Predictive Model of Anxiety and Depression Perception in Multiple Sclerosis Patients: Possible Implications for Clinical Treatment
Abstract
1. Introduction
1.1. Emotional Disturbances, Anxiety and Depression
1.2. Variables Related to Anxiety and Depression in MS
1.2.1. Prefrontal Brian Activity
1.2.2. Functional Activity
1.2.3. Psychological Variables
1.3. Aim
2. Materials and Methods
2.1. Sample
2.2. Instruments and Measurements
2.3. Design and Procedure
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
3.1. Descriptive Analysis
3.2. Confirmatory Factor Analysis
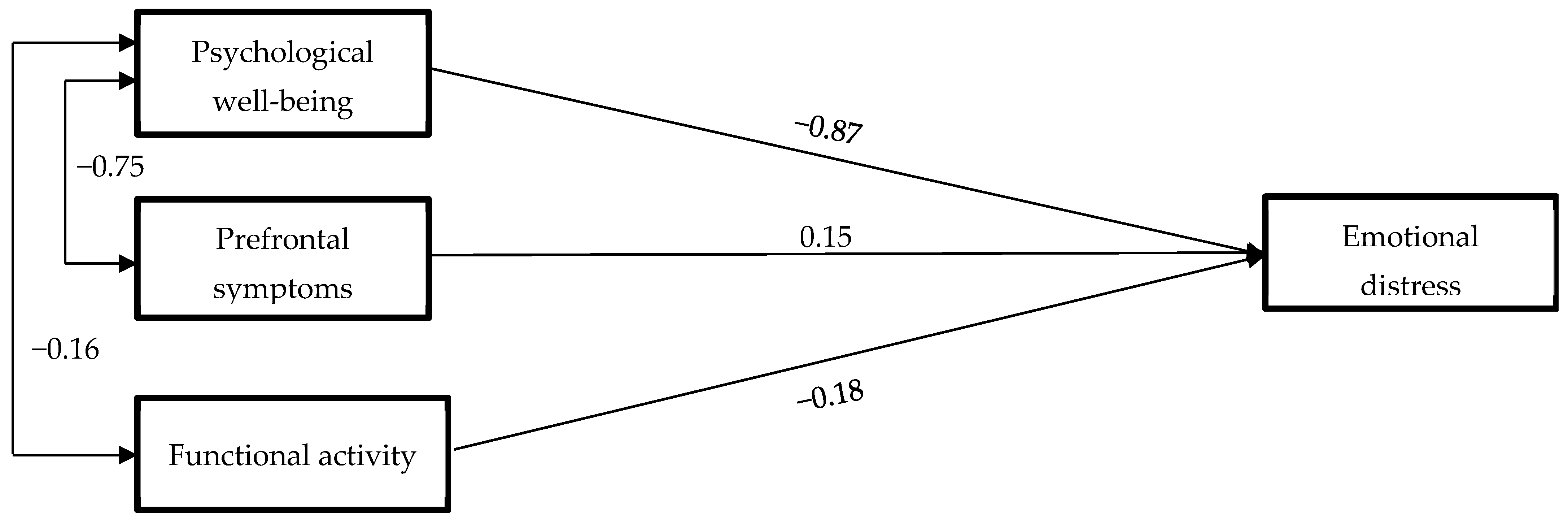
3.3. Predictive Model of Emotional Distress
4. Discussion
4.1. Prefrontal Symptoms
4.2. Functional Activity
4.3. Psychological Well-Being
4.4. Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reich, D.S.; Lucchinetti, C.F.; Calabresi, P.A. Multiple Sclerosis. N. Engl. J. Med. 2018, 11, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Carmona, N.; Fernández-Jover, E.; Pérez-Sempere, A. Epidemiología de la esclerosis múltiple en España. Rev. Neurol. 2019, 69, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Scalabrino, G. Newly Identified Deficiencies in the Multiple Sclerosis Central Nervous System and Their Impact on the Remyelination Failure. Biomedicine 2022, 10, 815. [Google Scholar] [CrossRef]
- Van Schependom, J.; D’hooghe, M.B.; Cleynhens, K.; D’hooge, M.; Haelewyck, J.; De Keyser, G.; Nagels, G. The Symbol Digit Modalities Test as sentinel test for cognitive impairment in multiple sclerosis. Eur. J. Neurol. 2014, 21, 1219–1225. [Google Scholar] [CrossRef]
- Lozano-Soto, E.; Cruz-López, Á.J.; Gutiérrez, R.; González, M.; Sanmartino, F.; Rashid-López, R.; Espinossa-Tosso, R.; Forero, L.; González-Rosa, J. Predicting Neuropsychological Impairment in Relapsing Remitting Multiple Sclerosis: The Role of Clinical Measures, Treatment, and Neuropsychiatry Symptoms. Arch. Clin. Neuropsychol. 2021, 36, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Teng, T.; Zhang, Y.; Del Giovane, C.; Furukawa, T.A.; Weisz, J.R.; Li, X.; Cuijpers, P.; Coghill, D.; Xiang, Y.; et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: A systematic review and network meta-analysis. Lancet Psychiatr. 2020, 7, 581–601. [Google Scholar] [CrossRef] [PubMed]
- Nathoo, N.; Mackie, A. Treating depression in multiple sclerosis with antidepressants: A brief review of clinical trials and exploration of clinical symptoms to guide treatment decisions. Mult. Scler. Relat. Disord. 2017, 18, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Fiest, K.M.; Walker, J.R.; Bernstein, C.N.; Graff, L.A.; Zarychanski, R.; Abou-Setta, A.M.; Patten, S.B.; Sareen, J.; Bolton, J.M.; Marriott, J.J.; et al. Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Systematic review and meta-analysis of interventions for depression and anxiety in persons with multiple sclerosis. Mult. Scler. Relat. Disord. 2016, 5, 12–26. [Google Scholar] [CrossRef]
- Mustač, F.; Pašić, H.; Medić, F.; Bjedov, B.; Vujević, L.; Alfirević, M.; Vidrih, B.; Tudor, K.I.; Bošnjak, P. Anxiety and Depression as Comorbidities of Multiple Sclerosis. Psychiatr. Danub. 2021, 33, 480–485. [Google Scholar]
- Brasanac, J.; Chien, C. A review on multiple sclerosis prognostic findings from imaging, inflammation, and mental health studies. Front. Hum. Neurosci. 2023, 17, 1151531. [Google Scholar] [CrossRef]
