Substrate Stiffness Modulates TGF-β Activation and ECM-Associated Gene Expression in Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Preparation of PDMS Substrates with Various Substrate Stiffness for Cell Culture
2.3. RNA Isolation and Real-Time PCR
2.4. CCL64 Assay
2.5. Western Blot Asaay
2.6. Immunocytochemistry
2.7. Zymography
2.8. Cell Cycle Analysis
2.9. Trypan Blue Exclusion Assay
2.10. Measurement of Cell and Nuclear Morphometric and Densitometric Analysis
2.11. Statistical Analysis
3. Results and Discussion
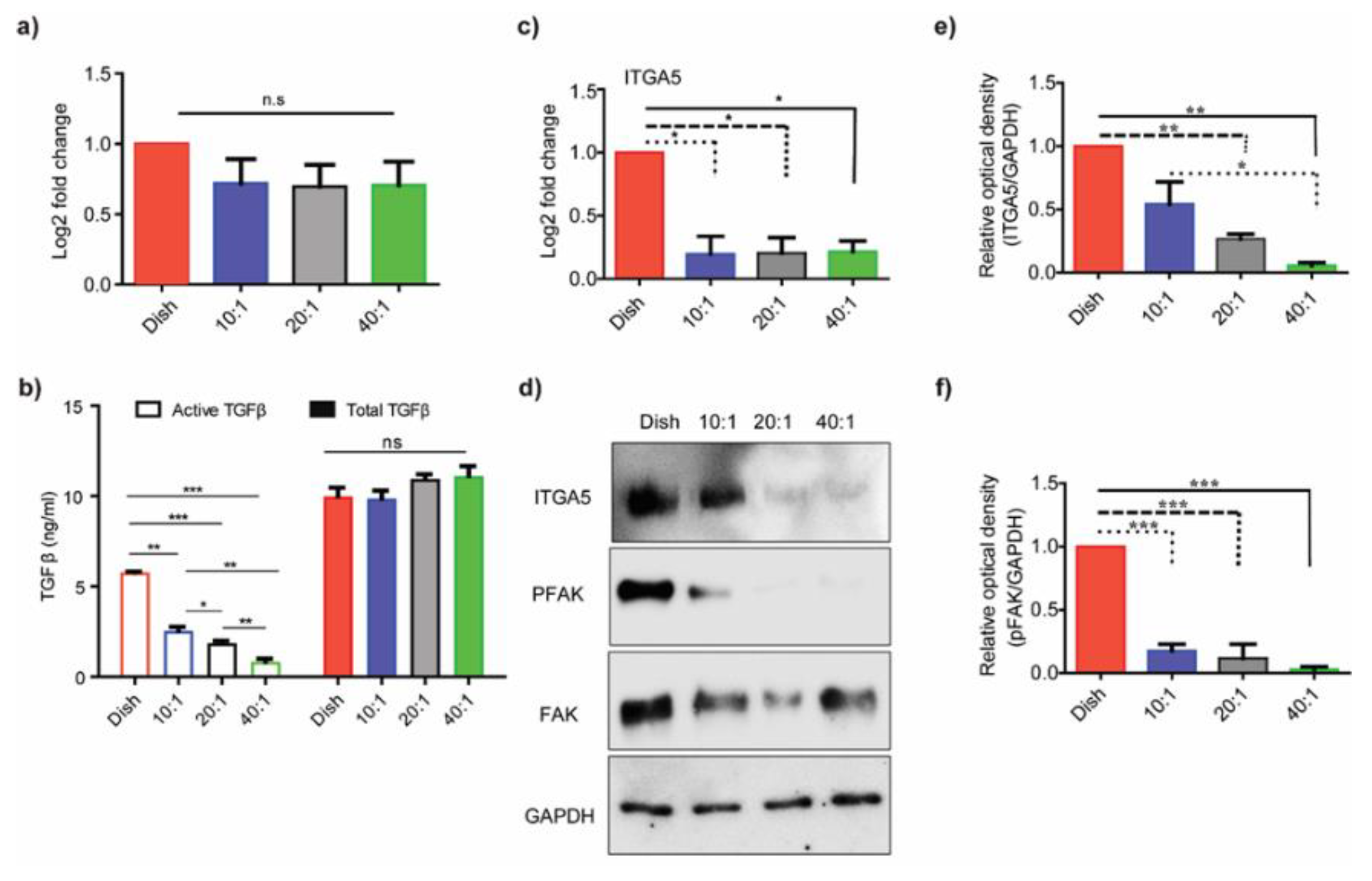
3.1. Substrate Stiffness Regulates TGF-β Activation
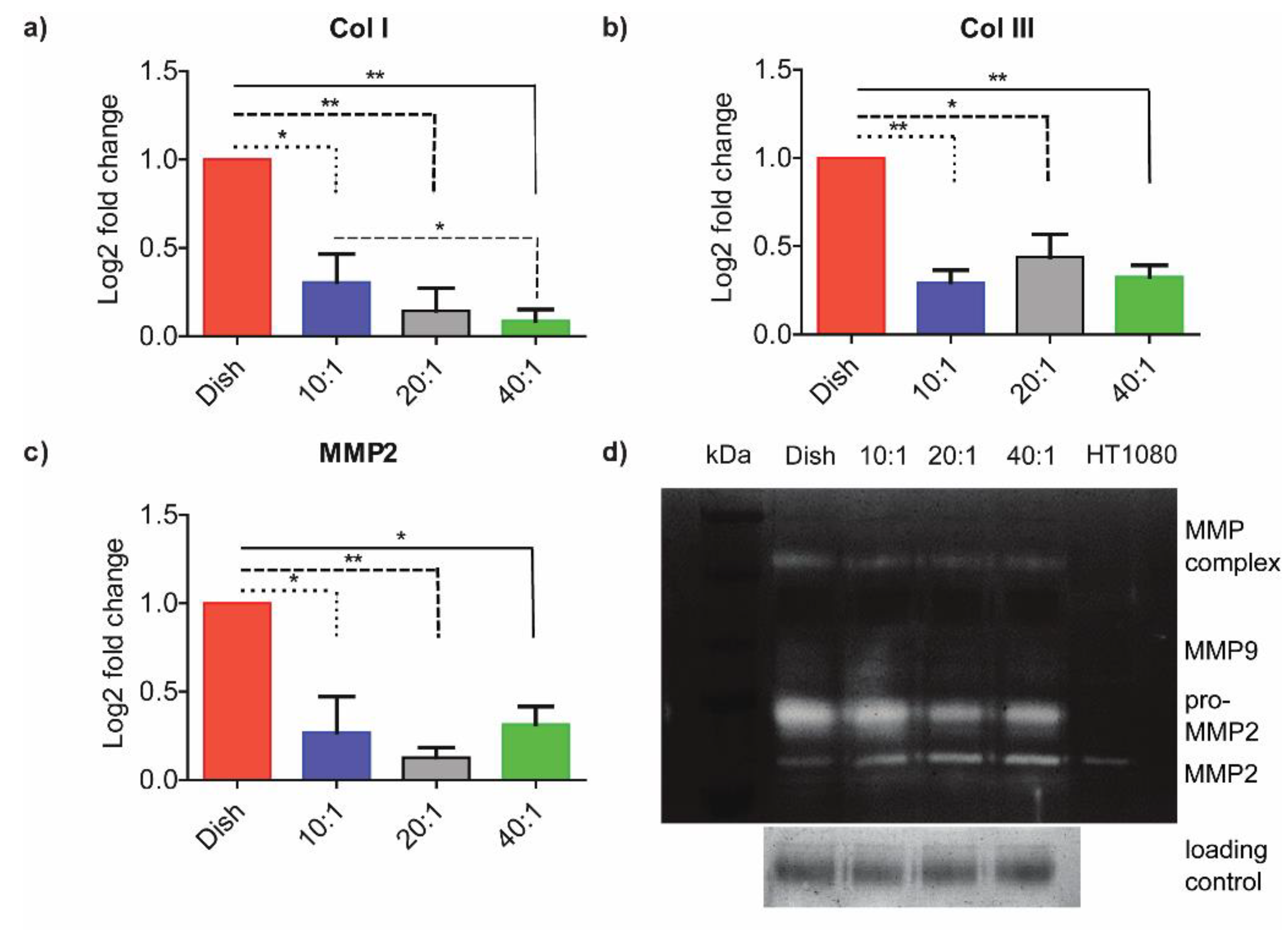
3.2. ECM-Associated Genes Are Downregulated at Lower Degrees of Stiffness
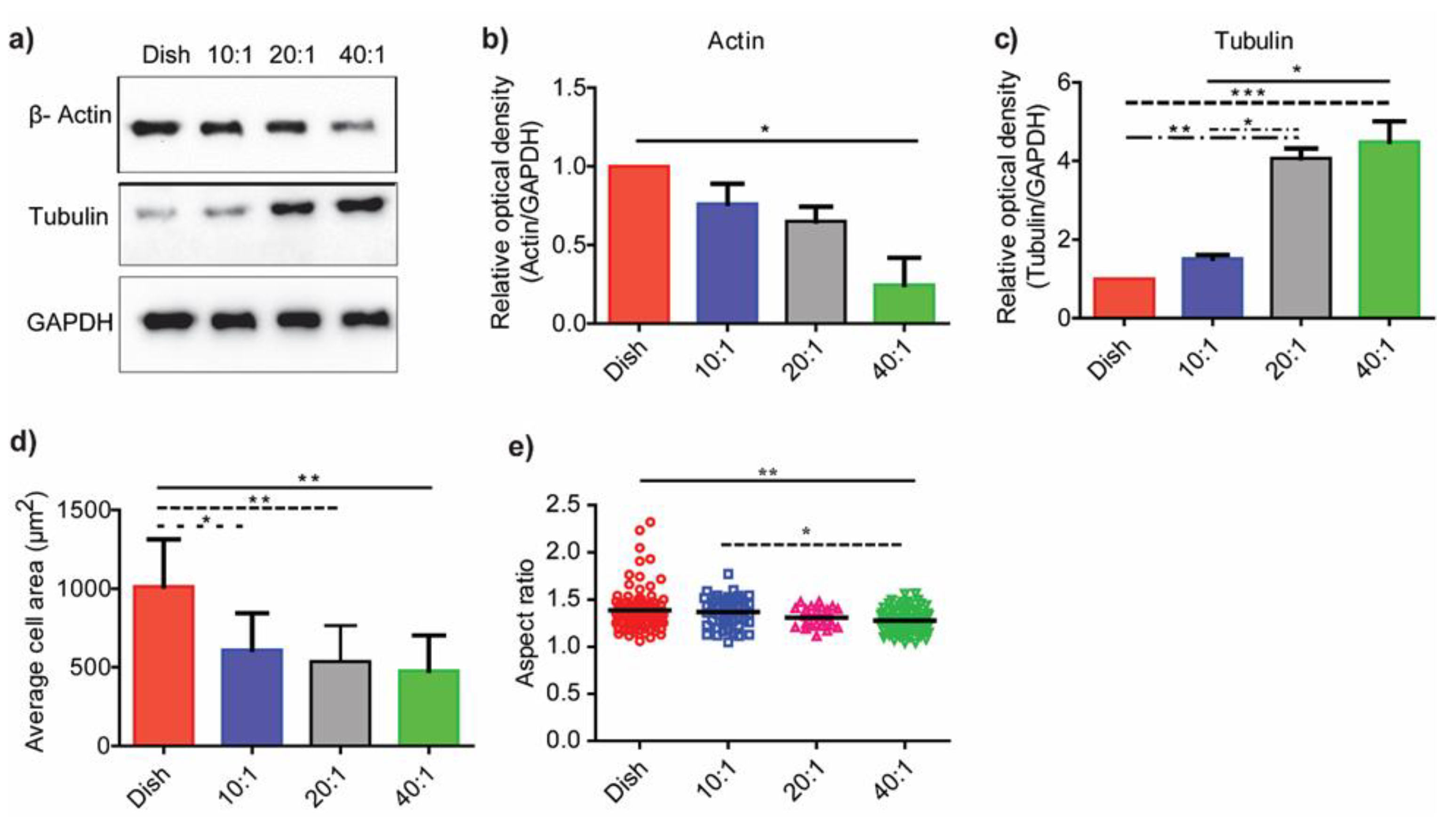
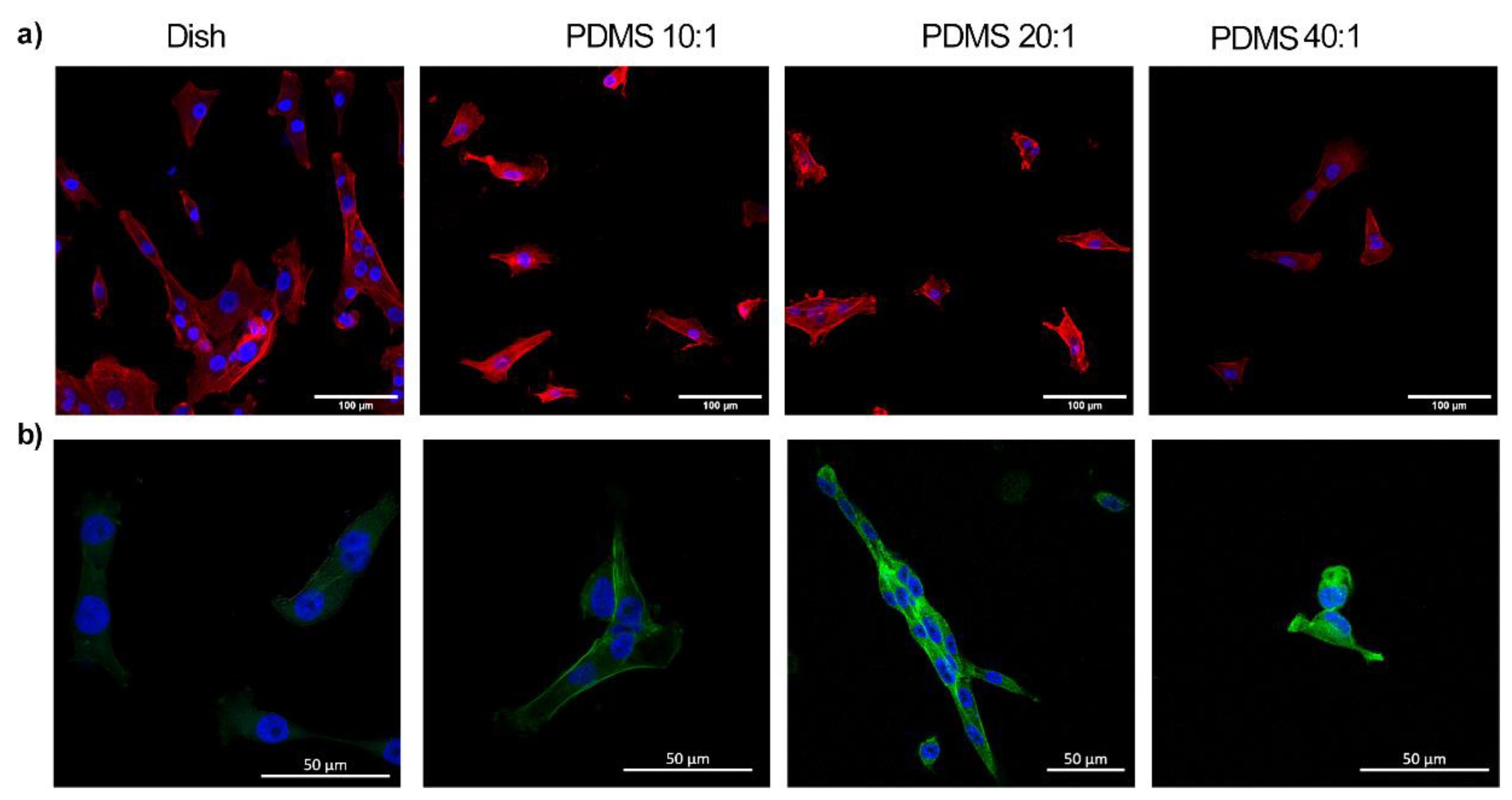
3.3. Substrate Stiffness Modulates Fibroblast Morphology and Proliferation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wynn, T.A. Cellular and Molecular Mechanisms of Fibrosis. J. Pathol. 2008, 214, 199. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and Mechano-Regulation of Connective Tissue Remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Babu, A.R.; Byju, A.G.; Gundiah, N. Biomechanical Properties of Human Ascending Thoracic Aortic Dissections. J. Biomech. Eng. 2015, 137, 081013. [Google Scholar] [CrossRef]
