The Effect of Pulling Angle on Rotator Cuff Mechanical Properties in a Canine In Vitro Model
Abstract
1. Introduction
2. Materials and Methods
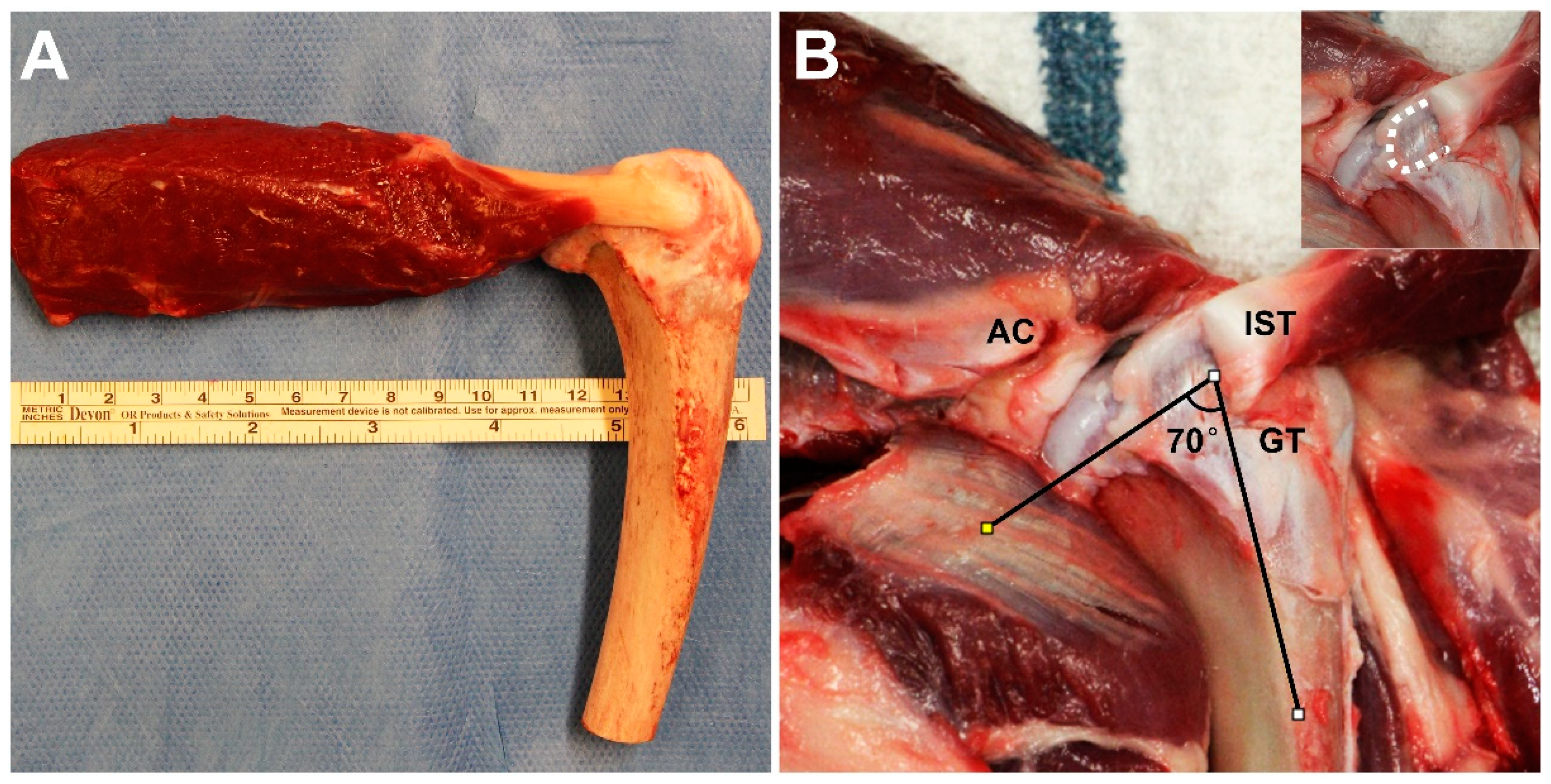
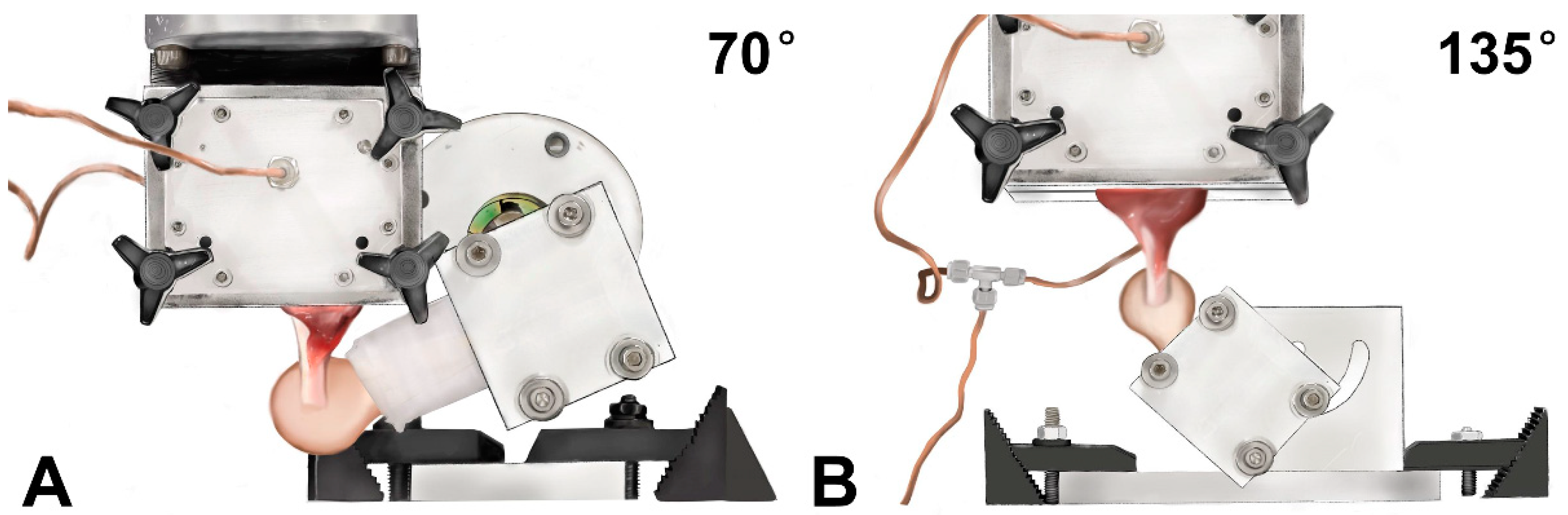
2.1. Experimental Design
2.2. Rotator Cuff Repair
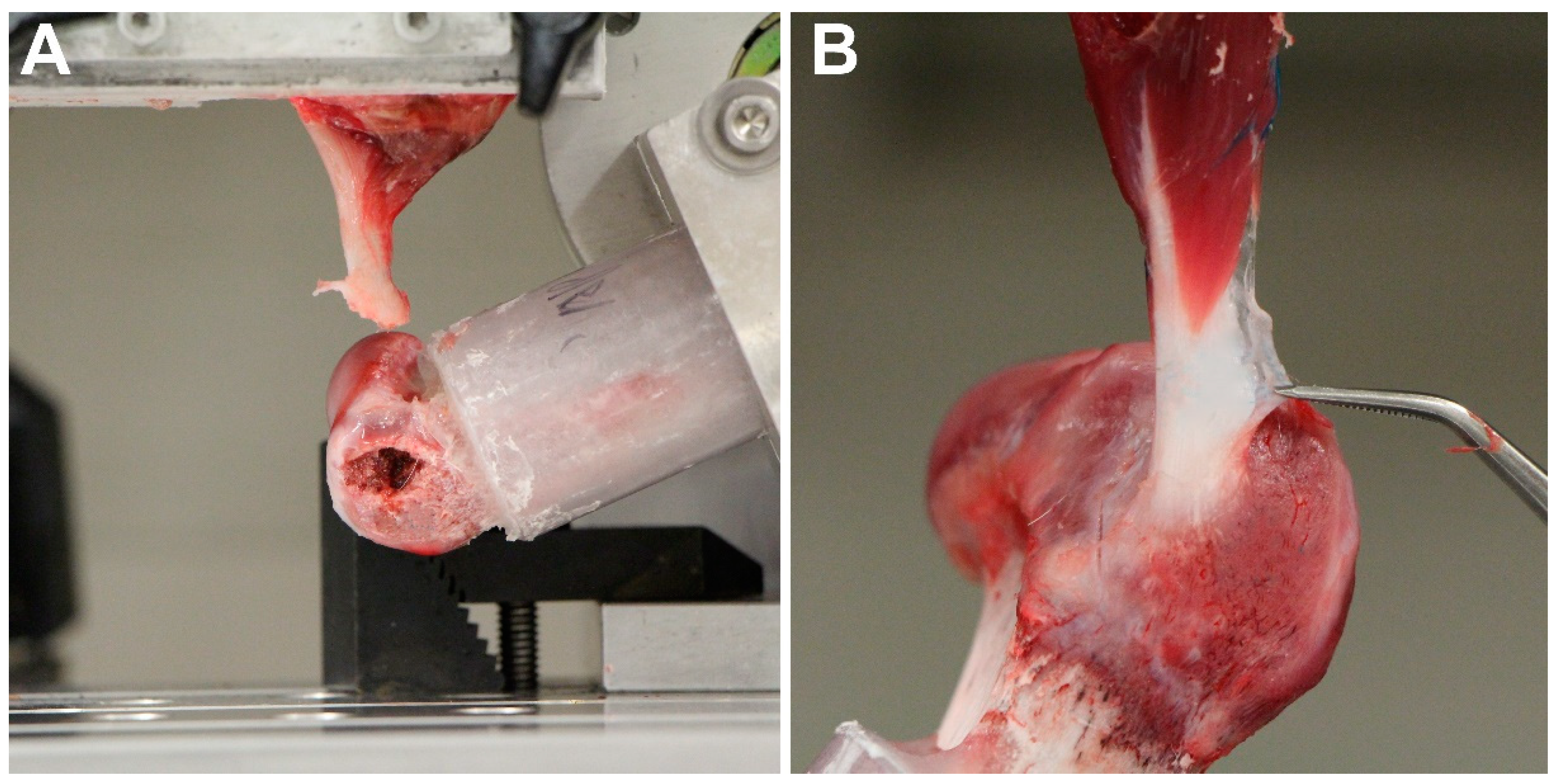
2.3. Biomechanical Testing
2.4. Statistical Analysis
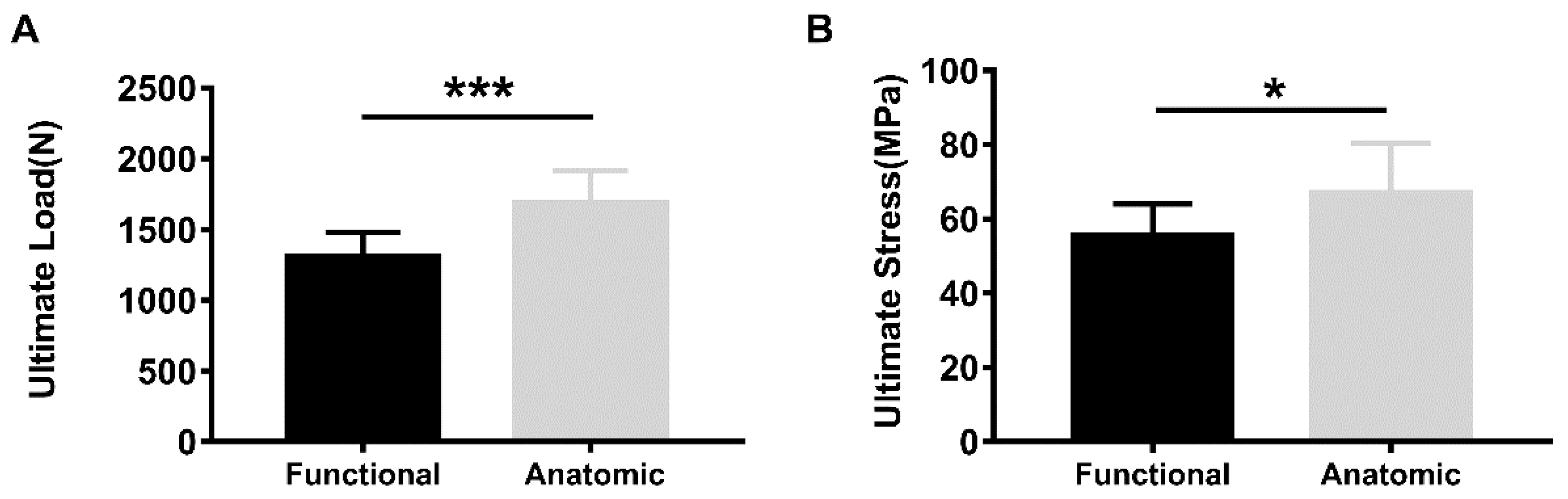
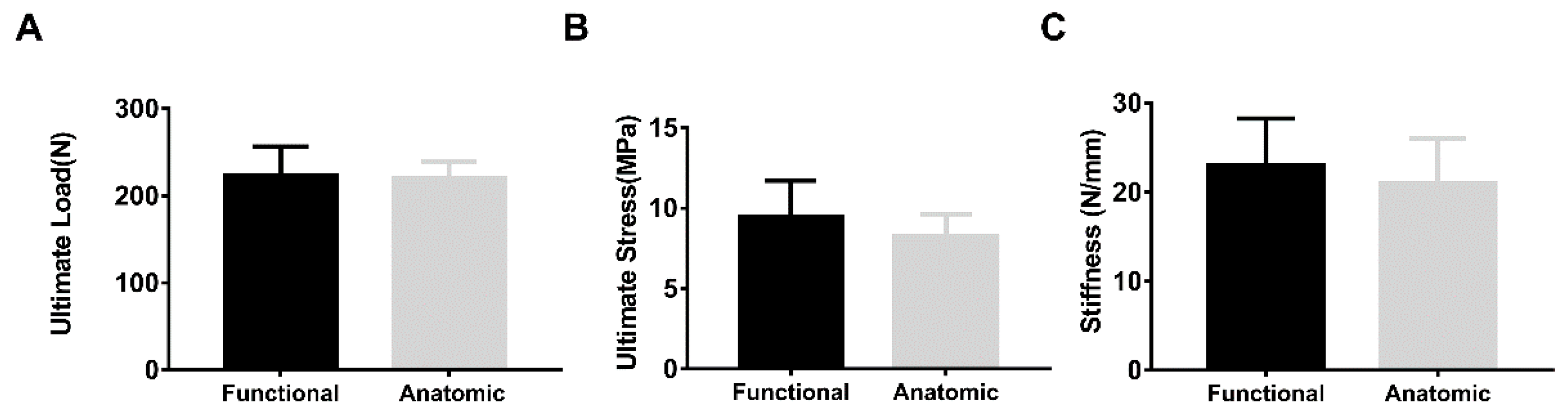
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deprés-Tremblay, G.; Chevrier, A.; Snow, M.; Hurtig, M.B.; Rodeo, S.; Buschmann, M.D. Rotator cuff repair: A review of surgical techniques, animal models, and new technologies under development. J. Shoulder Elb. Surg. 2016, 25, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Tirefort, J.; Schwitzguebel, A.J.; Collin, P.; Nowak, A.; Plomb-Holmes, C.; Ladermann, A. Postoperative Mobilization After Superior Rotator Cuff Repair: Sling Versus No Sling: A Randomized Prospective Study. J. Bone Jt. Surg. Am. 2019, 101, 494–503. [Google Scholar] [CrossRef]
- Hantes, M.E.; Ono, Y.; Raoulis, V.A.; Doxariotis, N.; Venouziou, A.; Zibis, A.; Vlychou, M. Arthroscopic Single-Row Versus Double-Row Suture Bridge Technique for Rotator Cuff Tears in Patients Younger Than 55 Years: A Prospective Comparative Study. Am. J. Sports Med. 2017, 46, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Park, J.S.; Rhee, S.M.; Park, J.H. Maximum Bridging Suture Tension Provides Better Clinical Outcomes in Transosse-ous-Equivalent Rotator Cuff Repair: A Clinical, Prospective Randomized Comparative Study. Am. J. Sports Med. 2020, 48, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Plachel, F.; Siegert, P.; Rüttershoff, K.; Thiele, K.; Akgün, D.; Moroder, P.; Scheibel, M.; Gerhardt, C. Long-term Results of Arthroscopic Rotator Cuff Repair: A Follow-up Study Comparing Single-Row Versus Double-Row Fixation Techniques. Am. J. Sports Med. 2020, 48, 1568–1574. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.P.; Chung, S.W.; Kim, J.Y.; Lee, B.J.; Kim, H.-S.; Kim, J.E.; Cho, J.H. Outcomes of Combined Bone Marrow Stimulation and Patch Augmentation for Massive Rotator Cuff Tears. Am. J. Sports Med. 2016, 44, 963–971. [Google Scholar] [CrossRef]
- Vastamäki, M.; Lohman, M.; Borgmästars, N. Rotator Cuff Integrity Correlates With Clinical and Functional Results at a Minimum 16 Years After Open Repair. Clin. Orthop. Relat. Res. 2013, 471, 554–561. [Google Scholar] [CrossRef]
- Mall, N.A.; Tanaka, M.J.; Choi, L.S.; Paletta, G.A., Jr. Factors Affecting Rotator Cuff Healing. J. Bone Jt. Surg. 2014, 96, 778–788. [Google Scholar] [CrossRef]
- Park, J.S.; Park, H.J.; Kim, S.H.; Oh, J.H. Prognostic Factors Affecting Rotator Cuff Healing After Arthroscopic Repair in Small to Medium-sized Tears. Am. J. Sports Med. 2015, 43, 2386–2392. [Google Scholar] [CrossRef]
- Mihata, T.; Watanabe, C.; Fukunishi, K.; Ohue, M.; Tsujimura, T.; Fujiwara, K.; Kinoshita, M. Functional and Structural Outcomes of Single-Row Versus Double-Row Versus Combined Double-Row and Suture-Bridge Repair for Rotator Cuff Tears. Am. J. Sports Med. 2011, 39, 2091–2098. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Kim, J.-Y.; Ki, S.-Y.; Chung, S.W. Influence of Smoking on the Expression of Genes and Proteins Related to Fat Infiltration, Inflammation, and Fibrosis in the Rotator Cuff Muscles of Patients with Chronic Rotator Cuff Tears: A Pilot Study. Arthroscopy 2019, 35, 3181–3191. