Electrochemical Devices in Cutaneous Wound Healing
Abstract
1. Introduction
2. Methods
3. Results
3.1. Dressings Containing Metal Ions
3.2. Devices
3.3. Mechanism
3.4. Clinical
3.5. Limitations
3.6. Wireless Electroceutical Dressings
3.7. Devices
3.8. Clinical
3.9. Limitations
3.10. Battery Powered Dressings
3.11. Devices
3.12. Mechanism
3.13. Clinical
3.14. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bois-Reymond, E.D. On Animal Electricity: Being an Abstract of the Discoveries of Emil Du Bois-Reymond; Kessinger Publishing, LLC: Whitefish, MT, USA, 1852. [Google Scholar]
- Levin, M.; Pezzulo, G.; Finkelstein, J.M. Endogenous Bioelectric Signaling Networks: Exploiting Voltage Gradients for Control of Growth and Form. Annu. Rev. Biomed. Eng. 2017, 19, 353–387. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, K.A.; Levin, M. Bioelectric Signaling in Regeneration: Mechanisms of Ionic Controls of Growth and Form. Dev. Biol. 2018, 433, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Heald, R.; Bennett, M.; Subramaniam, V.V.; Dusane, D.; Lochab, V.; Sundaram, P.M.; Salyer, S.; West, J.D.; Stoodley, P.; Prakash, S. Printed Electroceutical Dressings for the Inhibition of Biofilms and Treatment of Chronic Wounds. J. Microelectromech. Syst. 2020, 29, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Zhao, M. Electrical Fields in Wound Healing—An Overriding Signal That Directs Cell Migration. Semin. Cell Dev. Biol. 2009, 20, 674–682. [Google Scholar] [CrossRef]
- Blackiston, D.J.; McLaughlin, K.A.; Levin, M. Bioelectric Controls of Cell Proliferation: Ion Channels, Membrane Voltage and the Cell Cycle. Cell Cycle 2009, 8, 3527–3536. [Google Scholar] [CrossRef]
- Durant, F.; Morokuma, J.; Fields, C.; Williams, K.; Adams, D.S.; Levin, M. Long-Term, Stochastic Editing of Regenerative Anatomy via Targeting Endogenous Bioelectric Gradients. Biophys. J. 2017, 112, 2231–2243. [Google Scholar] [CrossRef]
- Adams, D.S.; Robinson, K.R.; Fukumoto, T.; Yuan, S.; Albertson, R.C.; Yelick, P.; Kuo, L.; McSweeney, M.; Levin, M. Early, H+-V-ATPase-Dependent Proton Flux Is Necessary for Consistent Left-Right Patterning of Non-Mammalian Vertebrates. Development 2006, 133, 1657–1671. [Google Scholar] [CrossRef]
- Pitcairn, E.; McLaughlin, K.A. Bioelectric Signaling Coordinates Patterning Decisions during Embryogenesis. Trends Dev. Biol. 2016, 9, 1–9. [Google Scholar]
- Lobo, D.; Lobikin, M.; Levin, M. Discovering Novel Phenotypes with Automatically Inferred Dynamic Models: A Partial Melanocyte Conversion in Xenopus. Sci. Rep. 2017, 7, 41339. [Google Scholar] [CrossRef]
- Aw, S.; Koster, J.C.; Pearson, W.; Nichols, C.G.; Shi, N.-Q.; Carneiro, K.; Levin, M. The ATP-Sensitive K+-Channel (KATP) Controls Early Left–Right Patterning in Xenopus and Chick Embryos. Dev. Biol. 2010, 346, 39–53. [Google Scholar] [CrossRef]
- Emmons-Bell, M.; Durant, F.; Hammelman, J.; Bessonov, N.; Volpert, V.; Morokuma, J.; Pinet, K.; Adams, D.S.; Pietak, A.; Lobo, D.; et al. Gap Junctional Blockade Stochastically Induces Different Species-Specific Head Anatomies in Genetically Wild-Type Girardia Dorotocephala Flatworms. Int. J. Mol. Sci. 2015, 16, 27865–27896. [Google Scholar] [CrossRef]
- Perathoner, S.; Daane, J.M.; Henrion, U.; Seebohm, G.; Higdon, C.W.; Johnson, S.L.; Nüsslein-Volhard, C.; Harris, M.P. Bioelectric Signaling Regulates Size in Zebrafish Fins. PLoS Genet. 2014, 10, e1004080. [Google Scholar] [CrossRef]
- Mathews, J.; Levin, M. Gap Junctional Signaling in Pattern Regulation: Physiological Network Connectivity Instructs Growth and Form. Dev. Neurobiol. 2017, 77, 643–673. [Google Scholar] [CrossRef]
- Inaba, M.; Jiang, T.-X.; Liang, Y.-C.; Tsai, S.; Lai, Y.-C.; Widelitz, R.B.; Chuong, C.M. Instructive Role of Melanocytes during Pigment Pattern Formation of the Avian Skin. Proc. Natl. Acad. Sci. USA 2019, 116, 6884–6890. [Google Scholar] [CrossRef]
- Naixin, J.; Jinrui, Y.; Jie, L.; Zhang, J. Electric Field: A Key Signal in Wound Healing. Chin. J. Plast. Reconstr. Surg. 2021, 3, 95–102. [Google Scholar]
- Nuccitelli, R.; Nuccitelli, P.; Li, C.; Narsing, S.; Pariser, D.M.; Lui, K. The Electric Field near Human Skin Wounds Declines with Age and Provides a Non-Invasive Indicator of Wound Healing. Wound Repair Regen. 2011, 19, 645–655. [Google Scholar] [CrossRef]
- Ud-Din, S.; Bayat, A. Electrical Stimulation and Cutaneous Wound Healing: A Review of Clinical Evidence. Healthcare 2014, 2, 445–467. [Google Scholar] [CrossRef]
