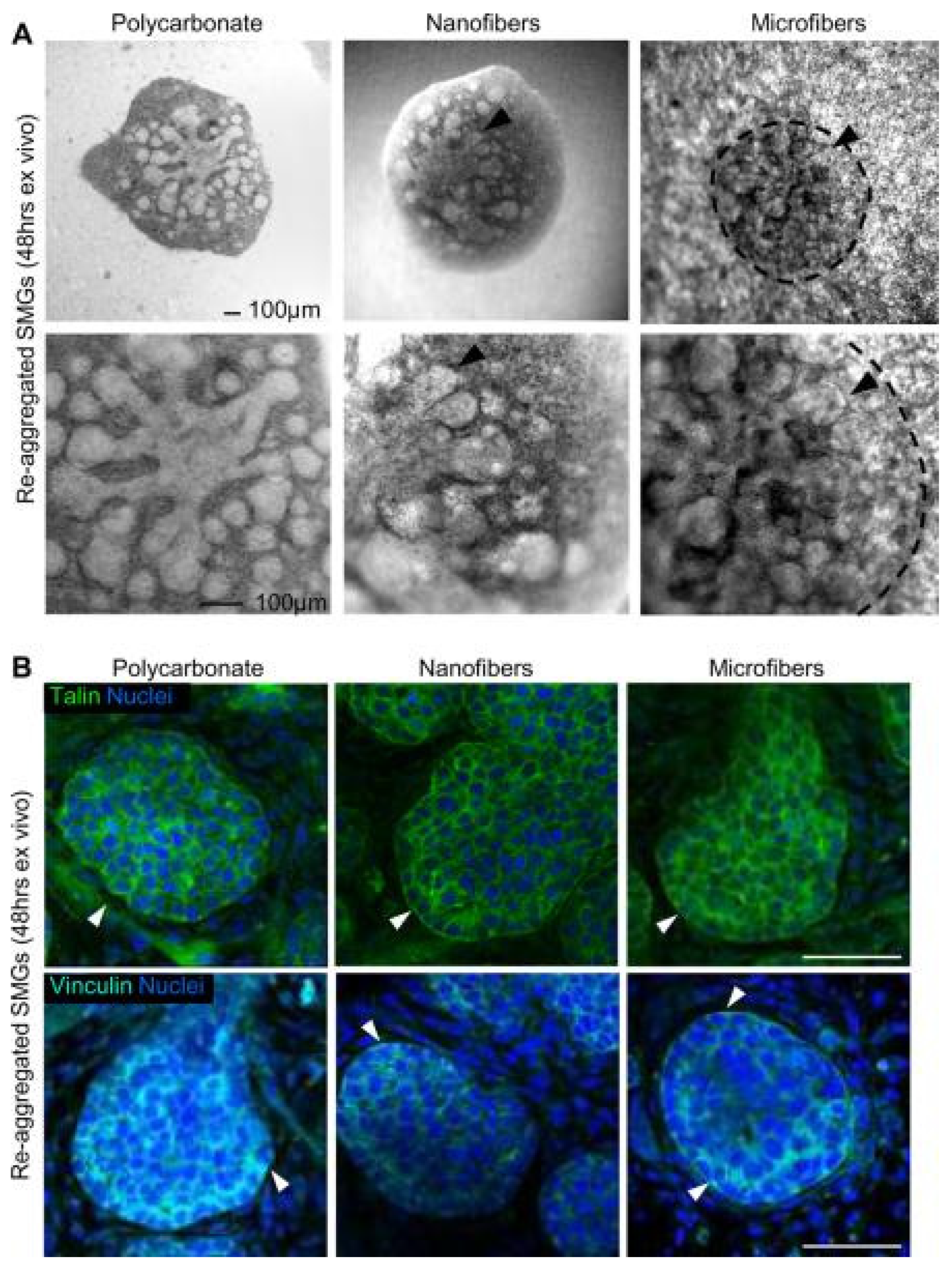


| Fabrication Method | Biomaterial and Dimensionality | Cell Type | Advantages and Disadvantages |
|---|---|---|---|
| Thermal molding | 2D PLLA and PGA (flat disks) | HSG | Pros: Biodegradable; able to form into coverslip-like disks suitable for cell seeding; versatile permitting melt-processing polymer pellets between sheets of aluminum foil using a Carver press at 350 °F, 450 °F, 175 °F, and 200 °F for PLLA, PGA, 50/50 PLGA, and 85/15 PLGA, respectively [232,233]. Cons: Require ECM coating to support cell attachment; lack 3D cues. |
| Electrospinning | PLGA (fibrous scaffolds) | SIMS | Pros: Provide topographic cues (e.g., nanofibers, curvature); exhibit more rounded and clustered cell shape vs. 2D flat disks; enhance cell-polarization effects and expression of water channel proteins [234,235]. Cons: Require laminin coating for cell polarization and tight junctions; lack in vivo-like viscoelasticity. |
| PLGA (fibrous scaffolds) | Par-C10 | ||
| Freeze-drying | Silk fibroin (porous scaffolds) | Primary salivary gland epithelial cells from rat SMG and parotid gland | Pros: Provide topographical cues; promote epithelial cell growth; facilitate the secretion of ECM proteins; retain the differentiated function [236,237]. Cons: Require fibronectin coating; mimic the basement membrane for epithelial cells but might not be ideal for stromal cell culture; lack in vivo-like viscoelasticity. |
| Hydrogel synthesis | HA hydrogels (cell culture insert) | Primary human salivary gland acinar-like cells from the parotid gland | Pros: Mimic the hydrogel component of ECM; exhibit acini-like structures with tight junctions, α-amylase expression, and an apoptotic central lumen on HA gels with an elastic modulus of 2000 Pa and incorporating peptide derived from domain IV of perlecan [238]. Cons: Require coupling bioactive peptides; or form acinar-like structures that are less organized or slowly growing in 2D or 2.5D than those in 3D HA hydrogels. |
| 3D HA/PEG hydrogels (cell culture insert) | Primary human salivary gland acinar-like cells from the parotid gland | Pros: Provide 3D microenvironment for encapsulated cells; facilitate cell self-assembly into acini-like spheroids of ~50 µm in size; demonstrate neurotransmitter-stimulated protein secretion and fluid production; integration in an in vivo rat model with no obvious signs of inflammation [239,240]. Cons: Indicate reverse polarity; lack essential machinery for full salivary restoration. | |
