Non-Invasive Hybrid Ultrasound Stimulation of Visual Cortex In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Preparation
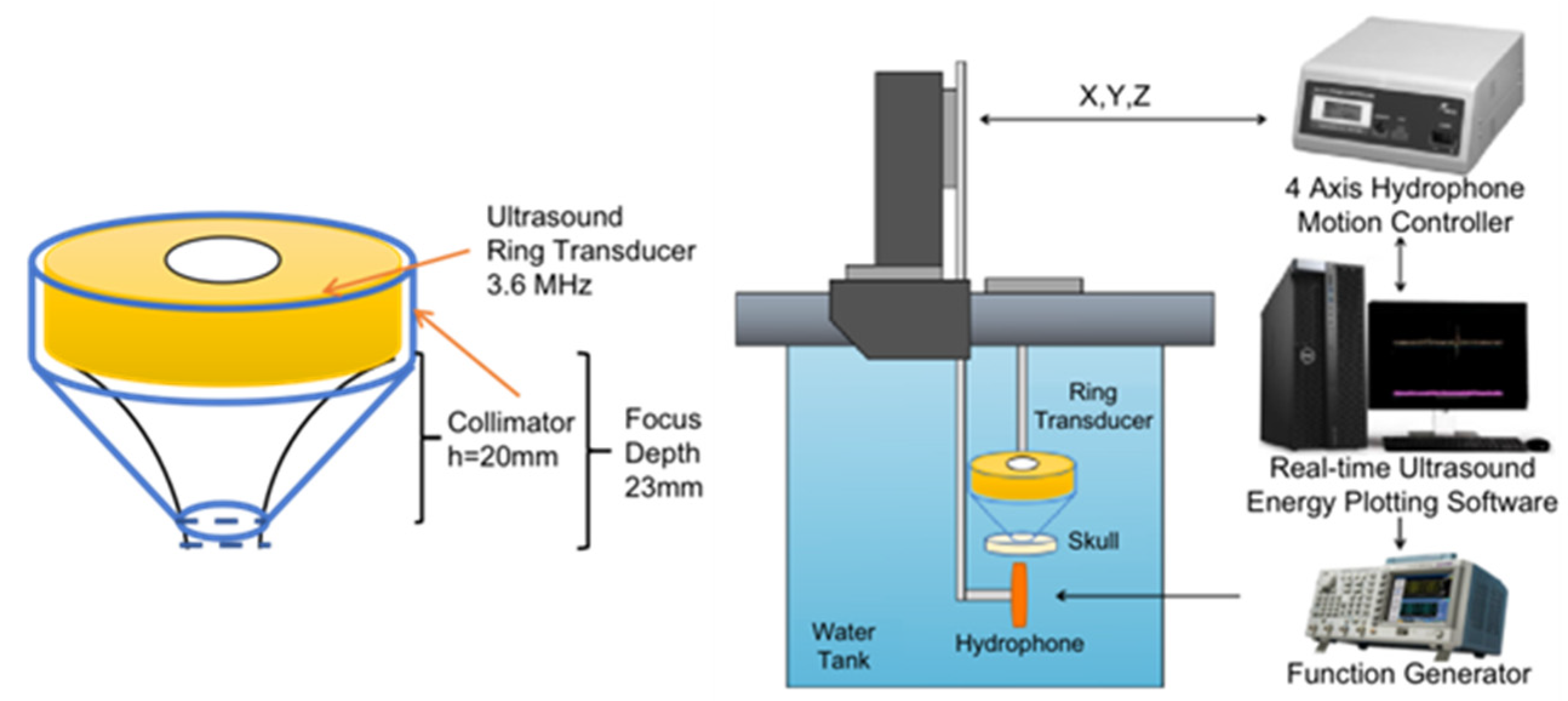
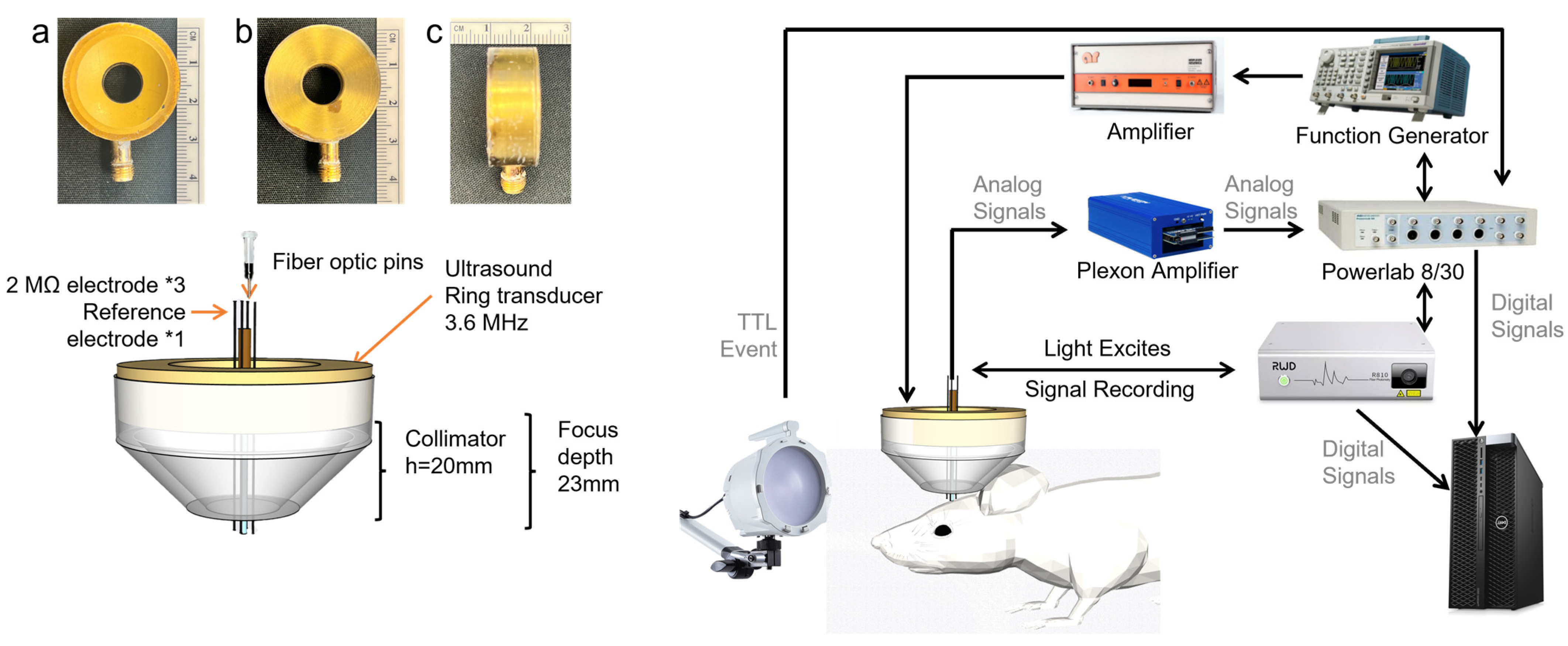
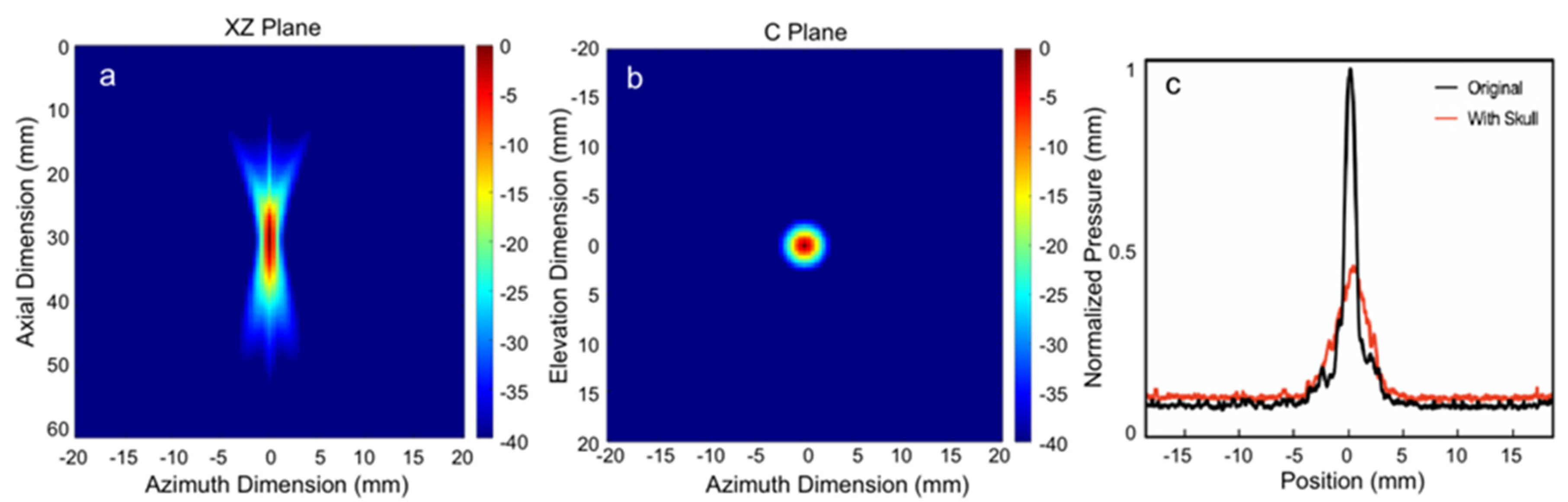
2.2. Transducer Design and Acoustic Field with Skull
2.3. The System Setup of Fiber Photometry Signal Acquisition and Electrophysiological Recordings
2.3.1. Fiber Photometry Signal Acquisition
2.3.2. Electrophysiological Signal Acquisition
2.4. Light and Ultrasound Stimulation and Signal Acquisition
3. Results
3.1. Acoustic Intensity Measurement
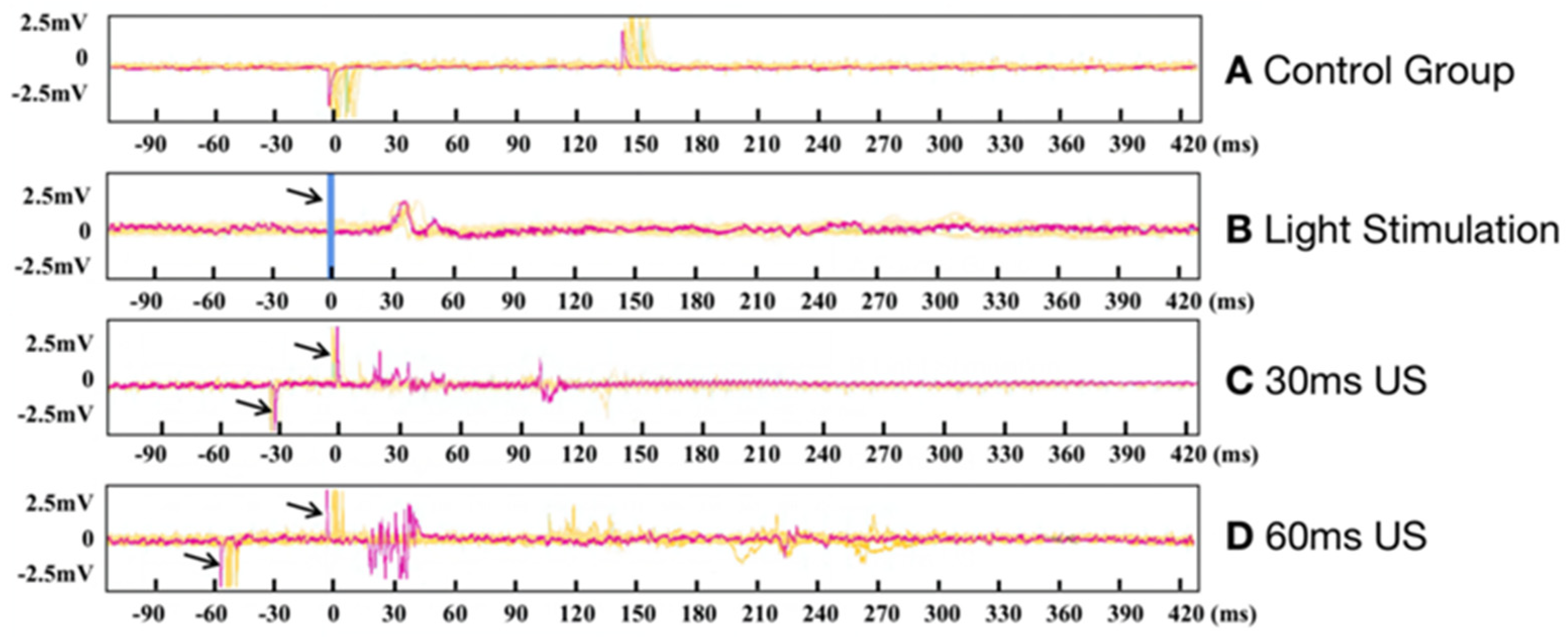
3.2. Electrophysiological Results of Ring Transducer Stimulation of the Visual Cortex
3.3. Photometry Results of Ring Transducer Stimulation of the Visual Cortex
4. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Curcio, C.A.; Medeiros, N.E.; Millican, C.L. Photoreceptor loss in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1236–1249. [Google Scholar]
- Riedl, S.; Cooney, L.; Grechenig, C.; Sadeghipour, A.; Pablik, E.; Seaman, J.W., 3rd; Waldstein, S.M.; Schmidt-Erfurth, U. Topographic Analysis of Photoreceptor Loss Correlated with Disease Morphology in Neovascular Age-Related Macular Degeneration. Retina 2020, 40, 2148–2157. [Google Scholar] [CrossRef] [PubMed]
- Krzyzanowska, I.; Toteberg-Harms, M. Dynamic properties of the eye could contribute to angle-closure glaucoma in addition to anatomy. Die Ophthalmol. 2023, 120, 330–331. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, M.; Virdee, J.; Giridharan, S.; Chavda, S.V.; Batra, R. Response of Recoverin-Positive Optic Neuritis to Chemotherapy, Steroid, and Plasma Exchange. J. Neuroophthalmol. 2022, 10, 1097. [Google Scholar] [CrossRef]
- Patel, A.U.; Patel, U.S.; May, E.F. Posterior Ischemic Optic Neuropathy Because of Hematologic Malignancy. J. Neuroophthalmol. 2022, 10, 1097. [Google Scholar] [CrossRef]