- Subramaniam, T.; Fauzi, M.B.; Lokanathan, Y.; Law, J.X. The Role of Calcium in Wound Healing. Int. J. Mol. Sci. 2021, 22, 6486. [Google Scholar] [CrossRef]
- Holyńska-Iwan, I.; Szewczyk-Golec, K. Analysis of Changes in Sodium and Chloride Ion Transport in the Skin. Sci. Rep. 2020, 10, 18094. [Google Scholar] [CrossRef]
- Mobaraki, M.; Abbasi, R.; Omidian Vandchali, S.; Ghaffari, M.; Moztarzadeh, F.; Mozafari, M. Corneal Repair and Regeneration: Current Concepts and Future Directions. Front. Bioeng. Biotechnol. 2019, 7, 135. [Google Scholar] [CrossRef]
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound Healing Phases. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Conde, E. Why Do We Use Topical Zinc on Wounds and Perilesional Skin? Ph.D. Thesis, Elena Conde Montero. 2019. [Google Scholar]
- Morris, C.; O’Donnell, M.J. Vacuolar H+-ATPase and Na+/K+-ATPase Energize Na+ Uptake Mechanisms in the Nuchal Organ of the Hyperregulating Freshwater Crustacean Daphnia Magna. J. Exp. Biol. 2021, 224, jeb242205. [Google Scholar] [CrossRef] [PubMed]
- Matoori, S.; Veves, A.; Mooney, D.J. Advanced Bandages for Diabetic Wound Healing. Sci. Transl. Med. 2021, 13, eabe4839. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Caruso, G.I.; De Pasquale, R.; Sortino, M.A.; Merlo, S. The Treatment of Impaired Wound Healing in Diabetes: Looking among Old Drugs. Pharmaceuticals 2020, 13, 60. [Google Scholar] [CrossRef]
- Anderson, D.J.; Podgorny, K.; Berríos-Torres, S.I.; Bratzler, D.W.; Dellinger, E.P.; Greene, L.; Nyquist, A.-C.; Saiman, L.; Yokoe, D.S.; Maragakis, L.L.; et al. Strategies to Prevent Surgical Site Infections in Acute Care Hospitals: 2014 Update. Infect. Control Hosp. Epidemiol. 2014, 35, 605–627. [Google Scholar] [CrossRef]
- Andersen, B.M. Prevention of Postoperative Wound Infections. In Prevention and Control of Infections in Hospitals; Springer: Berlin/Heidelberg, Germany, 2019; pp. 377–437. [Google Scholar]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Wound Healing Essentials: Let There Be Oxygen. Wound Repair Regen. 2009, 17, 1–18. [Google Scholar] [CrossRef]
- Alves, P.J.; Barreto, R.T.; Barrois, B.M.; Gryson, L.G.; Meaume, S.; Monstrey, S.J. Update on the Role of Antiseptics in the Management of Chronic Wounds with Critical Colonisation and/or Biofilm. Int. Wound J. 2021, 18, 342–358. [Google Scholar] [CrossRef]
- Carter, M.J.; Frykberg, R.G.; Oropallo, A.; Sen, C.K.; Armstrong, D.G.; Nair, H.K.; Serena, T.E. Efficacy of Topical Wound Oxygen Therapy in Healing Chronic Diabetic Foot Ulcers: Systematic Review and Meta-Analysis. Adv. Wound Care 2023, 12, 177–186. [Google Scholar] [CrossRef]
- Pugliese, G.; Liccardi, A.; Graziadio, C.; Barrea, L.; Muscogiuri, G.; Colao, A. Obesity and Infectious Diseases: Pathophysiology and Epidemiology of a Double Pandemic Condition. Int. J. Obes. 2022, 46, 449–465. [Google Scholar] [CrossRef]
- Ghaly, P.; Iliopoulos, J.; Ahmad, M. The Role of Nutrition in Wound Healing: An Overview. Br. J. Nurs. 2021, 30, S38–S42. [Google Scholar] [CrossRef]
- Almadani, Y.H.; Vorstenbosch, J.; Davison, P.G.; Murphy, A.M. Wound Healing: A Comprehensive Review. Semin. Plast. Surg. 2021, 35, 141–144. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Zhao, M.; Liu, G.; Wu, J. Advances of Hydrogel Dressings in Diabetic Wounds. Biomater. Sci. 2021, 9, 1530–1546. [Google Scholar] [CrossRef]
- Hawthorne, B.; Simmons, J.K.; Stuart, B.; Tung, R.; Zamierowski, D.S.; Mellott, A.J. Enhancing Wound Healing Dressing Development through Interdisciplinary Collaboration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1967–1985. [Google Scholar] [CrossRef]
- Sen, C.K.; Roy, S.; Mathew-Steiner, S.S.; Gordillo, G.M. Biofilm Management in Wound Care. Plast. Reconstr. Surg. 2021, 148, 275e–288e. [Google Scholar] [CrossRef]
- Sanjuan-Alberte, P.; Alexander, M.R.; Hague, R.J.M.; Rawson, F.J. Electrochemically Stimulating Developments in Bioelectronic Medicine. Bioelectron. Med. 2018, 4, 1. [Google Scholar] [CrossRef]
- McLister, A.; Phair, J.; Cundell, J.; Davis, J. Electrochemical Approaches to the Development of Smart Bandages: A Mini-Review. Electrochem. Commun. 2014, 40, 96–99. [Google Scholar] [CrossRef]
- Roy, S.; Prakash, S.; Mathew-Steiner, S.S.; Das Ghatak, P.; Lochab, V.; Jones, T.H.; Mohana Sundaram, P.; Gordillo, G.M.; Subramaniam, V.V.; Sen, C.K. Disposable Patterned Electroceutical Dressing (PED-10) Is Safe for Treatment of Open Clinical Chronic Wounds. Adv. Wound Care 2019, 8, 149–159. [Google Scholar] [CrossRef]
