Abstract
Chemodynamic therapy (CDT) has garnered significant interest as an innovative approach for cancer treatment, owing to its notable tumor specificity and selectivity, minimal systemic toxicity and side effects, and absence of the requirement for field stimulation during treatment. This treatment utilizes nanocatalytic medicines containing transitional metals to release metal ions within tumor cells, subsequently initiating Fenton and Fenton-like reactions. These reactions convert hydrogen peroxide (H2O2) into hydroxyl radical (•OH) specifically within the acidic tumor microenvironment (TME), thereby inducing apoptosis in tumor cells. However, insufficient endogenous H2O2, the overexpressed reducing substances in the TME, and the weak acidity of solid tumors limit the performance of CDT and restrict its application in vivo. Therefore, a variety of nanozymes and strategies have been designed and developed in order to potentiate CDT against tumors, including the application of various nanozymes and different strategies to remodel TME for enhanced CDT (e.g., increasing the H2O2 level in situ, depleting reductive substances, and lowering the pH value). This review presents an overview of the design and development of various nanocatalysts and the corresponding strategies employed to enhance catalytic drug targeting in recent years. Additionally, it delves into the prospects and obstacles that lie ahead for the future advancement of CDT.
1. Introduction
Cancer, as a major public health problem, causes approximately 10 million deaths worldwide every year due to its high incidence rate and mortality []. However, for patients with early-stage (stage I/II) cancer, traditional cancer treatments such as surgery, chemotherapy, and radiotherapy are less effective and have unavoidable side effects [,,]. Therefore, the development of innovative and effective cancer therapies with improved efficacy and fewer side effects is both urgent and challenging.
Reactive oxygen species (ROS) is a general term for a class of chemically active molecules or ions with high oxidation activity, including hydrogen peroxide (H2O2), superoxide anion (O2−), hydroxyl radical (•OH) and singlet oxygen (1O2), peroxy hydroxyl radical (•OOH), etc., which plays an important role in various physiological processes of biological systems []. A large number of studies have shown that the overgenerated ROS could break the redox hemostasis and cause a series of biochemical reactions, such as decreased mitochondrial membrane potential, DNA breakage, cytoskeleton contraction, chromatin condensation, etc., leading to cancer cells death [,]. Since cancer cells are more sensitive to ROS levels, ROS generation is considered to be an effective way to kill tumor cells selectively.
Currently, the applications of ROS-based tumor therapies mainly including photodynamic therapy (PDT), chemodynamic therapy (CDT), and sonodynamic therapy (SDT) have attracted significant attention from researchers [,]. Among these treatment modalities, various methods can generate excessive ROS. In PDT treatment, the photosensitizer generates ROS under the action of near-infrared irradiation, and the local ROS burst promotes cell apoptosis. The cavitation effect, sonoluminescence, and piezoelectric effect are the main mechanisms of ROS generation in SDT. At the same time, there are other ways to increase ROS levels in tumors. For instance, Wang et al. reported a kind of ultrathin two-dimensional metal-organic framework (Cu-TCPP), which was endowed with selectively producing 1O2 in the tumor microenvironment for tumor therapy []. Firstly, the acidic H2O2 peroxidized the tetrakis(4-carboxyphenyl) porphyrin (TCPP) ligand, and then it was reduced to peroxyl radicals under the action of peroxidase (POD)-like nanosheets and Cu2+. Finally, a spontaneous recombination reaction based on the Russell mechanism occurred to generate 1O2. In addition, Cu-TCPP also consumed glutathione (GSH) through a cyclic oxidation mechanism. Based on the above two “magic weapons”, Cu-TCPP nanosheets efficiently and selectively destroyed tumors.
CDT, proposed by Bu and co-workers in 2016, has emerged as a fascinating research area in recent years. This is primarily due to its remarkable specificity and selectivity for tumors, minimal systemic toxicity and side effects, and the absence of a requirement for field stimulation during treatment [,]. All the facts demonstrated that CDT not only had the potential to enhance the efficacy of cancer treatment but could also be used for bacterial infection treatments []. Generally, CDT utilizes peroxidase-like catalysis, metallic catalysis, or Fenton and Fenton-like reactions to significantly enhance intracellular ROS. Herein, transitional metal-based nanocatalytic medicines containing some metal ions (e.g., Fe, Cu, Mn, Co, V, Pd, Ag, Mo, Ru, W, and Ce) are utilized to release metal ions in tumor cells and then trigger Fenton and Fenton-like reactions to convert H2O2 into •OH with higher toxicity in an acidic tumor microenvironment (TME), thus inducing the death of tumor cells []. However, the performance of CDT is limited by some natural factors, which makes it difficult to be widely applied. Firstly, there are insufficient endogenous H2O2 in tumors with a content of 50–100 µmol L−1, which is insufficient to meet the ideal CDT therapeutic efficiency []. Secondly, the produced oxidative •OH could be captured and neutralized by the overexpressed reducing substances in the TME, such as GSH and hydrogen sulfide (H2S), resulting in an unsatisfactory therapeutic efficiency [,]. Thirdly, the weak acidity (pH 6.5–7.0) of solid tumors is not suitable for Fenton and Fenton-like reactions, which are required to be remodeled to provide more suitable reaction conditions []. Up to now, a variety of nanozymes and strategies have been designed and developed in order to solve these problems (Scheme 1) [,]. This review aims to provide an overview of the design and development of different types of nanozymes as well as strategies to improve CDT in recent years. Finally, the opportunities and challenges for the future development of CDT are also discussed to promote clinical translation.
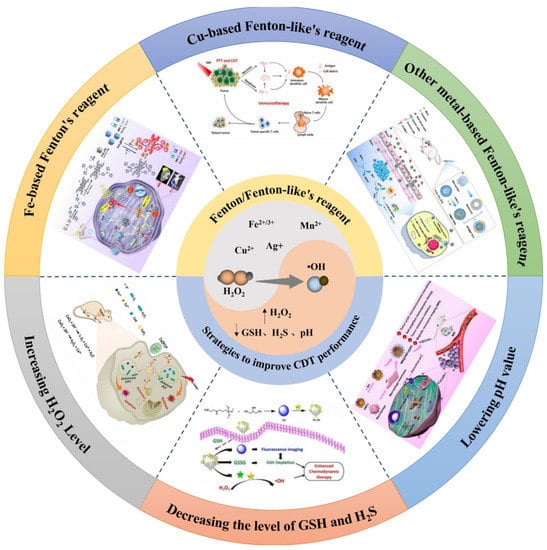
Scheme 1.
Schematic illustration of design of Fenton/Fenton–like reagent and strategies to improve CDT performance. Image for Fe–based Fenton’s regent: reproduced from publication of Li, D. et al. with permission, Copyright 2023, Elsevier Ltd. Image for Cu–based Fenton–like’s regent: reproduced from publication of Jiang, F. et al. with permission, Copyright 2021, Elsevier Ltd. Image for other metal–based Fenton–like’s regent: reproduced from publication of Xiao, T. et al. with permission, Copyright 2021, American Chemical Society. Image for lowering pH value: reproduced from publication of Dong, K. et al. with permission, Copyright 2022, Elsevier Ltd. Image for decreasing the level of GSH and H2S: reproduced from publication of Li, J. et al. with permission, Copyright 2022, Elsevier Ltd. Image for increasing H2O2 level: reproduced from publication of Shen, J. et al. with permission, Copyright 2021, WILEY-VCH.
2. Fenton/Fenton-like Reagent
Nowadays, the research into efficient nanozymes for Fenton and Fenton-like reactions has attracted extensive attention due to the wide application of CDT in cancer therapy. The choice of nanomaterials (iron-based materials, copper-based materials, and other metal-based materials) is crucial because of the irreplaceable role of catalysts in Fenton and Fenton-like reactions.
2.1. Iron-Based Fenton Reagent
In recent years, many nanozymes containing catalytically active ions have been designed based on the Fenton reaction and the Fenton-like reaction. So far, iron-based materials have been widely used in the biological field and have shown excellent biosafety and biocompatibility [,]. Various iron-based nanomaterials have been extensively studied to enhance the efficacy of CDT, such as ferroferric oxide (Fe3O4) [], ferrous disulfide (FeS2) [], ferric oxide (Fe2O3) [], Fe-metal organic frameworks (Fe-MOFs) [], and Fe-doped nanoagents []. A Fenton reaction is often catalyzed by Fe2+ or Fe3+, which decomposes H2O2 to produce radical •OH, resulting in the destruction of lipids, proteins, and DNA. The reactions (Equations (1) and (2)) are shown below:
Fe2+ + H2O2 → Fe3+ + •OH + OH−
Fe3+ + H2O2 → Fe2+ + •OOH + H+
As one of the most classical H2O2-responsive nanozymes, Fe3O4 nanoparticles become an effective POD-like enzyme with the Fe2+/Fe3+ redox cycle. As the reaction site of nanozymes, Fe2+ on the surface of Fe3O4 nanoparticles reacted with the overexpressed H2O2 in TME to produce •OH, which was then converted to Fe3+ in the oxidized state, leading to severe tumor damage. In this reaction process, the oxidation property of the generated •OOH was lower than that of the generated •OH, and the rate of the generated •OH was relatively faster [,]. For instance, Du et al. successfully constructed kinds of hollow Fe3O4 mesocrystals to realize the high level of •OH in the tumor region and a low expression of heat shock proteins, resulting in a self-enhanced antitumor efficacy between magnetic hyperthermia and CDT []. The therapeutic efficiency of nanomaterials was greatly restricted due to the low iron loading and cumbersome releasing route. Polydopamine (PDA) has been widely studied as a CDT agent owing to its high biocompatibility and radical scavenging strong chelation with Fe2+. Recently, Xiao et al. prepared a multifunctional hybrid nanozyme (PDA/Fe3O4), which was retained in infected sites via an external magnet (Figure 1a) []. PDA with redox-active catechol moieties supplied oxygen with electrons to produce H2O2, which was then decomposed by decorated ultrasmall Fe3O4 to generate more •OH. Surprisingly, PDA/Fe3O4 was equipped with simultaneous H2O2-self-sufficient •OH generation and GSH depletion to improve the CDT efficacy. In another work, Luo et al. synthesized a novel nanoplatform of Fe3O4@PDA@BSA-Bi2S3 nanoparticles via the amidation between the carboxyl and amino groups to promote the efficiency of Fenton reaction in cancer cells []. In this system, the Fe3O4 nanoparticles were not only capable of triggering Fenton reactions to generate highly •OH from the innate H2O2 in the TME but were also employed as the magnetic resonance imaging (MRI) contrast agent for the precise cancer diagnosis. Specifically, PDA promoted the long-term Fenton reactions and tumor apoptosis by preventing oxidation of Fe3O4, which reacted with intracellular H2O2 to produce •OH and further suppressed tumor growth. Moreover, computed tomography (CT) contrast was also enhanced due to the significant X-ray attenuation coefficient of Bi2S3, enabling the simultaneous CDT and PDT treatment of tumors. On the other hand, this also contributed to the regulation of the CDT treatment process, minimizing the biotoxicity and improving diagnostic and therapeutic capabilities. Eventually, the present study demonstrated a novel and viable approach for synthesizing composite theranostic nanoplatforms.
In addition to Fe3O4, other iron-based materials are also endowed with superb Fenton reaction catalysis. For instance, Wu et al. developed hollow porous carbon-coated FeS2 (HPFeS2@C) nanocatalysts for triple-modal imaging-guided synergistic tumor starvation therapy (ST), photothermal therapy (PTT), and CDT []. Interestingly, glucose oxidase (GOx) in the multifunctional nanocatalysts reacted with glucose in the TME to increase the H2O2 concentration, which improved the production of •OH. Meanwhile, tannic acid (TA) was effectively released for reducing Fe3+ to Fe2+ under near-infrared (NIR) laser exposure, thereby accelerating the Fenton reaction. In addition, the photothermal effect induced by NIR lasers also increased the catalytic efficiency. Furthermore, our group reported a phthalocyanine-iron-based complex FeS2@PcD, program-regulated by the dual factors of gluconic acid (H+) and H2O2 in the TME (Figure 1b) []. After the efficient internalization into cancer cells, FeS2@PcD reacted with excessive H+ to generate Fe2+. Fe2+ then catalyzed intracellular H2O2 to produce •OH for CDT and simultaneously generate Fe3+ for further MRI as well as activating the released building block PcD (Figure 1c). Interestingly, the nitrogen atom on the DPA group of PcD specifically chelated Fe3+, thereby recovering fluorescence and sonosensitizing activity. Moreover, in vitro data showed that the fluorescence intensity of FeS2@PcD increased 52.21-fold, resulting from the programmable response, whereas the signal of the H+ or H2O2 univariate factor was almost unchanged (Figure 1d). Moreover, FeS2@PcD combined with ultrasound irradiation had a significant inhibitory effect on HepG2 tumor-bearing mice tumor, with an inhibition rate of 87.15% (Figure 1e). In summary, our work achieved precise sonodynamic and chemodynamic therapies, providing a novel strategy for CDT-related clinical research. To date, dihydroartemisinin (DHA) has been regarded as a drug candidate for cancer therapy. This mechanism was based on the breakdown of weak endoperoxide bridges by Fe2+ to produce toxic ROS, which resulted in facilitating H2O2-mediated Fenton reactions to generate abundant ROS for effective CDT []. Xu et al. prepared an antibacterial nanoagent Fe2O3@DHA@MPN (FDM) for dual-augmented NIR and DHA antibacterial CDT []. As the core, α-Fe2O3 mesoporous nanorods provided both efficient DHA transport and an additional source of Fe2+ used for enhanced CDT. Furthermore, metal-polyphenol networks (MPN) shells released DHA in response to the microenvironment and led to the release of TA and Fe3+ simultaneously due to bacterial infection. Consequently, TA reduced Fe3+ to Fe2+ under NIR laser exposure, which was favorable for H2O2-mediated CDT as well as DHA-mediated CDT.
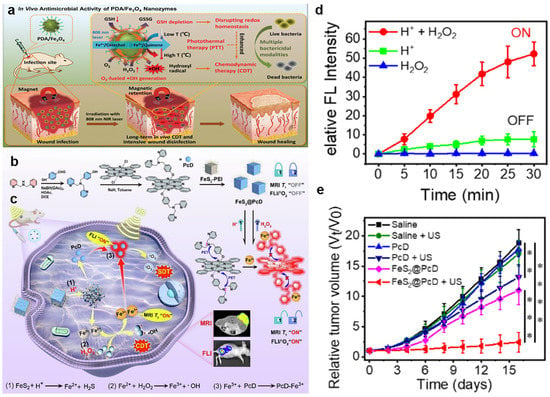
Figure 1.