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yang, J.; Kang, Y.; Hu, K.; Jiao, M.; Zhao, B.; Jiang, Y.; Liu, C.; Ding, F.; Yuan, B.; et al. Animal Models of Rotator Cuff Injury and Repair: A Systematic Review. Tissue Eng. Part. B Rev. 2022, 28, 1258–1273. [Google Scholar] [CrossRef] [PubMed]
- Lebaschi, A.; Deng, X.-H.; Zong, J.; Cong, G.-T.; Carballo, C.B.; Album, Z.M.; Camp, C.; Rodeo, S.A. Animal models for rotator cuff repair. Ann. N. Y. Acad. Sci. 2016, 1383, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Derwin, K.A.; Baker, A.R.; Iannotti, J.P.; McCarron, J.A. Preclinical Models for Translating Regenerative Medicine Therapies for Rotator Cuff Repair. Tissue Eng. Part B Rev. 2010, 16, 21–30. [Google Scholar] [CrossRef]
- Easley, J.; Puttlitz, C.; Hackett, E.; Broomfield, C.; Nakamura, L.; Hawes, M.; Getz, C.; Frankle, M.; Pierre, P.S.; Tashjian, R.; et al. A prospective study comparing tendon-to-bone interface healing using an interposition bioresorbable scaffold with a vented anchor for primary rotator cuff repair in sheep. J. Shoulder Elb. Surg. 2020, 29, 157–166. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Y.; Reisdorf, R.L.; Qi, J.; Lu, C.K.; Berglund, L.J.; Zhao, C. Engineered tendon-fibrocartilage-bone composite and bone mar-row-derived mesenchymal stem cell sheet augmentation promotes rotator cuff healing in a non-weight-bearing canine model. Biomaterials 2019, 192, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Rothrauff, B.B.; Smith, C.A.; Ferrer, G.A.; Novaretti, J.V.; Pauyo, T.; Chao, T.; Hirsch, D.; Beaudry, M.F.; Herbst, E.; Tuan, R.S.; et