Influence of Different Sterilization Methods on the Surface Chemistry and Electrochemical Behavior of Biomedical Alloys
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sterilization Processes
- Ethanol (EtOH): the samples were immersed in a 70% ethanol bath over 20 min.
- Autoclave sterilization (AC): after the EtOH step, samples were introduced in an autoclave at 121 °C at a pressure of 0.1 MPa over 20 min.
- UV-light sterilization (AC-UV): after the EtOH and AC steps, the samples were exposed to UV-Light (260 nm wavelength) over 20 min.
- Gamma radiation sterilization (G): the samples without any previous sterilization were exposed to a source of energy between 25–42 kGy.
2.3. Surface Analysis
2.4. Electrochemical Measurements
- -
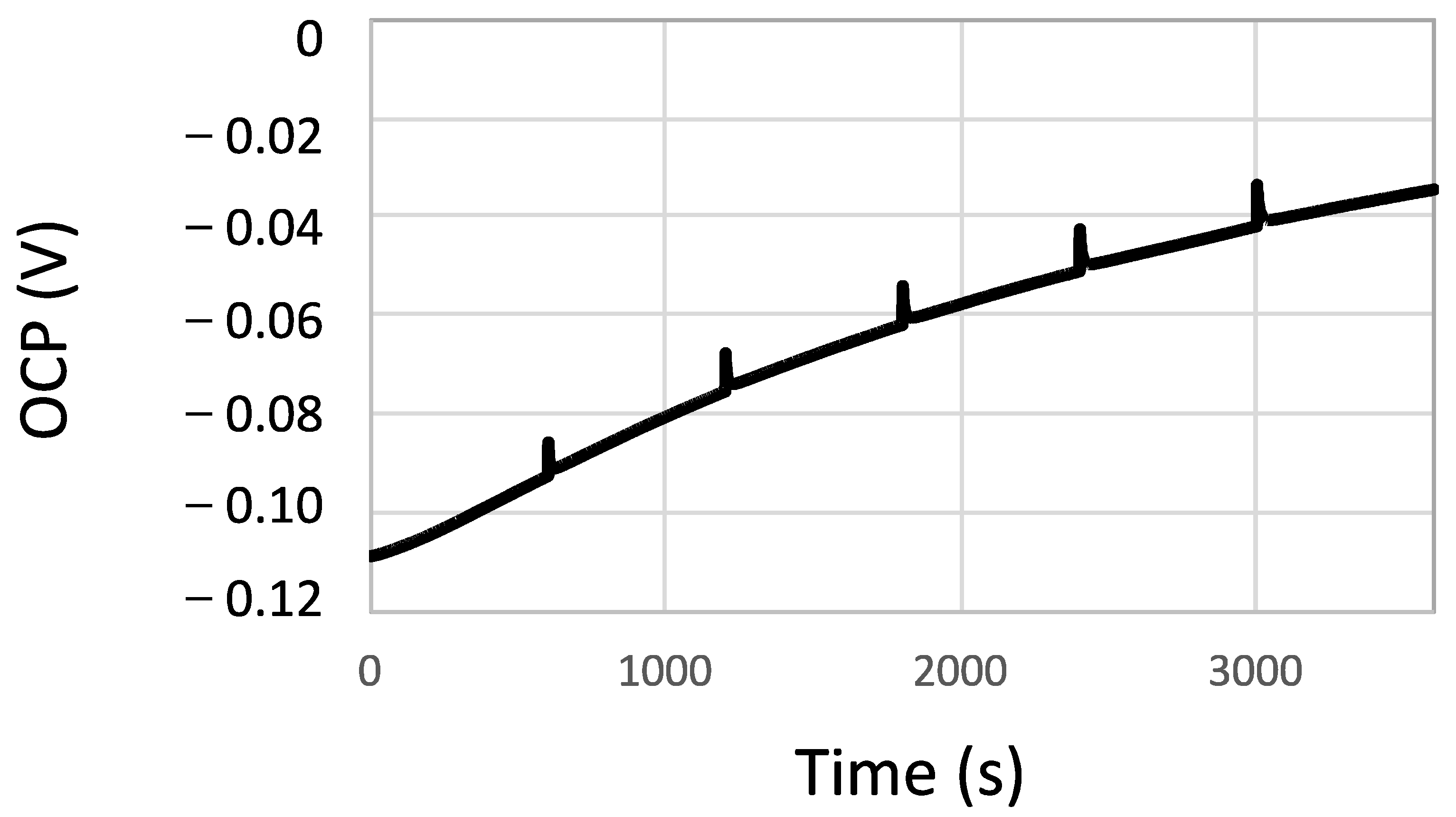
- The Open Circuit Potential (OCP) was continuously measured over 60 min.
- -
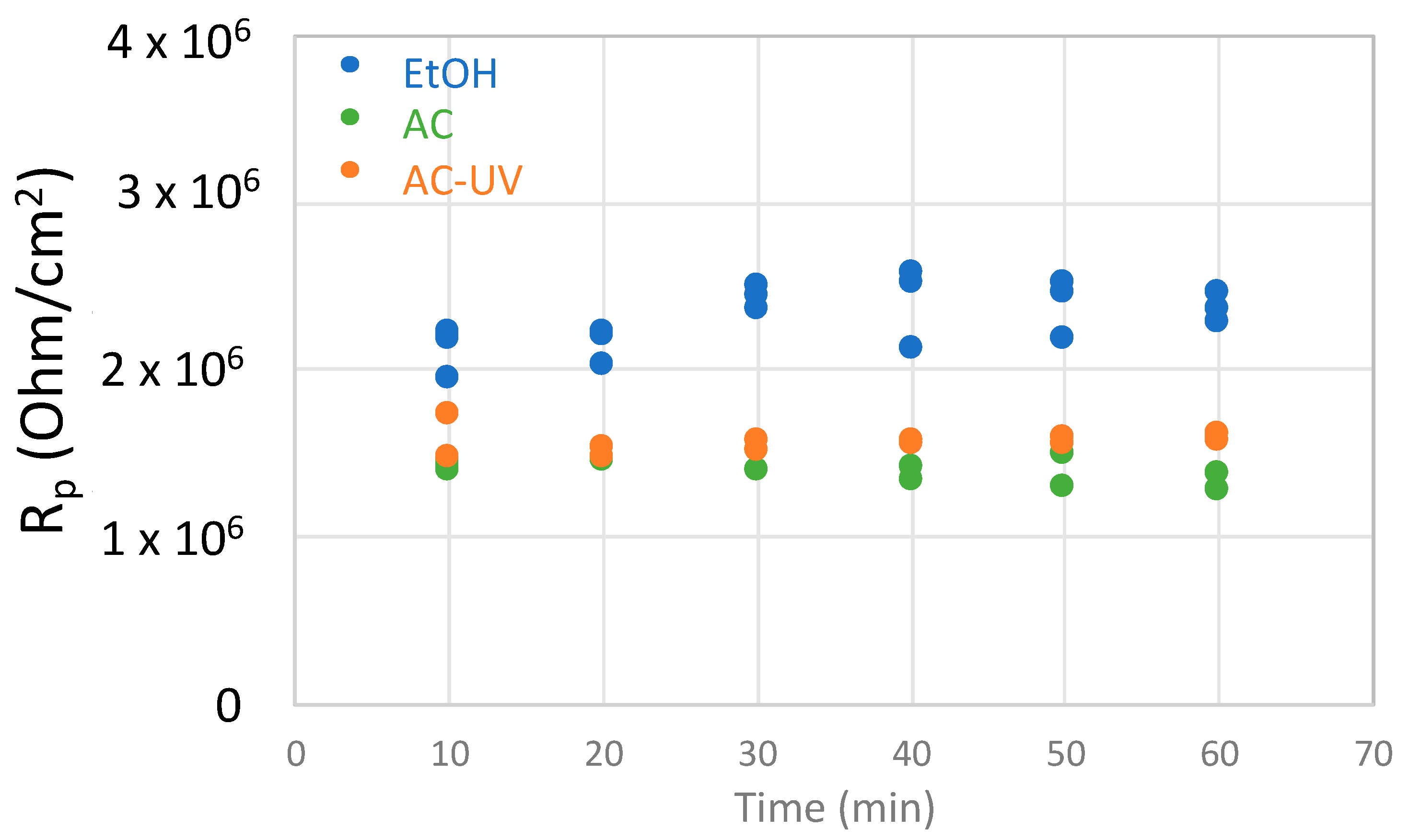
- During the OCP measurement, linear polarization measurements were carried out every 10 min from −10 mV to +10 mV with respect to the OCP at a scan rate of 2 mV/s in order to determine the polarization resistance (Rp). Rp is inversely proportional to the corrosion rate.