(a) The mechanism underlying the effects of PDA/Fe3O4 hybrid nanozymes as an enhanced CDT agent for bacterial elimination. Reproduced with permission []. Copyright 2021, WILEY–VCH. (b) Fabrication and programmable mechanism of the FeS2@PcD. (c) The action process of FeS2@PcD in HepG2 cells for sono/chemodynamic therapies. (d) Fluorescence intensity of FeS2@PcD in the presence of H+ and H2O2. (e) Curves showing HepG2 tumor volumes in mice treated with FeS2@PcD–mediated SDT. Data were expressed as mean ± SD, n = 5, ** p < 0.01, *** p < 0.001, **** p < 0.0001, analysed by Student’s t test. Reproduced with permission []. Copyright 2023, Elsevier Ltd.
Recently, MOFs, which are composed of metal ions and organic ligands, have attracted increasing attention in catalysis, energy storage, separation, and cancer therapy [,]. Owing to the large pore surfaces, good biodegradability, and biocompatibility, MOFs were widely used as nanoplatforms for drug delivery. Therein, Fe-MOFs (like MIL-101) showed an efficient catalytic effect as a Fenton nanocatalyst. For instance, Chen et al. prepared photosensitizer-integrated nanoagents of MIL-101(Fe)@TCPP for implementing the combined PDT and CDT []. In this system, the MIL-101(Fe) MOF transformed endogenous H2O2 into •OH via Fe ion-catalyzed Fenton reaction, resulting from the intrinsic POD-mimicking activity of MIL-101(Fe)@TCPP. Moreover, the photosensitizer TCPP was covalently connected with MIL-101(Fe) through amide bonds, leading to in situ 1O2 evolution at the tumor region under light irradiation and amplifying ROS-induced oxidative damage. Similarly, Yin et al. successfully synthesized a cascade biomimetic nanoplatform of CPPO@Fe-porphyrin-MOF@Cancer cell membrane-GOD (C1@M@C2G) by loading harbors bis(2-carbopentyloxy-3,5,6-trichlorophenyl) oxalate (CPPO) into the Fe-porphyrin-MOF nanoparticle, which was further coated with the cancer cell membrane and grafted GOD on the surface (Figure 2a) []. After accumulating in the tumor site, GOD-catalyzed glucose oxidation activated the therapy of starvation, thereby consuming the local glucose and further generating H+ and H2O2, further greatly decreasing the pH of the microenvironment and promoting the massive generation of •OH for efficient CDT. Simultaneously, CPPO, as an energetic reagent, reacted with some other H2O2 to form high energy states, which excited porphyrin photosensitizers and produced 1O2 for PDT in situ. Overall, this work combined CPPO-induced PDT, Fenton reaction-based CDT, and GOD-catalyzed ST to synergistically enhance cancer therapy with effective cascade catalysis, and also broaden anticancer pathways.
Additionally, due to properties such as prominent molecular recognition ability and accurate addressability of nucleic acids, DNA-based hydrogels have outstanding advantages in controlling the release of encapsulated cargoes for controlled drug delivery [,,]. Wang et al. prepared a kind of MOF-biomineralized DNA nanosphere (Fe-DNA@ZIF-8) via cascade reactions to implement the intracellular H2O2 level for enhanced CDT (Figure 2b) []. Fe-DNA was composed of Fe2+ and DNA molecules by coordination-driven self-assembly, which was then coated by zeolitic imidazolate framework-8 (ZIF-8) for mineralization. At the same time, GOx was encapsulated into ZIF-8 to further form Fe-DNA/GOx@ZIF-8. After being endocytosed by tumor cells, Fe-DNA and GOx were released in the acidic condition, providing Fe2+ and Fe3+ to activate the Fenton reaction and catalyzing glucose into H2O2 to enhance CDT effectiveness. In addition, multimetallic alloy nanostructures demonstrated higher catalytic activity than monometallic components owing to the electronic coordination effect, leading to its wide application. For example, Jana et al. fabricated a trimetallic (Pd, Cu, and Fe) alloy nanozyme (PCF-a NEs) with dynamic active-site synergism, which depleted intracellular overexpressed GSH and reduced the cellular self-defense antioxidant mechanism []. PCF-a NEs had the synergistic peroxidase-like property to promote •OH in the presence of Cu and Fe, thus boosting the effectiveness of CDT. This work pointed to the potential for using alloy nanozymes for tumor-specific treatments mediated by external stimulus.
To improve the application of iron-based nanoparticles for efficacious CDT, an effective method was to accelerate the conversion efficiency of Fe2+ and Fe3+. The low indirect band-gap (1.2–1.8 eV) of MoS2 made it an effective co-catalyst for the Fenton reaction, which converted Fe(III) into Fe(II). For example, our group synthesized gallic acid (GA)-modified MoS2 nanosheets coated with high Fe(III) (MoS2@GA-Fe), which were used for photoacoustic (PA) imaging and MRI-guided combined cancer treatment integrating •OH and H2S (Figure 2c) []. Firstly, GA-Fe(III) reacted with overexpressed GSH to produce GA-Fe(II), which promoted the generation of •OH in the TME. Moreover, after Mo (IV) was oxidized to Mo (VI), S-Mo-S was destroyed, exposing active sites for further reaction cycles and converting unsaturated sulfur atoms to H2S with selective cytotoxicity to cancer cells. Additionally, PA imaging for BALB/c nude tumor-bearing mice after intravenous injection of MoS2@GA-Fe showed that the signal of the tumor area was enhanced and remained for 24 h, resulting from the structure-based enhanced permeability and retention (EPR) effect (Figure 2d,e). Thus, the TME-responsive “Fenton Nanoreactor” was used as a promising nanomedicine to treat cancer or other diseases and improve patient health.
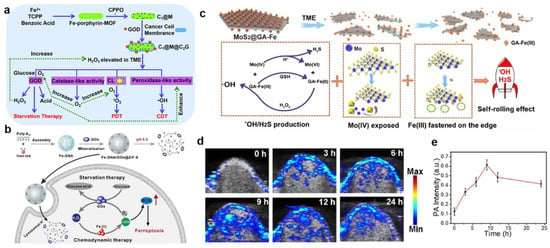

Figure 2.
(a) Schematic representation of the fabrication procedure of C1@M@C2G NSs and their working mechanism of CL–induced PDT, Fenton reaction–based CDT, and GOD–mediated ST. Reproduced with permission []. Copyright 2021, Royal Society of Chemistry. (b) Scheme of Fe–DNA/GOx@ZIF–8 for enhanced CDT. Reproduced with permission []. Copyright 2021, Elsevier Ltd. (c) Theranostic mechanism of the MoS2@GA–Fe NPs for PA/MR imaging–guided HCC treatment. (d) The PA images and (e) intensity of tumor–bearing mice after intravenous injection with MoS2@GA-Fe. Reproduced with permission []. Copyright 2021, Elsevier Ltd.
2.2. Copper-Based Fenton-like Reagent
In addition to ferrous iron ions, other transition metal ions like Cu2+ also have catalytic effects in Fenton and Fenton-like reactions. As a potential alternative to Fe-based nanomaterials, Cu-based nanomaterials have found a variety of applications in many CDT research fields. Cu-catalyzed Fenton-like reactions occur in neutral and very mild acidic conditions, which are less violent than that of Fe-catalyzed Fenton reactions []. Moreover, the valence transition between Cu2+ and Cu+ is usually accompanied by depletion of the excessive GSH in the TME, enhancing the production of •OH to achieve highly efficient CDT []. Hence, Cu-based nanomaterials have been extensively employed as Fenton reagents, such as copper sulfide (CuS), copper oxide (CuO), Cu-based MOF, and Cu-doped nanoagents [,,,]. The Cu-based catalytic reactions (Equations (3) and (4)) are shown below.
Cu+ + H2O2 → Cu2+ + •OH + OH−
Cu2+ + H2O2 → Cu+ + •OOH + H+
More and more attention has been paid to CuS nanoparticles because of their low toxicity, their ability to penetrate into biological tissues after being stimulated by NIR light, and their PA and Fenton-like properties [,,]. For instance, Kong and co-workers reported a smart biomimetic enzyme system of CuGP/G, which was composed of the CuS nanoparticle, generation 5 poly (amidoamine) (G5) dendrimer, and GOD for the combination of CDT and PTT (Figure 3a) []. Specifically, CuS cores with Fenton-like catalysts were entrapped by positively charged G5, which simultaneously bonded to the negatively charged GOD via electrostatic interactions as a carrier. Additionally, H2O2 was produced by GOD to generate •OH in the sequential Fenton-like reaction, resulting in efficient tumor treatment. Moreover, the Michaelis–Menten kinetic curve demonstrated that the Michaelis–Menten constant (Km) was 3.75 × 10−3 M, and the maximum reaction velocity (Vm) was calculated at 3.6 × 10−7 M s−1 after incorporating GOD with CuGP (Figure 3b,c), compared to a Fe3O4-GOD system with the Km of 10.93 × 10−3 M and the Vm of 4.22 × 10−8 M s−1, indicating that the Fenton-like catalytic activity of CuGP/G was better than the Fe3O4-GOD-based system []. Nevertheless, CuS was usually trapped by the endothelial reticular system or rapidly cleared by the kidney, resulting in poor accumulation in tumors. To efficiently transport CuS to tumor cells, Li et al. used macrophages to phagocytose large supramolecular aggregates of CuS for the specific-release nanomedicines in the tumor region (Figure 3d) []. The supramolecular aggregates were composed of CuS nanoparticles, which were capped by β-cyclodextrin (β-CD) and ferrocene (Fc), respectively, and self-assembled via β-CD-Fc host–guest interactions. Since macrophages were attracted to tumor tissue with inflammatory properties, nanomedicines were successfully delivered through macrophages and then the β-CD-Fc host–guest pair were dissociated by the oxidation of ferrocene, inducing the disassemble of CuS aggregates into small CuS nanoparticles for further photothermal-enhanced CDT. This work proposed a feasible strategy for specific cancer therapy and new insights into new cell-based medicine carriers.
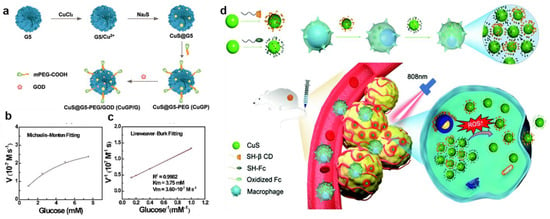
Figure 3.
(a) Synthetic route of CuGP/G. (b) The Michaelis–Menten kinetic curve and (c) Lineweaver–Burk plotting curve that indicate the catalytic activity of CuGP/G with the addition of GOD. Reproduced with permission []. Copyright 2020, WILEY–VCH. (d) Preparation of the supramolecular aggregates composed of CD–CuS and Fc–CuS and its action mechanism of tumor-targeted PTT/CDT. Reproduced with permission []. Copyright 2021, Royal Society of Chemistry.
CuO nanoparticles have aroused great interest in recent years, benefiting from the large surface area, reactive morphology, high catalytic reusability, and superb chemical and thermal stability [,]. As mentioned earlier, the rate of •OH production was relatively faster relative to •OOH in Cu-mediated CDT. As a representative CuO-based Fenton-like nanocatalyst in CDT, Jiang et al. reported the synthesis of semiconductor CuO and MoS2 nanoflowers to construct MoS2-CuO heteronanocomposites through a two-step hydrothermal method (Figure 4a) []. MoS2-CuO heteronanocomposites were then loaded with bovine serum albumin (BSA) and immunoadjuvant imiquimod (R837) on its surface to further form MoS2-CuO@BSA/R837 (MCBR) nanoplatforms, which had an excellent ability to eliminate the primary tumor and release tumor-associated antigens (TAAs). In this system, CuO responded to overexpressed H2O2 in tumor cells and produced a mass of •OH through Fenton-like reactions. The reactions were further improved by the effect of MoS2 due to its high photothermal conversion efficiency. And more importantly, MCBR released TAAs under 808 nm NIR laser irradiation, which combined with R837 to act as an immune-stimulating adjuvant to boost dendritic cells maturation and the release of effector T cells from the lymph node. Consequently, this work achieved an efficient and tumor-specific therapy, providing a meaningful paradigm for future clinical applications. In another study, Xiao et al. prepared an intelligent nanoplatform of CuO2@mPDA/DOX-HA (CPPDH) with self-sufficient H2O2 and GSH depletion for the synergistic chemotherapy, PTT, and CDT (Figure 4b) []. The surface of CuO2 nanospheres was decorated with mesoporous polydopamine (mPDA) with seed-mediated microemulsion and further loaded with doxorubicin (DOX) via π-π stacking. Moreover, the obtained CuO2@mPDA/DOX was constructed by hyaluronic acid (HA) using electrostatic adsorption to finally acquire CPPDH. After entering the intracellular HA environment, CuO2 was released to generate Cu2+ and H2O2 triggered by the acidic environment. The Cu2+ was then reduced to Cu+ by GSH, and subsequently Cu+ reacted with intracellular H2O2 to produce massive •OH for enhanced CDT. Interestingly, CPPDH had excellent targeting ability to tumor cells resulting from the specific binding affinity of HA, whose bonds overexpressed CD44 receptor in the tumor region. Furthermore, the nanoparticles were endowed with a superb effect of PTT due to the strong NIR absorption capacity of PDA, and the light-to-heat conversion efficiency was calculated as 38.39%, which was significantly higher than that of reported photothermal reagents like AuNRs@SiO2 (20.8%) [], MS-BSA (21.8%) [], and Au-Cu9S5 nanoparticles (37%) [].