al. The effect of adipose-derived stem cells on enthesis healing after repair of acute and chronic massive rotator cuff tears in rats. J. Shoulder Elb. Surg. 2019, 28, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Cook, J.L.; Kuroki, K.; Jayabalan, P.S.; Cook, C.R.; Pfeiffer, F.M.; Waters, N.P. Comparison of a Novel Bone-Tendon Allograft with a Human Dermis–Derived Patch for Repair of Chronic Large Rotator Cuff Tears Using a Canine Model. Arthrosc. J. Arthrosc. Relat. Surg. 2011, 28, 169–177. [Google Scholar] [CrossRef]
- Yoon, J.P.; Chung, S.W.; Jung, J.W.; Lee, Y.S.; Kim, K.I.; Park, G.Y.; Choi, J.H. Is a Local Administration of Parathyroid Hormone Effective to Tendon-to-Bone Healing in a Rat Rotator Cuff Repair Model? J. Orthop. Res. 2020, 38, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.P.; Lee, C.H.; Jung, J.W.; Lee, H.J.; Lee, Y.S.; Kim, J.Y.; Chung, S.W. Sustained Delivery of Transforming Growth Factor beta1 by Use of Absorbable Alginate Scaffold Enhances Rotator Cuff Healing in a Rabbit Model. Am. J. Sports Med. 2018, 46, 1441–1450. [Google Scholar] [CrossRef]
- Derwin, K.A.; Baker, A.R.; Codsi, M.J.; Iannotti, J.P. Assessment of the canine model of rotator cuff injury and repair. J. Shoulder Elb. Surg. 2007, 16, S140–S148. [Google Scholar] [CrossRef] [PubMed]
- Derwin, K.A.; Codsi, M.J.; Milks, R.A.; Baker, A.R.; McCarron, J.A.; Iannotti, J.P. Rotator Cuff Repair Augmentation in a Canine Model with Use of a Woven Poly-L-Lactide Device. J. Bone Jt. Surg. 2009, 91, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, T.F.; Hawkins, R.J.; Lewis, C.W.; Turner, A.S. An