- -
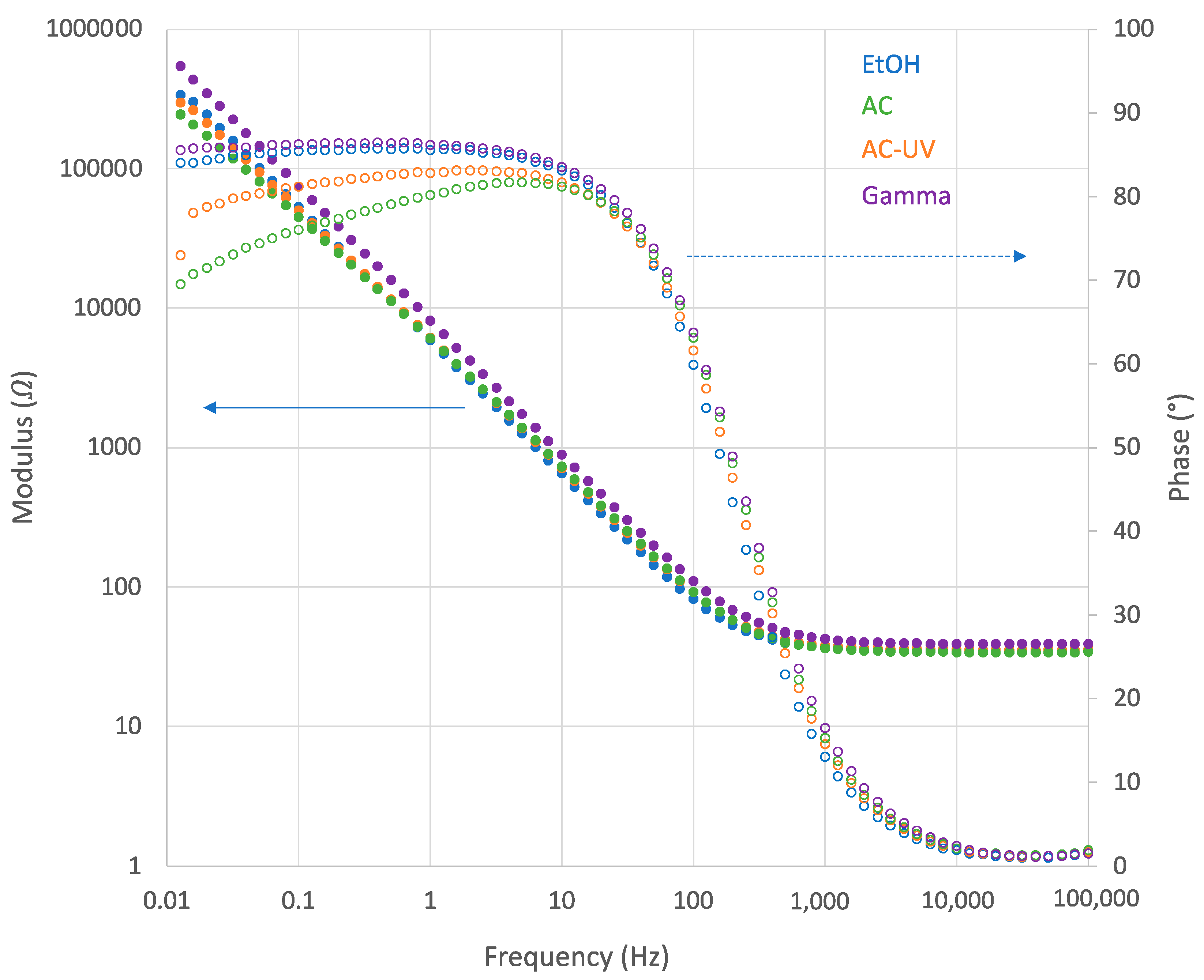
- Electrochemical impedance spectroscopy (EIS) was carried out at OCP after 60 min of immersion in a frequency range from 105 to 0.01 Hz with an amplitude of 10 mV. This measurement allowed for characterization of the interface biomaterial/electrolyte.