In addition to the use of CuS and CuO, Cu-MOF also exhibited superb catalytic performance to achieve highly efficient CDT. For example, Tian et al. reported a nanoplatform integrating vitamin K3 into the MOF-199(Cu) to implement a CDT-mediated tumor therapy []. The hollow structures derived from MOFs have the virtue of drug loading and delivery with outstanding mass transfer and loading capacity. In a recent study, Cheng et al. proposed a strategy to incorporate Cu2+ into the precursors of ZIF-90 to form the Cu+/2+ mixed-valence hollow porous structure (Cu/Zn-MOF) through heating treatment, and then Cu/Zn-MOF was integrated with Mn2+/MnO2 using manganese(II) acetylacetonate(Mn(acac)2) to construct a mixed-metal and mixed-valence (Cu+/2+/Mn2+/4+) structure, which was finally loaded by the photosensitizer indocyanine green (ICG) to prepare ICG@Mn/Cu/Zn-MOF@MnO2 (Figure 4c,d) []. After being endocytosed by tumor cells, Cu2+ and MnO2 oxidized GSH to generate a great mass of ROS and Cu+ and produced Mn2+, which was used to “turn on” MRI. Subsequently, the obtained Cu+ and Mn2+ catalyzed H2O2 to produce •OH for effective CDT via the Fenton-like reaction and O2 through a synergistic catalytic effect to relieve the hypoxia for the enhanced PDT. Then, the aggregated ICG in the system was not only employed for photothermal imaging (PTI) with excellent photothermal capacity, but also achieved “turn on” fluorescence imaging (FLI) after its release in tumor cells. Irradiated with a single 808 nm NIR, ICG@Mn/Cu/Zn-MOF@MnO2 exhibited an obvious temperature increase with an excellent photothermal conversion efficiency of 30.1% and converted the O2 generated in situ into 1O2 for enhancing the effect of PDT. ICG@Mn/Cu/Zn-MOF@MnO2 was expected as a versatile platform of PTI/FLI/MRI trimodality imaging due to the high photothermal conversion, ICG release capability, and the generation of Mn2+ that responded to TME.
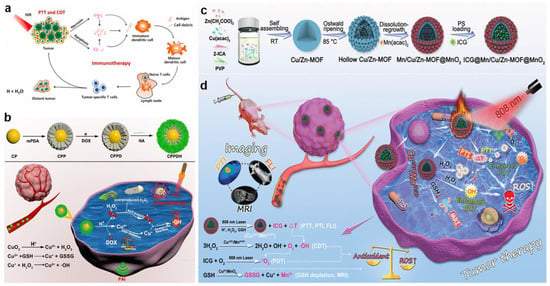

Figure 4.
(a) Schematic illustration of the therapeutic mechanisms of MoS2–CuO@BSA/R837 nanoplatforms for synergistic PTT/CDT/immunotherapy. Reproduced with permission []. Copyright 2021, Elsevier Ltd. (b) Schematic illustration of the synthesis of CPPDH and the schematic diagram of the therapeutic mechanism. Reproduced with permission []. Copyright 2021, American Chemical Society. (c) Schematic illustration of the synthesis of ICG@Mn/Cu/Zn–MOF@MnO2. (d) The scheme of therapeutic mechanism for PTI/FLI/MRI–guided ROS–augmented synergistic PTT/PDT/CDT. Reproduced with permission []. Copyright 2021, WILEY–VCH.
2.3. Other Metal-Based Fenton-like Reagents
In addition to Fe-and Cu-based Fenton reagents, other metals have also been used in Fenton and Fenton-like reactions, such as Mn [], Ti [], Ag [], Pt [], V [], and Ru []. For Mn-based Fenton reagents, manganese oxide (MnOx) triggers the generation of oxygen, a unique behavior that alleviates the hypoxia of TME, making it the most commonly used Mn-based Fenton nanomaterial in biological applications []. Moreover, as a classical Fenton-like reagent, MnOx can enhance CDT effectiveness by depleting intracellular GSH. Meanwhile, Mn2+ has been demonstrated as an effective contrast agent for tumor T1-weighted magnetic resonance imaging (T1-MRI), and it also has excellent PTI, photoacoustic imaging (PAI), and ultrasound imaging capabilities [,,].
In a recent study, Xiao and co-workers reported kinds of macrophage membrane-coated polymer nanogels (MPM@P NGs), which were co-loaded with MnO2 nanoparticles and cisplatin to achieve specific targeted chemotherapy and CDT (Figure 5a) []. In this nanoplatform, redox-responsive poly (N-vinylcaprolactam) nanogels containing disulfide bond (S-S) cross-linkers were disintegrated in TME to specifically release cisplatin and consume GSH to enhance CDT. Furthermore, MnO2 simultaneously consumed the highly concentrated endogenous GSH to promote the production of •OH, and the produced Mn2+ catalyzed the decomposition of H2O2 to generate ROS for tumor apoptosis and was also employed for T1-MRI (Figure 5b). In addition, the outer macrophage membrane enabled the polymer nanogels to penetrate the blood–brain barrier (BBB) due to the overexpressed macrophage-1 antigen, integrin α4, and β1 on the surface for effective glioma targeting. Consequently, this nanoplatform represented a feasible way to enhance MRI-guided synergetic antitumor effect. To realize more accurate cancer imaging and effective treatment, Wang et al. employed poly(lactic-co-glycolic acid) (PLGA) nanoparticles with porous structure as templates to prepare hollow MnO2 (HMnO2) shells in situ, which were further utilized to deliver bufalin and then cloaked with platelet membrane (Figure 5c) []. Bufalin and platelet modification were able to inhibit cancer angiogenesis, block cancer cell growth, and improve tumor targeting mediated by specific binding of P-selectin to the CD44 receptor of cancer cells. Moreover, under conditions of acidic tumor pH and relatively high GSH concentration, HMnO2 nanoparticles were rapidly degraded to allow the controlled release of bufalin, while Mn2+ was obtained for magnetic resonance monitoring and further targeted chemotherapy and CDT.
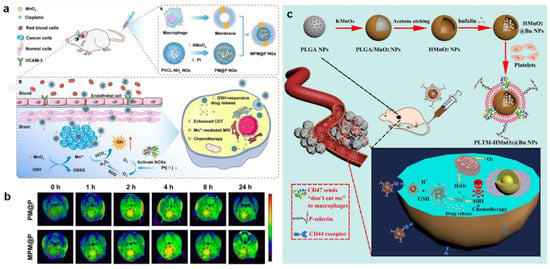
Figure 5.
(a) Schematic synthetic process of macrophage membrane–moated and the therapeutic mechanisms of MPM@P NGs for MRI–guided combination chemotherapy/CDT. (b) T1–weighted MR images of the orthotopic glioma before injection and after intravenous injection of PM@P NGs or MPM@P NGs. Reproduced with permission []. Copyright 2021, American Chemical Society. (c) Schematic synthetic process of PLTM–HMnO2@Bu NPs and the schematic illustration of the therapeutic mechanisms for in vivo MRI–monitored combination chemotherapy/CDT. Reproduced with permission []. Copyright 2020, Elsevier Ltd.
Recent reports have especially shown that multicomponent metallic composites improved activity and selectivity compared to single components, which was called the “synergetic catalytic effect” [,]. Zhou et al. synthesized cerium peroxide with good stability and then in situ doped Fenton-type metallic peroxides (Fe2+, Cu2+, Mn2+, Ce4+, Cr3+, etc.) to successfully prepare cerium peroxide matrix (M-CeOx) as a novel Fenton-type bimetallic peroxide nanomaterial []. Interestingly, the results of 3,3′,5,5′-tetramethylbenzidine (TMB) assays exhibited that the maximum absorbance increase of TMB increased 40–60-fold at pH 7.0 in the presence of M-CeOx, suggesting that the relatively high pH stimulated the generation of •OH and M-CeOx had excellent catalytic performance. In this prepared M-CeOx, 10% doping of Mn had much higher catalyst efficiency, and Mn-CeOx represented superb anticancer activity at the relatively high pH, due to the in situ self-generated H2O2 supplement and the multimetal-mediated synergistic-enhanced catalytic activity. In addition, in a recent work by Wu’s group, a novel type of organosilica hybrid micelles (Mn3O4@PDOMs-GOD) was designed and constructed by co-loading Mn3O4 and GOD for enhanced ST and CDT []. After the entry of Mn3O4@PDOMs-GOD in the tumor cells, GOD effectively depleted glucose and produced a large amount of H2O2 at the same time. Under the condition of acidic TME, H2O2 was decomposed into O2 by Mn3O4, breaking the vicious cycle of hypoxia and simultaneously enhancing the GOD-mediated ST effect through the regeneration of H2O2. Meanwhile, Mn3O4 also reacted with overexpressed GSH to release Mn2+, thereby converting the increased H2O2 into highly toxic •OH. Eventually, the Mn3O4@PDOMs-GOD nanosystem exhibited excellent cascade catalytic performance in the TME, which endowed it with a superior ability of sustained and stable delivery of O2 and H2O2 in situ. Overall, the novel type of organosilica hybrid micelles was a promising approach to improve the efficiency of O2-dependent ST and H2O2-dependent CDT.
3. Different Strategies to Improve CDT Performance
Although CDT has unique advantages over cancer treatments, its wide application in vivo is still limited by factors such as harsh Fenton reaction conditions, complex physiological environment, and limited reaction efficiency. Accordingly, to tackle the main obstacles existing in CDT, several strategies have been proposed to change this dilemma in the past few years. In general, the strategies to improve CDT performance are as follows: (1) CDT efficacy is improved by generating more H2O2; (2) oxidative stress at the tumor cells is enhanced by decreasing the reducing substances amount; and (3) CDT efficiency is improved by reducing the pH value.
3.1. Increasing H2O2 Level
The concentration of H2O2 plays an important role in the efficiency of CDT. In response to the limitation of endogenous H2O2 deficiency in the tumor region, an earlier approach was to directly deliver exogenous H2O2 into the body []. For example, Li et al. prepared a PLGA polymersome to encapsulate H2O2 into a hydrophilic core for direct delivery of exogenous H2O2 []. However, supplementing H2O2 from an external source had a risk of leakage and was difficult to implement. Therefore, several approaches for production in situ of endogenous H2O2 have been designed to overcome the limitation of H2O2 insufficiency in the tumor cells, such as the application of H2O2-related enzymes, metal peroxides, and photocatalysts.
GOx, which was able to specifically oxidize β-D-glucose into H2O2 and H+, was ideal for increasing tumor acidity and H2O2 level []. Zhang et al. synthesized an adenosine triphosphate (ATP)-responsive autocatalytic Fenton nanoagent (GOx@ZIF@MPN) for tumor therapy (Figure 6a) []. The nanoagents had cores which were formed by GOx and then incorporated with ZIF and MPN shells. Interestingly, the MPN shell was decomposed into TA and Fe3+ by the overexpression of ATP existing in the tumor cells, resulting in the exposure of internal GOx. Subsequently, the GOx consumed endogenous glucose to generate abundant H2O2. And TA then reacted with Fe3+ to produce Fe2+, which converted H2O2 to •OH and finally exerted good antitumor efficacy. It is widely known that GOx, as an aerobic enzyme, cannot efficiently catalyze the production of H2O2 in situ due to the hypoxic condition of cancer cells. To address this situation, Feng et al. employed MnO2 as an oxygen donor to compose core-shell structured nanoplatform Fe5C2-GOD@MnO2, which generated O2 through the weakly acidic TME-specific response, thereby enhancing the process of GOx to catalyze the production of H2O2 from glucose and O2 (Figure 6b) []. Tao et al. designed a new CDT nanosystem of RBC@Hb@GOx that effectively overcame the restriction of BBB by using GOx to elevate H2O2 concentration at the tumor site for the treatment of glioblastoma multiforme. Specifically, hemoglobin (Hb) and GOx were combined as an alternative synthetic Fenton catalyst and then encapsulated with red blood cell (RBC) membranes to achieve longer retention time and less immunogenic responses.
ROS-regulated nanozymes, such as superoxide dismutase (SOD) and POD, are employed to regulate intracellular and extracellular ROS concentrations. Hence, materials possessing SOD-like or POD-like enzyme activity are extensively utilized to enhance the level of ROS in TME [,]. In a study by Sang and her collaborators, the nanoparticle zeolitic imidazole framework-67 (ZIF-67) was constructed by assembling cobalt ions with 2-methylimidazole []. Subsequently, small-molecule inhibitor 3-amino-1,2,4-triazole (3-AT) and COOH-PEG-COOH were modified on the surface of ZIF-67 to fabricate the final product PZIF67-AT. PZIF67-AT with SOD-like activity could break the homeostasis of H2O2 and convert plenty of O2•− into H2O2. At the same time, the small molecule inhibitors released in the acidic microenvironment effectively inhibited the activity of catalase (CAT) and reduced the self-consumption of H2O2. In a separate study, Dong et al. constructed a nanozyme with tunable multi-enzymatic activities named PHMZCO-AT, which was composed of a hollow mesoporous Mn/Zr-doped CeO2 and 3-AT (Figure 6c) []. The nanozyme PHMZCO-AT, with enhanced SOD-like and POD-like activities, as well as inhibited CAT-like activity, allowed more H2O2 to participate in the Fenton reaction, thereby generating massive hydroxyl radicals to induce tumor cells apoptosis and death.
In addition to the SOD-like activity materials, cisplatin and β-Lapachone have also been demonstrated to be involved in affecting the related enzymes responsible for the generation of H2O2. For example, cisplatin was an extensively used broad-spectrum chemotherapy drug for cancer therapy. It was reported that nicotinamide adenine dinucleotide phosphate (NADPH) oxidase existing in cancer cells could be specifically activated by cisplatin and, subsequently, an O2 molecule was converted into O2•− after accepting an electron provided by the oxidation of NADPH, which was further catalyzed by SOD to produce the final H2O2 []. Ren et al. utilized this mechanism to design and synthesize the nanodrug of PTCG, which was composed of phenolic platinum (IV) prodrug, epigallocatechin-3gallate, and copolymer (PEG-b-PPOH) []. The study demonstrated that activated cisplatin was employed to increase the intracellular H2O2 level obviously via a cascade reaction in vitro and in vivo. Moreover, β-Lapachone, a new antitumor drug, was catalyzed by NAD(P)H: quinone oxidoreductase1 (NQO1) to generate H2O2 []. For instance, Wang et al. combined β-Lapachone, iron oxide nanoparticles, and camptothecin (CPT) to form pH- and H2O2-dual-responsive nanoplatform LaCIONPs (Figure 6d) []. The research indicated that β-Lapachone could generate large amounts of H2O2 through tumor-specific NAD(P)H: NQO1 catalysis. Interestingly, the product H2O2 not only participated in the Fenton reaction but also specifically activated the release of camptothecin from LaCIONPs for chemotherapy.