- Ovington, L. The Value of Silver in Wound Management. Podiatry Today 1999, 12, 59–62. [Google Scholar]
- Khansa, I.; Schoenbrunner, A.R.; Kraft, C.T.; Janis, J.E. Silver in Wound Care—Friend or Foe? A Comprehensive Review. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2390. [Google Scholar] [CrossRef]
- Banerjee, J.; Ghatak, P.D.; Roy, S.; Khanna, S.; Sequin, E.K.; Bellman, K.; Dickinson, B.C.; Suri, P.; Subramaniam, V.V.; Chang, C.J.; et al. Improvement of Human Keratinocyte Migration by a Redox Active Bioelectric Dressing. PLoS ONE 2014, 9, e89239. [Google Scholar] [CrossRef]
- The Metis Foundation A Prospective, Randomized, Controlled Study to Determine the Superiority of a Fabric-Based Wireless Electroceutical Dressing, Procellera® Compared to Standard of Care Treatment in Mitigating Biofilm Formation in Acute Trauma and Burn Wounds. Available online:https://clinicaltrials.gov (accessed on 18 October 2022).
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Umair, M.; Hussain, I.; Javed, I. Nanosilver at the Interface of Biomedical Applications, Toxicology, and Synthetic Strategies. Met. Nanopart. Drug. Deliv. Diagn. Appl. 2020, 119–139. [Google Scholar] [CrossRef]
- Fong, J.; Wood, F.; Fowler, B. A Silver Coated Dressing Reduces the Incidence of Early Burn Wound Cellulitis and Associated Costs of Inpatient Treatment: Comparative Patient Care Audits. Burns 2005, 31, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Morris, C. Bio-Electrical Stimulation Therapy Using POSiFECT® RD. Wounds UK 2006, 2, 112. [Google Scholar]
- Butcher, M. How to Use POSiFECT® Bio-Electric Stimulation Therapy in Chronic Wounds. Wound Essent. 2007, 2, 186–193. [Google Scholar]
- Kloth, L. Wound Healing with Conductive Electrical Stimulation—It’s the Dosage That Counts. J. Wound Technol. 2009, 6, 30–37. [Google Scholar]
- Nešporová, K.; Pavlík, V.; Šafránková, B.; Vágnerová, H.; Odráška, P.; Žídek, O.; Císařová, N.; Skoroplyas, S.; Kubala, L.; Velebný, V. Effects of Wound Dressings Containing Silver on Skin and Immune Cells. Sci. Rep. 2020, 10, 15216. [Google Scholar] [CrossRef]
- Coutts, P.; Gary Sibbald, R. The Effect of a Silver-Containing Hydrofiber® Dressing on Superficial Wound Bed and Bacterial Balance of Chronic Wounds. Int. Wound J. 2005, 2, 348–356. [Google Scholar] [CrossRef]
- Vanscheidt, W.; Lazareth, I.; Routkovsky-Norval, C. Safety Evaluation of a New Ionic Silver Dressing in the Management of Chronic Ulcers. Wounds-A Compend. Clin. Res. Pract. 2003, 15, 371–378. [Google Scholar]
- Leaper, D.J. Silver Dressings: Their Role in Wound Management. Int. Wound J. 2006, 3, 282–294. [Google Scholar] [CrossRef]
- Banerjee, J.; Ghatak, P.D.; Roy, S.; Khanna, S.; Hemann, C.; Deng, B.; Das, A.; Zweier, J.L.; Wozniak, D.; Sen, C.K. Silver-Zinc Redox-Coupled Electroceutical Wound Dressing Disrupts Bacterial Biofilm. PLoS ONE 2015, 10, e0119531. [Google Scholar] [CrossRef]
- Yu, C.; Xu, Z.-X.; Hao, Y.-H.; Gao, Y.-B.; Yao, B.-W.; Zhang, J.; Wang, B.; Hu, Z.-Q.; Peng, R.-Y. A Novel Microcurrent Dressing for Wound Healing in a Rat Skin Defect Model. Mil. Med. Res. 2019, 6, 22. [Google Scholar] [CrossRef]
- Turner, N.; Ovens, L. The Results of a Clinical Evaluation of Accel-Heal® Electroceutical Treatment in a Large NHS Trust. Wounds UK 2017, 13, 92–99. [Google Scholar]
- Young, S.; Hampton, S.; Tadej, M. Study to Evaluate the Effect of Low-Intensity Pulsed Electrical Currents on Levels of Oedema in Chronic Non-Healing Wounds. J. Wound Care 2011, 20, 368–373. [Google Scholar] [CrossRef]
- Ashrafi, M.; Alonso-Rasgado, T.; Baguneid, M.; Bayat, A. The Efficacy of Electrical Stimulation in Lower Extremity Cutaneous Wound Healing: A Systematic Review. Exp. Dermatol. 2017, 26, 171–178. [Google Scholar] [CrossRef]
- Borkow, G.; Melamed, E. Copper, an Abandoned Player Returning to the Wound Healing Battle. In Recent Advances in Wound Healing; IntechOpen: London, UK, 2021; ISBN 978-1-83968-573-6. [Google Scholar]
- Melamed, E.; Kiambi, P.; Okoth, D.; Honigber, I.; Tamir, E.; Borkow, G. Healing of Chronic Wounds by Copper Oxide-Impregnated Wound Dressings—Case Series. Medicina 2021, 57, 296. [Google Scholar] [CrossRef]
- Schwartz, J.; Goss, S.; Facchin, F.; Gendics, C.; Lantis, J. Single-Use Negative Pressure Wound Therapy for the Treatment of Chronic Lower Leg Wounds. J. Wound Care 2015, 24, S4–S9. [Google Scholar] [CrossRef]