in vivo comparison of the modified Mason-Allen suture technique versus an inclined horizontal mattress suture technique with regard to tendon-to-bone healing: A biomechanical and histologic study in sheep. J. Shoulder Elb. Surg. 2007, 16, 115–121. [Google Scholar] [CrossRef]
- Smith, M.J.; Bozynski, C.C.; Kuroki, K.; Cook, C.R.; Stoker, A.M.; Cook, J.L. Comparison of biologic scaffolds for augmentation of partial rotator cuff tears in a canine model. J. Shoulder Elb. Surg. 2020, 29, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Pfeiffer, F.M.; Cook, C.R.; Kuroki, K.; Cook, J.L. Rotator cuff healing using demineralized cancellous bone matrix sponge interposition compared to standard repair in a preclinical canine model. J. Orthop. Res. 2017, 36, 906–912. [Google Scholar] [CrossRef]
- Nicholson, G.P.; Breur, G.J.; Van Sickle, D.; Yao, J.Q.; Kim, J.; Blanchard, C.R. Evaluation of a cross-linked acellular porcine dermal patch for rotator cuff repair augmentation in an ovine model. J. Shoulder Elb. Surg. 2007, 16, S184–S190. [Google Scholar] [CrossRef]
- Pulatkan, A.; Anwar, W.; Ayık, O.; Bozdag, E.; Yildirim, A.N.; Kapicioglu, M.; Tuncay, I.; Bilsel, K. Tear Completion Versus In Situ Repair for 50% Partial-Thickness Bursal-Side Rotator Cuff Tears: A Biomechanical and Histological Study in an Animal Model. Am. J. Sports Med. 2020, 48, 1818–1825. [Google Scholar] [CrossRef]
- Yea, J.-H.; Bae, T.S.; Kim, B.J.; Cho, Y.W.; Jo, C.H. Regeneration of the rotator cuff tendon-to-bone interface using umbilical cord-derived mesenchymal stem cells and gradient extracellular matrix scaffolds from adipose tissue in a rat model. Acta Biomater. 2020, 114, 104–116. [Google Scholar] [CrossRef]