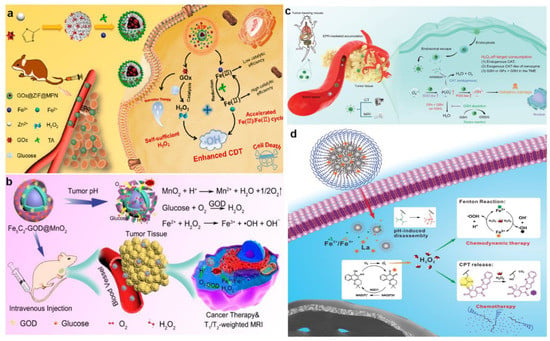
Figure 6.
(a) Schematic illustration of the synthesis of GOx@ZIF@MPN and its detailed therapeutic mechanisms for synergetic CDT and ST. Reproduced with permission []. Copyright 2018, American Chemical Society. (b) Schematic illustration of the therapeutic mechanisms of Fe5C2–GOD@MnO2. Reproduced with permission []. Copyright 2018, American Chemical Society. (c) The action mechanism of PHMZCO–AT nanozymes. Reproduced with permission []. Copyright 2021, WILEY–VCH. (d) The detailed therapeutic mechanisms of LaCIONPs for chemo/chemodynamic combination therapy. Reproduced with permission []. Copyright 2019, WILEY–VCH.
Metal peroxides were conventionally composed of metal ions and peroxo groups. Previous studies have shown that metal peroxides, especially in acidic solutions, more easily react with H2O to generate H2O2, which makes them more suitable for tumor therapy through the acidic TME response []. Meanwhile, with the rapid development of nanotheranostic technology in recent years, many nanostructured metal peroxides have been designed and fabricated for biomedical research and have achieved good therapeutic outcomes [,,,]. As a highly biocompatible metal peroxide, calcium peroxide (CaO2) was rapidly hydrolyzed in the acidic TME to produce O2 and H2O2 while releasing large amounts of Ca2+. The production of H2O2 not only strengthened the effect of CDT, but also caused the abnormal function of intracellular calcium channels, which eventually led to calcium overload-mediated cell necrosis. In addition, to prevent the disintegration of CaO2 during blood circulation in vivo, Shen et al. utilized pH-responsive ZIF-90 as a protective layer to encapsulate CaO2 (Figure 7a) []. CaO2@ZIF-Fe/Ce6@PEG was co-synthesized by Fe2+ and photosensitizer chlorin e6 (Ce6). CaO2@ZIF-Fe/Ce6@PEG achieved structural disassembly in the weakly acidic TME, and the exposed CaO2 was further hydrolyzed to produce O2 and H2O2, which enhanced Ce6-mediated PDT and Fe2+-induced CDT. HA, another material that prevents the breakdown of CaO2 during circulation, has been widely used as a tumor-targeting delivery carrier due to its good biocompatibility, biodegradability, and specific binding ability to CD44 receptors []. Meanwhile, HA exhibited strong affinity to CaO2 and Fe3O4 based on the coordinating properties of carboxyl groups with Ca2+ and Fe3+. Therefore, Han et al. prepared CaO2-Fe3O4@HA nanoparticles utilizing HA as a stabilizer and tumor-targeting block to deliver CaO2 (Figure 7b) []. In addition, there are several other metal peroxides that have shown the ability to generate H2O2. For instance, ZnO2 nanoparticles generated exogenous H2O2 and Zn2+ under the weakly acidic TME, and the generated Zn2+ could further promote the generation of endogenous O2•− and H2O2 after entering cancer cells [,].
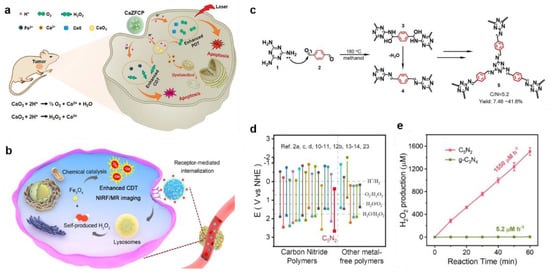
Figure 7.
(a) The therapeutic mechanisms of pH–responsive CaZFCP for enhanced CDT/PDT. Reproduced with permission []. Copyright 2021, WILEY–VCH. (b) The action mechanism of CaO2–Fe3O4@HA NPs in vivo. Reproduced with permission []. Copyright 2019, American Chemical Society. (c) The synthesis of C5N2 photocatalyst via Schiff base reactions. (d) CB/VB positions of photocatalysts reported by C5N2 et al. (e) Photocatalytic generation of H2O2 by C5N2 or g-C3N4 in water. Reproduced with permission []. Copyright 2022, WILEY–VCH.
In the fields of energy and the environment, artificial photocatalysis is a common approach to produce H2O2. In order to apply the approach of photocatalytic synthesis of H2O2 to tumor treatment, it urgently needed to be considered that the low efficiency of existing photocatalysts to generate H2O2 under the low oxygen condition, as well as the critical characteristic of the tumor microenvironment (pO2 ≤ 5 mmHg), retrained its application. Therefore, Ma et al. proposed a C5N2 photocatalyst with a conjugated C=N chain by Schiff base reaction for stable and sufficient H2O2 production in both hypoxic and normoxic conditions (Figure 7c) []. The results showed that the CB/VB position of C5N2 was downshifted remarkably due to the reinforced delocalization of π-electrons, which contributed to a favorable CB and VB band position for H2O2 photosynthesis in kinetics and thermodynamics (Figure 7d). Encouragingly, the H2O2 production rate of C5N2 was 1550 μmol L−1 per hour, which was approximately 298 times higher than that of traditional g-C3N4 (5.2 μmol L−1 per hour), indicating that C5N2 had high photocatalytic H2O2 production performance (Figure 7e). Since H2O2 did not have a direct strong killing effect on cancer cells, the original C5N2 was exfoliated into nanosheets by ultrasonication, and then Fe3+ was doped into C5N2 nanosheets to achieve effective CDT. Overall, this finding proposed a promising approach for improving CDT efficiency.
3.2. Decreasing the Level of Reducing Substances
The high expression of GSH in tumor cells hinders the production of ROS, further weakening the antitumor effect of CDT owing to the balance of oxidation and reduction being broken in the presence of high-level GSH in the TME. Therefore, downregulation of GSH in the TME was required to enhance the effectiveness of CDT.
One strategy for eliminating GSH was to deplete the existing GSH in tumor cells. Metals in the oxidized state were the most commonly used oxidants in existing GSH consumption because they could directly interact with GSH and consume it rapidly [,]. For instance, Chen et al. designed carrier-free Fe(III)-artemisinin (ART)-coordinated nanoparticles (Fe(III)-ART NPs) via coordination-driven self-assembly for self-enhanced MRI-guided CDT (Figure 8a) []. After cellular endocytosis, Fe(III)-ART NPs decomposed in an acidic and redox environment of endo/lysosomes to release Fe3+ and ART. The Fe3+ was then reduced to Fe2+ by intracellular GSH, catalyzing the endoperoxide of ART to generate toxic C-centered free radicals for efficacious CDT. Moreover, significant toxicities were detected in A549 cells co-incubated with Fe(III)-ART NPs. In vitro studies demonstrated that after being treated with 200 μg mL−1 Fe(III)-ART NPs for 24 h, the cell viability decreased to 34%, resulting from DNA damage and lipid and protein oxidation (Figure 8b). In addition, FLI was performed using ICG-labeled Fe(III)-ART NPs. The experiment showed that signals at the tumor site were detected within 8 h and maximized at 48 h. Furthermore, T2-weighted MRI signals in tumors were significantly enhanced at 32 h after intravenous administration of Fe(III)-ART NPs, proving that Fe(III)-ART NPs successfully accumulated in tumor cells (Figure 8c). Notably, the free radical generation process of Fe(III)-ART NPs was without reliance on pH or endogenous H2O2, thereby breaking through the bottlenecks of traditional CDT. Carbon dots (CDs) are small fluorescent carbon nanoparticles less than 10 nm in diameter, and have high chemical stability, luminescence, and broadband optical absorption. Existing studies have shown that CDs easily and rapidly formed iron-containing nanoparticles with Fe3+ and then quench the fluorescence. Subsequently, GSH continues to react with the nanoparticles to restore fluorescence while oxidizing into GSSG. In this way, GSH in the tumor region was consumed, and CDs-based GSH FLI was realized for further cancer therapy [,]. In recent work, Li et al. combined fluorescence-imaging CD and Fe3+ to produce a TME stimuli-responsive therapeutic nanodrug (Fe-CD) for FLI of glutathione depletion and tumor therapy (Figure 8d) []. After the entry of Fe-CD into the tumor cells, Fe-CD reacted with GSH and then released Fe3+, which not only acted as a quencher but was also an important factor in enhanced CDT. In this process, GSH was converted to GSSG, while Fe3+ was simultaneously reduced to Fe2+, making CDT more efficient. Moreover, this important process was also monitored by enhancing the fluorescence intensity of Fe-CD. In vitro data showed little change in GSH content when the A549 cells were treated with CD. However, when treated with Fe-CD, GSH content was significantly reduced, by 31.8%, due to the introduction of Fe3+, suggesting that Fe-CD had a significant GSH consumption potential (Figure 8e). In addition, this process restored the bright fluorescence of CD, while the reduction of intracellular GSH content was qualitatively indicated by the increase of fluorescence intensity.
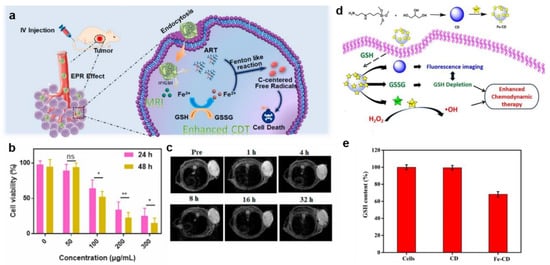
Figure 8.
(a) The therapeutic mechanisms of Fe(III)–ART NPs. (b) The viability of A549 cells treated with Fe(III)–ART NPs. Data were expressed as mean ± SD, n = 3, ns: no significance, * p < 0.05, ** p < 0.01, analysed by Student’s t test. (c) T2–weighted MR imaging of A549 tumor-bearing mice. Reproduced with permission []. Copyright 2020, Elsevier Ltd. (d) The synthesis of Fe–CD and its action mechanism in cancer cells. (e) GSH content of A549 cells treated with CD and Fe–CD. Reproduced with permission []. Copyright 2022, Elsevier Ltd.
The other strategy for more effectively eliminating GSH was to inhibit the synthesis of GSH utilizing chemical inhibitors. However, the use of inhibitors was limited by their high toxicity to normal cells and short half-life. In order to overcome these shortcomings and more effectively eliminate GSH in the tumor, Huang et al. synthesized a multistage GSH exhaustion nanomedicine, named TDMH (TP/2-DG @HMnO2@HA), to achieve high-efficiency CDT (Figure 9a) []. HA, which was modified on the surface of nanoparticles, enhanced tumor recognition by binding to the CD44 receptor on tumor cells. After HMnO2 entered the TME, it underwent a redox reaction with GSH to generate Mn2+ and GSSG, thereby quickly depleting the original GSH in the tumor. The generated Mn2+ catalyzed the formation of •OH from H2O2 in the presence of bicarbonate (HCO3−), which was abundant in the physiological medium. On the other hand, TP and 2-DG were released due to the collapse of the HMnO2 framework. Notably, the released TP targeted Nrf2 (nuclear factor (erythroid-derived 2)-like 2)/SLC7A11, thereby preventing de novo synthesis of GSH. As a commonly used glycolysis inhibitor, 2-DG blocked ATP production in the glycolytic pathway, which provides energy for tumor cells. Consequently, these reaction processes enabled multistage GSH depletion and amplified differences in the susceptibility of tumor cells and normal cells to oxidative stress.
High endogenous H2S expression is unique to the colon cancer tumor microenvironment compared to other tumor types []. As such, it is the target of many colon cancer treatments. The reducing capacity of endogenous H2S (currently 0.3–3.4 mM) in colon cancer is much stronger than that of GSH []. Hence, the efficacy of CDT in colon cancer is limited by endogenous H2S, which has a strong reducing ability and can scavenge the generated hydroxyl radicals. To solve the problem, Liu et al. synthesized H2S-activated CuFe2O4 nanoparticles using the characteristic that H2S could react with metal oxides and accelerate the valence transformation of Fenton or Fenton-like reagents for efficacious CDT of colon cancer (Figure 9b) []. Firstly, CuFe2O4 nanoparticles reacted with endogenous H2S in situ in the TME, leading to the depletion of endogenous H2S in colon cancer. Meanwhile, Cu9Fe9S16 nanoparticles with strong NIR absorption were obtained by CuFe2O4, which were used as photothermal-enhanced CDT agents and intelligent PAI reagents (Figure 9c,d). In addition, parts of Cu2+ and Fe3+ in CuFe2O4 nanoparticles were reduced to Cu+ and Fe2+ by endogenous H2S to form hydroxyl radicals via Fenton or Fenton-like reactions. Eventually, CuFe2O4 had a wide range of promising applications in anticancer drug delivery for colon cancer.
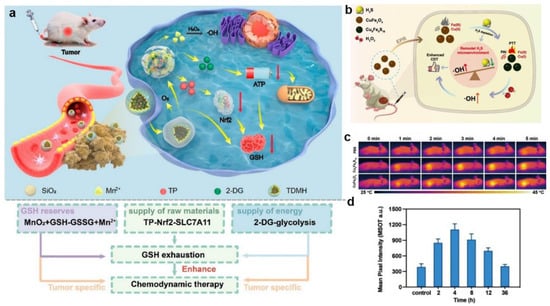

Figure 9.
(a) The therapeutic mechanisms of TDMH nanomedicine. Reproduced with permission []. Copyright 2022, American Chemical Society. (b) The action mechanism of CuFe2O4 nanoparticles in cancer cells. (c) Thermal imaging of different groups under 808 nm laser irradiation. (d) Corresponding PA signals of HCT116 tumor-bearing mice. Reproduced with permission []. Copyright 2021, Elsevier Ltd.