- Li, R.; Qian, X.; Gong, C.; Zhang, J.; Liu, Y.; Xu, B.; Humayun, M.S.; Zhou, Q. Simultaneous Assessment of the Whole Eye Biomechanics Using Ultrasonic Elastography. IEEE Trans. Biomed. Eng. 2022, 70, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Yu, K.; He, B. Transcranial focused ultrasound induces sustained synaptic plasticity in rat hippocampus. Brain Stimul. 2022, 15, 352–359. [Google Scholar] [CrossRef]
- Qian, X.; Li, R.; Lu, G.; Jiang, L.; Kang, H.; Kirk Shung, K.; Humayun, M.S.; Zhou, Q. Ultrasonic elastography to assess biomechanical properties of the optic nerve head and peripapillary sclera of the eye. Ultrasonics 2021, 110, 106263. [Google Scholar] [CrossRef]
- Qian, X.; Li, R.; Li, Y.; Lu, G.; He, Y.; Humayun, M.S.; Chen, Z.; Zhou, Q. In vivo evaluation of posterior eye elasticity using shaker-based optical coherence elastography. Exp. Biol. Med. 2020, 245, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Humayun, M.S.; Dorn, J.D.; Ahuja, A.K.; Caspi, A.; Filley, E.; Dagnelie, G.; Salzmann, J.; Santos, A.; Duncan, J.; daCruz, L.; et al. Preliminary 6 month results from the Argus II epiretinal prosthesis feasibility study. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 4566–4568. [Google Scholar] [CrossRef]
- da Cruz, L.; Dorn, J.D.; Humayun, M.S.; Dagnelie, G.; Handa, J.; Barale, P.O.; Sahel, J.A.; Stanga, P.E.; Hafezi, F.; Safran, A.B.; et al. Five-Year Safety and Performance Results from the Argus II Retinal Prosthesis System Clinical Trial. Ophthalmology 2016, 123, 2248–2254. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, G.; Zeng, Y.; Sun, Y.; Kang, H.; Burford, J.; Gong, C.; Humayun, M.S.; Chen, Y.; Zhou, Q. Flexible ultrasound-induced retinal stimulating piezo-arrays for biomimetic visual prostheses. Nat. Commun. 2022, 13, 3853. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Qian, X.; Gong, C.; Ji, J.; Thomas, B.B.; Humayun, M.S.; Zhou, Q. Ultrasound Retinal Stimulation: A Mini-Review of Recent Developments. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2022, 69, 3224–3231. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Chen, P.; Xu, Z.; Mo, X.; Jin, H.; Yang, W.; Wang, S.; Duan, J.; Hu, B.; Luo, Z.; et al. Hybrid-Piezoelectret Based Highly Efficient Ultrasonic Energy Harvester for Implantable Electronics. Adv. Funct. Mater. 