- Roßbach, B.P.; Gülecyüz, M.F.; Kempfert, L.; Pietschmann, M.F.; Ullamann, T.; Ficklscherer, A.; Niethammer, T.R.; Zhang, A.; Klar, R.M.; Müller, P.E. Rotator Cuff Repair with Au-tologous Tenocytes and Biodegradable Collagen Scaffold: A Histological and Biomechanical Study in Sheep. Am. J. Sports Med. 2020, 48, 450–459. [Google Scholar] [CrossRef]
- Ji, X.; Chen, Q.; Thoreson, A.R.; Qu, J.; An, K.N.; Amadio, P.C.; Zhao, C. Rotator cuff repair with a tendon-fibrocartilage-bone composite bridging patch. Clin. Biomech. 2015, 30, 976–980. [Google Scholar] [CrossRef]
- Zhang, T.; Hatta, T.; Thoreson, A.R.; Lu, C.; Steinmann, S.P.; Moran, S.L.; Zhao, C. Rotator cuff repair with a novel mesh suture: An ex vivo assessment of mechanical properties. J. Orthop. Res. 2017, 36, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Newton, M.D.; Davidson, A.A.; Pomajzl, R.; Seta, J.; Kurdziel, M.D.; Maerz, T. The influence of testing angle on the biomechanical properties of the rat supraspinatus tendon. J. Biomech. 2016, 49, 4159–4163. [Google Scholar] [CrossRef] [PubMed]
- Mathewson, M.A.; Kwan, A.; Eng, C.M.; Lieber, R.L.; Ward, S.R. Comparison of rotator cuff muscle architecture between humans and other selected vertebrate species. J. Exp. Biol. 2013, 217, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-W.; Kim, D.-H.; Kang, S.-H.; Lee, J.-H. Arthroscopic modified Mason-Allen technique for large U- or L-shaped rotator cuff tears. Knee Surg. Sports Traumatol. Arthrosc. 2016, 25, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Yang, D.S.; Lee, G.S.; Ma, C.H.; Choy, W.S. Clinical outcomes and repair integrity after arthroscopic full-thickness rotator cuff repair: Suture-bridge versus double-row modified Mason-Allen technique. J. Shoulder Elb. Surg. 2018, 27, 1953–1959. [Google Scholar] [CrossRef]