- Gitarja, W.S.; Jamaluddin, A.; Wibisono, A.; Megawati, V.; Fajar, K. Wound Care Management in Indonesia: Issues and Challenges in Diabetic Foot Ulceration. Wounds Asia 2018, 1, 13–17. [Google Scholar]
- Thompson, M.W. Regulation of Zinc-Dependent Enzymes by Metal Carrier Proteins. Biometals 2022, 35, 187–213. [Google Scholar] [CrossRef]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Mlyniec, K.; Librowski, T. Antioxidant and Anti-Inflammatory Effects of Zinc. Zinc-Dependent NF-ΚB Signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef]
- Tekiner, H.; Karamanou, M. The Unna Boot: A Historical Dressing for Varicose Ulcers. Acta Dermatovenerol. Croat. ADC 2019, 27, 273–274. [Google Scholar]
- Lin, P.-H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2017, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Roth, T.; Kalinkovich, A. Wide Spectrum Potent Antimicrobial Efficacy of Wound Dressings Impregnated with Cuprous Oxide Microparticles. Microbiol. Res. 2022, 13, 366–376. [Google Scholar] [CrossRef]
- Ofstead, C.L.; Buro, B.L.; Hopkins, K.M.; Eiland, J.E. The Impact of Continuous Electrical Microcurrent on Acute and Hard-to-Heal Wounds: A Systematic Review. J. Wound Care 2020, 29, S6–S15. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, P.D.; Schlanger, R.; Ganesh, K.; Lambert, L.; Gordillo, G.M.; Martinsek, P.; Roy, S. A Wireless Electroceutical Dressing Lowers Cost of Negative Pressure Wound Therapy. Adv. Wound Care 2015, 4, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs Are Unique: Opportunities and Challenges of Discovery and Development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]
- Betts, H.D.; Whitehead, C.; Harris, H.H. Silver in Biology and Medicine: Opportunities for Metallomics Researchers. Metallomics 2021, 13, mfaa001. [Google Scholar] [CrossRef]
- Irnawati, I. Case Study: The Use of Metcovazin in Curing Burns. St. Med. 2019, 15, 106–112. [Google Scholar] [CrossRef]
- Rybka, M.; Mazurek, Ł.; Konop, M. Beneficial Effect of Wound Dressings Containing Silver and Silver Nanoparticles in Wound Healing—From Experimental Studies to Clinical Practice. Life 2023, 13, 69. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Kirketerp-Møller, K.; Kristiansen, S.; Phipps, R.; Nielsen, A.K.; Jensen, P.Ø.; Høiby, N.; Givskov, M. Silver against Pseudomonas Aeruginosa Biofilms. APMIS 2007, 115, 921–928. [Google Scholar] [CrossRef]
- Erci, F.; Cakir-Koc, R.; Yontem, M.; Torlak, E. Synthesis of Biologically Active Copper Oxide Nanoparticles as Promising Novel Antibacterial-Antibiofilm Agents. Prep. Biochem. Biotechnol. 2020, 50, 538–548. [Google Scholar] [CrossRef]
- Ansarifard, E.; Zareshahrabadi, Z.; Sarafraz, N.; Zomorodian, K. Evaluation of Antimicrobial and Antibiofilm Activities of Copper Oxide Nanoparticles within Soft Denture Liners against Oral Pathogens. Bioinorg. Chem. Appl. 2021, 2021, 9939275. [Google Scholar] [CrossRef]
- Agarwala, M.; Choudhury, B.; Yadav, R.N.S. Comparative Study of Antibiofilm Activity of Copper Oxide and Iron Oxide Nanoparticles against Multidrug Resistant Biofilm Forming Uropathogens. Indian. J. Microbiol. 2014, 54, 365–368. [Google Scholar] [CrossRef]
- Wu, C.; Labrie, J.; Tremblay, Y.D.N.; Haine, D.; Mourez, M.; Jacques, M. Zinc as an Agent for the Prevention of Biofilm Formation by Pathogenic Bacteria. J. Appl. Microbiol. 2013, 115, 30–40. [Google Scholar] [CrossRef]
- Abdelghafar, A.; Yousef, N.; Askoura, M. Zinc Oxide Nanoparticles Reduce Biofilm Formation, Synergize Antibiotics Action and Attenuate Staphylococcus Aureus Virulence in Host; an Important Message to Clinicians. BMC Microbiol. 2022, 22, 244. [Google Scholar] [CrossRef]
- Tredget, E.E.; Shankowsky, H.A.; Groeneveld, A.; Burrell, R. A Matched-Pair, Randomized Study Evaluating the Efficacy and Safety of Acticoat Silver-Coated Dressing for the Treatment of Burn Wounds. J. Burn. Care Rehabil. 1998, 19, 531–537. [Google Scholar] [CrossRef]
- Caruso, D.M.; Foster, K.N.; Hermans, M.H.; Rick, C. Aquacel Ag® in the Management of Partial-Thickness Burns: Results of a Clinical Trial. J. Burn. Care Rehabil. 2004, 25, 89–97. [Google Scholar] [CrossRef]
- MedCu Technologies Ltd. Pilot Efficacy Study of MedCu Wound Dressings with Copper Oxide in Treating Pressure Sores and Post-Op Wounds. Available online:http://clinicaltrials.gov (accessed on 18 October 2022).