- Vacaras, V.; Văcăraș, V.; Nistor, C.; Văcăraș, D.; Opre, A.; Blaga, P.; Mureșanu, D. The Influence of Depression and Anxiety on Neurological Disability in Multiple Sclerosis Patients. Behav. Neurol. 2020, 2020, 6738645. [Google Scholar] [CrossRef]
- Ozdemir, P.G.; Milanlioglu, A.; Boysan, M.; Cilingir, V.; Aydin, N.; Atli, A. Relations between mood characteristics, circadian preferences, and functionality in multiple sclerosis. Int. J. Psychiatry Clin. Pract. 2015, 19, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Sabanagic-Hajric, S.; Memic-Serdarevic, A.; Sulejmanpasic, G.; Salihovic-Besirovic, D.; Kurtovic, A.; Bajramagic, N.; Mehmedika-Suljic, E. Cognitive Imapirment in Multiple Sclerosis: Relation to Dysability, Duration and Type of Disease. Mater. Sociomed. 2023, 35, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.E.; Gencöz, T.; Wells, A. Unique contributions of metacognition and cognition to depressive symptoms. J. Genl. Psychol. 2015, 142, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Tyszka, E.; Bozinov, N.; Briggs, F. Characterizing Relationships Between Cognitive, Mental, and Physical Health and Physical Activity Levels in Persons with Multiple Sclerosis. Int. J. MS Care 2022, 24, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Peres, D.; Rodrigues, P.; Viero, F.; Frare, J.; Kudsi, S.; Meira, G.; Trevisan, G. Prevalence of depression and anxiety in the different clinical forms of multiple sclerosis and associations with disability: A systematic review and meta-analysis. Brain Behav. Immun. Health 2022, 24, 100484. [Google Scholar] [CrossRef]
- Davis, W.; van Rensburg, S.; Cronje, F.; Whati, L.; Fisher, L.; van der Merwe, L.; Geiger, D.; Hassan, M.; Matsha, T.; Erasmus, R.; et al. The fat mass and obesity-associated FTO rs9939609 polymorphism is associated with elevated homocysteine levels in patients with multiple sclerosis screened for vascular risk factors. Metab. Brain Dis. 2014, 29, 409–419. [Google Scholar] [CrossRef]
- Meyer-Arndt, L.; Kuchling, J.; Brasanac, J.; Hermann, A.; Asseyer, S.; Bellmann-Strobl, J.; Paul, F.; Gold, S.M.; Weygandt, M. Prefrontal-amygdala emotion regulation and depression in multiple sclerosis. Brain Commun. 2022, 13, fcac152. [Google Scholar] [CrossRef]
- Bogosian, A.; Hughes, A.; Norton, S.; Silber, E.; Moss-Morris, R. Potential treatment mechanisms in a mindfulness-based intervention for people with progressive multiple sclerosis. Br. J. Health Psychol. 2016, 21, 859–880. [Google Scholar] [CrossRef]
- Potter, K.; Golijana-Moghaddam, N.; Evangelou, N.; Mhizha-Murira, J.; das Nair, R. Self-help Acceptance and Commitment Therapy for Carers of People with Multiple Sclerosis: A Feasibility Randomised Controlled Trial. J. Clin. Psychol. Med. Settings 2021, 28, 279–294. [Google Scholar] [CrossRef]
- Rochefort, C.; Baldwin, A.S.; Chmielewski, M. Experiential Avoidance: An Examination of the Construct Validity of the AAQ-II and MEAQ. Behav. Ther. 2018, 49, 435–449. [Google Scholar] [CrossRef]
- Edwards, V.; Vari, C.; Rose, M.; Graham, C.D.; O’Connell, N.; Taylor, E.; McCracken, L.; Radunovic, A.; Rakowicz, W.; Norton, S.; et al. Participant experiences of guided self-help Acceptance and Commitment Therapy for improving quality of life in muscle disease: A nested qualitative study within the ACTMus randomized controlled trial. Front. Psychol. 2023, 14, 1233526. [Google Scholar] [CrossRef]