- Midwood, K.S.; Williams, L.V.; Schwarzbauer, J.E. Tissue Repair and the Dynamics of the Extracellular Matrix. Int. J. Biochem. Cell Biol. 2004, 36, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.E. Mechanistic Insight into Contextual TGF-β Signaling. Curr. Opin. Cell Biol. 2018, 51, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Munger, J.S.; Sheppard, D. Cross Talk among TGF-β Signaling Pathways, Integrins, and the Extracellular Matrix. Cold Spring Harb. Perspect. Biol. 2011, 3, a005017. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. TGFβ Signalling in Context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef]
- Daley, W.P.; Peters, S.B.; Larsen, M. Extracellular Matrix Dynamics in Development and Regenerative Medicine. J. Cell Sci. 2008, 121, 255–264. [Google Scholar] [CrossRef]
- Solon, J.; Levental, I.; Sengupta, K.; Georges, P.C.; Janmey, P.A. Fibroblast Adaptation and Stiffness Matching to Soft Elastic Substrates. Biophys. J. 2007, 93, 4453–4461. [Google Scholar] [CrossRef]
- Evans, N.D.; Minelli, C.; Gentleman, E.; LaPointe, V.; Patankar, S.N.; Kallivretaki, M.; Chen, X.; Roberts, C.J.; Stevens, M.M. Substrate Stiffness Affects Early Differentiation Events in Embryonic Stem Cells. Eur. Cell. Mater. 2009, 18, 1–13. [Google Scholar] [CrossRef]
- Seo, B.R.; Chen, X.; Ling, L.; Song, Y.H.; Shimpi, A.A.; Choi, S.; Gonzalez, J.; Sapudom, J.; Wang, K.; Eguiluz, R.C.A.; et al. Collagen Microarchitecture Mechanically Controls Myofibroblast Differentiation. Proc. Natl. Acad. Sci. USA 2020, 117, 11387–11398. [Google Scholar] [CrossRef] [PubMed]