- Khona, D.K.; Roy, S.; Ghatak, S.; Huang, K.; Jagdale, G.; Baker, L.A.; Sen, C.K. Ketoconazole Resistant Candida Albicans Is Sensitive to a Wireless Electroceutical Wound Care Dressing. Bioelectrochemistry 2021, 142, 107921. [Google Scholar] [CrossRef]
- Blount, A.L.; Foster, S.; Rapp, D.A.; Wilcox, R. The Use of Bioelectric Dressings in Skin Graft Harvest Sites: A Prospective Case Series. J. Burn. Care Res. 2012, 33, 354–357. [Google Scholar] [CrossRef]
- Housler, G.J.; Cross, S.; Marcel, V.; Kennedy, D.O.; Husband, M.; Register, A.; Roberts, T.; Grubbs, S.; Dudewicz, D.; Setka, N.; et al. A Prospective Randomized Controlled Two-Arm Clinical Study Evaluating the Efficacy of a Bioelectric Dressing System for Blister Management in US Army Ranger Recruits. J. Spec. Oper. Med. 2017, 17, 25. [Google Scholar] [CrossRef]
- Whitcomb, E.; Monroe, N.; Hope-Higman, J.; Campbell, P. Demonstration of a Microcurrent-Generating Wound Care Device for Wound Healing Within a Rehabilitation Center Patient Population. J. Am. Coll. Clin. Wound Spec. 2013, 4, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.I. Resolving Long-Standing Uncertainty about the Clinical Efficacy of Transcutaneous Electrical Nerve Stimulation (TENS) to Relieve Pain: A Comprehensive Review of Factors Influencing Outcome. Medicina 2021, 57, 378. [Google Scholar] [CrossRef] [PubMed]
