A Proposal for the Classification of Temporomandibular Joint Disc Deformity in Hemifacial Microsomia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CT, 3D Reconstruction, and MRI
2.3. Statistical Analysis
3. Results
3.1. Classification of the OMENS+C
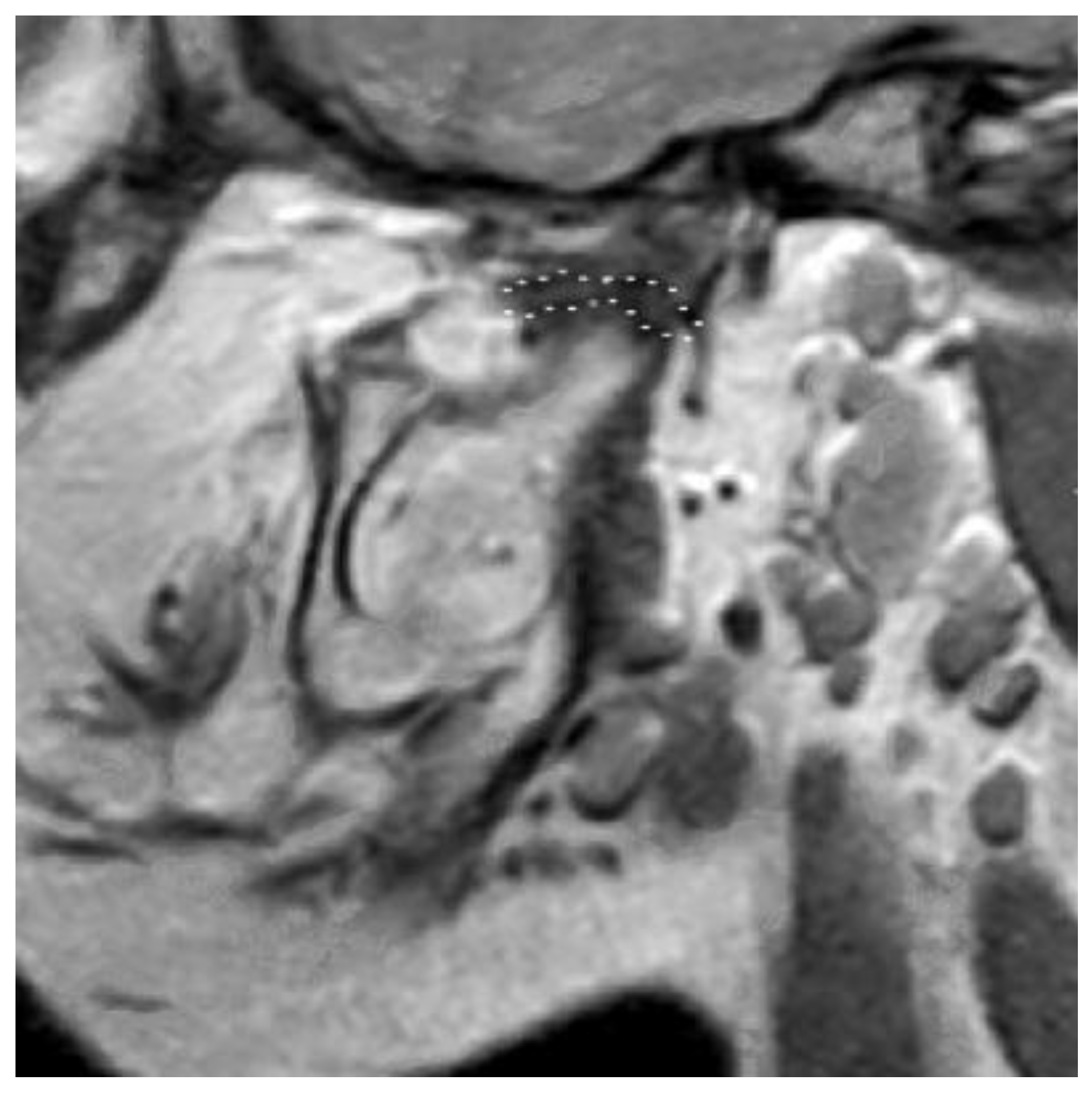
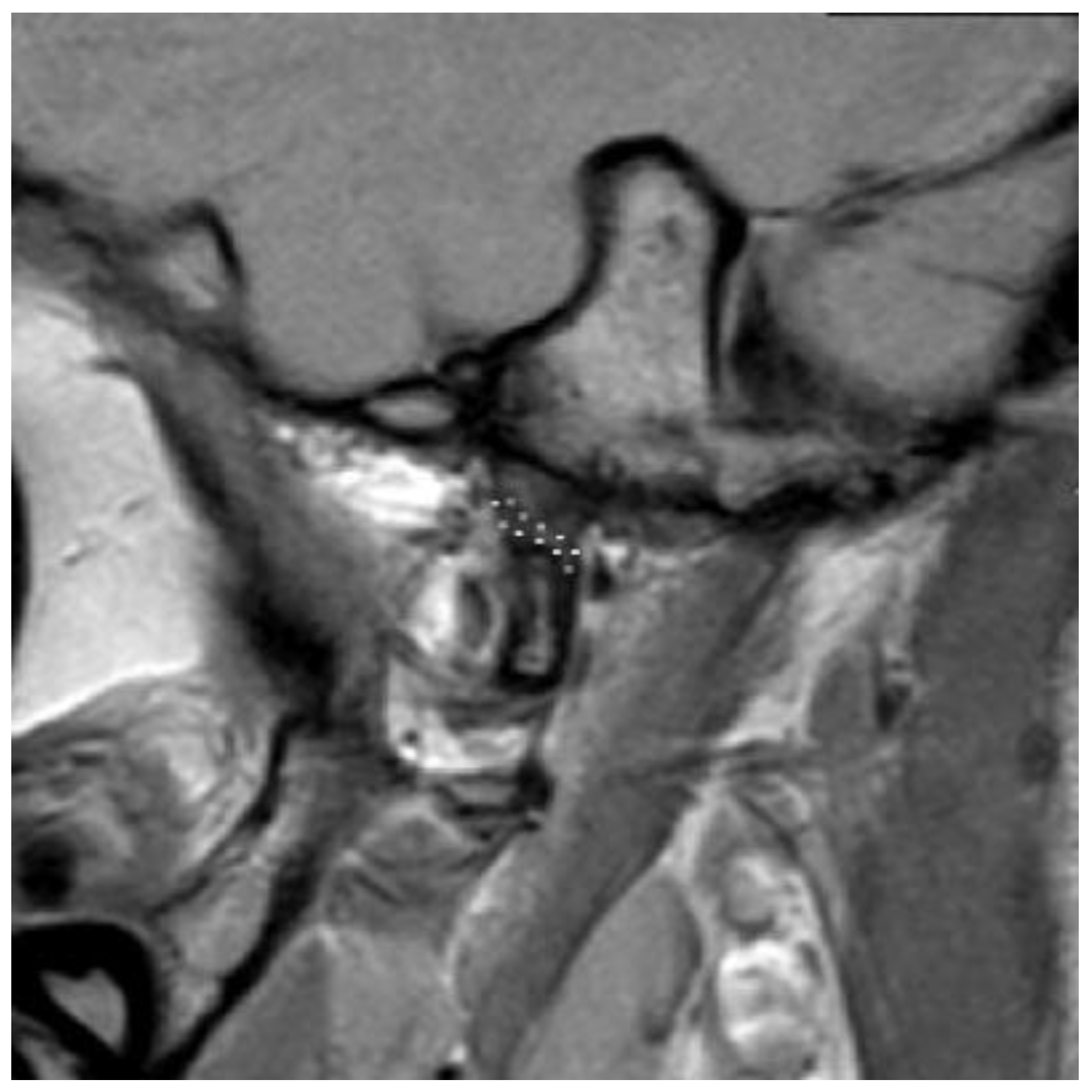
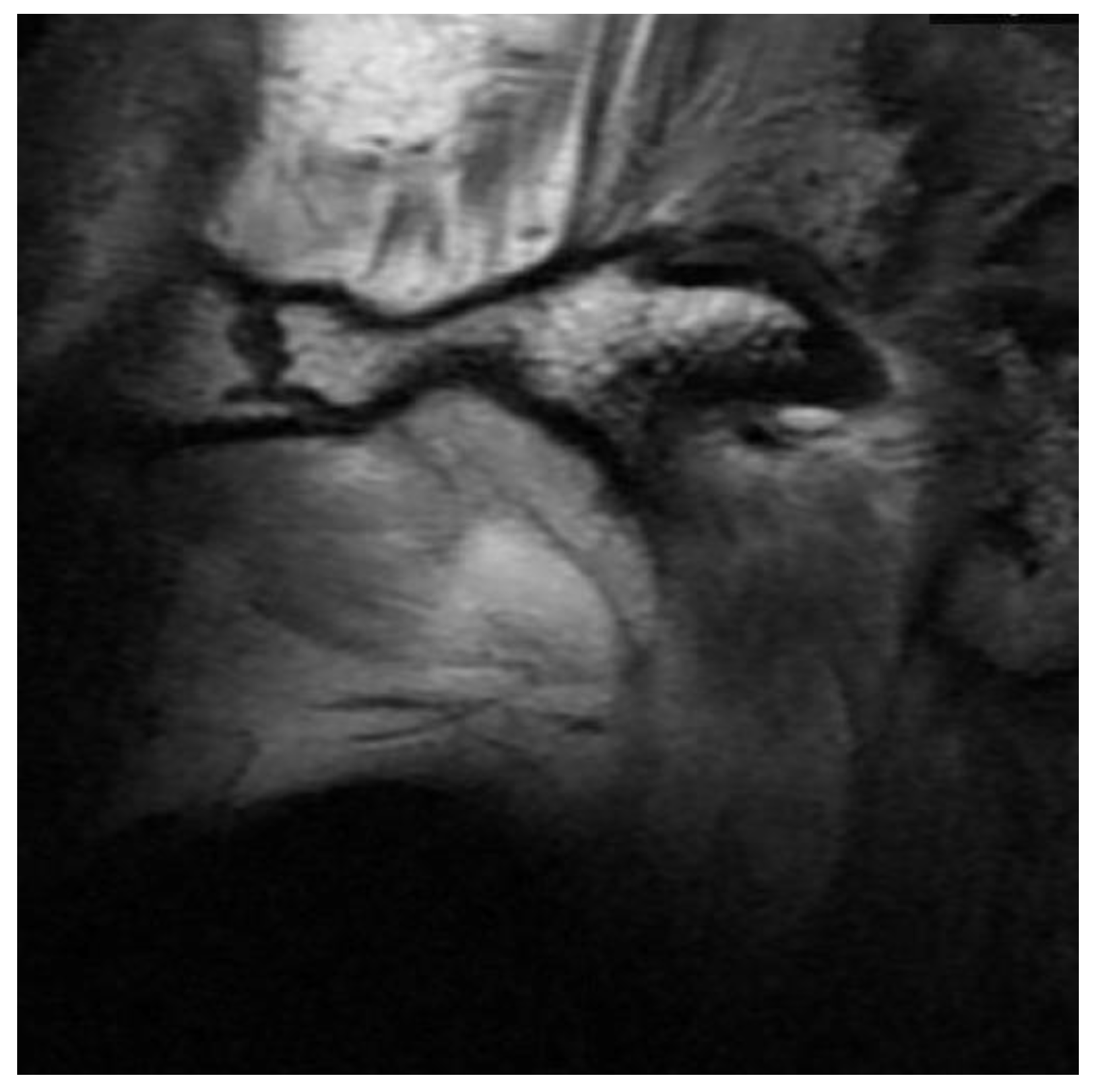
3.2. Definition of Disc Classification
3.3. Sample Distribution
3.4. Correlation between OMENS+C and Disc Classifications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gougoutas, A.J.; Singh, D.J.; Low, D.W.; Bartlett, S.P. Hemifacial Microsomia: Clinical Features and Pictographic Representations of the OMENS Classification System. Plast. Reconstr. Surg. 2007, 120, 112e–120e. [Google Scholar] [CrossRef] [PubMed]
- Hartsfield, J.K. Review of the etiologic heterogeneity of the oculo-auriculo-vertebral spectrum (Hemifacial Microsomia). Orthod. Craniofac. Res. 2007, 10, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Tuin, A.J.; Tahiri, Y.; Paine, K.M.; Paliga, J.T.; Taylor, J.A.; Bartlett, S.P. Clarifying the Relationships among the Different Features of the OMENS+ Classification in Craniofacial Microsomia. Plast. Reconstr. Surg. 2015, 135, 149e–156e. [Google Scholar] [CrossRef] [PubMed]
- Bogusiak, K.; Puch, A.; Arkuszewski, P. Goldenhar syndrome: Current perspectives. World J. Pediatr. 2017, 13, 405–415. [Google Scholar] [CrossRef]
- Horgan, J.E.; Padwa, B.L.; Labrie, R.A.; Mulliken, J.B. OMENS-Plus: Analysis of Craniofacial and Extracraniofacial Anomalies in Hemifacial Microsomia. Cleft Palate-Craniofac. J. 1995, 32, 405–412. [Google Scholar] [CrossRef]
- Pierpont, M.E.M.; Moller, J.H.; Gorlin, R.J.; Edwards, J.E. Congenital cardiac, pulmonary, and vascular malformations in oculoauriculovertebral dysplasia. Pediatr. Cardiol. 1982, 2, 297–302. [Google Scholar] [CrossRef]