- Gałdyszyńska, M.; Radwańska, P.; Szymański, J.; Drobnik, J. The Stiffness of Cardiac Fibroblast Substrates Exerts a Regulatory Influence on Collagen Metabolism via A2β1 Integrin, FAK and Src Kinases. Cells 2021, 10, 3506. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Qu, J.; He, L.; Zhu, Y.; Yang, S.Z.; Zhang, F.; Guo, T.; Peng, H.; Chen, P.; Zhou, Y. Stiff Matrix Instigates Type I Collagen Biogenesis by Mammalian Cleavage Factor I Complex-Mediated Alternative Polyadenylation. JCI Insight 2020, 5, e133972. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Wang, X.; Li, S.; Tang, L. Substrate Stiffness Drives Epithelial to Mesenchymal Transition and Proliferation through the NEAT1-Wnt/β-Catenin Pathway in Liver Cancer. Int. J. Mol. Sci. 2021, 22, 12066. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.D.; Hallur, P.M.; Karkisaval, A.G.; Gudipati, A.; Rajendiran, S.; Dhavale, V.; Ramachandran, B.; Jayaprakash, A.; Gundiah, N.; Chaubey, A. Gelatin Methacrylate Hydrogels as Biomimetic Three-Dimensional Matrixes for Modeling Breast Cancer Invasion and Chemoresponse in Vitro. ACS Appl. Mater Interfaces 2016, 8, 22005–22017. [Google Scholar] [CrossRef]
- Pang, M.; Teng, Y.; Huang, J.; Yuan, Y.; Lin, F.; Xiong, C. Substrate Stiffness Promotes Latent TGF-Β1 Activation in Hepatocellular Carcinoma. Biochem. Biophys. Res. Commun. 2017, 483, 553–558. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, K.; Gu, X.; Leong, K.W. Biophysical Regulation of Cell Behavior—Cross Talk between Substrate Stiffness and Nanotopography. Engineering 2017, 3, 36–54. [Google Scholar] [CrossRef]
- Babu, A.R.; Gundiah, N. Role of Crosslinking and Entanglements in the Mechanics of Silicone Networks. Exp. Mech. 2014, 54, 1177–1187. [Google Scholar] [CrossRef]