- -
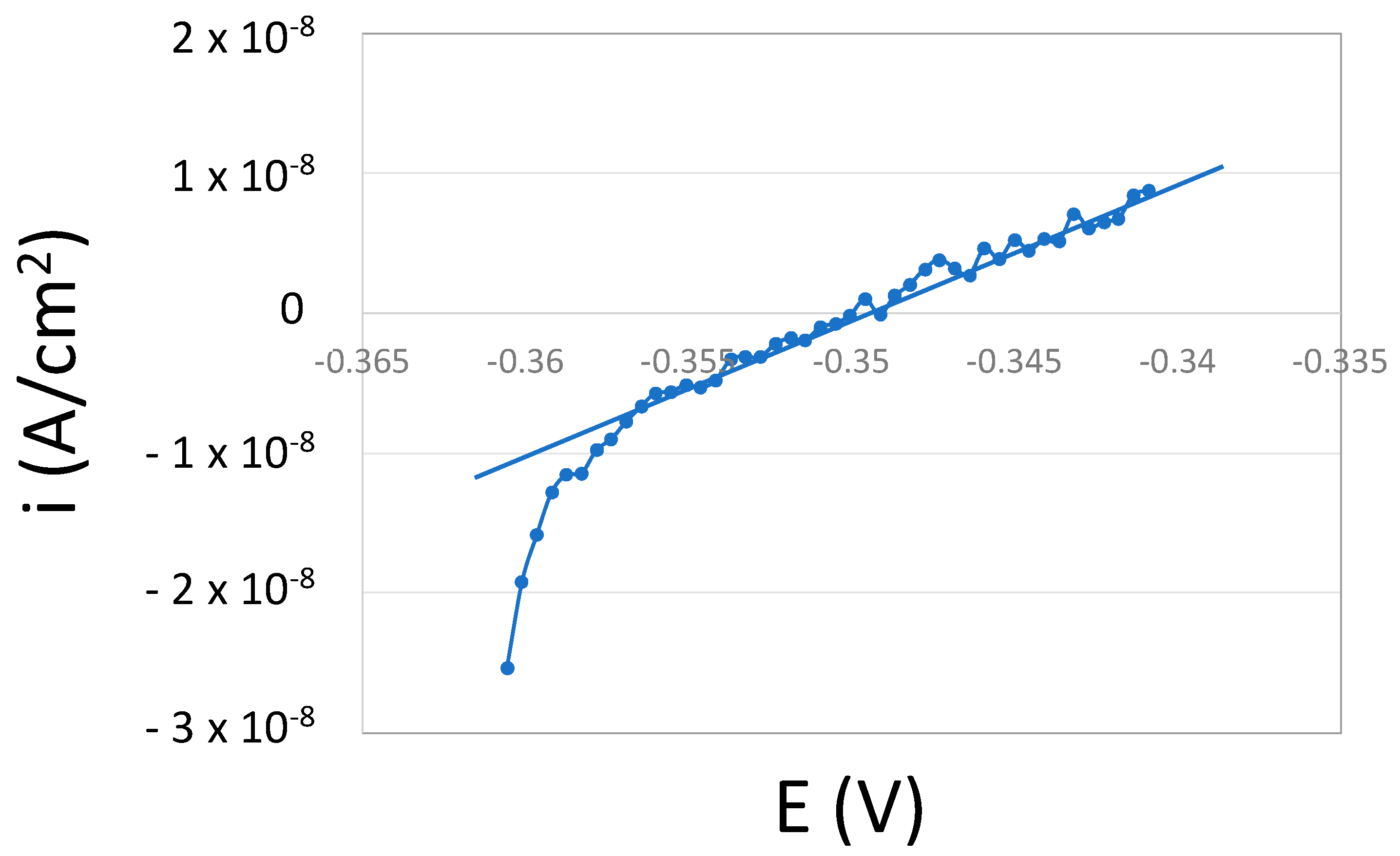
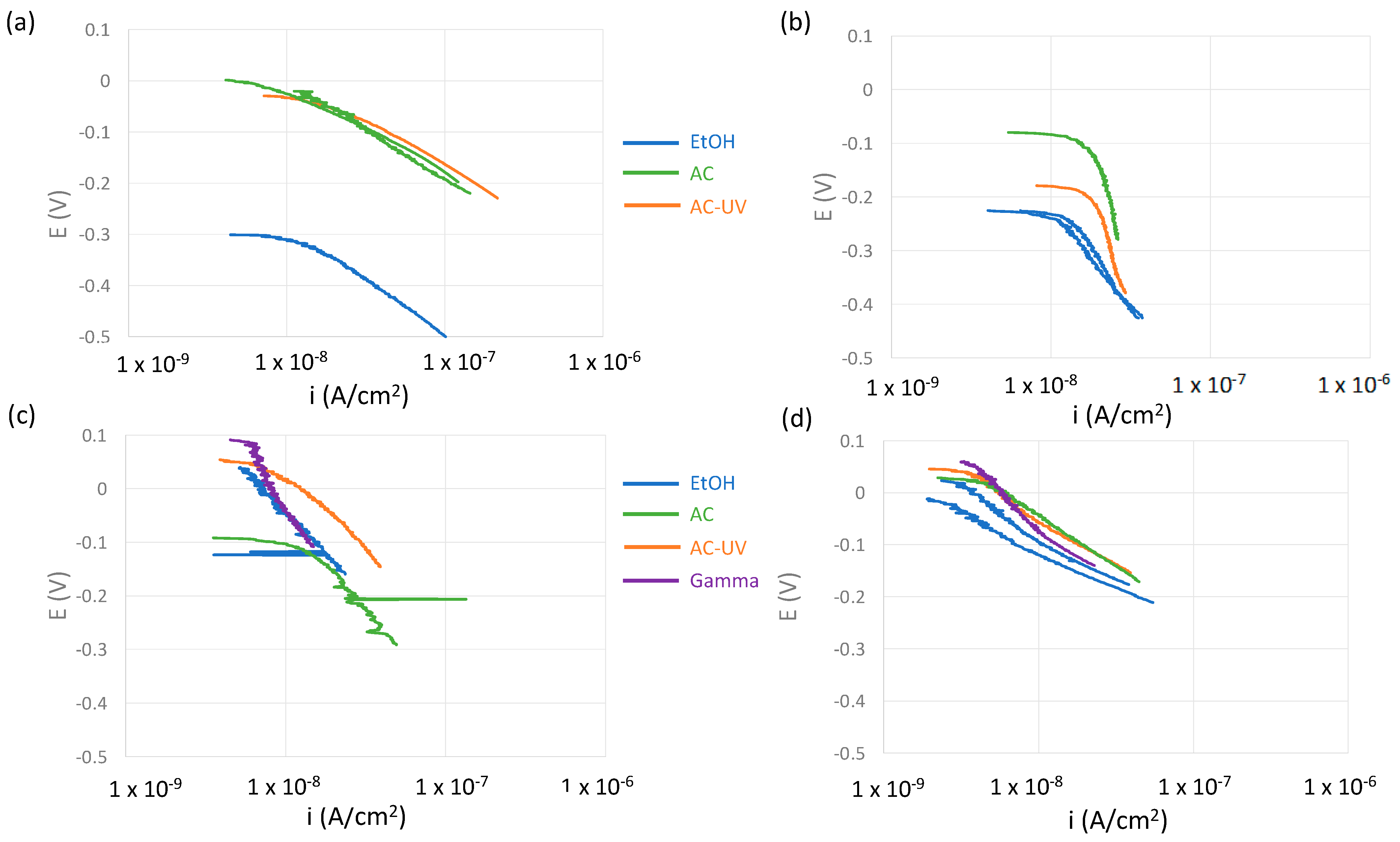
- A cathodic potentiodynamic scan was carried out from OCP towards −200 mV vs. OCP at 2 mV/s. Cathodic kinetic parameters were extracted from those curves.