- David, D.J.; Mahatumarat, C.; Cooter, R.D. Hemifacial microsomia—A multisystem classification. Plast. Reconstr. Surg. 1987, 80, 525–535. [Google Scholar] [CrossRef]
- Rollnick, B.R.; Kaye, C.I.; Nagatoshi, K.; Hauck, W.; Martin, A.O.; Reynolds, J.F. Oculoauriculovertebral dysplasia and variants: Phenotypic characteristics of 294 patients. Am. J. Med Genet. 1987, 26, 361–375. [Google Scholar] [CrossRef]
- Birgfeld, C.; Heike, C. Craniofacial Microsomia. Clin. Plast. Surg. 2019, 46, 207–221. [Google Scholar] [CrossRef]
- Pruzansky, S. Not all dwarfed mandibles are alike. Birth Defects 1969, 1, 120–129. [Google Scholar]
- Kaban, L.B.; Moses, M.H.; Mulliken, J.B. Surgical correction of hemifacial microsomia in the growing child. Plast. Reconstr. Surg. 1988, 82, 9–19. [Google Scholar] [CrossRef]
- Lauritzen, C.; Munro, I.R.; Ross, R.B. Classification and treatment of hemifacial microsomia. Scand. J. Plast. Reconstr. Surg. 1985, 19, 33–39. [Google Scholar] [CrossRef]
- Meurman, Y. Congenital Microtia and Meatal Atresia: Observations and Aspects of Treatment. AMA Arch. Otolaryngol. Neck Surg. 1957, 66, 443–463. [Google Scholar] [CrossRef]
- Murray, J.E.; Kaban, L.B.; Mulliken, J.B. Analysis and Treatment of Hemifacial Microsomia. Plast. Reconstr. Surg. 1984, 74, 186–199. [Google Scholar] [CrossRef]
- Vento, A.R.; LaBrie, R.A.; Mulliken, J.B. The O.M.E.N.S. classification of hemifacial microsomia. Cleft Palate Craniofac. J. 1991, 28, 68–76. [Google Scholar] [CrossRef]
- Birgfeld, C.B.; Heike, C. Craniofacial microsomia. Semin. Plast. Surg. 2012, 26, 91–104. [Google Scholar] [CrossRef]
- Brandstetter, K.A.; Patel, K.G. Craniofacial Microsomia. Facial. Plast. Surg. Clin. N. Am. 2016, 24, 495–515. [Google Scholar] [CrossRef]