- Yeung, T.; Georges, P.C.; Flanagan, L.A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.; Weaver, V.; Janmey, P.A. Effects of Substrate Stiffness on Cell Morphology, Cytoskeletal Structure, and Adhesion. Cell Motil. Cytoskelet. 2005, 60, 24–34. [Google Scholar] [CrossRef]
- Chakravarthy, A.; Khan, L.; Bensler, N.P.; Bose, P.; de Carvalho, D.D. TGF-β-Associated Extracellular Matrix Genes Link Cancer-Associated Fibroblasts to Immune Evasion and Immunotherapy Failure. Nat. Commun. 2018, 9, 4692. [Google Scholar] [CrossRef]
- Buxboim, A.; Ivanovska, I.L.; Discher, D.E. Matrix Elasticity, Cytoskeletal Forces and Physics of the Nucleus: How Deeply Do Cells “feel” Outside and In? J. Cell Sci. 2010, 123, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Ling, J.Y.; Chen, W.C.; Lin, H.H.; Tang, M.J. Mechanotransduction of Matrix Stiffness in Regulation of Focal Adhesion Size and Number: Reciprocal Regulation of Caveolin-1 and Β1 Integrin. Sci. Rep. 2017, 7, 15008. [Google Scholar] [CrossRef] [PubMed]
- MacKay, K.; Kondaiah, P.; Danielpour, D.; Austin, H.A.; Brown, P.D. Expression of Transforming Growth Factor-Beta 1 and Beta 2 in Rat Glomeruli. Kidney Int. 1990, 38, 1095–1100. [Google Scholar] [CrossRef]
- Patil, S.S.; Railkar, R.; Swain, M.; Atreya, H.S.; Dighe, R.R.; Kondaiah, P. Novel Anti IGFBP2 Single Chain Variable Fragment Inhibits Glioma Cell Migration and Invasion. J. Neurooncol. 2015, 123, 225–235. [Google Scholar] [CrossRef]
- Peterson, T. Densitometric Analysis Using NIH Image. N. Am. Vasc. Biol. Organ. NAVB Enewslett. 2010, 16. [Google Scholar]