2022, 32, 2200589. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Q.; Wan, X.; Yang, M.; Liu, C.; Xu, C.; Hu, B.; Feng, J.; Luo, Z. Wireless electrical stimulation of the vagus nerves by ultrasound-responsive programmable hydrogel nanogenerators for anti-inflammatory therapy in sepsis. Nano Energy 2021, 89, 106327. [Google Scholar] [CrossRef]
- Xu, Z.; Wan, X.; Mo, X.; Lin, S.; Chen, S.; Chen, J.; Pan, Y.; Zhang, H.; Jin, H.; Duan, J.; et al. Electrostatic Assembly of Laminated Transparent Piezoelectrets for Epidermal and Implantable Electronics. Nano Energy 2021, 89, 106450. [Google Scholar] [CrossRef]
- Foerster, O. Contributions to the pathophysiology of the visual pathway and visual sphere. J. Psychol. Neurol. 1929, 39, 435–463. [Google Scholar]
- Xiao, X.; Yin, J.; Shen, S.; Che, Z.; Wan, X.; Wang, S.; Chen, J. Advances in solid-state fiber batteries for wearable bioelectronics. Curr. Opin. Solid State Mater. Sci. 2022, 26, 101042. [Google Scholar] [CrossRef]
- Che, Z.; O’Donovan, S.; Xiao, X.; Wan, X.; Chen, G.; Zhao, X.; Zhou, Y.; Yin, J.; Chen, J. Implantable Triboelectric Nanogenerators for Self-Powered Cardiovascular Healthcare. Small 2023, 2207600. [Google Scholar] [CrossRef]
- Qian, X.; Lu, G.; Thomas, B.B.; Li, R.; Chen, X.; Shung, K.K.; Humayun, M.; Zhou, Q. Noninvasive Ultrasound Retinal Stimulation for Vision Restoration at High Spatiotemporal Resolution. BME Front. 2022, 2022, 9829316. [Google Scholar] [CrossRef]
- Lee, W.; Lee, S.D.; Park, M.Y.; Foley, L.; Purcell-Estabrook, E.; Kim, H.; Fischer, K.; Maeng, L.S.; Yoo, S.S. Image-Guided Focused Ultrasound-Mediated Regional Brain Stimulation in Sheep. Ultrasound Med. Biol. 2016, 42, 459–470. [Google Scholar] [CrossRef]