- Ji, X.; Bao, N.; An, K.-N.; Amadio, P.C.; Steinmann, S.P.; Zhao, C. A Canine Non-Weight-Bearing Model with Radial Neurectomy for Rotator Cuff Repair. PLoS ONE 2015, 10, e0130576. [Google Scholar] [CrossRef]
- Derwin, K.A.; Galatz, L.M.; Ratcliffe, A.; Thomopoulos, S. Enthesis Repair: Challenges and Opportunities for Effective Ten-don-to-Bone Healing. J. Bone Jt. Surg. Am. 2018, 100, e109. [Google Scholar] [CrossRef]
- Sharma, P.; Maffulli, N. Biology of tendon injury: Healing, modeling and remodeling. J. Musculoskelet. Neuronal Interact. 2006, 6, 181–190. [Google Scholar]
- Miller, K.S.; Connizzo, B.K.; Feeney, E.; Soslowsky, L.J. Characterizing local collagen fiber re-alignment and crimp behavior throughout mechanical testing in a mature mouse supraspinatus tendon model. J. Biomech. 2012, 45, 2061–2065. [Google Scholar] [CrossRef]
- Miller, K.S.; Edelstein, L.; Connizzo, B.K.; Soslowsky, L.J. Effect of Preconditioning and Stress Relaxation on Local Collagen Fiber Re-Alignment: Inhomogeneous Properties of Rat Supraspinatus Tendon. J. Biomech. Eng. 2012, 134, 031007. [Google Scholar] [CrossRef]
- Gomoll, A.H.; Katz, J.N.; Warner, J.J.P.; Millett, P.J. Rotator cuff disorders: Recognition and management among patients with shoulder pain. Arthritis Rheum. 2004, 50, 3751–3761. [Google Scholar] [CrossRef] [PubMed]