- Feedar, J.A.; Kloth, L.C.; Gentzkow, G.D. Chronic Dermal Ulcer Healing Enhanced with Monophasic Pulsed Electrical Stimulation. Phys. Ther. 1991, 71, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Gentzkow, G.D. Healing of Refractory Stage III and IV Pressure Ulcers by a New Electrical Stimulation Device. Wounds 1993, 5, 160–172. [Google Scholar]
- Peters, E.J.; Lavery, L.A.; Armstrong, D.G.; Fleischli, J.G. Electric Stimulation as an Adjunct to Heal Diabetic Foot Ulcers: A Randomized Clinical Trial. Arch. Phys. Med. Rehabil. 2001, 82, 721–725. [Google Scholar] [CrossRef]
- Malin, E.W.; Galin, C.M.; Lairet, K.F.; Huzar, T.F.; Williams, J.F.; Renz, E.M.; Wolf, S.E.; Cancio, L.C. Silver-Coated Nylon Dressing plus Active DC Microcurrent for Healing of Autogenous Skin Donor Sites. Ann Plast Surg 2013, 71, 481–484. [Google Scholar] [CrossRef]
- Lucchese, A.; Gherlone, E.; Portelli, M.; Bertossi, D. Tooth Orthodontic Movement after Maxillofacial Surgery. Eur. J. Inflamm. 2012, 10, 227–232. [Google Scholar] [CrossRef]
- Rosso, M.; Blasi, G.; Gherlone, E.; Rosso, R. Effect of Granulocyte-Macrophage Colony-Stimulating Factor on Prevention of Mucositis in Head and Neck Cancer Patients Treated with Chemo-Radiotherapy. J. Chemother. 1997, 9, 382–385. [Google Scholar] [CrossRef]
- Tecco, S.; Grusovin, M.; Sciara, S.; Bova, F.; Pantaleo, G.; Capparé, P. The Association between Three Attitude-Related Indexes of Oral Hygiene and Secondary Implant Failures: A Retrospective Longitudinal Study. Int. J. Dent. Hyg. 2018, 16, 372–379. [Google Scholar] [CrossRef]