- Pakenham, K.I.; Fleming, M. Relations between acceptance of multiple sclerosis and positive and negative adjustments. Psychol. Health 2011, 26, 1292–1309. [Google Scholar] [CrossRef] [PubMed]
- Farb, N.; Anderson, A.; Ravindran, A.; Hawley, L.; Irving, J.; Mancuso, E.; Gulamani, T.; Williams, G.; Ferguson, A.; Segal, Z.V. Prevention of relapse/recurrence in major depressive disorder with either mindfulness-based cognitive therapy or cognitive therapy. J. Consult. Clin. Psychol. 2018, 86, 200–204. [Google Scholar] [CrossRef]
- Fresco, D.; Moore, M.; van Dulmen, M.; Segal, Z.; Ma, S.; Teasdale, J.; Williams, J. Initial psychometric properties of the experiences questionnaire: Validation of a self-report measure of decentering. Behav. Ther. 2007, 38, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Lo, P.-H.; Chen, S.-M. Electrochemical selective determination of ascorbic acid at redox active polymer modified electrode derived from direct blue 71. Biosens. Bioelectron. 2008, 24, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Strauss, C.; Taylor, B.; Gu, J.; Kuyken, W.; Baer, R.; Jones, F.; Cavanagh, K. What is compassion and how can we measure it? A review of definitions and measures. Clin. Psychol. Rev. 2016, 47, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Neff, K. The development and validation of a scale to measure self-compassion. Self. Identity 2003, 2, 223–250. [Google Scholar] [CrossRef]
- Lopez, A.; Sanderman, R.; Ranchor, A.V.; Schroevers, M. Compassion for others and self-compassion: Levels, correlates, and relationship with psychological well- being. Mindfulness 2018, 9, 325–331. [Google Scholar] [CrossRef]
- Hofmann, S.; Gómez, A. Mindfulness-Based Interventions for Anxiety and Depression. Psychiatr. Clin. N. Am. 2017, 40, 739–749. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating Neurologic Impairment in Multiple Sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983, 33, 1444. [Google Scholar] [CrossRef] [PubMed]
- Confavreux, C.; Vukusic, S. Natural history of multiple sclerosis: A unifying concept. Brain 2006, 129 Pt 3, 606–616. [Google Scholar] [CrossRef]
- Pfeffer, R.I.; Kurosaki, T.T.; Harrah, C.H., Jr.; Chance, J.M.; Filos, S. Measurement of functional activities in older adults in the community. J. Gerontol. 1982, 37, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Contador, I.; Fernández-Calvo, B.; Rueda-Revé, L.; Olazarán, J.; Bermejo-Pareja, F. Characterizing functional alterations in instrumental activities of daily living using latent class analysis: A population-based study (NEDICES). Aging Ment. Health 2020, 24, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Bond, F.; Hayes, S.; Baer, R.; Carpenter, K.; Guenole, N.; Orcutt, H.; Waltz, T.; Zettle, R. Preliminary psychometric properties of the Acceptance and Action Questionnaire-II: A revised measure of psychological inflexibility and experiential avoidance. Behav. Ther. 2011, 42, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, F.; Langer Herrera, Á.; Luciano, C.; Cangas, A.; Beltrán, I. Measuring experiential avoidance and psychological inflexibility: The Spanish version of the Acceptance and Action Questionnaire-II. Psicothema 2013, 25, 123–129. [Google Scholar] [CrossRef]
- Soler, J.; Franquesa, A.; Feliu-Soler, A.; Cebolla, A.; García-Campayo, J.; Tejedor, R.; Portella, M. Assessing decentering: Validation, psychometric properties, and clinical usefulness of the experiences questionnaire in a Spanish sample. Behav. Ther. 2014, 45, 863–871. [Google Scholar] [CrossRef]