- Nebbe, B.; Major, P.W.; Prasad, N.G.N.; Hatcher, D. Quantitative assessment of temporomandibular joint disk status. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 598–607. [Google Scholar] [CrossRef]
- Xie, Q.; Yang, C.; He, D.; Cai, X.; Ma, Z. Is mandibular asymmetry more frequent and severe with unilateral disc displacement? J. Craniomaxillofac. Surg. 2015, 43, 81–86. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, J.; Li, B.; Liu, Z.; Shen, S.; Wang, X. Accuracy of a new custom-made bone-supported osteotomy and repositioning guide system for reconstruction of the mandibular ramus using costochondral grafts: A preliminary study. Br. J. Oral Maxillofac. Surg. 2020, 58, 51–56. [Google Scholar] [CrossRef]
- Li, B.; Sun, H.; Zeng, F.; Zhang, T.; Wang, X. Accuracy of a CAD/CAM surgical template for mandibular distraction: A preliminary study. Br. J. Oral Maxillofac. Surg. 2018, 56, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.K.; Yang, C.; Xie, Q.Y. Changes in disc status in the reducing and nonreducing anterior disc displacement of temporomandibular joint: A longitudinal retrospective study. Sci. Rep. 2016, 6, 34253. [Google Scholar] [CrossRef] [PubMed]
- Kitai, N.; Murakami, S.; Takashima, M.; Furukawa, S.; Kreiborg, S.; Takada, K. Evaluation of Temporomandibular Joint in Patients With Hemifacial Microsomia. Cleft Palate Craniofac. J. 2004, 41, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Al-Moraissi, E.A.; Wolford, L.M. Does Temporomandibular Joint Pathology with or without Surgical Management Affect the Stability of Counterclockwise Rotation of the Maxillomandibular Complex in Orthognathic Surgery? A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2017, 75, 805–821. [Google Scholar] [CrossRef]