- Grill, W.M. Safety considerations for deep brain stimulation: Review and analysis. Expert Rev. Med. Devices 2005, 2, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Amon, A.; Alesch, F. Systems for deep brain stimulation: Review of technical features. J. Neural Transm. 2017, 124, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Hamilton, M., 2nd; Offutt, S.J.; Gloeckner, C.D.; Li, T.; Kim, Y.; Legon, W.; Alford, J.K.; Lim, H.H. Ultrasound Produces Extensive Brain Activation via a Cochlear Pathway. Neuron 2018, 98, 1020–1030.e4. [Google Scholar] [CrossRef]
- Lu, G.X.; Qian, X.J.; Castillo, J.; Li, R.Z.; Jiang, L.M.; Lu, H.T.; Shung, K.K.; Humayun, M.S.; Thomas, B.B.; Zhou, Q.F. Transcranial Focused Ultrasound for Noninvasive Neuromodulation of the Visual Cortex. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, K.; Zhang, Y.; Yan, J.; Wang, Z.; Wang, X.; Liu, M.; Li, X. The Effect of Low-Intensity Transcranial Ultrasound Stimulation on Neural Oscillation and Hemodynamics in the Mouse Visual Cortex Depends on Anesthesia Level and Ultrasound Intensity. IEEE Trans. Biomed. Eng. 2021, 68, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Provansal, M.; Labernède, G.; Joffrois, C.; Rizkallah, A.; Goulet, R.; Valet, M.; Deschamps, W.; Ferrari, U.; Chaffiol, A.; Dalkara, D.; et al. Functional ultrasound imaging of the spreading activity following optogenetic stimulation of the rat visual cortex. Sci. Rep. 2021, 11, 12603. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, Y.; Lan, L.; Ge, X.; Cheng, R.; Zhan, Y.; Chen, G.; Shi, L.; Wang, R.; Zheng, N.; et al. Optically-generated focused ultrasound for noninvasive brain stimulation with ultrahigh precision. Light Sci. Appl. 2022, 11, 321. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Zhao, J.; Roberts, N.; Kong, D.; Qi, X.; Xing, H.; Gong, Q. Transcranial magnetic stimulation (TMS) localization by co-registration of facial point clouds. Brain Stimul. 2023, 16, 79–81. [Google Scholar] [CrossRef]
- Bortoletto, M.; Veniero, D.; Julkunen, P.; Hernandez-Pavon, J.C.; Mutanen, T.P.; Zazio, A.; Bagattini, C. T4TE: Team for TMS-EEG to improve reproducibility through an open collaborative initiative. Brain Stimul. 2022, 16, 20–22. [Google Scholar] [CrossRef]