- Garcia-Campayo, J.; Navarro-Gil, M.; Andrés, E.; Montero-Marin, J.; López-Artal, L.; Marcos, M.; Demarzo, P. Validación de las versiones en español de 26 items y 12 items de la escala de autocompasión. Health Qual. Life Outcomes 2014, 12, 1–9. [Google Scholar]
- Raes, F.; Pommier, E.; Neff, K.D.; Van Gucht, D. Construction and factorial validation of a short form of the self-compassion scale. Clin. Psychol. Psychother. 2011, 18, 250–255. [Google Scholar] [CrossRef]
- Neff, K.; Pisitsungkagarn, K.; Hsieh, Y. Self-compassion and self-construal in the United States, Thailand, and Taiwan. J. Cross-Cult. Psychol. 2008, 39, 267–285. [Google Scholar] [CrossRef]
- Sanz, J.; Vázquez, C. Fiabilidad, validez y datos normativos del inventario de depresión de Beck. Psicothema 1998, 10, 303–318. [Google Scholar]
- Spielberger, C. State-Trait Anxiety Inventory. In The Corsini Encyclopedia of Psychology; Wiley Online Library: Hoboken, NJ, USA, 2010. [Google Scholar]
- Spielberger, C.; Díaz-Guerrero, R. Idare: Inventario de Ansiedad: Rasgo-Estado; Editorial El Manual Moderno: Mexico City, Mexico, 1975. [Google Scholar]
- Knowles, K.; Olatunji, B. Specificity of trait anxiety in anxiety and depression: Meta-analysis of the State-Trait Anxiety Inventory. Clin. Psychol. Rev. 2020, 82, 101928. [Google Scholar] [CrossRef] [PubMed]
- Pedrero-Pérez, E.; Ruiz, J.; Rojo, G.; Morales, S.; Pedrero-Aguilar, J.; Lorenzo, I.; González, A. Inventario de síntomas prefrontales (ISP): Validez ecológica y convergencia con medidas neuropsicológicas. Rev. Neurol. 2016, 63, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, J.L. SPSS User’s Guide; Amos: Sidney, OH, USA, 2006. [Google Scholar]
- Bentler, P.; Bonett, D. Significance tests and goodness of fit in the analysis of covariance structures. Acad. Psychol. 1980, 88, 588–606. [Google Scholar] [CrossRef]
- Jöreskog, K.; Sörbom, D. LISREL 8: User’s Guide; Scientific Software International: Chapel Hill, NC, USA, 1993. [Google Scholar]
- Hu, L.; Bentler, P. Cutoff criteria for fi t indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. A Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Steiger, J. Structural model evaluation modification: An interval estimation approach. Multivar. Behav. Res. 1990, 25, 173–180. [Google Scholar] [CrossRef]
- Hair, J.; Anderson, R.; Tatham, R.; Black, W. Análisis Multivariante; Prentice Hall: Hoboken, NY, USA, 1999. [Google Scholar]
- Bentler, P. Comparative fit indexes in structural models. Psychol. Bull. 1990, 107, 238–246. [Google Scholar] [CrossRef]
- Tucker, L.; Lewis, C. A reliability coefficient for maximum likelihood factor analysis. Psychometrika 1973, 38, 1–10. [Google Scholar] [CrossRef]
- Byrne, B. Structural Equation Modeling with AMOS Basic Concepts, Applications, and Programming, 3rd ed.; Lawrence Erlbaum: Mahwah, NY, USA, 2016. [Google Scholar]
- MacCallum, R.; Widaman, K.; Zhang, S.; Hong, S. Sample size in factor analysis. Psychol. Methods 1999, 4, 84–99. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- West, S.; Finch, J.; Curran, P. Structural Equation Models with Non-Normal Variables. In Structural Equation Modeling: Concepts, Issues and Applications; Hoyle, R.H., Ed.; Sage: New York, NY, USA, 1995; pp. 56–75. [Google Scholar]