- Goncalves, J.R.; Cassano, D.S.; Wolford, L.M.; Santos-Pinto, A.; Marquez, I.M. Postsurgical stability of counterclockwise maxillomandibular advancement surgery: Affect of articular disc repositioning. J. Oral Maxillofac. Surg. 2008, 66, 724–738. [Google Scholar] [CrossRef]
- Wan, J.; Meara, J.G.; Kovanlikaya, A.; Nelson, M.D.; Don, D. Clinical, Radiological, and Audiological Relationships in Hemifacial Microsomia. Ann. Plast. Surg. 2003, 51, 161–166. [Google Scholar] [CrossRef]
- Park, J.U.; Do, T.H.; Kwon, G.Y.; Choi, T.H.; Kim, S. Statistical analysis using the OMENS classification in Oriental patients with hemifacial microsomia: A comparative analysis with Western centers. Ann. Plast. Surg. 2014, 72, 50–55. [Google Scholar] [CrossRef]
- Gillies, H.D. Plastic surgery of the face. Lancet 1920, 196, 177–192. [Google Scholar] [CrossRef]
- Klein, C.; Howaldt, H.-P. Correction of Mandibular Hypoplasia by Means of Bidirectional Callus Distraction. J. Craniofac. Surg. 1996, 7, 258–266. [Google Scholar] [CrossRef]
- McCarthy, J.G.; Stelnicki, E.J.; Mehrara, B.J.; Longaker, M.T. Distraction Osteogenesis of the Craniofacial Skeleton. Plast. Reconstr. Surg. 2001, 107, 1812–1824. [Google Scholar] [CrossRef]
- Mommaerts, M.Y.; Nagy, K. Is early osteodistraction a solution for the ascending ramus compartment in hemifacial microsomia? A literature study. J. Cranio-Maxillofac. Surg. 2002, 30, 201–207. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Zhang, Z.; Li, X.; Ye, B.; Li, J. Comprehensive consideration and design with the virtual surgical planning-assisted treatment for hemifacial microsomia in adult patients. J. Cranio-Maxillofacial Surg. 2018, 46, 1268–1274. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, Y.; Shen, G.; Dai, J. Etiology and Pathogenesis of Hemifacial Microsomia. J. Dent. Res. 2018, 97, 1297–1305. [Google Scholar] [CrossRef]