3. Results
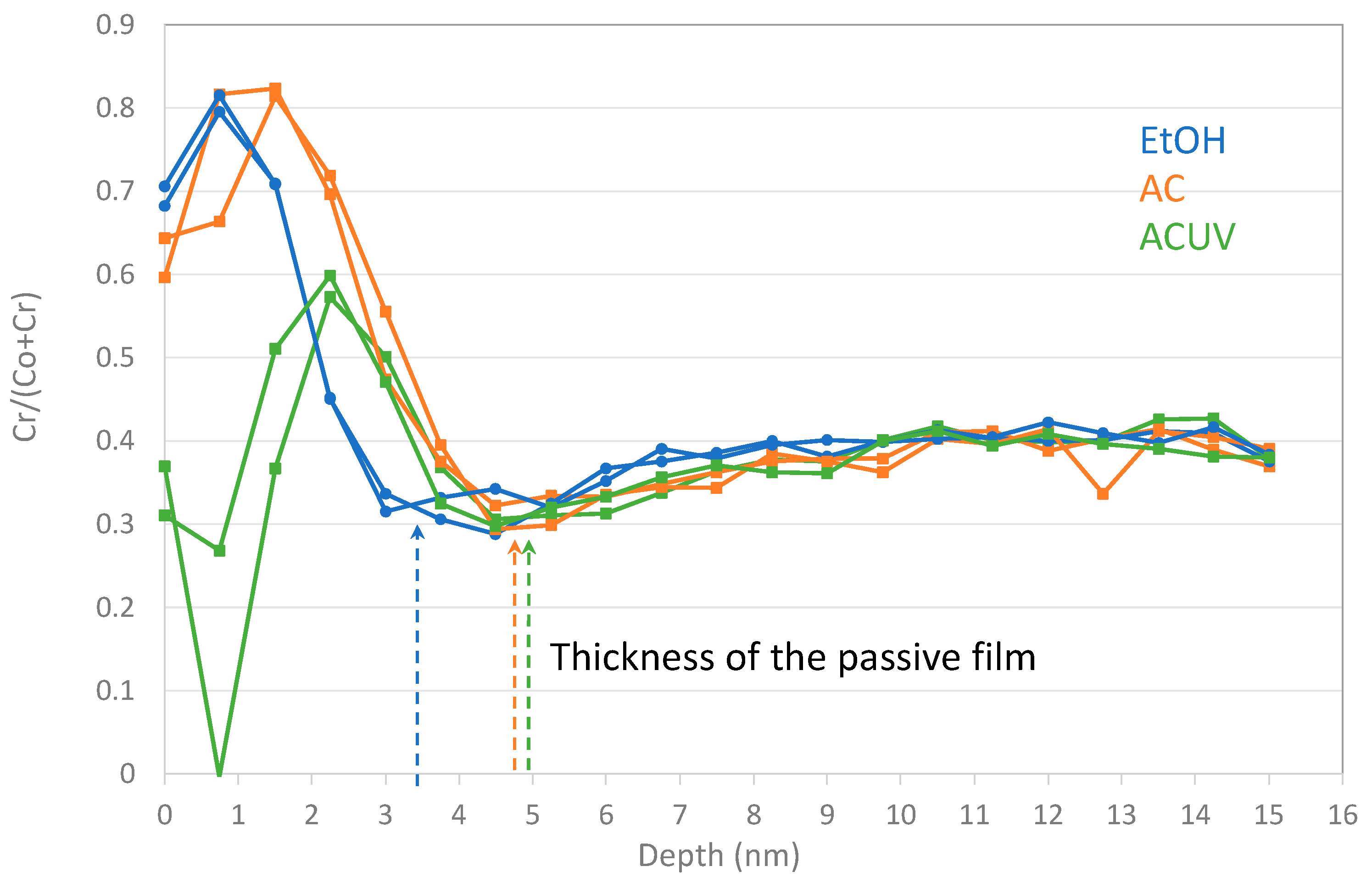
3.1. Surface Analysis
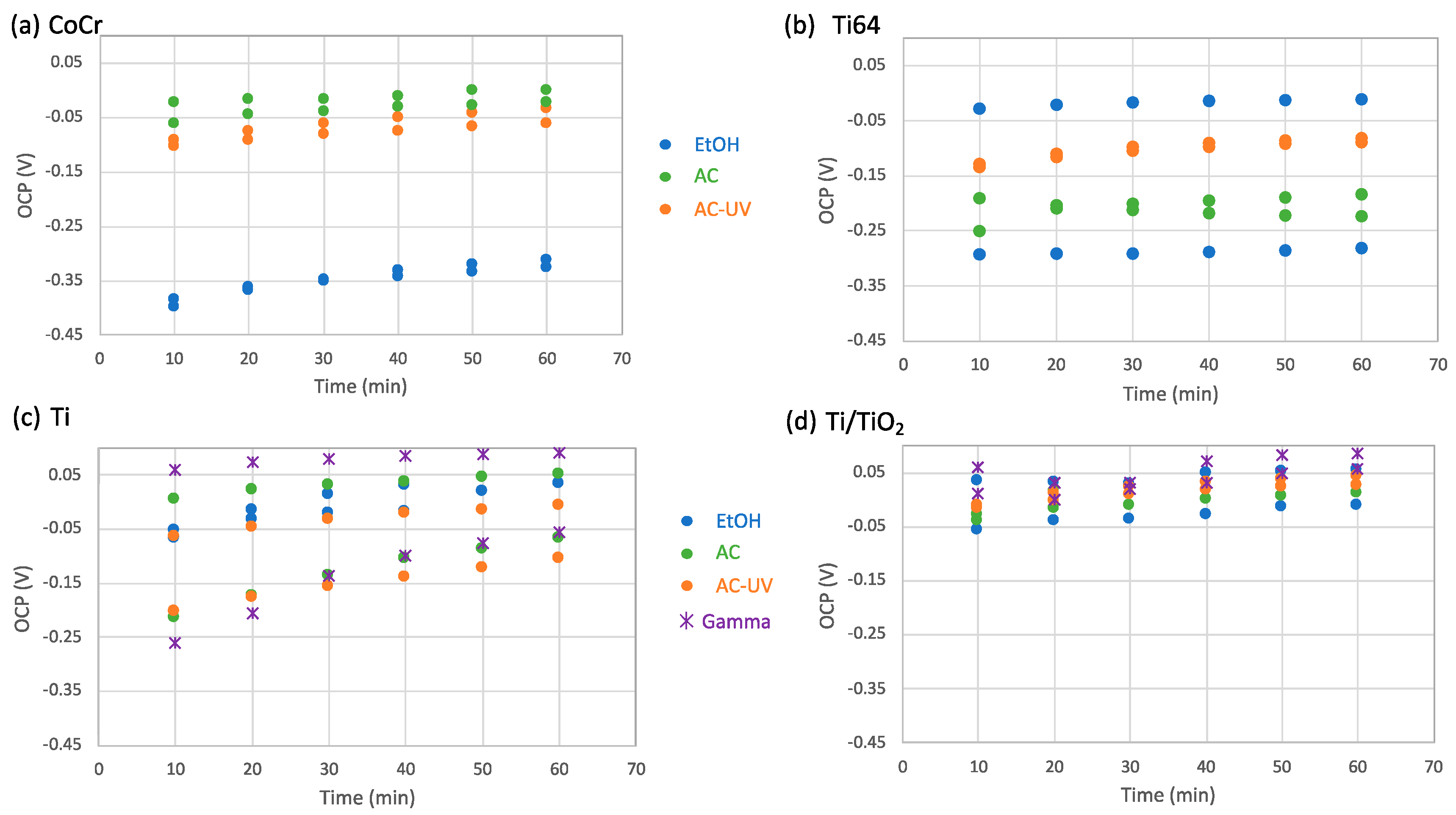
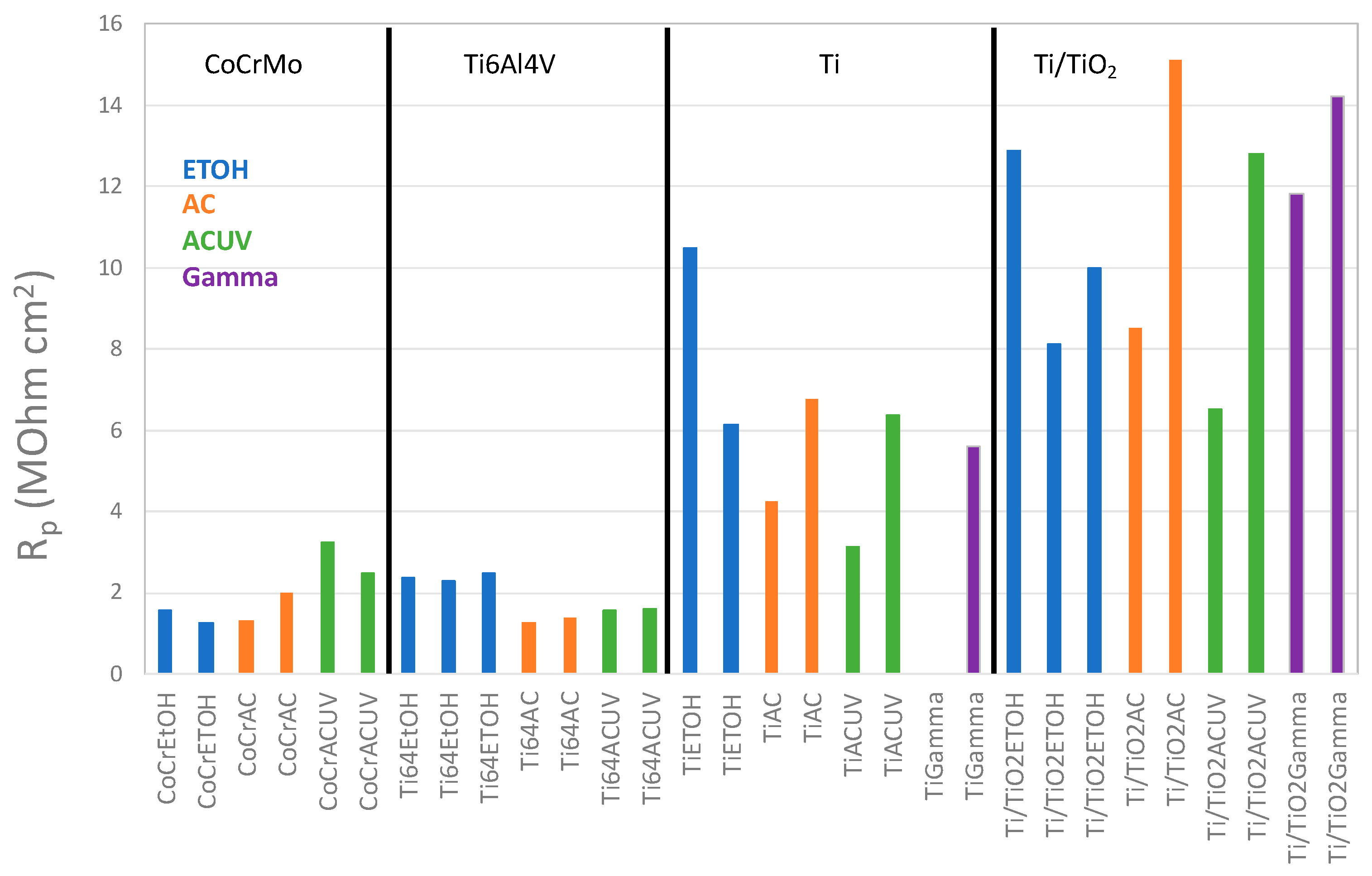
3.2. Electrochemical Measurements
4. Discussion
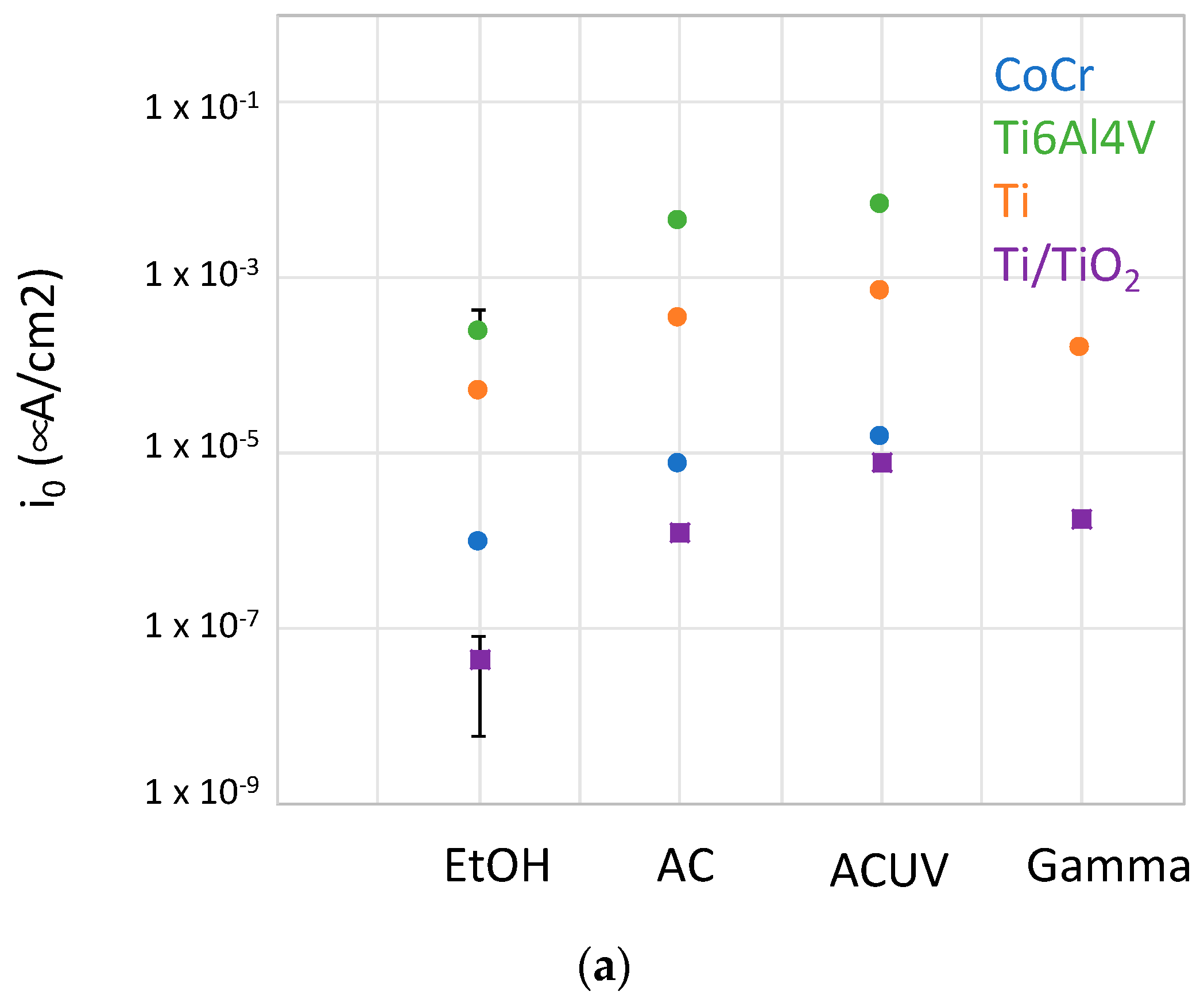
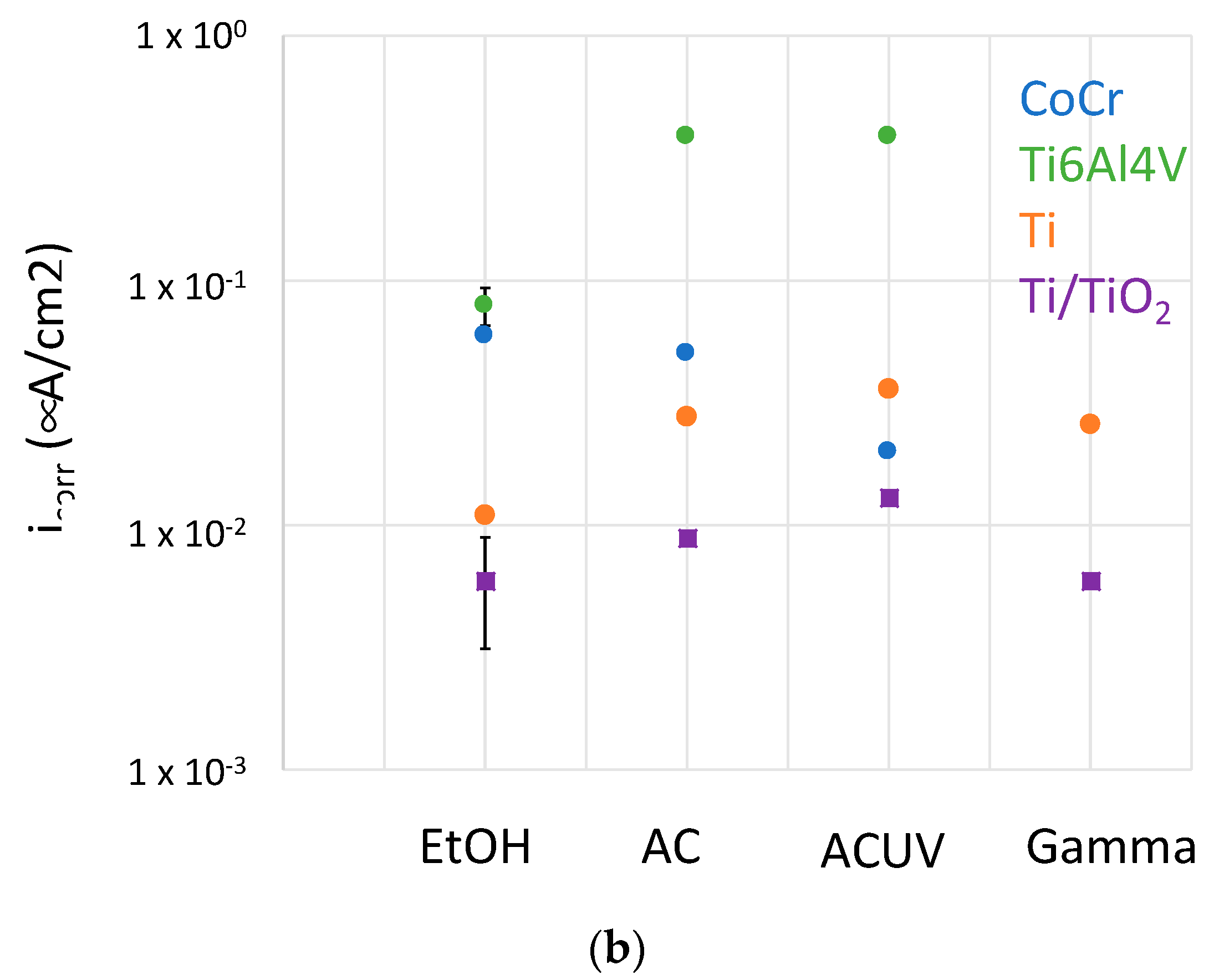
4.1. Oxygen Reduction Kinetics
4.2. Effect of Sterilization on the Electrochemical Behavior of the CoCrMo Biomedical Alloy
4.3. Effect of Sterilization on the Electrochemical Behavior of Titanium and Titanium Alloy Biomedical Alloy
5. Conclusions
- For CoCrMo alloys, sterilization by AC and ACUV significantly modified the surface composition (thicker and chromium rich passive film) and improved the corrosion resistance compared to simple sterilization in EtOH. The modified surface composition was found to inhibit the oxygen reduction reaction, thus reducing the corrosion rate.
- For titanium and titanium alloys, the sterilization has a negative impact on the corrosion resistance. Indeed, the corrosion behavior was found to be controlled by the oxygen reduction reaction rate, which is enhanced by the AC and ACUV sterilization methods. On the contrary, gamma irradiation slightly increases the corrosion resistance of the investigated titanium and titanium alloy.
- Sterilization methods potentially lead to different reactivities of biomedical alloys and therefore their further biological and clinical performance.