- Bollen, K.; Stine, R. Bootstrapping Goodness-of-Fit Measures in Structural Equation Models. In Testing Structural Equation Models; Bollen, K.A., Long, J.S., Eds.; Sage: New York, NY, USA, 1993; pp. 111–135. [Google Scholar]
- Wallis, O.; Bol, Y.; Köhler, S.; Van Heugten, C. Anxiety in multiple sclerosis is related to depressive symptoms and cognitive complaints. Act. Neurol. Scand. 2020, 141, 212–218. [Google Scholar] [CrossRef]
- Leavitt, V.M.; Brandstadter, R.; Fabian, M.; Sand, I.K.; Klineova, S.; Krieger, S.; Lewis, C.; Lublin, F.; Miller, A.; Pelle, G.; et al. Dissociable cognitive patterns related to depression and anxiety in multiple sclerosis. Mult. Scler. J. 2019, 26, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Beier, M.; Amtmann, D.; Ehde, D. Beyond depression: Predictors of self-reported cognitive function in adults living with MS. Rehabil. Psychol. 2015, 60, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Lo Buono, V.; Corallo, F.; Bonanno, L.; Pria, D.; Di Cara, M.; Palmeri, R.; D’Aleo, G.; Rifici, C.; Sessa, E.; Marino, S.; et al. Psychological symptoms in Multiple Sclerosis and the role of marital status: Results from a retrospective single-center study. Mult. Scler. Relat. Disord. 2023, 79, 105051. [Google Scholar] [CrossRef] [PubMed]
- Alswat, A.; Altirkistani, B.; Alserihi, A.; Baeshen, O.; Alrushid, E.; Alkhudair, J.; Aldbas, A.; Wadaan, O.; Alsaleh, A.; Al Malik, Y.; et al. The prevalence of major depression and generalized anxiety disorder in patients with multiple sclerosis in Saudi Arabia: A cross-sectional multicentered study. Front. Psychiatr. 2023, 29, 1195101. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Moock, S.; Feng, Y.S.; Maeurer, M.; Dippel, F.W.; Kohlmann, T. Systematic literature review and validity evaluation of the expanded disability status scale (EDSS) and the multiple sclerosis functional composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 2014, 14, 58. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Wei, Z.; Chen, X.; Zhuang, X.; Yi, L. The prevalence and risk factors of anxiety in multiple sclerosis: A systematic review and meta-analysis. Front. Neurosci. 2023, 17, 1120541. [Google Scholar] [CrossRef]
- Rezapour, A.; Almasian Kia, A.; Goodarzi, S.; Hasoumi, M.; Nouraei Motlagh, S.; Vahedi, S. The impact of disease characteristics on multiple sclerosis patients’ quality of life. Epidemiol. Health 2017, 39, e2017008. [Google Scholar] [CrossRef]
- McCracken, L.; Gutiérrez-Martínez, O.; Smyth, C. “Decentering” reflects psychological flexibility in people with chronic pain and correlates with their quality of functioning. Health Psychol. 2013, 32, 820. [Google Scholar] [CrossRef]
- Karekla, M.; Forsyth, J.; Kelly, M. Emotional avoidance and panicogenic responding to a biological challenge procedure. Behav. Ther. 2004, 35, 725–746. [Google Scholar] [CrossRef]
- Roemer, L.; Lee, J.; Salters-Pedneault, K.; Erisman, S.; Orsillo, S.; Mennin, D. Mindfulness and emotion regulation difficulties in generalized anxiety disorder: Preliminary evidence for independent and overlapping contributions. Behav. Ther. 2009, 40, 142–154. [Google Scholar] [CrossRef]