- Zielinski, D.; Markus, B.; Sheikh, M.; Gymrek, M.; Chu, C.; Zaks, M.; Srinivasan, B.; Hoffman, J.D.; Aizenbud, D.; Erlich, Y. OTX2 Duplication Is Implicated in Hemifacial Microsomia. PLoS ONE 2014, 9, e96788. [Google Scholar] [CrossRef]
- Digilio, M.C.; McDonald-McGinn, D.M.; Heike, C.; Catania, C.; Dallapiccola, B.; Marino, B.; Zackai, E.H. Three patients with oculo-auriculo-vertebral spectrum and microdeletion 22q11.2. Am. J. Med Genet. Part A 2009, 149A, 2860–2864. [Google Scholar] [CrossRef]
- Xu, J.; Fan, Y.S.; Siu, V.M. A child with features of Goldenhar syndrome and a novel 1.12 Mb deletion in 22q11.2 by cytogenetics and oligonucleotide array CGH: Is this a candidate region for the syndrome? Am. J. Med. Genet. A 2008, 146A, 1886–1889. [Google Scholar] [CrossRef]
- Tan, T.Y.; Collins, A.; James, P.; McGillivray, G.; Stark, Z.; Gordon, C.T.; Leventer, R.J.; Pope, K.; Forbes, R.; Crolla, J.A.; et al. Phenotypic variability of distal 22q11.2 copy number abnormalities. Am. J. Med Genet. Part A 2011, 155, 1623–1633. [Google Scholar] [CrossRef]
- Timberlake, A.T.; Griffin, C.; Heike, C.L.; Hing, A.V.; Cunningham, M.L.; Chitayat, D.; Davis, M.R.; Doust, S.J.; Drake, A.F.; Duenas-Roque, M.M.; et al. Haploinsufficiency of SF3B2 causes craniofacial microsomia. Nat. Commun. 2021, 12, 4680. [Google Scholar] [CrossRef]
- Wang, Y.; Ping, L.; Luan, X.; Chen, Y.; Fan, X.; Li, L.; Liu, Y.; Wang, P.; Zhang, S.; Zhang, B.; et al. A Mutation in VWA1, Encoding von Willebrand Factor A Domain-Containing Protein 1, Is Associated with Hemifacial Microsomia. Front. Cell Dev. Biol. 2020, 8, 571004. [Google Scholar] [CrossRef]