3.3. Lowering pH Value
The pH range of the TME was between 6.5 and 7.0, while the optimal pH for Fenton reactions ranged from 2 to 4 []. Since the high pH value inhibited the decomposition of nanomaterials and the release of metal ions with catalytic activity, lowering the pH value of TME could effectively improve the catalytic efficiency of the Fenton reaction and enhance the therapeutic efficiency of CDT. In general, introducing exogenous acids or other substances was an efficient and common approach to regulating the pH of the TME. GOx was able to oxidize glucose to H2O2 and H+, which effectively increased the acidity and hydrogen peroxide in the tumor region, thus improving the efficiency of CDT treatment []. For instance, Liu et al., for the first time, designed a ternary pillar[6]arene-based nanocatalyst (GOx@T-NPs) employing pillar[6]arene and ferrocene via host–guest interactions to realize the combination of chemotherapy, ST, and CDT (Figure 10a) []. After being internalized and endocytosed by cancer cells, the intratumoral glucose was catalyzed by GOx@T-NPs into H+ and H2O2, thereby reducing the pH value inside cancer cells and further achieving high toxicity toward tumor cells. Meanwhile, overexpressed GSH in tumors was consumed by disulfide bonds of GOx@T-NPs, inducing the massive •OH production and the simultaneous release of the antitumor drug CPT. Li et al. reported a cascade catalytic nanoplatform (GOx-NCs/Fe3O4) for antibacterial CDT toward Escherichia coli (Figure 10b) []. Specifically, GOx-NCs/Fe3O4 was constructed by loading GOx onto the surface of covalent-assembled polymer capsules (NCs) through electrostatic interactions, which was successfully achieved to efficiently encapsulate Fe3O4 and immobilize GOx. Furthermore, GOx was oxidized into H2O2 in the presence of glucose, increasing in H+ concentration. After that, H2O2 was converted into •OH triggered by Fe3O4 nanoparticles through the Fenton reaction, thereby destroying the lipoproteins of the bacterial cell wall and causing the death of pathogens. The results of plate counting showed that GOx-NCs/Fe3O4 represented much higher inhibition efficiency compared with GOx and GOx-NCs, demonstrating its potential for efficient antimicrobial activity (Figure 10c). As a result, this strategy demonstrated that GOx-NCs/Fe3O4 improved the efficacy of the Fe3O4-mediated and GOx-introduced CDT for antibacterial applications.
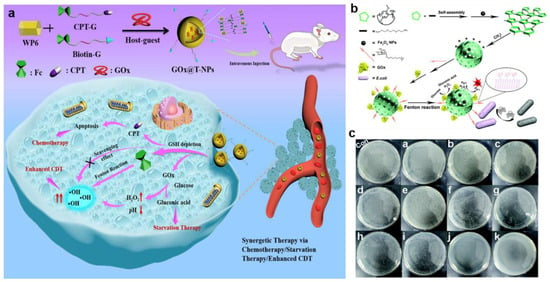
Figure 10.
(a) The therapeutic mechanisms of GOx@T–NPs. Reproduced with permission []. Copyright 2021, American Chemical Society. (b) The synthesis of Gox–NCs/Fe3O4 and its antibacterial action mechanism. (c) Plate coating results. con, Control.((a) glucose; (b) NCs; (c) GOx (10 μg mL−1) without glucose; (d,e) GOx–NCs (10 μg mL−1) and GOx–NCs/Fe3O4 (GOx: 10 μg mL−1; Fe3O4: 50 μg mL−1) without glucose; (f,g) GOx (5 μg mL−1and 10 μg mL−1) incubated with glucose; (h,i) GOx–NCs (5 μg mL−1 and 10 μg mL−1) incubated with glucose; (j) Gox–NCs/Fe3O4(GOx: 5 μg mL−1 and Fe3O4: 25 μg mL−1) and (k) GOx–NCs/Fe3O4 (GOx: 10 μg mL−1 and Fe3O4 50 μg mL−1) incubated with glucose). Reproduced with permission []. Copyright 2021, Royal Society of Chemistry.
However, the uncontrollable reaction between GOx and glucose during blood circulation could cause damage to normal tissue, so Shi et al. synthesized an acidity-unlocked nanoplatform (FePt@-FeOx@TAM-PEG) in order to selectively produce ROS within tumors while remaining silent in normal tissue (Figure 11a) []. Since FePt@FeOx@TAM-PEG had a hydrophobic shell, it remained silent in blood and normal tissues. After reaching the tumor area, FePt@FeOx@TAM-PEG was decomposed and further reacted with H2O2 to generate •OH due to the pH-responsive transition from hydrophobic to hydrophilic tamoxifen (TAM). Notably, TAM, an anti-estrogen drug, inhibited mitochondrial complex I and further triggered the adenosine monophosphate-activated protein kinase signaling pathway. This process promoted the decomposition of glucose and the accumulation of lactic acid, which increased the acidity of the cancer cells, thus overcoming the limitations of the weak acidity of the tumor. The increased intracellular acidity boosted the release of Fe2+ and Fe3+ from FePt@FeOx nanoparticles, thereby accelerating the H2O2 degradation and enhancing the antitumor potential of the nanoplatform. Therefore, the problem of insufficient CDT efficiency and potential side effects on normal cells have been effectively solved by the design of the nanodrug.
Carbonic anhydrase IX (CA IX) is a pH-regulating enzyme overexpressed on the cell membrane of most hypoxic tumors and can catalyze the reversible hydration of CO2 to generate H2CO3 and H+, participate in tumor extracellular acidosis, and accelerate tumor metastases []. CA IX inhibitors (CAI) inhibit extracellular HCO3−/H+ formation, leading to intracellular glycolytic H+ accumulation. Inspired by this, Zuo et al. designed and constructed kinds of hollow mesoporous ferric oxide (HMFe) nanocatalysts (MM@HMFe@BS), which were camouflaged by macrophage membrane as well as loaded with CAI to decrease intracellular pH in the TME for self-amplified CDT (Figure 11b) []. The camouflage of macrophage cell membrane enabled the nanocatalysts to have immune evasion ability and recognize tumor endothelium cells and cancer cells via α4/VCAM-1 interaction, further promoting tumor chemotactic aggregation. After the efficient internalization into cancer cells, MM@HMFe@BS was specifically degraded to release HMFe and 4-(2-aminoethyl) benzene sulfonamide (BS). Interestingly, the released HMFe not only acted as a highly active atom-exposing Fenton agent to enhance CDT but was also used with high-efficiency MRI to monitor the biodistribution and treatment progress of MM@HMFe@BS. With the specific release of the CAI, CA IX was inhibited by BS, thus inducing intracellular H+ accumulation to accelerate the Fenton reaction. Dong and co-workers reported a multifunctional nanosystem of TPI@PPCAI to remodel the TME and reinforce the in situ Fenton reaction, which was composed of the inner TPI micelles-loading iron-oxide nanoparticles (IONs) and the outer poly (dopamine-co-protocatechuic acid) (PDA-PA, PP) coating modified with the CAI []. Firstly, 4-(2-aminoethyl) benzenesulfonamide, a CA IX inhibitor, inhibited the overexpressed CA IX, thus leading to intracellular acidification. Next, the PP coating was degraded, triggered by the acidification and NIR irradiation, resulting in the disintegration of the inner micellar structure to further release TPGS-PPh3 and IONs. Research showed that TPGS-PPh3, as the endogenous ROS amplifier, was able to target the mitochondria and then interfere with the function, thereby increasing the intracellular ROS basal level for Fenton reactions [,,]. Additionally, based on the good photothermal conversion performance of PDA, the temperature of TPI@PPCAI nanoparticles solution increased by more than 15 °C and reached 46.5 °C at a concentration of 0.5 mg mL−1 after NIR irradiation for 8 min, indicating that TPI@PPCAI had good photothermal performance to enhance Fenton catalytic efficiency and improve the efficiency of CDT.
Jiang et al. synthesized a nanomedicine of FeCNB with high catalytic activity in acidic/neutral conditions, which overcame the limit of firm acidity required for the traditional Fenton reaction (Figure 11c) []. The nanomedicine FeCNB was composed of a Fe/Fe3C core and mesoporous graphite carbon shell modified by biotin to realize effective CDT by improving the efficiency of the Fenton reaction in acidic/neutral conditions. Specifically, graphitic carbon received electrons from Fe0 via a reduction reaction based on the principle of galvanic cells. Meanwhile, Fe0 in FeCNB as a reducing agent was oxidized to Fe2+, thus consuming H2O2 to produce •OH in neutral and acidic environments. Moreover, the experimental results confirmed that the photothermal conversion efficiency of FeCNB was 31.4% with 808 nm laser irradiation, revealing that it had an efficient photothermal conversion function to achieve effective PTT. This work offered a new perspective to remodel the pH in the TME for the development of ideal CDT nanomedicine.
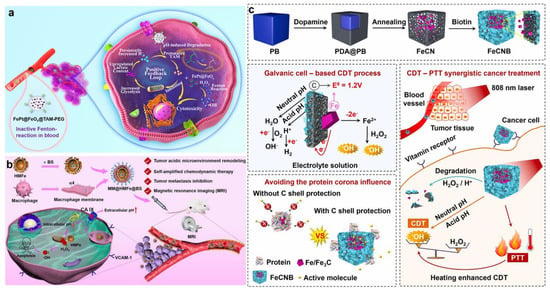

Figure 11.
(a) The therapeutic mechanisms of FePt@–FeOx@TAM–PEG. Reproduced with permission []. Copyright 2021, WILEY–VCH. (b) Schematic diagram of the therapeutic mechanisms of MM@HMFe@BS nanomaterials in vivo. Reproduced with permission []. Copyright 2022, American Chemical Society. (c) Schematic illustrations of the construction and the therapeutic mechanism of FeCNB []. Copyright 2022, Elsevier Ltd.
4. Conclusions and Future Directions
Cancer has remained a major killer that endangers human life and health. However, traditional cancer treatment techniques such as chemotherapy, radiotherapy, and surgery suffer from poor treatment efficiency, large toxic side effects and easy recurrence, and metastasis, which limit their application in the clinic. Many researchers have focused on exploring more efficient and simpler cancer treatments with important clinical value. Based on Fenton/Fenton-like reactions, CDT is attracting increasing attention as a catalyst-driven modality for cancer therapy because of its remarkable specificity and selectivity for tumors, minimal systemic toxicity and side effects, and the absence of a requirement for field stimulation during treatment. So far, many studies have reported that a large number of metal-based nanocatalytic medicines have been developed to initiate in situ Fenton or Fenton-like reactions in tumor cells, while various nanomaterials have also been used to modulate the tumor microenvironment to improve the therapeutic efficiency of CDT and achieve better tumor treatment effects. Here, Table 1 shows commonly used metal-based catalysts and their applications in biomedicine.

Table 1.
Summary of various Fenton nanocatalysts for enhanced chemodynamic therapy.
However, CDT is still in its early stages. More research is needed for the clinical translation of CDT, as several important scientific questions need to be addressed. Typical questions are listed below. (1) The target activity of anticancer medicines needs to improve. Metal-based compounds are widely utilized to enhance the performance of CDT based on Fenton and Fenton-like reactions. However, the targeted delivery via the EPR effect is limited, so modifying the surface of nanoparticles for active targeting to recognize tumors selectively could be considered. (2) It is also an urgent issue to reduce the toxic side effects of CDT. Some of these metal ions remain in vivo after therapy, which has potential side effects on the body. Hence, it is necessary to improve the catalytic efficiency of the catalyst and to deepen its ADME (absorption, distribution, metabolism, and excretion) performance study. (3) New smart combinatorial therapeutic nanosystems with high therapeutic efficiency need to be developed. The efficiency of most nanocatalytic therapies depends largely on the tumor microenvironment. The H2O2 and reductive substances levels, and the pH value in the TME, greatly restrict the efficiency of CDT. Moreover, regulation of the CDT treatment process is also extremely important. Therefore, smart combinatorial therapeutic nanosystems with diagnostic and therapeutic capabilities capable of modulating the tumor microenvironment are a future prospect [,]. (4) The efficacy of a single treatment is limited. The further development and design of CDT-induced combination cancer therapies will, to some degree, overcome the shortcomings of CDT, which has a stronger therapeutic performance than any single therapy or their theoretical combinations, such as CDT-PTT, CDT-PDT, CDT-ST, CDT-chemotherapy, CDT-SDT, and CDT-immunotherapy. For example, the Fenton and Fenton-like reactions in CDT are used to produce large amounts of •OH in combination with immunotherapy, and the Russell mechanism in CDT is used to produce a number of 1O2 to overcome the deficiencies of photodynamic therapy.
CDT is a fascinating research frontier that still requires further development. It is recommended to develop more metal-based and metal-free CDT nanomaterials and to improve the targeting and biological safety of these drugs. At the same time, metal-based nanocatalytic medicines are more likely to progress to the clinical stage, due to their multimodal diagnostic and therapeutic capabilities combined with advanced diagnostic methods like CT imaging and MRI. These nanomedicines could accurately diagnose diseases in real-time while simultaneously providing treatment. Moreover, the ability to monitor the therapeutic effect and adjust the dosage regimen during treatment enables optimal therapeutic outcomes and promotes the application of CDT nanomaterials in clinical transformation [,]. Additionally, an in-depth understanding and exploration of the anticancer pathways of CDT at the genetic and molecular levels are needed, which would help deepen the understanding of the anticancer mechanism of CDT and lead to the design of more effective agents.