- Yela, J.; Crego, A.; Buz, J.; Sánchez-Zaballos, E.; Gómez-Martínez, M.Á. Reductions in experiential avoidance explain changes in anxiety, depression and well-being after a mindfulness and self-compassion (MSC) training. Psychol. Psychot. 2022, 95, 402–422. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Pinto-Gouveia, J. Experiential avoidance and self-compassion in chronic pain. J. Appl. Soc. Psychol. 2013, 43, 1578–1591. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, C.; Coto-Lesmes, R.; Martínez-Loredo, V.; Cuesta-Izquierdo, M. Psychological Inflexibility, Anxiety and Depression: The Moderating Role of Cognitive Fusion, Experiential Avoidance and Activation. Psicothema 2022, 34, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.; Gomes, C.; Rodrigues, A.; da Motta, C. Neuropathic pain, cognitive fusion, and alexithymia in patients with multiple sclerosis: Cross-sectional evidence for an explanatory model of anxiety symptoms. J. Clin. Psychol. 2023, 79, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Eghbali, B.B.; Saadat, S.; Hasanzadeh, K.; Pourramzani, A.; Khatami, S.S.; Saberi, A.; Jafroudi, M. Relationship between self-compassion and psychological well-being with the mediating role of resilience in people with multiple sclerosis. Postep. Psychiatr. Neurol. 2022, 31, 43–51. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Zhang, G.; Jin, J.; Zheng, Z. The effect of CBT and its modifications for relapse prevention in major depressive disorder: A systematic review and meta-analysis. BMC Psychiatry 2018, 18, 50. [Google Scholar] [CrossRef]
- Newby, J.M.; Williams, A.D.; Andrews, G. Reductions in negative repetitive thinking and metacognitive beliefs during transdiagnostic internet cognitive behavioural therapy (iCBT) for mixed anxiety and depression. Behav. Res. Ther. 2014, 59, 52–60. [Google Scholar] [CrossRef]
- Dragan, M.; Dragan, W. Temperament and anxiety: The mediating role of metacognition. J. Psychopathol. Behav. Assess 2014, 36, 246–254. [Google Scholar] [CrossRef]
- Getch, J.; Kessel, R.; Forkmann, T.; Gaugel, S.; Drueke, B.; Scherer, A.; Mainz, V. A mediational model of mindfulness and decentering: Sequential psychological constructs or one and the same? Biomed. Psychol. 2014, 2, 1–13. [Google Scholar] [CrossRef]
- Dickens, H.; Bruehl, S.; Rao, U.; Myers, H.; Goodin, B.; Huber, F.; Nag, S.; Carter, C.; Karlson, C.; Kinney, K.; et al. Cognitive-Affective-Behavioral Pathways Linking Adversity and Discrimination to Daily Pain in African-American Adults. J. Racial. Ethn. Health Disparities 2023, 10, 2718–2730. [Google Scholar] [CrossRef]
- Igarashi, N.; Karam, C.; Afonso, R.; Carneiro, F.; Lacerda, S.; Santos, B.; Kozasa, E.; Rangel, É. The effects of a short-term meditation-based mindfulness protocol in patients receiving hemodialysis. Psychol. Health Med. 2022, 27, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, C.; Baker, B. Effects of Mindful Self-Compassion Program on Psychological Well-being and Levels of Compassion in People Affected by Breast Cancer. Altern. Ther. Health Med. 2023, 29, 36–41. [Google Scholar] [PubMed]
- Pinto-Gouveia, J.; Duarte, C.; Matos, M.; Fraguas, S. The protective role of self-compassion in relation to psychopathology symptoms and quality of life in chronic and in cancer patients. Clin. Psychol. Psychother. 2014, 21, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Ozonder, I.; Ordu, C. Alexithymia, Self-Compassion, Emotional Resilience, and Cognitive Emotion Regulation: Charting the Emotional Journey of Cancer Patients. Curr. Oncol. 2023, 30, 8872–8887. [Google Scholar] [CrossRef] [PubMed]