- Su, P.-H.; Liu, Y.-F.; Yu, J.-S.; Chen, J.-Y.; Chen, S.-J.; Lai, Y.-J. Facial asymmetry and clinical manifestations in patients with novel insertion of theTCOF1gene. Clin. Genet. 2012, 82, 460–465. [Google Scholar] [CrossRef]
- Naora, H.; Kimura, M.; Otani, H.; Yokoyama, M.; Koizumi, T.; Katsuki, M.; Tanaka, O. Transgenic Mouse Model of Hemifacial Microsomia: Cloning and Characterization of Insertional Mutation Region on Chromosome 10. Genomics 1994, 23, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Cousley, R.; Naora, H.; Yokoyama, M.; Kimura, M.; Otani, H. Validity of theHfmTransgenic Mouse as a Model for Hemifacial Microsomia. Cleft Palate-Craniofac. J. 2002, 39, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Gendron-Maguire, M.; Mallo, M.; Zhang, M.; Gridley, T. Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell 1993, 75, 1317–1331. [Google Scholar] [CrossRef]
- Santagati, F.; Minoux, M.; Ren, S.-Y.; Rijli, F.M. Temporal requirement of Hoxa2 in cranial neural crest skeletal morphogenesis. Development 2005, 132, 4927–4936. [Google Scholar] [CrossRef] [PubMed]
- Quiat, D.; Timberlake, A.T.; Curran, J.J.; Cunningham, M.L.; McDonough, B.; Artunduaga, M.A.; DePalma, S.R.; Duenas-Roque, M.M.; Gorham, J.M.; Gustafson, J.A.; et al. Damaging variants in FOXI3 cause microtia and craniofacial microsomia. Genet Med. 2023, 25, 143–150. [Google Scholar] [CrossRef]
- Luquetti, D.V.; Heike, C.L.; Zarante, I.; Timms, A.E.; Gustafson, J.; Pachajoa, H.; Porras-Hurtado, G.L.; Ayala-Ramirez, P.; Duenas-Roque, M.M.; Jimenez, N.; et al. MYT1 role in the microtia-craniofacial microsomia spectrum. Mol. Genet. Genomic Med. 2020, 8, e1401. [Google Scholar] [CrossRef]
- Poswillo, D. The pathogenesis of the first and second branchial arch syndrome. Oral Surg. Oral Med. Oral Pathol. 1973, 35, 302–328. [Google Scholar] [CrossRef]

| O (Orbit) | |
| O0 | normal orbital size and position |