Author Contributions
Conceptualization, D.L., P.W. and G.L.; writing—original draft preparation, B.C. and D.L.; resources, C.L. and B.C.; data curation, Z.Z.; project administration, D.L. and P.W.; writing—review and editing, D.L., P.W. and G.L.; supervision, G.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Major State Basic Research Development Program of China (2017YFA0205201), the National Natural Science Foundation of China (NSFC) (81925019, 81901876 and U1705281), the Fundamental Research Funds for the Central Universities (20720190088 and 20720200019), and the Program for New Century Excellent Talents in University, China (NCET-13-0502).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| reactive oxygen species | ROS |
| hydrogen peroxide | H2O2 |
| superoxide anion | O2− |
| hydroxyl radical | •OH |
| singlet oxygen | 1O2 |
| peroxy hydroxyl radical | •OOH |
| photodynamic therapy | PDT |
| chemodynamic therapy | CDT |
| sonodynamic therapy | SDT |
| tetrakis(4-carboxyphenyl) porphyrin | TCPP |
| peroxidase | POD |
| glutathione | GSH |
| tumor microenvironment | TME |
| hydrogen sulfide | H2S |
| ferroferric oxide | Fe3O4 |
| ferrous disulfide | FeS2 |
| ferric oxide | Fe2O3 |
| Fe-metal organic frameworks | Fe-MOFs |
| polydopamine | PDA |
| magnetic resonance imaging | MRI |
| computed tomography | CT |
| starvation therapy | ST |
| photothermal therapy | PTT |
| glucose oxidase | GOx |
| glucose oxidase | GOD |
| tannic acid | TA |
| near-infrared | NIR |
| gluconic acid | H+ |
| dihydroartemisinin | DHA |
| metal-polyphenol networks | MPN |
| bis(2-carbopentyloxy-3,5,6-trichlorophenyl) oxalate | CPPO |
| zeolitic imidazolate framework-8 | ZIF-8 |
| gallic acid | GA |
| photoacoustic | PA |
| enhanced permeability and retention | EPR |
| copper sulfide | CuS |
| copper oxide | CuO |
| bovine serum albumin | BSA |
| immunoadjuvant imiquimod | R837 |
| tumor-associated antigens | TAAs |
| mesoporous polydopamine | mPDA |
| doxorubicin | DOX |
| hyaluronic acid | HA |
| generation 5 poly (amidoamine) | G5 |
| Michaelis–Menten constant | Km |
| velocity | Vm |
| β-cyclodextrin | β-CD |
| ferrocene | Fc |
| indocyanine green | ICG |
| photothermal imaging | PTI |
| fluorescence imaging | FLI |
| manganese oxide | MnOx |
| T1-weighted magnetic resonance imaging | T1-MRI |
| photoacoustic imaging | PAI |
| blood–brain barrier | BBB |
| poly(lactic-co-glycolic acid) | PLGA |
| hollow MnO2 | HMnO2 |
| cerium peroxide matrix | M-CeOx |
| 3,3′,5,5′-tetramethylbenzidine | TMB |
| adenosine triphosphate | ATP |
| hemoglobin | Hb |
| red blood cell | RBC |
| superoxide dismutase | SOD |
| zeolitic imidazole framework-67 | ZIF-67 |
| 3-amino-1,2,4-triazole | 3-AT |
| catalase | CAT |
| nicotinamide adenine dinucleotide phosphate | NADPH |
| quinone oxidoreductase1 | NQO1 |
| camptothecin | CPT |
| calcium peroxide | CaO2 |
| chlorin e6 | Ce6 |
| artemisinin | ART |
| carbon dots | CDs |
| bicarbonate | HCO3– |
| tamoxifen | TAM |
| carbonic anhydrase IX | CA IX |
| CA IX inhibitor | CAI |
| hollow mesoporous ferric oxide | HMFe |
| 4-(2-aminoethyl) benzene sulfonamide | BS |
| iron-oxide nanoparticles | IONs |
| poly (dopamine-co-protocatechuic acid) | PP |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar]
- Faguet, G.B. A brief history of cancer: Age-old milestones underlying our current knowledge database. Int. J. Cancer 2015, 136, 2022–2036. [Google Scholar]
- Gotwals, P.; Cameron, S.; Cipolletta, D.; Cremasco, V.; Crystal, A.; Hewes, B.; Mueller, B.; Quaratino, S.; Sabatos-Peyton, C.; Petruzzelli, L.; et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat. Rev. Cancer 2017, 17, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Jure-Kunkel, M.; Masters, G.; Girit, E.; Dito, G.; Lee, F.; Hunt, J.T.; Humphrey, R. Synergy between chemotherapeutic agents and CTLA-4 blockade in preclinical tumor models. Cancer Immunol. Immunother. 2013, 62, 1533–1545. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Li, D.; Li, C.; Zhuang, Z.; Chu, C.; Ostrikov, K.; Thompson, E.W.; Liu, G.; Wang, P. A review on reactive oxygen species (ROS)-inducing nanoparticles activated by uni- or multi-modal dynamic treatment for oncotherapy. Nanoscale 2023, 15, 11813–11833. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shan, X.; Shi, S.; Jiang, C.; Li, T.; Wei, S.; Zhang, X.; Sun, G.; Liu, J. Tumor microenvironment-responsive polydopamine-based core/shell nanoplatform for synergetic theranostics. J. Mater. Chem. B 2020, 8, 4056–4066. [Google Scholar] [CrossRef]
- Li, D.; Yang, Y.; Li, D.; Pan, J.; Chu, C.; Liu, G. Organic Sonosensitizers for Sonodynamic Therapy: From Small Molecules and Nanoparticles toward Clinical Development. Small 2021, 17, 2101976. [Google Scholar] [CrossRef]
- Chu, C.; Ren, E.; Zhang, Y.; Yu, J.; Lin, H.; Pang, X.; Zhang, Y.; Liu, H.; Qin, Z.; Cheng, Y.; et al. Zinc(II)-Dipicolylamine Coordination Nanotheranostics: Toward Synergistic Nanomedicine by Combined Photo/Gene Therapy. Angew. Chem. Int. Ed. 2019, 58, 269–272. [Google Scholar] [CrossRef]
- Wang, C.; Cao, F.; Ruan, Y.; Jia, X.; Zhen, W.; Jiang, X. Specific Generation of Singlet Oxygen through the Russel Mechanism in Hypoxic Tumors and GSH Depletion by Cu-TCPP Nanosheets for Cancer Therapy. Angew. Chem. Int. Ed. 2019, 58, 9846–9850. [Google Scholar] [CrossRef]
- Zhang, C.; Bu, W.; Ni, D.; Zhang, S.; Li, Q.; Yao, Z.; Zhang, J.; Yao, H.; Wang, Z.; Shi, J. Synthesis of Iron Nanometallic Glasses and Their Application in Cancer Therapy by a Localized Fenton Reaction. Angew. Chem. Int. Ed. 2016, 55, 2101–2106. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, X.; Liu, Z.; Cheng, L. Recent progress of chemodynamic therapy-induced combination cancer therapy. Nano Today 2020, 35, 100946. [Google Scholar] [CrossRef]
- Li, D.; Pan, J.; Xu, S.; Fu, S.; Chu, C.; Liu, G. Activatable Second Near-Infrared Fluorescent Probes: A New Accurate Diagnosis Strategy for Diseases. Biosensors 2021, 11, 436. [Google Scholar] [CrossRef]
- Jia, C.; Guo, Y.; Wu, F.G. Chemodynamic Therapy via Fenton and Fenton-Like Nanomaterials: Strategies and Recent Advances. Small 2022, 18, 2103868. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Clement, M.V.; Long, L.H. Hydrogen peroxide in the human body. FEBS Lett. 2000, 486, 10–13. [Google Scholar] [CrossRef]
- Liang, K.; Sun, H.; Yang, Z.; Yu, H.; Shen, J.; Wang, X.; Chen, H. Breaking the Redox Homeostasis: An Albumin-Based Multifunctional Nanoagent for GSH Depletion-Assisted Chemo-/Chemodynamic Combination Therapy. Adv. Funct. Mater. 2021, 31, 2100355. [Google Scholar] [CrossRef]
- Ren, Z.; Sun, S.; Sun, R.; Cui, G.; Hong, L.; Rao, B.; Li, A.; Yu, Z.; Kan, Q.; Mao, Z. A Metal-Polyphenol-Coordinated Nanomedicine for Synergistic Cascade Cancer Chemotherapy and Chemodynamic Therapy. Adv. Mater. 2020, 32, 1906024. [Google Scholar] [CrossRef]
- Hu, T.; Yan, L.; Wang, Z.; Shen, W.; Liang, R.; Yan, D.; Wei, M. A pH-responsive ultrathin Cu-based nanoplatform for specific photothermal and chemodynamic synergistic therapy. Chem. Sci. 2021, 12, 2594–2603. [Google Scholar] [CrossRef]
- Li, S.L.; Jiang, P.; Jiang, F.L.; Liu, Y. Recent Advances in Nanomaterial-Based Nanoplatforms for Chemodynamic Cancer Therapy. Adv. Funct. Mater. 2021, 31, 2100243. [Google Scholar] [CrossRef]
- Park, S.C.; Kim, N.H.; Yang, W.; Nah, J.W.; Jang, M.K.; Lee, D. Polymeric micellar nanoplatforms for Fenton reaction as a new class of antibacterial agents. J. Control. Release 2016, 221, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, X.; Zhang, T.; Ghosal, A.; Zhang, G.; Fan, H.M.; Zhao, L. Iron nanoparticles augmented chemodynamic effect by alternative magnetic field for wound disinfection and healing. J Control. Release 2020, 324, 598–609. [Google Scholar] [CrossRef]
- Qin, X.; Wu, C.; Niu, D.; Qin, L.; Wang, X.; Wang, Q.; Li, Y. Peroxisome inspired hybrid enzyme nanogels for chemodynamic and photodynamic therapy. Nat. Commun. 2021, 12, 5243. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Li, M.; Dai, R.; Xiao, S.; Tang, J.; Zhang, X.; Chen, B.; Liu, J. Multifunctional FeS2@SRF@BSA nanoplatform for chemo-combined photothermal enhanced photodynamic/chemodynamic combination therapy. Biomater. Sci. 2021, 10, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Liu, C.; Xu, Y.; Si, W.; Wang, W.; Zhong, L.; Zhao, Y.; Dong, X. gamma-Fe2O3 Loading Mitoxantrone and Glucose Oxidase for pH-Responsive Chemo/Chemodynamic/Photothermal Synergistic Cancer Therapy. Adv. Healthc. Mater. 2022, 11, 2102632. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Z.; Wei, J.; Xiao, Y.; She, Y.; Su, Q.; Zhao, T.; Li, J.; Shao, J. Juglone-loaded metal-organic frameworks for H2O2 self-modulating enhancing chemodynamic therapy against prostate cancer. Chem. Eng. J. 2022, 430, 133057. [Google Scholar] [CrossRef]
- Lei, H.; Wang, X.; Bai, S.; Gong, F.; Yang, N.; Gong, Y.; Hou, L.; Cao, M.; Liu, Z.; Cheng, L. Biodegradable Fe-Doped Vanadium Disulfide Theranostic Nanosheets for Enhanced Sonodynamic/Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 52370–52382. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Jiang, G.; Wan, Y.; Liu, J.; Pi, F. Nanomaterials-modulated Fenton reactions: Strategies, chemodynamic therapy and future trends. Chem. Eng. J. 2023, 466, 142960. [Google Scholar]
- Tang, Z.; Zhao, P.; Wang, H.; Liu, Y.; Bu, W. Biomeicine Meets Fenton Chemistry. Chem. Rev. 2021, 121, 1981–2019. [Google Scholar] [CrossRef]
- Du, W.; Liu, T.; Xue, F.; Cai, X.; Chen, Q.; Zheng, Y.; Chen, H. Fe3O4 Mesocrystals with Distinctive Magnetothermal and Nanoenzyme Activity Enabling Self-Reinforcing Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 19285–19294. [Google Scholar] [CrossRef]
- Xiao, J.; Hai, L.; Li, Y.; Li, H.; Gong, M.; Wang, Z.; Tang, Z.; Deng, L.; He, D. An Ultrasmall Fe3O4—Decorated Polydopamine Hybrid Nanozyme Enables Continuous Conversion of Oxygen into Toxic Hydroxyl Radical via GSH-Depleted Cascade Redox Reactions for Intensive Wound Disinfection. Small 2022, 18, 2105465. [Google Scholar] [CrossRef]
- Luo, K.; Zhao, J.; Jia, C.; Chen, Y.; Zhang, Z.; Zhang, J.; Huang, M.; Wang, S. Integration of Fe3O4 with Bi2S3 for Multi-Modality Tumor Theranostics. ACS Appl. Mater. Interfaces 2020, 12, 22650–22660. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, Q.; Zhang, M.; Sun, B.; She, Z.; Ge, M.; Lu, T.; Chu, X.; Wang, Y.; Wang, J.; et al. Hollow Porous Carbon Coated FeS2-Based Nanocatalysts for Multimodal Imaging-Guided Photothermal, Starvation, and Triple-Enhanced Chemodynamic Therapy of Cancer. ACS Appl. Mater. Interfaces 2020, 12, 10142–10155. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Pan, J.; Xu, S.; Cheng, B.; Wu, S.; Dai, Q.; Ke, M.-R.; Zheng, B.-Y.; Chu, C.; Liu, C.; et al. Programmable phthalocyanine-iron-based nanoreactor for fluorescence/magnetic resonance dual-modality imaging-guided sono/chemodynamic therapies. Chem. Eng. J. 2023, 452, 139330. [Google Scholar] [CrossRef]
- Haynes, R.K.; Chan, W.C.; Lung, C.M.; Uhlemann, A.C.; Eckstein, U.; Taramelli, D.; Parapini, S.; Monti, D.; Krishna, S. The Fe2+—Mediated decomposition, PfATP6 binding, and antimalarial activities of artemisone and other artemisinins: The unlikelihood of C-centered radicals as bioactive intermediates. ChemMedChem 2007, 2, 1480–1497. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, L.; Chen, J.; Wu, Q.; Yu, W.; Zeng, W.; Shi, Y.; Lu, Y.; Liu, Y. alpha-Fe2O3 based nanotherapeutics for near-infrared/dihydroartemisinin dual-augmented chemodynamic antibacterial therapy. Acta Biomater. 2022, 150, 367–379. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, K.; Yang, F.; Dai, W.; Lu, H.; Dong, H.; Zhang, X. Biodegradable Metal-Organic Frameworks Power DNAzyme for in Vivo Temporal-Spatial Control Fluorescence Imaging of Aberrant MicroRNA and Hypoxic Tumor. Anal. Chem. 2020, 92, 8333–8339. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Xu, H.; Xu, C.; Tong, Z.; Zhang, S.; Bai, Y.; Chen, Y.; Xu, Q.; Zhou, L.; Ding, H.; et al. A Nanomedicine Fabricated from Gold Nanoparticles-Decorated Metal-Organic Framework for Cascade Chemo/Chemodynamic Cancer Therapy. Adv. Sci. 2020, 7, 2001060. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Niu, H.; Wang, Y.; Wu, A.; Shu, C.; Zhu, Y.; Bian, Y.; Lin, K. Metal-Organic Framework-Based Nanoagents for Effective Tumor Therapy by Dual Dynamics-Amplified Oxidative Stress. ACS Appl. Mater. Interfaces 2021, 13, 45201–45213. [Google Scholar] [CrossRef]