- Lee, W.; Kim, H.-C.; Jung, Y.; Chung, Y.A.; Song, I.-U.; Lee, J.-H.; Yoo, S.-S. Transcranial focused ultrasound stimulation of human primary visual cortex. Sci. Rep. 2016, 6, 34026. [Google Scholar] [CrossRef]
- Yoo, S.; Mittelstein, D.R.; Hurt, R.C.; Lacroix, J.; Shapiro, M.G. Focused ultrasound excites cortical neurons via mechanosensitive calcium accumulation and ion channel amplification. Nat. Commun. 2022, 13, 493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Murgoitio-Esandi, J.; Qian, X.; Li, R.; Gong, C.; Nankali, A.; Hao, L.; Xu, B.Y.; Kirk Shung, K.; Oberai, A.; et al. High-Frequency Ultrasound Elastography to Assess the Nonlinear Elastic Properties of the Cornea and Ciliary Body. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2022, 69, 2621–2629. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.B.; Aramant, R.B.; Sadda, S.R.; Seiler, M.J. Light response differences in the superior colliculus of albino and pigmented rats. Neurosci. Lett. 2005, 385, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, V.; Pawlak, V.; Wallace, D.J.; Kerr, J.N.D. Impact of visual callosal pathway is dependent upon ipsilateral thalamus. Nat. Commun. 2020, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hirschberg, A.W.; Xu, H.; Slingsby-Smith, Z.; Lecomte, A.; Scholten, K.; Song, D.; Meng, E. A Parylene Neural Probe Array for Multi-Region Deep Brain Recordings. J. Microelectromech. Syst. 2020, 29, 499–513. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.A.; Muller, A.; Hynynen, K. Ultrasound Insertion Loss of Rat Parietal Bone Appears to Be Proportional to Animal Mass at Submegahertz Frequencies. Ultrasound Med. Biol. 2011, 37, 1930–1937. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hynynen, K. Focusing of therapeutic ultrasound through a human skull: A numerical study. J. Acoust. Soc. Am. 1998, 104, 1705–1715. [Google Scholar] [CrossRef]
- Hynynen, K.; Jolesz, F.A. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med. Biol. 1998, 24, 275–283. [Google Scholar] [CrossRef]
- Qi, X.; Lyu, K.; Meng, L.; Li, C.; Zhang, H.; Niu, L.; Lin, Z.; Zheng, H.; Tang, J. Low-Intensity Ultrasound Causes Direct Excitation of Auditory Cortical Neurons. Neural Plast. 2021, 2021, 8855055. [Google Scholar] [CrossRef]