- Dahmardeh, H.; Sadooghiasl, A.; Mohammadi, E.; Kazemnejad, A. The experiences of patients with multiple sclerosis of self-compassion: A qualitative content analysis. Biomedicine 2021, 11, 35–42. [Google Scholar] [CrossRef]
- Carletto, S.; Cavalera, C.; Sadowski, I.; Rovaris, M.; Borghi, M.; Khoury, B.; Ostacoli, L.; Pagnini, F. Mindfulness-Based Interventions for the Improvement of Well-Being in People with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Psychosom. Med. 2020, 82, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Kabat-Zinn, J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness; Dell Publishing: New York, NY, USA, 1990. [Google Scholar]
- Ortí, J.E.; Cuerda-Ballester, M.; Drehmer, E.; Carrera-Juliá, S.; Motos-Muñoz, M.; Cunha-Pérez, C.; Benlloch, M.; López-Rodríguez, M.M. Vitamin B1 Intake in Multiple Sclerosis Patients and its Impact on Depression Presence: A Pilot Study. Nutrients 2020, 12, 2655. [Google Scholar] [CrossRef]
- Platero, J.L.; Cuerda-Ballester, M.; Sancho-Cantus, D.; Benlloch, M.; Ceron, J.J.; Peres, C.; García-Pardo, M.P.; López-Rodríguez, M.M.; de la Rubia Ortí, J.E. The Impact of Epigallocatechin Gallate and Coconut Oil Treatment on Cortisol Activity and Depression in Multiple Sclerosis Patients. Life 2021, 11, 353. [Google Scholar] [CrossRef]
- Platero, J.L.; Cuerda-Ballester, M.; Ibáñez, V.; Sancho-Cantus, D.; Lopez-Rodríguez, M.M.; Drehmer, E.; Ortí, J.E. The Impact of Coconut Oil and Epigallocatechin Gallate on the Levels of IL-6, Anxiety and Disability in Multiple Sclerosis Patients. Nutrients 2020, 12, 305. [Google Scholar] [CrossRef]
- Ishwarya, M.; Narendhirakannan, R.T. The Advances in Neurobiology. Adv. Neurobiol. 2016, 12, 293–306. [Google Scholar] [CrossRef]
- Mabrouk, M.; El Ayed, M.; Démosthènes, A.; Aissouni, Y.; Aouani, E.; Daulhac-Terrail, L.; Mokni, M.; Bégou, M. Antioxidant effect of grape seed extract corrects experimental autoimmune encephalomyelitis behavioral dysfunctions, demyelination, and glial activation. Front. Immunol. 2022, 13, 960355. [Google Scholar] [CrossRef]
- Ensari, I.; Motl, R.W.; Pilutti, L.A. Exercise training improves depressive symptoms in people with multiple sclerosis: Results of a meta-analysis. J. Psychosom. Res. 2014, 76, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.; Pilutti, L. The effect of exercise training in adults with multiple sclerosis with severe mobility disability: A systematic review and future research directions. Mult. Scler. Relat. Disord. 2017, 16, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Latimer-Cheung, A.; Ginis, K.; Hicks, A.; Motl, R.; Pilutti, L.; Duggan, M.; Wheeler, G.; Persad, R.; Smith, K. Development of evidence-informed physical activity guidelines for adults with multiple sclerosis. Arch. Phys. Med. Rehabil. 2013, 94, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Tarakci, E.; Yeldan, I.; Huseyinsinoglu, B.; Zenginler, Y.; Eraksoy, M. Group exercise training for balance, functional status, spasticity, fatigue and quality of life in multiple sclerosis: A randomized controlled trial. Clin. Rehabil. 2013, 27, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Carling, A.; Forsberg, A.; Gunnarsson, M.; Nilsagård, Y. CoDuSe group exercise programme improves balance and reduces falls in people with multiple sclerosis: A multi-centre, randomized, controlled pilot study. Mult. Scler. J. 2016, 23, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Learmonth, Y.; Marshall-McKenna, R.; Paul, L.; Mattison, P.; Miller, L. A qualitative exploration of the impact of a 12-week group exercise class for those moderately affected with multiple sclerosis. Disabil. Rehabil. 2013, 35, 81–88. [Google Scholar] [CrossRef]