| [PEG(RGD)-C12]n microfibers | Human primary salivary gland myoepithelial cells | Pros: Fabricate meter-long multiblock copolymer microfibers via straightforward interfacial bioorthogonal polymerization; provide guidance cues for the attachment and elongation of myoepithelial cells [17]. Cons: Cannot use for cell encapsulation; culture cells on the surface two dimensionally rather inside the microfiber three dimensionally. | |
| 3D PEG hydrogels | A mixture of primary acinar and ductal cells from mouse SMG | Pros: Improve cell viability and proliferation and facilitate cell–cell contacts by encapsulation of pre-assembled spheroids [241]. Cons: Remain as single cells without forming organized acini-like structures after cell encapsulation in 3D PEG hydrogels. | |
| 2D polyacrylamide gels | Mouse E13 SMG | Pros: Promote branching morphogenesis; partially rescue acini structure and differentiation by transferring glands from stiff to soft gels or by adding exogenous TGFβ1 [242] Cons: Require addition of exogenous TGFβ1 to polyacrylamide gels for partial acini structure rescue; lack 3D cues. | |
| 2D RGD-modified alginate hydrogel sheet | Mouse E13 mesenchymal cells and SMG | Pros: Promote mesenchymal (not epithelial) cell adhesion by RGD surface modification; enhance the bud expansion and cleft formation in SMG by softer gels, whereas stiffer gels attenuate them and decrease gene expression of FGF7 and FGF10; partially rescue acini structure and differentiation by adding exogenous FGF7 or FGF10, or by transferring SMGs from stiff to soft gels [243]. Cons: Stiffer RGD-modified alginate hydrogel sheets attenuate bud expansion and decrease gene expression of FGF7 and FGF10. | |
| 3D Alginate hydrogel microtubes | Co-culture of SIMS with mouse NIH 3T3 fibroblasts or E16 mesenchyme cells | Pros: Provide 3D microenvironment in hydrogel that is easy to handle; allow for high density cell growth; facilitate 3D mesenchymal-epithelial interaction; allow salivary gland epithelial cell organization into 3D cavitated structures with lumen formation; exhibit potential for formation of uniform organoids and functional units [244]. Cons: Require 3D arrangement of microtubes with organoids and additional elements to construct the full machinery of the salivary gland. | |
| 2D Fibrin-based hydrogels | Par-C10 | Pros: Support differentiation of salivary gland cell clusters with mature lumens [245] Cons: 2D culture on the hydrogel surface; require laminin-111 peptide-modification. | |