- Sato, T.; Shapiro, M.G.; Tsao, D.Y. Ultrasonic Neuromodulation Causes Widespread Cortical Activation via an Indirect Auditory Mechanism. Neuron 2018, 98, 1031–1041.e5. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Yan, S.M.; Ding, Y.; Zhou, C.; Qian, S.W.; Wang, Z.Q.; Gong, C.; Zhang, M.; Zhang, Y.J.; Zhao, Y.D.; et al. Anterior Auditory Field Is Needed for Sound Categorization in Fear Conditioning Task of Adult Rat. Front. Neurosci. 2019, 13, 1374. [Google Scholar] [CrossRef]
- Chang, W.H.-S.; Sun, J.-S.; Chang, S.-P.; Lin, J.C. Study of thermal effects of ultrasound stimulation on fracture healing. Bioelectromagnetics 2002, 23, 256–263. [Google Scholar] [CrossRef]
- Kubanek, J.; Shukla, P.; Das, A.; Baccus, S.A.; Goodman, M.B. Ultrasound Elicits Behavioral Responses through Mechanical Effects on Neurons and Ion Channels in a Simple Nervous System. J. Neurosci. 2018, 38, 3081–3091. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wu, P.; Wan, X.; Wang, Q.; Xu, C.; Yang, M.; Feng, J.; Hu, B.; Luo, Z. Ultrasound-driven electrical stimulation of peripheral nerves based on implantable piezoelectric thin film nanogenerators. Nano Energy 2021, 86, 106123. [Google Scholar] [CrossRef]
- Menz, M.D.; Ye, P.; Firouzi, K.; Nikoozadeh, A.; Pauly, K.B.; Khuri-Yakub, P.; Baccus, S.A. Radiation Force as a Physical Mechanism for Ultrasonic Neurostimulation of the Ex Vivo Retina. J. Neurosci. 2019, 39, 6251–6264. [Google Scholar] [CrossRef]
- Rudenko, O.V.; Sarvazyan, A.P.; Emelianov, S.Y. Acoustic radiation force and streaming induced by focused nonlinear ultrasound in a dissipative medium. J. Acoust. Soc. Am. 1996, 99, 2791–2798. [Google Scholar] [CrossRef]
- Tyler, W.J.; Lani, S.W.; Hwang, G.M. Ultrasonic modulation of neural circuit activity. Curr. Opin. Neurobiol. 2018, 50, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.H.; Kala, S.; Guo, J.H.; Xian, Q.X.; Zhu, J.J.; Zhu, T.; Hou, X.D.; Wong, K.F.; Yang, M.Y.; Wang, H.R.; et al. Targeted Neurostimulation in Mouse Brains with Non-invasive Ultrasound. Cell Rep. 2020, 32, 108033. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, Y.; Chen, R.; Lu, G.; Li, R.; Li, D.; Humayun, M.S.; Shung, K.K.; Zhu, J.; Chen, Y.; et al. Flexible piezoelectric ultrasonic energy harvester array for bio-implantable wireless generator. Nano Energy 2019, 56, 216–224. [Google Scholar] [CrossRef]
- Kang, H.; Sun, Y.; Wodnicki, R.; He, Q.; Zeng, Y.; Lu, G.; Yeom, J.-Y.; Yang, Y.; Zhou, Q. 2-D Array Design and Fabrication With Pitch-Shifting Interposer at Frequencies From 4 MHz up to 10 MHz. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2022, 69, 3382–3391. [Google Scholar] [CrossRef]
- Lu, J.Y.; Lu, G.; Humayun, M.; Zhou, Q. Concave 2D Ring Array Transducer for Ultrasound Visual Stimulation of the Brain. In Proceedings of the IEEE International Ultrasonics Symposium (IUS), Venice, Italy, 10–13 October 2022. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, C.; Li, R.; Lu, G.; Ji, J.; Zeng, Y.; Chen, J.; Chang, C.; Zhang, J.; Xia, L.; Nair, D.S.R.; et al. Non-Invasive Hybrid Ultrasound Stimulation of Visual Cortex In Vivo. Bioengineering 2023, 10, 577. https://doi.org/10.3390/bioengineering10050577
Gong C, Li R, Lu G, Ji J, Zeng Y, Chen J, Chang C, Zhang J, Xia L, Nair DSR, et al. Non-Invasive Hybrid Ultrasound Stimulation of Visual Cortex In Vivo. Bioengineering. 2023; 10(5):577. https://doi.org/10.3390/bioengineering10050577
Chicago/Turabian StyleGong, Chen, Runze Li, Gengxi Lu, Jie Ji, Yushun Zeng, Jiawen Chen, Chifeng Chang, Junhang Zhang, Lily Xia, Deepthi S. Rajendran Nair, and et al. 2023. "Non-Invasive Hybrid Ultrasound Stimulation of Visual Cortex In Vivo" Bioengineering 10, no. 5: 577. https://doi.org/10.3390/bioengineering10050577
APA StyleGong, C., Li, R., Lu, G., Ji, J., Zeng, Y., Chen, J., Chang, C., Zhang, J., Xia, L., Nair, D. S. R., Thomas, B. B., Song, B. J., Humayun, M. S., & Zhou, Q. (2023). Non-Invasive Hybrid Ultrasound Stimulation of Visual Cortex In Vivo. Bioengineering, 10(5), 577. https://doi.org/10.3390/bioengineering10050577