- Goldyn, A.M.; Rioja, B.A.; Spatz, J.P.; Ballestrem, C.; Kemkemer, R. Force-Induced Cell Polarisation Is Linked to RhoA-Driven Microtubule-Independent Focal-Adhesion Sliding. J. Cell Sci. 2009, 122, 3644–3651. [Google Scholar] [CrossRef] [PubMed]
- Haage, A.; Schneider, I.C. Cellular Contractility and Extracellular Matrix Stiffness Regulate Matrix Metalloproteinase Activity in Pancreatic Cancer Cells. FASEB J. 2014, 28, 3589–3599. [Google Scholar] [CrossRef]
- Walker, E.J.; Heydet, D.; Veldre, T.; Ghildyal, R. Transcriptomic Changes during TGF-β-Mediated Differentiation of Airway Fibroblasts to Myofibroblasts. Sci. Rep. 2019, 9, 20377. [Google Scholar] [CrossRef]
- Lachowski, D.; Cortes, E.; Rice, A.; Pinato, D.; Rombouts, K.; del Rio Hernandez, A. Matrix Stiffness Modulates the Activity of MMP-9 and TIMP-1 in Hepatic Stellate Cells to Perpetuate Fibrosis. Sci. Rep. 2019, 9, 7299. [Google Scholar] [CrossRef]
- Giménez, A.; Duch, P.; Puig, M.; Gabasa, M.; Xaubet, A.; Alcaraz, J. Dysregulated Collagen Homeostasis by Matrix Stiffening and TGF-Β1 in Fibroblasts from Idiopathic Pulmonary Fibrosis Patients: Role of FAK/Akt. Int. J. Mol. Sci. 2017, 18, 2431. [Google Scholar] [CrossRef]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple Functions of P21 in Cell Cycle, Apoptosis and Transcriptional Regulation after DNA Damage. DNA Repair 2016, 42, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Boontheekul, T.; Hill, E.E.; Kong, H.J.; Mooney, D.J. Regulating Myoblast Phenotype through Controlled Gel Stiffness and Degradation. Tissue Eng. 2007, 13, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.A.; Yin, L.; Kothapalli, D.; Castagnino, P.; Byfield, F.J.; Xu, T.; Levental, I.; Hawthorne, E.; Janmey, P.A.; Assoian, R.K. Cell-Cycle Control by Physiological Matrix Elasticity and in Vivo Tissue Stiffening. Curr. Biol. 2009, 19, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.; Kukkurainen, S.; Hytönen, V.P.; Wehrle-Haller, B. Cell Adhesion by Integrins. Physiol. Rev. 2019, 99, 1655–1699. [Google Scholar] [CrossRef]
- Noguchi, S.; Saito, A.; Mikami, Y.; Urushiyama, H.; Horie, M.; Matsuzaki, H.; Takeshima, H.; Makita, K.; Miyashita, N.; Mitani, A.; et al. TAZ Contributes to Pulmonary Fibrosis by Activating Profibrotic Functions of Lung Fibroblasts. Sci. Rep. 2017, 7, 42595. [Google Scholar] [CrossRef]
- Bordeleau, F.; Mason, B.N.; Lollis, E.M.; Mazzola, M.; Zanotelli, M.R.; Somasegar, S.; Califano, J.P.; Montague, C.; LaValley, D.J.; Huynh, J.; et al. Matrix Stiffening Promotes a Tumor Vasculature Phenotype. Proc. Natl. Acad. Sci. USA 2017, 114, 492–497. [Google Scholar] [CrossRef]
- Chen, C.S.; Mrksich, M.; Huang, S.; Whitesides, G.M.; Ingber, D.E. Geometric Control of Cell Life and Death. Science 1997, 276, 1425–1428. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Cortese, B.; D’Amone, S.; Manca, M.; Viola, I.; Cingolani, R.; Gigli, G. Superhydrophobicity Due to the Hierarchical Scale Roughness of PDMS Surfaces. Langmuir 2008, 24, 2712–2718. [Google Scholar] [CrossRef]
- Brown, X.Q.; Ookawa, K.; Wong, J.Y. Evaluation of Polydimethylsiloxane Scaffolds with Physiologically-Relevant Elastic Moduli: Interplay of Substrate Mechanics and Surface Chemistry Effects on Vascular Smooth Muscle Cell Response. Biomaterials 2005, 26, 3123–3129. [Google Scholar] [CrossRef]