| O1 | abnormal orbital size |
| O2↓ | inferior orbital displacement |
| O2↑ | superior orbital displacement |
| O3 | abnormal orbital size and position |
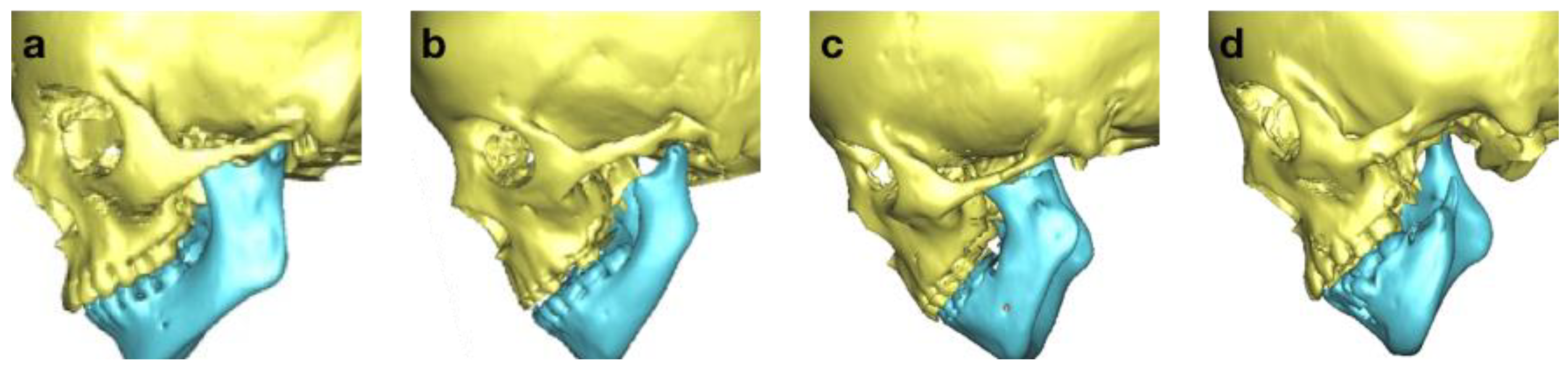
| M (Mandible) | |
| M0 | normal mandible |
| M1 | small mandible and glenoid fossa with short ramus |
| M2a | abnormally shaped and short ramus (glenoid fossa in acceptable position) |
| M2b | abnormally shaped and short ramus (glenoid fossa is inferiorly, medially, and anteriorly displaced with severe hypoplasia of condyle) |
| M3 | absence of ramus and glenoid fossa |
| E (Ear) | |
| E0 | normal auricle |
| E1 | mild hypoplasia and cupping with presence of all structures |
| E2 | absence of external canal with variable hypoplasia of concha |
| E3 | malpositioned lobule with absent auricle; lobular remnant typically inferiorly and anteriorly displaced |
| N (Nerve) | |
| N0 | no facial nerve involvement |
| N1 | temporal and/or zygomatic branch involvement |
| N2 | buccal and/or mandibular and/or cervical branch involvement |
| N3 | all branches affected |
| S (Soft tissue) | |
| S0 | no soft tissue deficiency |
| S1 | minimal soft tissue deficiency |
| S2 | moderate soft tissue deficiency (between S1 and S3) |
| S3 | severe soft tissue deficiency |
| C (Macrostomia/Cleft) | |
| C0 | no cleft |
| C1 | cleft terminates medially to anterior border of masseter |
| C2 | cleft terminates laterally to anterior border of masseter |
| Orbit | n | % |
|---|---|---|
| O0 | 35 | 32.4% |
| O1 | 24 | 22.2% |
| O2 | 33 | 30.6% |
| O3 | 16 | 14.8% |
| Total | 108 | |
| Mandible | n | % |
| M1 | 15 | 13.9% |
| M2a | 35 | 32.4% |
| M2b | 45 | 41.7% |
| M3 | 13 | 12.0% |
| Total | 108 | |
| Ear | n | % |
| E0 | 36 | 33.3% |
| E1 | 12 | 11.1% |
| E2 | 27 | 25.0% |
| E3 | 33 | 30.6% |