| Fibrin-based hydrogels | Acellular, laminin I peptide functionalized | Pros: Mitigate the risk of tumor development; results in restoration of functional salivary tissue [246]. Cons: Require decoration with laminin-1 peptides; require injection of liquid followed by internal gelation to avoid hydrogel clogging the needle. | |
| Gelatin-based hydrogel sheet | Acellular, controlled release of growth factors (EGF, FGF, NGF) | Pros: Demonstrate atrophy and regeneration of the SMG; enable observation of effects of sustained release of physiologically active substances contained within an implanted hydrogel sheet. Cons: Collapse of the hydrogel mesh began by day 7, in conjunction with invasion of surrounding fibrotic connective tissue, without regeneration of the salivary gland tissue [247]. | |
| Cryoelectrospinning | 3D Alginate-elastin cryoelectrospinning scaffolds | NIH 3T3 fibroblasts | Pros: Produce porous nanofiber-sponge scaffolds that recapitulate the topography and viscoelasticity of salivary gland ECM; allow cell penetration deeply and 3D culture; support stromal cell viability and homeostatic marker expression [230]. Cons: Require dynamic seeding to achieve high seeding efficiency; require dynamic culture to achieve high density cell growth. |
| Bioprinting | Magnetic 3D bioprinting (M3DB) | Neural crest-derived MSCs and human dental pulp stem cells | Pros: Develop innervated secretory epithelial organoids in the presence of FGF using cells tagged with magnetic nanoparticles that are ordered using magnetic dots. Cons: Require magnetic nanoparticles; challenge to determine an apicobasal polarization due to tightly packed epithelial cells; exhibit limited vascularization in the organoids [248]. |
| Category | Details and Strategies |
|---|---|
| Origins and development |
|
| Histological features of hypofunctioning tissue |
|
| Fabrication strategies for scaffolds |
|
| Major challenges |
|
| Emerging technologies |
|
| Outlook |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rose, S.C.; Larsen, M.; Xie, Y.; Sharfstein, S.T. Salivary Gland Bioengineering. Bioengineering 2024, 11, 28. https://doi.org/10.3390/bioengineering11010028
Rose SC, Larsen M, Xie Y, Sharfstein ST. Salivary Gland Bioengineering. Bioengineering. 2024; 11(1):28. https://doi.org/10.3390/bioengineering11010028
Chicago/Turabian StyleRose, Stephen C., Melinda Larsen, Yubing Xie, and Susan T. Sharfstein. 2024. "Salivary Gland Bioengineering" Bioengineering 11, no. 1: 28. https://doi.org/10.3390/bioengineering11010028
APA StyleRose, S. C., Larsen, M., Xie, Y., & Sharfstein, S. T. (2024). Salivary Gland Bioengineering. Bioengineering, 11(1), 28. https://doi.org/10.3390/bioengineering11010028