- Further analysis in more complex simulated body fluids (i.e., containing proteins and other organic molecules, controlled oxygen content) together with in vivo measurements could be carried out to optimize the sterilization procedures allowing for minimization of metal ion release in patients.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lerouge, S. Sterilisation and cleaning of metallic implants. In Metals for Biomedical Devices; Niinomi, M., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2010. [Google Scholar]
- Matusiewicz, H. Potential Release of in Vivo Trace Metals from Metallic Medical Implants in the Human Body: From Ions to Nanoparticles-A Systematic Analytical Review. Acta Biomater. 2014, 10, 2379–2403. [Google Scholar] [CrossRef] [PubMed]
- Forsthoefel, C.W.; Brown, N.M.; Barba, M.L. Comparison of Metal Ion Levels in Patients with Hip Resurfacing versus Total Hip Arthroplasty. J. Orthop. 2017, 14, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Magone, K.; Luckenbill, D.; Goswami, T. Metal Ions as Inflammatory Initiators of Osteolysis. Arch. Orthop. Trauma. Surg. 2015, 135, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Olsson, C.O.A.; Igual Muñoz, A.; Mischler, S. Adsorption of organic matter on titanium surfaces with nano- and micro-scale roughness studied with the electrochemical quartz crystal microbalance dissipation technique. Biointerphases 2021, 16, 051001. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Olivares-Navarrete, R.; Baier, R.E.; Meyer, A.E.; Tannenbaum, R.; Boyan, B.D.; Schwartz, Z. Effect of cleaning and sterilization on titanium implant surface properties and cellular response. Acta Biomater. 2012, 8, 1966–1975. [Google Scholar] [CrossRef]
- Thierry, B.; Tabrizian, M.; Savadogo, O.; Yahia, L. Effects of sterilization processes on NiTi alloy: Surface characterization. J. Biomed. Mater. Res. 1998, 49, 88–98. [Google Scholar] [CrossRef]
- Kilpadi, D.V.; Weimer, J.J.; Lemons, J.E. Effect of passivation and dry heat- sterilization on surface energy and topography of unalloyed titanium implants. Colloids Surf. A Physicochem. Eng. Asp. 1998, 135, 89–101. [Google Scholar] [CrossRef]
- Zhao, L.Z.; Mei, S.L.; Wang, W.; Chu, P.K.; Wu, Z.F.; Zhang, Y.M. The role of sterilization in the cytocompatibility of titania nanotubes. Biomaterials 2010, 31, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Vezeau, P.J.; Koorbusch, G.F.; Draughn, R.A.; Keller, J.C. Effects of multiple sterilization on surface characteristics and in vitro bio- logic responses to titanium. J. Oral. Maxillofac. Surg. 1996, 54, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Junkar, I.; Kulkarni, M.; Drasåler, B.; Rugelj, N.; Mazare, A.; Flasåker, A.; Drobne, D.; HumpoliÅcåek, P.; Resnik, M.; Schmuki, P.; et al. Influence of various sterilization procedures on TiO2 nanotubes used for biomedical devices. Bioelectrochemistry 2016, 109, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Brammer, K.S.; Moon, K.-S.; Bae, J.-M.; Jin, S. Influence of sterilization methods on cell behavior and functionality of osteoblasts cultured on TiO2 nanotubes. Mater. Sci. Eng. C 2011, 31, 873–879. [Google Scholar] [CrossRef]
- Kummer, K.M.; Taylor, E.N.; Durmas, N.G.; Tarquinio, K.M.; Ercan, B.; Webste, T.J. Effects of different sterilization techniques and varying anodized TiO2 nanotube dimensions on bacteria growth. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101B, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Thierry, B.; Tabrizian, M.; Trepanier, C.; Savadogo, O.; Yahia, L.H. Effect of surface treatment and sterilization processes on the corrosion behavior of NiTi shape memory alloy. J. Biomed. Mater. Res. 2000, 51, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Igual Muñoz, A.; Olsson, C.O.A.; Jolles, B.M.; Mischler, S. The electrochemical behavior of Ti in human synovial fluids. Materials 2022, 15, 1726. [Google Scholar] [CrossRef] [PubMed]
- Mischler, S.; Mathieu, H.J.; Landolt, D. Investigation of a passive film on an iron-chromium alloy by AES and XPS. Surf. Interface Anal. 1988, 11, 182. [Google Scholar] [CrossRef]