- Rothrauff, B.B.; Pauyo, T.; Debski, R.E.; Rodosky, M.W.; Tuan, R.S.; Musahl, V. The Rotator Cuff Organ: Integrating Developmental Biology, Tissue Engineering, and Surgical Considerations to Treat Chronic Massive Rotator Cuff Tears. Tissue Eng. Part B Rev. 2017, 23, 318–335. [Google Scholar] [CrossRef] [PubMed]
| Intact | Repaired | |||
|---|---|---|---|---|
| Failure Modes | Functional (n = 10) | Anatomic (n = 10) | Functional (n = 8) | Anatomic (n = 8) |
| Bone avulsion at insertion | 0 | 7 | - | - |
| Proximal bone fracture | 0 | 3 | - | - |
| Soft tissue & | 10 | 0 | - | - |
| Suture pullout from tendon | - | - | 1 | 4 |
| Suture pullout from bone | - | - | 3 | 2 |
| Suture break | - | - | 4 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Qi, J.; Zhu, W.; Thoreson, A.R.; An, K.-N.; Steinmann, S.P.; Zhao, C. The Effect of Pulling Angle on Rotator Cuff Mechanical Properties in a Canine In Vitro Model. Bioengineering 2023, 10, 599. https://doi.org/10.3390/bioengineering10050599
Liu Q, Qi J, Zhu W, Thoreson AR, An K-N, Steinmann SP, Zhao C. The Effect of Pulling Angle on Rotator Cuff Mechanical Properties in a Canine In Vitro Model. Bioengineering. 2023; 10(5):599. https://doi.org/10.3390/bioengineering10050599
Chicago/Turabian StyleLiu, Qian, Jun Qi, Weihong Zhu, Andrew R. Thoreson, Kai-Nan An, Scott P. Steinmann, and Chunfeng Zhao. 2023. "The Effect of Pulling Angle on Rotator Cuff Mechanical Properties in a Canine In Vitro Model" Bioengineering 10, no. 5: 599. https://doi.org/10.3390/bioengineering10050599
APA StyleLiu, Q., Qi, J., Zhu, W., Thoreson, A. R., An, K.-N., Steinmann, S. P., & Zhao, C. (2023). The Effect of Pulling Angle on Rotator Cuff Mechanical Properties in a Canine In Vitro Model. Bioengineering, 10(5), 599. https://doi.org/10.3390/bioengineering10050599