- Sikder, P.; Nagaraju, P.; Naganaboyina, H.P.S. 3D-Printed Piezoelectric Porous Bioactive Scaffolds and Clinical Ultrasonic Stimulation Can Help in Enhanced Bone Regeneration. Bioengineering 2022, 9, 679. [Google Scholar] [CrossRef]
- Kocak-Topbas, N.; Kamburoğlu, K.; Ertürk-Avunduk, A.T.; Ozemre, M.O.; Eratam, N.; Çakmak, E.E. Clinical Performance of Diagnostic Methods in Third Molar Teeth with Early Occlusal Caries. Diagnostics 2023, 13, 284. [Google Scholar] [CrossRef]
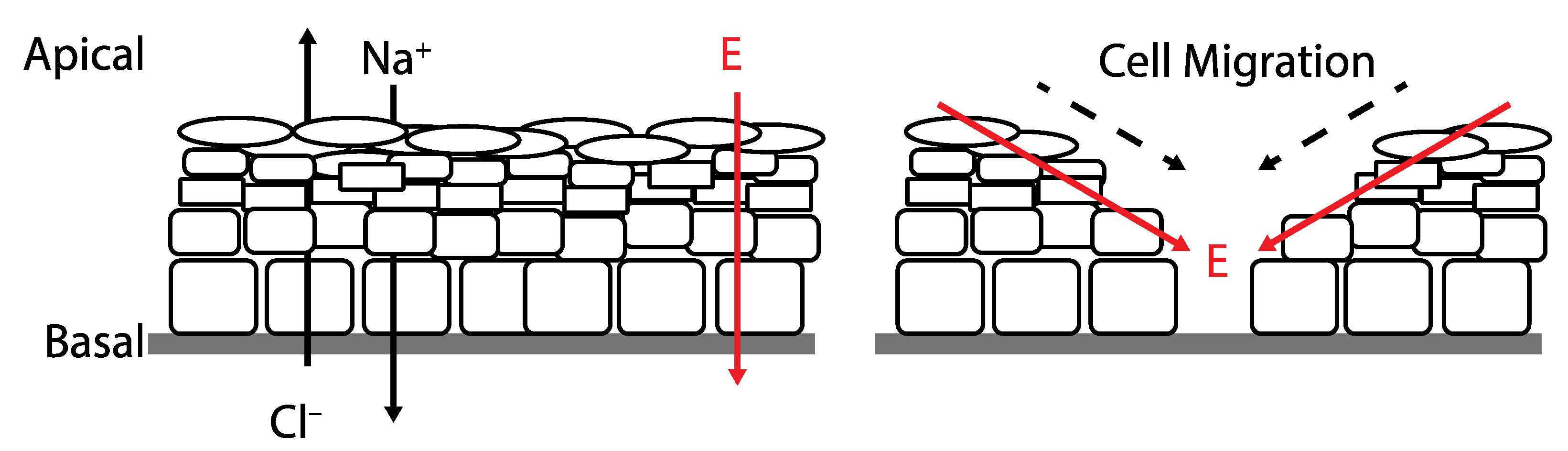
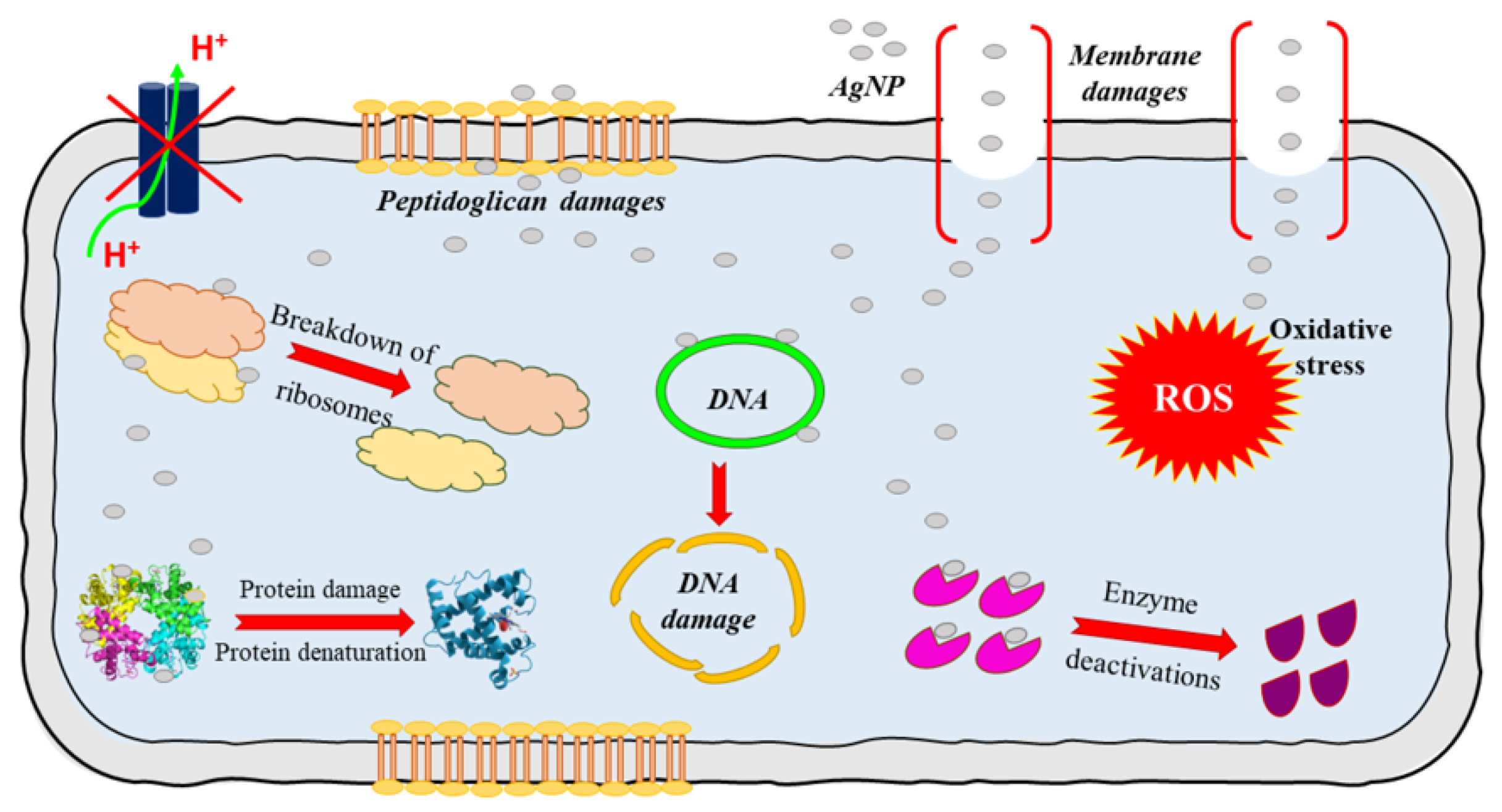
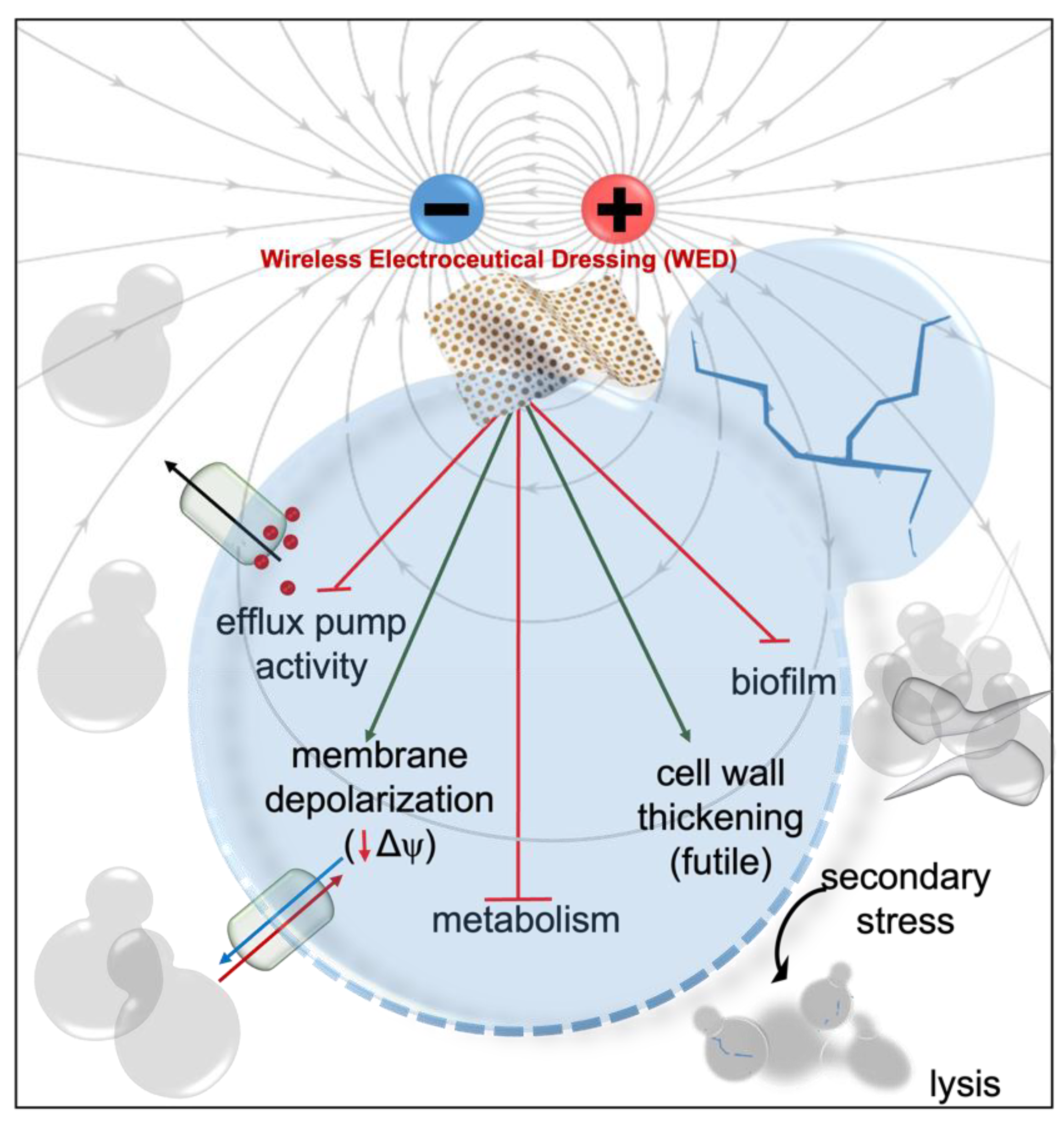
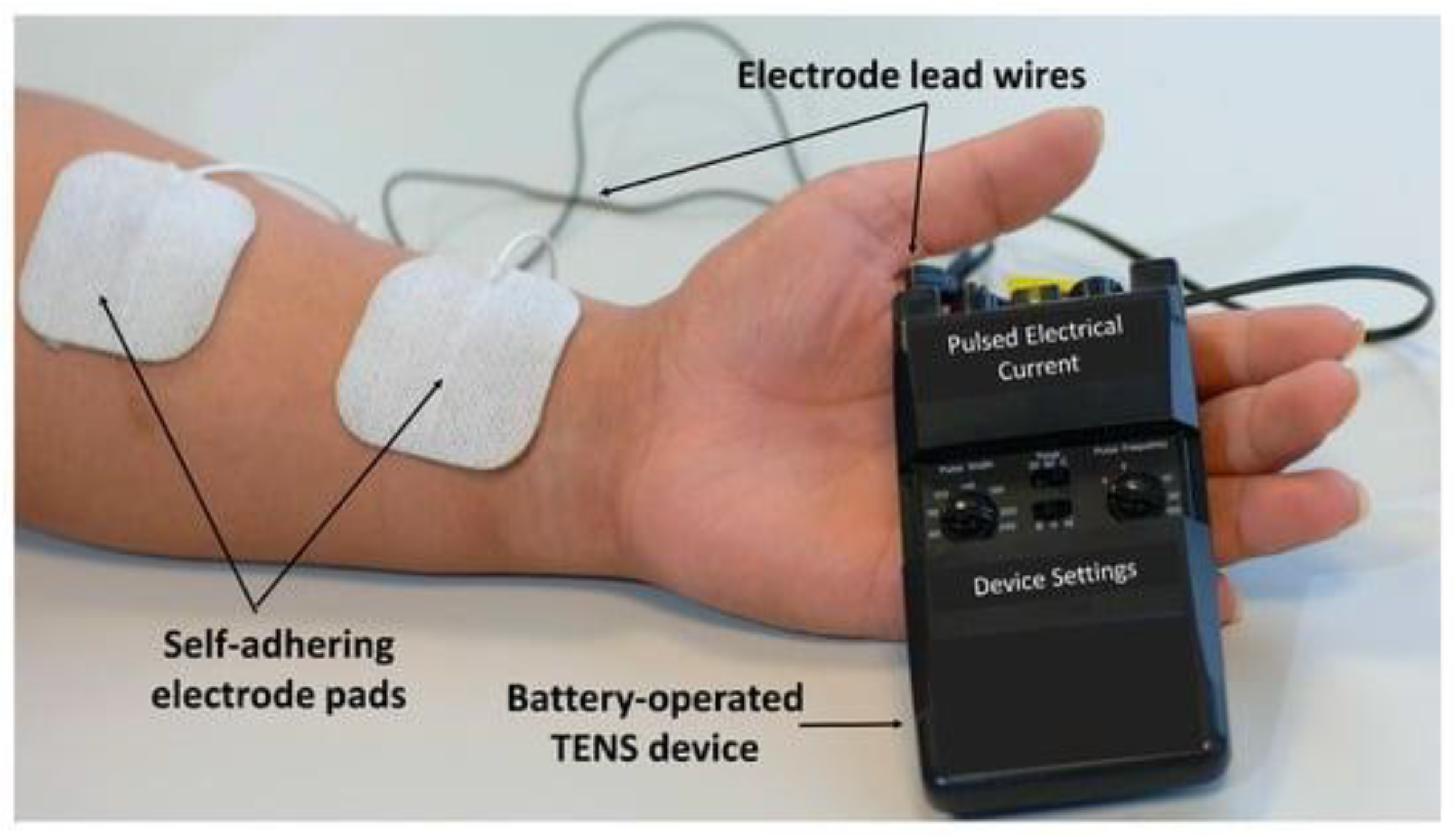
| Ionic | Wireless | Battery-Powered | |||
|---|---|---|---|---|---|
| The electrochemical gradient drives ions to the bacterial cell. Where the local electrochemical gradient is high enough, ions are driven into the cell where they disrupt the respiratory system and bind to DNA. | The electrochemical potential difference creates an electric field that is maintained by ion transport between the anode and cathode until the electrochemical reaction reaches equilibrium. | An electric potential is applied to an inert electrode and the field is maintained until the battery depletes. | |||
| Commercial name | Reference(s) | Commercial name | Reference(s) | Commercial name | Reference(s) |
| Silver sulfadiazine | [43,44] | Procellera | [45,46] | Patterned electroceutical dressing | [42] |
| Acticoat | [47,48] | ||||
| Actisorb | [49] | PosiFectRD | [6,19,50,51,52] | ||
| Aqucel-Ag | [53,54,55] | ||||