- Growcock, F.; Jasinski, R.J. Time-resolved impedance spectroscopy of mild steel in concentrated hydrochloric acid. J. Electrochem. Soc. 1989, 136, 2310. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of effective capacitance and film thickness from constant-phase-element parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Della Rovere, C.A.; Alano, J.H.; Silva, R.; Nascente, P.A.P.; Otubo, J.; Kuri, S.E. Characterization of passive films on shape memory stainless steels. Corros. Sci. 2012, 57, 154–161. [Google Scholar] [CrossRef]
- Wei, Y.; Pan, Z.; Fu, Y.; Yu, W.; He, S.; Yuan, Q.; Luo, H.; Li, X. Effect of annealing temperatures on microstructural evolution and corrosion behavior of Ti-Mo titanium alloy in hydrochloric acid. Corros. Sci. 2022, 197, 110079. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Peng, X.; Li, Y.; Liu, Z.; Liu, C.; Ya, J.; Huang, Y. Photoinduced superhydrophilicity of TiO2 thin film with hierarchical Cu doping. Sci. Technol. Adv. Mater. 2012, 13, 025001. [Google Scholar] [CrossRef] [PubMed]
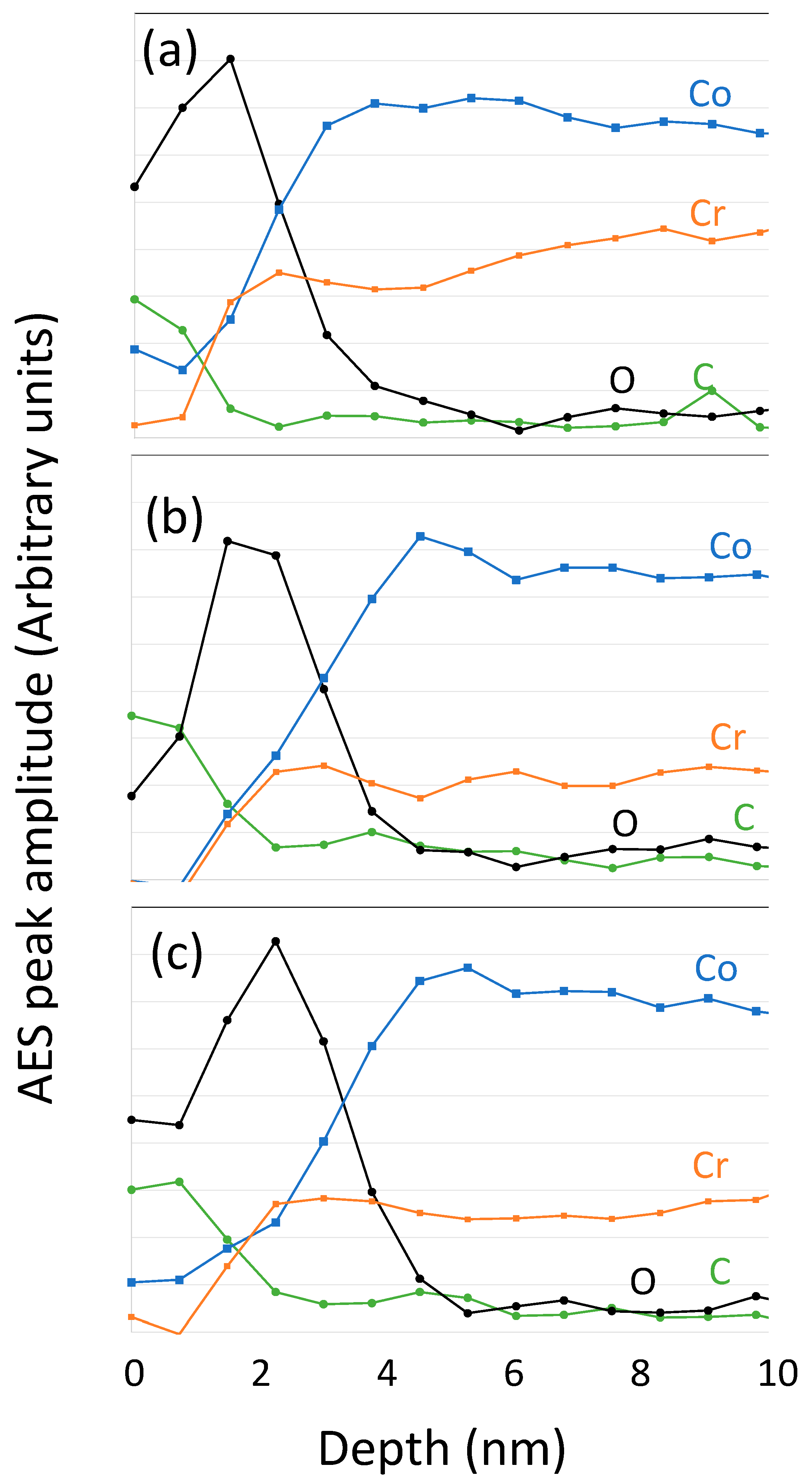


| Element | Peak | Kinetic Energy (eV) | IMFP (nm) |
|---|---|---|---|
| C | KLL | 273 | 0.7 |
| O | KLL | 503 | 1.0 |
| Cr | LMM | 528 | 1.1 |
| Co | LMM | 778 | 1.4 |
| Ti | LMM | 421 | 1.0 |
| Sample | Sterilization | OCP (V) | Cox (μF/cm2) |
|---|---|---|---|
| CoCr | EtOH | −0.321 | 4 |
| −0.305 | 4 | ||
| AC | −0.029 | 1.5 | |
| −0.058 | 0.9 | ||
| AC-UV | 0 | 0.8 | |
| −0.235 | 1.5 | ||
| Ti64 | EtOH | −0.278 | 1.8 |
| −0.009 | 1.8 | ||
| −0.225 | 2.2 | ||
| AC | −0.178 | 1.5 | |
| −0.146 | 1.5 | ||
| AC-UV | −0.079 | 2 | |
| −0.085 | 1.8 | ||
| Ti | ETOH | 0.040 | 3 |
| 0.057 | 3.1 | ||
| AC | 0.055 | 2.5 | |
| −0.059 | 2.5 | ||
| AC-UV | −0.091 | 2.4 | |
| −0.008 | 2.7 | ||
| Gamma | 0.090 | 2.1 | |
| −0.040 | 2.1 | ||
| Ti/TiO2 | ETOH | −0.011 | 1 |
| 0.055 | 1.2 | ||
| 0.024 | 1.1 | ||
| AC | 0.046 | 1.1 | |
| 0.017 | 1.1 | ||
| AC-UV | 0.030 | 1.2 | |
| 0.045 | 0.9 | ||
| Gamma | 0.060 | 0.7 | |
| 0.090 | 0.7 |
| Sample | βc (V/Decade) | icorr (nA/cm2) | i0 (μA/cm2) |
|---|---|---|---|
| CoCrEtOH | 0.092 | 59 | 9.4 × 10−7 |
| CoCrAC | 0.077 | 50 | 7.1 × 10−6 |
| CoCrACUV | 0.086 | 20 | 1.4 × 10−5 |
| CoCrACUV | 0.080 | 25 | 1.7 × 10−5 |
| Ti64EtOH | 0.167/0.122 | 70 | 6.3 × 10−5 |
| Ti64EtOH | 0.225/0.163 | 90 | 3.8 × 10−4 |
| Ti64AC | 0.498/0.291 | 389 | 4.1 × 10−3 |
| Ti64ACUV | 0.610 | 386 | 6.5 × 10−3 |
| TiETOH | 0.116 | 11 | 4.9 × 10−5 |
| TiAC | 0.121 | 28 | 3.2 × 10−4 |
| TiACUV | 0.114 | 36 | 6.8 × 10−4 |
| TiGamma | 0.100/0.146 | 26 | 1.5 × 10−4 |
| Ti/TiO2EtOH | 0.075/0.052 | 4 | 1.7 × 10−8 |
| Ti/TiO2EtOH | 0.090/0.056 | 8 | 6.9 × 10−8 |
| Ti/TiO2AC | 0.100/0.070 | 9 | 1.2 × 10−6 |
| Ti/TiO2ACUV | 0.086 | 13 | 7.9 × 10−6 |
| Ti/TiO2Gamma | 0.123/0.075 | 6 | 1.7 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Igual-Munoz, A.; Genilloud, J.-L.; Jolles, B.M.; Mischler, S. Influence of Different Sterilization Methods on the Surface Chemistry and Electrochemical Behavior of Biomedical Alloys. Bioengineering 2023, 10, 749. https://doi.org/10.3390/bioengineering10070749
Igual-Munoz A, Genilloud J-L, Jolles BM, Mischler S. Influence of Different Sterilization Methods on the Surface Chemistry and Electrochemical Behavior of Biomedical Alloys. Bioengineering. 2023; 10(7):749. https://doi.org/10.3390/bioengineering10070749
Chicago/Turabian StyleIgual-Munoz, Anna, Jean-Ludovic Genilloud, Brigitte M. Jolles, and Stefano Mischler. 2023. "Influence of Different Sterilization Methods on the Surface Chemistry and Electrochemical Behavior of Biomedical Alloys" Bioengineering 10, no. 7: 749. https://doi.org/10.3390/bioengineering10070749
APA StyleIgual-Munoz, A., Genilloud, J.-L., Jolles, B. M., & Mischler, S. (2023). Influence of Different Sterilization Methods on the Surface Chemistry and Electrochemical Behavior of Biomedical Alloys. Bioengineering, 10(7), 749. https://doi.org/10.3390/bioengineering10070749