- Del Ser, T.; Sánchez, F.; García, M.; Otero, A.; Zunzunegui, M.; Muñoz, D. Spanish version of the 7 Minute screening neurocognitive battery. Normative data of an elderly population sample over 70. Neurologia 2004, 19, 344–358. [Google Scholar]
- Reitan, R.M. Validity of the Trail Making Test as an indicator of organic brain damage. Percept. Mot. Ski. 1958, 8, 271–276. [Google Scholar] [CrossRef]


| Measurements | M | SD | Asymmetry | Kurtosis | h2 Models |
|---|---|---|---|---|---|
| Functional Activity | 5.77 | 8.67 | 1.92 | 3.10 | |
| Acceptance Action Questionnaire | 23.98 | 10.24 | 0.42 | −0.62 | 1.00 |
| Self-Compassion Scale | 36.90 | 6.33 | −0.63 | 1.83 | 0.08 |
| Mindfulness Questionnaire | 44.50 | 7.52 | −0.39 | 0.99 | 0.07 |
| Experiences Questionnaire | 38.41 | 7.52 | 0.05 | 0.18 | 0.27 |
| Inventory of Prefrontal Symptoms | 54.48 | 27.91 | 0.28 | −0.65 | |
| State Anxiety | 20.02 | 13.07 | 0.78 | 0.38 | 0.46 |
| Trait Anxiety | 25.64 | 12.69 | 0.33 | −0.71 | 1.00 |
| Depression | 13.52 | 9.82 | 0.76 | −0.21 | 0.71 |
| χ2/df | GFI | NFI | CFI | TLI | RMSEA | SRMR | Residues ≥ |±2.58| | |
|---|---|---|---|---|---|---|---|---|
| Confirmatory model a | 2.761 | 0.979 | 0.968 | 0.978 | 0.866 | 0.167 | 0.090 | 0.00% |
| Confirmatory model b | 9.314 | 0.916 | 0.926 | 0.933 | 0.798 | 0.363 | 0.060 | 0.00% |
| Predictive model | 1.981 | 0.985 | 0.992 | 0.996 | 0.977 | 0.125 | 0.082 | 0.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuerda-Ballester, M.; Bustos, A.; Sancho-Cantus, D.; Martínez-Rubio, D.; Privado, J.; Alarcón-Jiménez, J.; Villarón-Casales, C.; de Bernardo, N.; Navarro Illana, E.; de la Rubia Ortí, J.E. Predictive Model of Anxiety and Depression Perception in Multiple Sclerosis Patients: Possible Implications for Clinical Treatment. Bioengineering 2024, 11, 100. https://doi.org/10.3390/bioengineering11010100
Cuerda-Ballester M, Bustos A, Sancho-Cantus D, Martínez-Rubio D, Privado J, Alarcón-Jiménez J, Villarón-Casales C, de Bernardo N, Navarro Illana E, de la Rubia Ortí JE. Predictive Model of Anxiety and Depression Perception in Multiple Sclerosis Patients: Possible Implications for Clinical Treatment. Bioengineering. 2024; 11(1):100. https://doi.org/10.3390/bioengineering11010100
Chicago/Turabian StyleCuerda-Ballester, María, Antonio Bustos, David Sancho-Cantus, David Martínez-Rubio, Jesús Privado, Jorge Alarcón-Jiménez, Carlos Villarón-Casales, Nieves de Bernardo, Esther Navarro Illana, and José Enrique de la Rubia Ortí. 2024. "Predictive Model of Anxiety and Depression Perception in Multiple Sclerosis Patients: Possible Implications for Clinical Treatment" Bioengineering 11, no. 1: 100. https://doi.org/10.3390/bioengineering11010100
APA StyleCuerda-Ballester, M., Bustos, A., Sancho-Cantus, D., Martínez-Rubio, D., Privado, J., Alarcón-Jiménez, J., Villarón-Casales, C., de Bernardo, N., Navarro Illana, E., & de la Rubia Ortí, J. E. (2024). Predictive Model of Anxiety and Depression Perception in Multiple Sclerosis Patients: Possible Implications for Clinical Treatment. Bioengineering, 11(1), 100. https://doi.org/10.3390/bioengineering11010100