| Total | 108 | |
| Nerve | n | % |
| N0 | 84 | 77.8% |
| N1 | 10 | 9.3% |
| N2 | 11 | 10.2% |
| N3 | 3 | 2.8% |
| Total | 108 | |
| Soft tissue | n | % |
| S0 | 30 | 27.8% |
| S1 | 41 | 38.0% |
| S2 | 31 | 28.7% |
| S3 | 6 | 5.6% |
| Total | 108 | |
| Cleft | n | % |
| C0 | 78 | 72.2% |
| C1 | 21 | 19.4% |
| C2 | 9 | 8.3% |
| Total | 108 | |
| Disc | n | % |
| D0 | 31 | 28.7% |
| D1 | 17 | 15.7% |
| D2 | 36 | 33.3% |
| D3 | 24 | 22.2% |
| Total | 108 |
| Disc | Orbit | Mandible | Ear | Nerve | Soft Tissue | |
|---|---|---|---|---|---|---|
| Orbit | −0.016 | |||||
| Mandible | 0.614 ** | 0.099 | ||||
| Ear | 0.242 * | 0.122 | 0.137 | |||
| Nerve | −0.056 | 0.106 | −0.025 | 0.234 | ||
| Soft tissue | 0.291 ** | 0.245 * | 0.332 ** | 0.340 ** | 0.166 | |
| Cleft | 0.320 ** | −0.012 | 0.332 ** | −0.032 | 0.036 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, X.; Liu, Z.; Wei, H.; Wang, X. A Proposal for the Classification of Temporomandibular Joint Disc Deformity in Hemifacial Microsomia. Bioengineering 2023, 10, 595. https://doi.org/10.3390/bioengineering10050595
Xue X, Liu Z, Wei H, Wang X. A Proposal for the Classification of Temporomandibular Joint Disc Deformity in Hemifacial Microsomia. Bioengineering. 2023; 10(5):595. https://doi.org/10.3390/bioengineering10050595
Chicago/Turabian StyleXue, Xiaochen, Zhixu Liu, Hongpu Wei, and Xudong Wang. 2023. "A Proposal for the Classification of Temporomandibular Joint Disc Deformity in Hemifacial Microsomia" Bioengineering 10, no. 5: 595. https://doi.org/10.3390/bioengineering10050595
APA StyleXue, X., Liu, Z., Wei, H., & Wang, X. (2023). A Proposal for the Classification of Temporomandibular Joint Disc Deformity in Hemifacial Microsomia. Bioengineering, 10(5), 595. https://doi.org/10.3390/bioengineering10050595