| Contreet Foam | [43,44,53,56,57] | Yu et al. | [58] | Accel-Heal | [59,60,61] |
| Urgotul | [43,44,53,56,57] | ||||
| MedCu | [62,63] | WoundEL | [60,64] | ||
| Metcovazin | [24,65,66,67,68] | ||||
| Mode of action | Mode of action | Mode of action | |||
 Silver circles represent silver ions, green ovals represent bacteria, and black arrows represent the electrochemical gradient. | 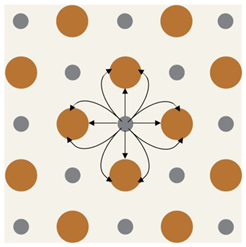 Silver circles represent silver electrodes, copper circles represent copper electrodes, and black arrows represent the electrochemical gradient. | 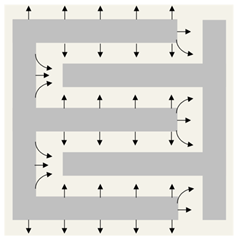 Silver rectangles represent silver electrodes, beige represents the inert dressing, and black arrows represent the electrochemical gradient. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, J.P.; Sen, C.K. Electrochemical Devices in Cutaneous Wound Healing. Bioengineering 2023, 10, 711. https://doi.org/10.3390/bioengineering10060711
Evans JP, Sen CK. Electrochemical Devices in Cutaneous Wound Healing. Bioengineering. 2023; 10(6):711. https://doi.org/10.3390/bioengineering10060711
Chicago/Turabian StyleEvans, J. Parker, and Chandan K. Sen. 2023. "Electrochemical Devices in Cutaneous Wound Healing" Bioengineering 10, no. 6: 711. https://doi.org/10.3390/bioengineering10060711
APA StyleEvans, J. P., & Sen, C. K. (2023). Electrochemical Devices in Cutaneous Wound Healing. Bioengineering, 10(6), 711. https://doi.org/10.3390/bioengineering10060711