- Yin, S.Y.; Liu, W.; Yang, J.; Li, J. Synergistically enhanced multienzyme catalytic nanoconjugates for efficient cancer therapy. J. Mater. Chem. B 2021, 9, 5877–5886. [Google Scholar] [CrossRef]
- Xiao, M.; Lai, W.; Wang, F.; Li, L.; Fan, C.; Pei, H. Programming Drug Delivery Kinetics for Active Burst Release with DNA Toehold Switches. J. Am. Chem. Soc. 2019, 141, 20354–20364. [Google Scholar] [CrossRef]
- Li, J.; Zheng, C.; Cansiz, S.; Wu, C.; Xu, J.; Cui, C.; Liu, Y.; Hou, W.; Wang, Y.; Zhang, L.; et al. Self-assembly of DNA nanohydrogels with controllable size and stimuli-responsive property for targeted gene regulation therapy. J. Am. Chem. Soc. 2015, 137, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lee, M.; Kim, T.; Na, J.; Jung, Y.; Jung, G.Y.; Kim, S.; Park, N. A RNA producing DNA hydrogel as a platform for a high performance RNA interference system. Nat. Commun. 2018, 9, 4331. [Google Scholar] [CrossRef]
- Wang, P.; Xiao, M.; Pei, H.; Xing, H.; Luo, S.-H.; Tsung, C.-K.; Li, L. Biomineralized DNA nanospheres by metal organic framework for enhanced chemodynamic therapy. Chem. Eng. J. 2021, 415, 129036. [Google Scholar] [CrossRef]
- Jana, D.; Wang, D.; Bindra, A.K.; Guo, Y.; Liu, J.; Zhao, Y. Ultrasmall Alloy Nanozyme for Ultrasound- and Near-Infrared Light-Promoted Tumor Ablation. ACS Nano 2021, 15, 7774–7782. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ma, B.; Shi, Y.; Dai, Q.; Li, D.; Ren, E.; Zhu, J.; Liu, J.; Chen, H.; Yin, Z.; et al. Tumor microenvironment-triggered MoS2@GA-Fe nanoreactor: A self-rolling enhanced chemodynamic therapy and hydrogen sulfide treatment for hepatocellular carcinoma. Chem. Eng. J. 2021, 406, 126888. [Google Scholar]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.; Jin, Y.; Zhen, W.; Wang, Y.; Liu, J.; Jin, L.; Zhang, S.; Zhao, Y.; Song, S.; et al. Copper(I) Phosphide Nanocrystals for In Situ Self-Generation Magnetic Resonance Imaging-Guided Photothermal-Enhanced Chemodynamic Synergetic Therapy Resisting Deep-Seated Tumor. Adv. Funct. Mater. 2019, 29, 1904678. [Google Scholar] [CrossRef]
- Hu, R.; Fang, Y.; Huo, M.; Yao, H.; Wang, C.; Chen, Y.; Wu, R. Ultrasmall Cu2−xS nanodots as photothermal-enhanced Fenton nanocatalysts for synergistic tumor therapy at NIR-II biowindow. Biomaterials 2019, 206, 101–114. [Google Scholar] [CrossRef]
- Lin, L.S.; Huang, T.; Song, J.; Ou, X.Y.; Wang, Z.; Deng, H.; Tian, R.; Liu, Y.; Wang, J.F.; Liu, Y.; et al. Synthesis of Copper Peroxide Nanodots for H2O2 Self-Supplying Chemodynamic Therapy. J. Am. Chem. Soc. 2019, 141, 9937–9945. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Hu, Y.; Liu, J.; Guo, M.; Wei, H.; Zheng, S.; Jiang, T.; Sun, X.; Ma, Z.; et al. Photothermal conversion-coordinated Fenton-like and photocatalytic reactions of Cu2−xSe-Au Janus nanoparticles for tri-combination antitumor therapy. Biomaterials 2020, 255, 120167. [Google Scholar] [CrossRef]
- Ni, K.; Aung, T.; Li, S.; Fatuzzo, N.; Liang, X.; Lin, W. Nanoscale Metal-Organic Framework Mediates Radical Therapy to Enhance Cancer Immunotherapy. Chem 2019, 5, 1892–1913. [Google Scholar] [CrossRef]
- Tee, S.Y.; Ye, E.; Teng, C.P.; Tanaka, Y.; Tang, K.Y.; Win, K.Y.; Han, M.Y. Advances in photothermal nanomaterials for biomedical, environmental and energy applications. Nanoscale 2021, 13, 14268–14286. [Google Scholar] [CrossRef]
- Yan, H.; Chen, J.; Li, Y.; Bai, Y.; Wu, Y.; Sheng, Z.; Song, L.; Liu, C.; Zhang, H. Ultrasmall hybrid protein-copper sulfide nanoparticles for targeted photoacoustic imaging of orthotopic hepatocellular carcinoma with a high signal-to-noise ratio. Biomater. Sci. 2018, 7, 92–103. [Google Scholar] [CrossRef]
- Ding, K.; Zeng, J.; Jing, L.; Qiao, R.; Liu, C.; Jiao, M.; Li, Z.; Gao, M. Aqueous synthesis of PEGylated copper sulfide nanoparticles for photoacoustic imaging of tumors. Nanoscale 2015, 7, 11075–11081. [Google Scholar] [CrossRef]
- Kong, L.; Yuan, F.; Huang, P.; Yan, L.; Cai, Z.; Lawson, T.; Wu, W.; Chou, S.; Liu, Y. A Metal-Polymer Hybrid Biomimetic System for use in the Chemodynamic-Enhanced Photothermal Therapy of Cancers. Small 2020, 16, 2004161. [Google Scholar] [CrossRef]
- Zhang, R.; Le, B.; Xu, W.; Guo, K.; Sun, X.; Su, H.; Huang, L.; Huang, J.; Shen, T.; Liao, T.; et al. Magnetic “Squashing” of Circulating Tumor Cells on Plasmonic Substrates for Ultrasensitive NIR Fluorescence Detection. Small Methods 2019, 3, 1800474. [Google Scholar] [CrossRef]
- Li, J.; Cheng, Q.; Yue, L.; Gao, C.; Wei, J.; Ding, Y.; Wang, Y.; Zheng, Y.; Wang, R. Macrophage-hitchhiking supramolecular aggregates of CuS nanoparticles for enhanced tumor deposition and photothermal therapy. Nanoscale Horiz. 2021, 6, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Udayabhanu; Nethravathi, P.C.; Pavan Kumar, M.A.; Suresh, D.; Lingaraju, K.; Rajanaika, H.; Nagabhushana, H.; Sharma, S.C. Tinospora cordifolia mediated facile green synthesis of cupric oxide nanoparticles and their photocatalytic, antioxidant and antibacterial properties. Mater. Sci. Semicond. Process. 2015, 33, 81–88. [Google Scholar] [CrossRef]
- Dipankar, C.; Murugan, S. The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf. B Biointerfaces 2012, 98, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Ding, B.; Liang, S.; Zhao, Y.; Cheng, Z.; Xing, B.; Ma, P.; Lin, J. Intelligent MoS2-CuO heterostructures with multiplexed imaging and remarkably enhanced antitumor efficacy via synergetic photothermal therapy/chemodynamic therapy/immunotherapy. Biomaterials 2021, 268, 120545. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zuo, W.; Chen, L.; Wu, L.; Liu, N.; Liu, J.; Jin, Q.; Zhao, Y.; Zhu, X. H2O2 Self-Supplying and GSH-Depleting Nanoplatform for Chemodynamic Therapy Synergetic Photothermal/Chemotherapy. ACS Appl. Mater. Interfaces 2021, 13, 43925–43936. [Google Scholar] [CrossRef]
- Hu, X.; Mandika, C.; He, L.; You, Y.; Chang, Y.; Wang, J.; Chen, T.; Zhu, X. Construction of Urokinase-Type Plasminogen Activator Receptor-Targeted Heterostructures for Efficient Photothermal Chemotherapy against Cervical Cancer to Achieve Simultaneous Anticancer and Antiangiogenesis. ACS Appl. Mater. Interfaces 2019, 11, 39688–39705. [Google Scholar] [CrossRef]
- Duan, H.; Guo, H.; Zhang, R.; Wang, F.; Liu, Z.; Ge, M.; Yu, L.; Lin, H.; Chen, Y. Two-dimensional silicene composite nanosheets enable exogenous/endogenous-responsive and synergistic hyperthermia-augmented catalytic tumor theranostics. Biomaterials 2020, 256, 120206. [Google Scholar] [CrossRef]
- Ding, X.; Liow, C.H.; Zhang, M.; Huang, R.; Li, C.; Shen, H.; Liu, M.; Zou, Y.; Gao, N.; Zhang, Z.; et al. Surface plasmon resonance enhanced light absorption and photothermal therapy in the second near-infrared window. J. Am. Chem. Soc. 2014, 136, 15684–15693. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, M.; Jin, G.; Jiang, Y.; Luan, Y. Cu-MOF chemodynamic nanoplatform via modulating glutathione and H2O2 in tumor microenvironment for amplified cancer therapy. J. Colloid Interface Sci. 2021, 587, 358–366. [Google Scholar] [CrossRef]
- Cheng, Y.; Wen, C.; Sun, Y.Q.; Yu, H.; Yin, X.B. Mixed-Metal MOF-Derived Hollow Porous Nanocomposite for Trimodality Imaging Guided Reactive Oxygen Species-Augmented Synergistic Therapy. Adv. Funct. Mater. 2021, 31, 2104378. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, X.; Luo, Q.; Wang, Q.; Zhao, L.; Deng, G.; Ge, R.; Zhang, L.; Hu, J.; Lu, J. Tumor environment responsive degradable CuS@mSiO2@MnO2/DOX for MRI guided synergistic chemo-photothermal therapy and chemodynamic therapy. Chem. Eng. J. 2020, 389, 124450. [Google Scholar] [CrossRef]
- Bai, S.; Yang, N.; Wang, X.; Gong, F.; Dong, Z.; Gong, Y.; Liu, Z.; Cheng, L. Ultrasmall Iron-Doped Titanium Oxide Nanodots for Enhanced Sonodynamic and Chemodynamic Cancer Therapy. ACS Nano 2020, 14, 15119–15130. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.Y.; Wang, Y.J.; Liu, J.W.; Wang, Y.M.; Li, N.; Jiang, J.H. Tumor-selective catalytic nanosystem for activatable theranostics. Chem. Commun. 2018, 54, 8214–8217. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, X.; Gong, F.; Yin, K.; Zhu, W.; Yang, N.; Bai, S.; Liao, F.; Shao, M.; Cheng, L. Silicon nanowires decorated with platinum nanoparticles were applied for photothermal-enhanced sonodynamic therapy. Theranostics 2021, 11, 9234–9242. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Zhong, X.; Li, G.; Yang, Z.; Gong, Y.; Liu, Z.; Cheng, L. V-TiO2 nanospindles with regulating tumor microenvironment performance for enhanced sonodynamic cancer therapy. Appl Phys. Rev. 2020, 7, 041411. [Google Scholar] [CrossRef]
- Cong, C.; Li, C.; Cao, G.; Liu, C.; Yuan, Y.; Zhang, X.; Wang, D.; Gao, D. Dual-activity nanozyme to initiate tandem catalysis for doubly enhancing ATP-depletion anti-tumor therapy. Biomater. Adv. 2022, 143, 213181. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Pu, Y.; Lu, X.; Lin, H.; Shi, J. Transitional Metal-Based Noncatalytic Medicine for Tumor Therapy. Adv. Healthc. Mater. 2021, 10, 2001819. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, W.; Liu, X.; Chen, K.; Zhu, S.; Shi, P.; Chen, Y.; Shi, J. Mesoporous manganese silicate coated silica nanoparticles as multi-stimuli-responsive T1-MRI contrast agents and drug delivery carriers. Acta Biomater. 2016, 30, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Dai, Y.; Yang, P.; Xu, J.; Yang, D.; Gai, S.; He, F.; An, G.; Zhong, C.; Lin, J. Glutathione and H2O2 consumption promoted photodynamic and chemotherapy based on biodegradable MnO2–Pt@Au25 nanosheets. Chem. Eng. J. 2019, 356, 543–553. [Google Scholar] [CrossRef]
- Ding, B.; Zheng, P.; Ma, P.; Lin, J. Manganese Oxide Nanomaterials: Synthesis, Properties, and Theranostic Applications. Adv. Mater. 2020, 32, 1905823. [Google Scholar] [CrossRef]
- Xiao, T.; He, M.; Xu, F.; Fan, Y.; Jia, B.; Shen, M.; Wang, H.; Shi, X. Macrophage Membrane-Camouflaged Responsive Polymer Nanogels Enable Magnetic Resonance Imaging-Guided Chemotherapy/Chemodynamic Therapy of Orthotopic Glioma. ACS Nano 2021, 15, 20377–20390. [Google Scholar] [CrossRef]
- Wang, H.; Bremner, D.H.; Wu, K.; Gong, X.; Fan, Q.; Xie, X.; Zhang, H.; Wu, J.; Zhu, L.-M. Platelet membrane biomimetic bufalin-loaded hollow MnO2 nanoparticles for MRI-guided chemo-chemodynamic combined therapy of cancer. Chem. Eng. J. 2020, 382, 122848. [Google Scholar] [CrossRef]
- Shi, J. On the synergetic catalytic effect in heterogeneous nanocomposite catalysts. Chem. Rev. 2013, 113, 2139–2181. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J. Magnetic nanoscaled Fe3O4/CeO2 composite as an efficient Fenton-like heterogeneous catalyst for degradation of 4-chlorophenol. Environ. Sci. Technol. 2012, 46, 10145–10153. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Niu, D.; Li, Y.; Liu, X.; Li, C.; Si, W.; Cao, J.; Song, Y.; Wen, G.; et al. Ultrasensitive Chemodynamic Therapy: Bimetallic Peroxide Triggers High pH-Activated, Synergistic Effect/H2O2 Self-Supply-Mediated Cascade Fenton Chemistry. Adv. Healthc. Mater. 2021, 10, 2002126. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.; Liu, S.; Wang, Q.; Cao, Y.; Hao, J.N.; Li, Y. GSH-Responsive Organosilica Hybrid Nanosystem as a Cascade Promoter for Enhanced Starvation and Chemodynamic Therapy. Adv. Healthc. Mater. 2023, 12, 2201262. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Hu, Q.; Wang, X.; Li, X.; Hu, J.; Zheng, B.; Ke, M.; Huang, J. A non-aggregated silicon(IV) phthalocyanine-lactose conjugate for photodynamic therapy. Bioorg. Med. Chem. Lett. 2020, 30, 127164. [Google Scholar] [CrossRef]