- Lee, J.N.; Jiang, X.; Ryan, D.; Whitesides, G.M. Compatibility of Mammalian Cells on Surfaces of Poly(Dimethylsiloxane). Langmuir 2004, 20, 11684–11691. [Google Scholar] [CrossRef]
- Bodas, D.; Khan-Malek, C. Formation of More Stable Hydrophilic Surfaces of PDMS by Plasma and Chemical Treatments. Microelectron. Eng. 2006, 83, 1277–1279. [Google Scholar] [CrossRef]
- Janmey, P.A.; Fletcher, D.A.; Reinhart-King, C.A. Stiffness Sensing by Cells. Physiol. Rev. 2020, 100, 695–724. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yamanaka, M.; Tokeshi, M.; Morishima, K.; Kitamori, T. Microchip-Based Enzyme-Linked Immunosorbent Assay (ELISA) System. In Proceedings of the µTAS 2002 Symposium, Nara, Japan, 3–7 November 2002; pp. 190–192. [Google Scholar] [CrossRef]
- Wheeler, A.R.; Throndset, W.R.; Whelan, R.J.; Leach, A.M.; Zare, R.N.; Liao, Y.H.; Farrell, K.; Manger, I.D.; Daridon, A. Microfluidic Device for Single-Cell Analysis. Anal. Chem. 2003, 75, 3581–3586. [Google Scholar] [CrossRef] [PubMed]
- Takayama, S.; Ostuni, E.; LeDuc, P.; Naruse, K.; Ingber, D.E.; Whitesides, G.M. Subcellular Positioning of Small Molecules. Nature 2001, 411, 1016. [Google Scholar] [CrossRef]
- Hung, P.J.; Lee, P.J.; Sabounchi, P.; Lin, R.; Lee, L.P. Continuous Perfusion Microfluidic Cell Culture Array for High-Throughput Cell-Based Assays. Biotechnol. Bioeng. 2005, 89, 1–8. [Google Scholar] [CrossRef]
- Mao, X.; Tan, Y.; Wang, H.; Li, S.; Zhou, Y. Substrate Stiffness Regulates Cholesterol Efflux in Smooth Muscle Cells. Front. Cell. Dev. Biol. 2021, 9, 648715. [Google Scholar] [CrossRef]
- Chen, Y.F.; Li, Y.-S.J.; Chou, C.-H.; Chiew, M.Y.; Huang, H.-D.; Ho, J.H.-C.; Chien, S.; Lee, O.K. Control of matrix stiffness promotes endodermal lineage specification by regulating SMAD2/3 via lncRNA LINC00458. Sci. Adv. 2020, 6, eaay0264. [Google Scholar] [CrossRef]
- Sharma, S.; Goswami, R.; Zhang, D.X.; Rahaman, S.O. TRPV4 regulates matrix stiffness and TGFβ1-induced epithelial-mesenchymal transition. J. Cell. Mol. Med. 2018, 23, 761–774. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, B.K.; Chatterjee, A.; Kondaiah, P.; Gundiah, N. Substrate Stiffness Modulates TGF-β Activation and ECM-Associated Gene Expression in Fibroblasts. Bioengineering 2023, 10, 998. https://doi.org/10.3390/bioengineering10090998
Verma BK, Chatterjee A, Kondaiah P, Gundiah N. Substrate Stiffness Modulates TGF-β Activation and ECM-Associated Gene Expression in Fibroblasts. Bioengineering. 2023; 10(9):998. https://doi.org/10.3390/bioengineering10090998
Chicago/Turabian StyleVerma, Brijesh Kumar, Aritra Chatterjee, Paturu Kondaiah, and Namrata Gundiah. 2023. "Substrate Stiffness Modulates TGF-β Activation and ECM-Associated Gene Expression in Fibroblasts" Bioengineering 10, no. 9: 998. https://doi.org/10.3390/bioengineering10090998
APA StyleVerma, B. K., Chatterjee, A., Kondaiah, P., & Gundiah, N. (2023). Substrate Stiffness Modulates TGF-β Activation and ECM-Associated Gene Expression in Fibroblasts. Bioengineering, 10(9), 998. https://doi.org/10.3390/bioengineering10090998