- Li, W.P.; Su, C.H.; Chang, Y.C.; Lin, Y.J.; Yeh, C.S. Ultrasound-Induced Reactive Oxygen Species Mediated Therapy and Imaging Using a Fenton Reaction Activable Polymersome. ACS Nano 2016, 10, 2017–2027. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.H.; Qi, C.; Lin, J.; Huang, P. Catalytic chemistry of glucose oxidase in cancer diagnosis and treatment. Chem. Soc. Rev. 2018, 47, 6454–6472. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, S.S.; Li, C.X.; Xu, L.; Cheng, H.; Zhang, X.Z. An Adenosine Triphosphate-Responsive Autocatalytic Fenton Nanoparticle for Tumor Ablation with Self-Supplied H2O2 and Acceleration of Fe(III)/Fe(II) Conversion. Nano Lett. 2018, 18, 7609–7618. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Xie, R.; Wang, C.; Gai, S.; He, F.; Yang, D.; Yang, P.; Lin, J. Magnetic Targeting, Tumor Microenvironment-Responsive Intelligent Nanocatalysts for Enhanced Tumor Ablation. ACS Nano 2018, 12, 11000–11012. [Google Scholar] [CrossRef]
- Phan, N.M.; Nguyen, T.L.; Kim, J. Nanozyme-Based Enhanced Cancer Immunotherapy. Tissue Eng. Regen. Med. 2022, 19, 237–252. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Sang, Y.; Cao, F.; Li, W.; Zhang, L.; You, Y.; Deng, Q.; Dong, K.; Ren, J.; Qu, X. Bioinspired Construction of a Nanozyme-Based H2O2 Homeostasis Disruptor for Intensive Chemodynamic Therapy. J. Am. Chem. Soc. 2020, 142, 5177–5183. [Google Scholar] [CrossRef]
- Dong, S.; Dong, Y.; Liu, B.; Liu, J.; Liu, S.; Zhao, Z.; Li, W.; Tian, B.; Zhao, R.; He, F.; et al. Guiding Transition Metal-Doped Hollow Cerium Tandem Nanozymes with Elaborately Regulated Multi-Enzymatic Activities for Intensive Chemodynamic Therapy. Adv. Mater. 2022, 34, 2107054. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H.; Kim, S.J.; Oh, G.S.; Moon, H.D.; Kwon, K.B.; Park, C.; Park, B.H.; Lee, H.K.; Chung, S.Y.; et al. Roles of NADPH oxidases in cisplatin-induced reactive oxygen species generation and ototoxicity. J. Neurosci. 2010, 30, 3933–3946. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Motea, E.A.; Moore, Z.R.; Yao, J.; Dong, Y.; Chakrabarti, G.; Kilgore, J.A.; Silvers, M.A.; Patidar, P.L.; Cholka, A.; et al. Leveraging an NQO1 Bioactivatable Drug for Tumor-Selective Use of Poly(ADP-ribose) Polymerase Inhibitors. Cancer Cell 2016, 30, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Z.; Yu, G.; Zhou, Z.; Jacobson, O.; Liu, Y.; Ma, Y.; Zhang, F.; Chen, Z.Y.; Chen, X. Tumor-Specific Drug Release and Reactive Oxygen Species Generation for Cancer Chemo/Chemodynamic Combination Therapy. Adv. Sci. 2019, 6, 1801986. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yu, L.; Qian, X.; Chen, Y.; Chen, B.; Li, Y. Chemoreactive Nanotherapeutics by Metal Peroxide Based Nanomedicine. Adv. Sci. 2020, 8, 2000494. [Google Scholar] [CrossRef]
- Lin, L.S.; Wang, J.F.; Song, J.; Liu, Y.; Zhu, G.; Dai, Y.; Shen, Z.; Tian, R.; Song, J.; Wang, Z.; et al. Cooperation of endogenous and exogenous reactive oxygen species induced by zinc peroxide nanoparticles to enhance oxidative stress-based cancer therapy. Theranostics 2019, 9, 7200–7209. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Song, S.; Zhang, H. Cascade-responsive nanobomb with domino effect for anti-tumor synergistic therapies. Natl. Sci. Rev. 2022, 9, nwab139. [Google Scholar] [CrossRef]
- Tang, Z.M.; Liu, Y.Y.; Ni, D.L.; Zhou, J.J.; Zhang, M.; Zhao, P.R.; Lv, B.; Wang, H.; Jin, D.Y.; Bu, W.B. Biodegradable Nanoprodrugs: “Delivering” ROS to Cancer Cells for Molecular Dynamic Therapy. Adv. Mater. 2020, 32, 1904011. [Google Scholar] [CrossRef]
- Shen, J.; Yu, H.; Shu, Y.; Ma, M.; Chen, H. A Robust ROS Generation Strategy for Enhanced Chemodynamic/Photodynamic Therapy via H2O2/O2 Self-Supply and Ca2+ Overloading. Adv. Funct. Mater. 2021, 31, 2106106. [Google Scholar] [CrossRef]
- Xu, S.; Shi, X.; Ren, E.; Zhang, J.; Gao, X.; Mu, D.; Liu, C.; Liu, G. Genetically Engineered Nanohyaluronidase Vesicles: A Smart Sonotheranostic Platform for Enhancing Cargo Penetration of Solid Tumors. Adv. Funct. Mater. 2022, 32, 2112989. [Google Scholar] [CrossRef]
- Han, Y.; Ouyang, J.; Li, Y.; Wang, F.; Jiang, J.-H. Engineering H2O2 Self-Supplying Nanotheranostic Platform for Targeted and Imaging-Guided Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 12, 288–297. [Google Scholar] [CrossRef]
- Ma, J.; Peng, X.; Zhou, Z.; Yang, H.; Wu, K.; Fang, Z.; Han, D.; Fang, Y.; Liu, S.; Shen, Y.; et al. Extended Conjugation Tuning Carbon Nitride for Non-sacrificial H(2) O(2) Photosynthesis and Hypoxic Tumor Therapy. Angew. Chem. Int. Ed. 2022, 61, 202210856. [Google Scholar] [CrossRef]
- Ma, B.; Wang, S.; Liu, F.; Zhang, S.; Duan, J.; Li, Z.; Kong, Y.; Sang, Y.; Liu, H.; Bu, W.; et al. Self-Assembled Copper-Amino Acid Nanoparticles for in Situ Glutathione “AND” H2O2 Sequentially Triggered Chemodynamic Therapy. J. Am. Chem. Soc. 2019, 141, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, L.; Xu, X.; Bertrand, N.; Choi, W.I.; Yameen, B.; Shi, J.; Shah, V.; Mulvale, M.; MacLean, J.L.; et al. Hydrophobic Cysteine Poly(disulfide)-based Redox-Hypersensitive Nanoparticle Platform for Cancer Theranostics. Angew. Chem. Int. Ed. 2015, 54, 9218–9223. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Zhang, Y.; Zhang, S.; Liu, H.; Zhang, J.; Feng, H.; Li, B.; Wu, X.; Gao, Y.; et al. A redox-triggered C-centered free radicals nanogenerator for self-enhanced magnetic resonance imaging and chemodynamic therapy. Biomaterials 2021, 266, 120457. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Hu, D.; Wang, W.; Zhang, Q.; Meng, Z.; Jia, X.; Xi, K. Carbon dot cluster as an efficient “off–on” fluorescent probe to detect Au(III) and glutathione. Biosens. Bioelectron. 2015, 68, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, Y.; Yang, H.; Zhao, K.; Li, J.; Deng, A. Fluorescent nitrogen and sulfur co-doped carbon dots from casein and their applications for sensitive detection of Hg2+ and biothiols and cellular imaging. Anal. Chim. Acta 2017, 964, 150–160. [Google Scholar] [CrossRef]
- Li, J.; Hu, Z.E.; We, Y.J.; Liu, Y.H.; Wang, N.; Yu, X.Q. Multifunctional carbon quantum dots as a theranostic nanomedicine for fluorescence imaging-guided glutathione depletion to improve chemodynamic therapy. J. Colloid. Interface Sci. 2022, 606, 1219–1228. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, S.; Zhang, L.; Deng, Q.; Ren, J.; Qu, X. A Metabolic Multistage Glutathione Depletion Used for Tumor-Specific Chemodynamic Therapy. ACS Nano 2022, 16, 4228–4238. [Google Scholar] [CrossRef]
- Chen, S.; Yue, T.; Huang, Z.; Zhu, J.; Bu, D.; Wang, X.; Pan, Y.; Liu, Y.; Wang, P. Inhibition of hydrogen sulfide synthesis reverses acquired resistance to 5-FU through miR-215-5p-EREG/TYMS axis in colon cancer cells. Cancer Lett. 2019, 466, 49–60. [Google Scholar] [CrossRef]
- He, R.; Hu, B.; Zhong, H.; Jin, F.; Fan, J.; Hu, Y.H.; Jing, Z. Reduction of CO2 with H2S in a simulated deep-sea hydrothermal vent system. Chem. Commun. 2019, 55, 1056–1059. [Google Scholar] [CrossRef]
- Liu, D.; Liu, M.; Wan, Y.; Zhou, X.; Yang, S.; An, L.; Huang, G.; Tian, Q. Remodeling endogenous H2S microenvironment in colon cancer to enhance chemodynamic therapy. Chem. Eng. J. 2021, 422, 130098. [Google Scholar] [CrossRef]
- Da, X.; Wang, Z.; Jian, Y.; Zhang, C.; Hou, Y.; Yao, Y.; Wang, X.; Zhou, Q. A targeted and efficient CDT system with photocatalytic supplement of H2O2 and hydroxyl radical production at a neutral pH. Inorg. Chem. Front. 2022, 9, 2544–2556. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Meng, C.; Zhu, P.; Liu, X.; Qian, J.; Ling, S.; Zhang, Y.; Ling, Y. Pillar[6]arene-Based Supramolecular Nanocatalysts for Synergistically Enhanced Chemodynamic Therapy by the Intracellular Cascade Reaction. ACS Appl. Mater. Interfaces 2021, 13, 53574–53585. [Google Scholar] [CrossRef]
- Li, F.; Zang, M.; Hou, J.; Luo, Q.; Yu, S.; Sun, H.; Xu, J.; Liu, J. Cascade catalytic nanoplatform constructed by laterally-functionalized pillar[5]arenes for antibacterial chemodynamic therapy. J. Mater. Chem. B 2021, 9, 5069–5075. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, Y.; Zhang, C.; Zhao, Y.; Lu, C.; Yin, B.; Yang, Y.; Gong, X.; Teng, L.; Liu, Y.; et al. An Acidity-Unlocked Magnetic Nanoplatform Enables Self-Boosting ROS Generation through Upregulation of Lactate for Imaging-Guided Highly Specific Chemodynamic Therapy. Angew. Chem. Int. Ed. 2021, 60, 9562–9572. [Google Scholar] [CrossRef]
- Chafe, S.C.; Vizeacoumar, F.S.; Venkateswaran, G.; Nemirovsky, O.; Awrey, S.; Brown, W.S.; McDonald, P.C.; Carta, F.; Metcalfe, A.; Karasinska, J.M.; et al. Genome-wide synthetic lethal screen unveils novel CAIX-NFS1/xCT axis as a targetable vulnerability in hypoxic solid tumors. Sci. Adv. 2021, 7, eabj0364. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Chen, W.; Liu, J.; Huang, S.; Chen, L.; Liu, Q.; Liu, N.; Jin, Q.; Li, Y.; Wang, P.; et al. Macrophage-Mimic Hollow Mesoporous Fe-Based Nanocatalysts for Self-Amplified Chemodynamic Therapy and Metastasis Inhibition via Tumor Microenvironment Remodeling. ACS Appl. Mater. Interfaces 2022, 14, 5053–5065. [Google Scholar] [CrossRef]
- Dong, K.; Chen, W.; Zhao, Z.; Zhang, Y.; Wang, P.; Wang, K.; Xing, J.; Lu, T.; Dong, Y. Multifunctional nanosystems sequentially regulating intratumor Fenton chemistry by remodeling the tumor microenvironment to reinforce chemodynamic therapy. Biomater. Adv. 2022, 138, 212957. [Google Scholar] [CrossRef]
- Su, Z.; Chen, M.; Xiao, Y.; Sun, M.; Zong, L.; Asghar, S.; Dong, M.; Li, H.; Ping, Q.; Zhang, C. ROS-triggered and regenerating anticancer nanosystem: An effective strategy to subdue tumor’s multidrug resistance. J. Control. Release 2014, 196, 370–383. [Google Scholar]
- Almeida, J.; Ball, B.A. Effect of alpha-tocopherol and tocopherol succinate on lipid peroxidation in equine spermatozoa. Anim. Reprod. Sci. 2005, 87, 321–337. [Google Scholar] [CrossRef]
- Youk, H.; Lee, E.; Choi, M.; Lee, Y.; Chung, J.; Kim, S.; Lee, C.; Lim, S. Enhanced anticancer efficacy of α-tocopheryl succinate by conjugation with polyethylene glycol. J. Control. Release 2005, 107, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wang, L.; Wu, G.; Zhou, L.; Wei, S. Synergistic anticancer theragnostic study of a core-shell structured galvanic cell. Colloids Surf. B Biointerfaces 2022, 209, 112154. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Luo, Y.; Du, W.; Liu, Z.; Zhang, S.; Yang, J.; Yao, H.; Liu, T.; Ma, M.; Chen, H. Clearable Theranostic Platform with a pH-Independent Chemodynamic Therapy Enhancement Strategy for Synergetic Photothermal Tumor Therapy. ACS Appl. Mater. Interfaces 2019, 11, 18133–18144. [Google Scholar] [CrossRef]
- Wang, P.; Liang, C.; Zhu, J.; Yang, N.; Jiao, A.; Wang, W.; Song, X.; Dong, X. Manganese-Based Nanoplatform As Metal Ion-Enhanced ROS Generator for Combined Chemodynamic/Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 41140–41147. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Yin, J.; Yoon, J. Sonodynamic and chemodynamic therapy based on organic/organometallic sensitizers. Coord. Chem. Rev. 2021, 429, 213610. [Google Scholar]
- Wang, Y.; Ye, Z.; Song, G.; Liu, Z. Magnetic-Optical Imaging for Monitoring Chemodynamic Therapy. Chem. Res. Chin. Univ. 2022, 38, 481–492. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).