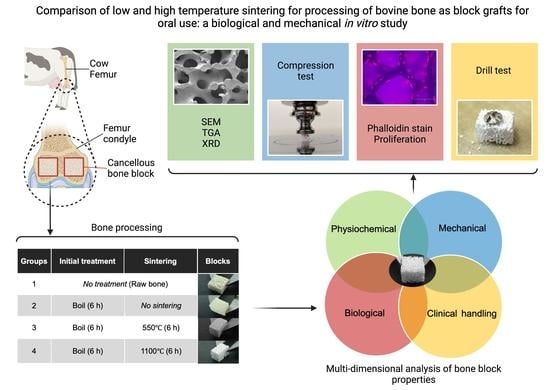
Comparison of Low and High Temperature Sintering for Processing of Bovine Bone as Block Grafts for Oral Use: A Biological and Mechanical In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Bone Sample Preparation
- Group 1: Control (untreated bone);
- Group 2: boiled for 6 h in a pressure multi-cooker (Crockpot, Model: CPE300, Boca Raton, FL, USA) with distilled H2O covering the specimens at a volume of 10 mL/mg bone and renewed every 2 h;
- Group 3: boiled for 6 h and then sintered in a dental furnace (MESTRA®, Txorierri Etorbidea, Spain) at 550 °C for 6 h;
- Group 4: boiled for 6 h and then sintered at 1100 °C for 6 h.
2.2. Residual Organic Content Analysis Using Thermogravimetric Analysis
2.3. Mechanical Strength Using Compression Testing
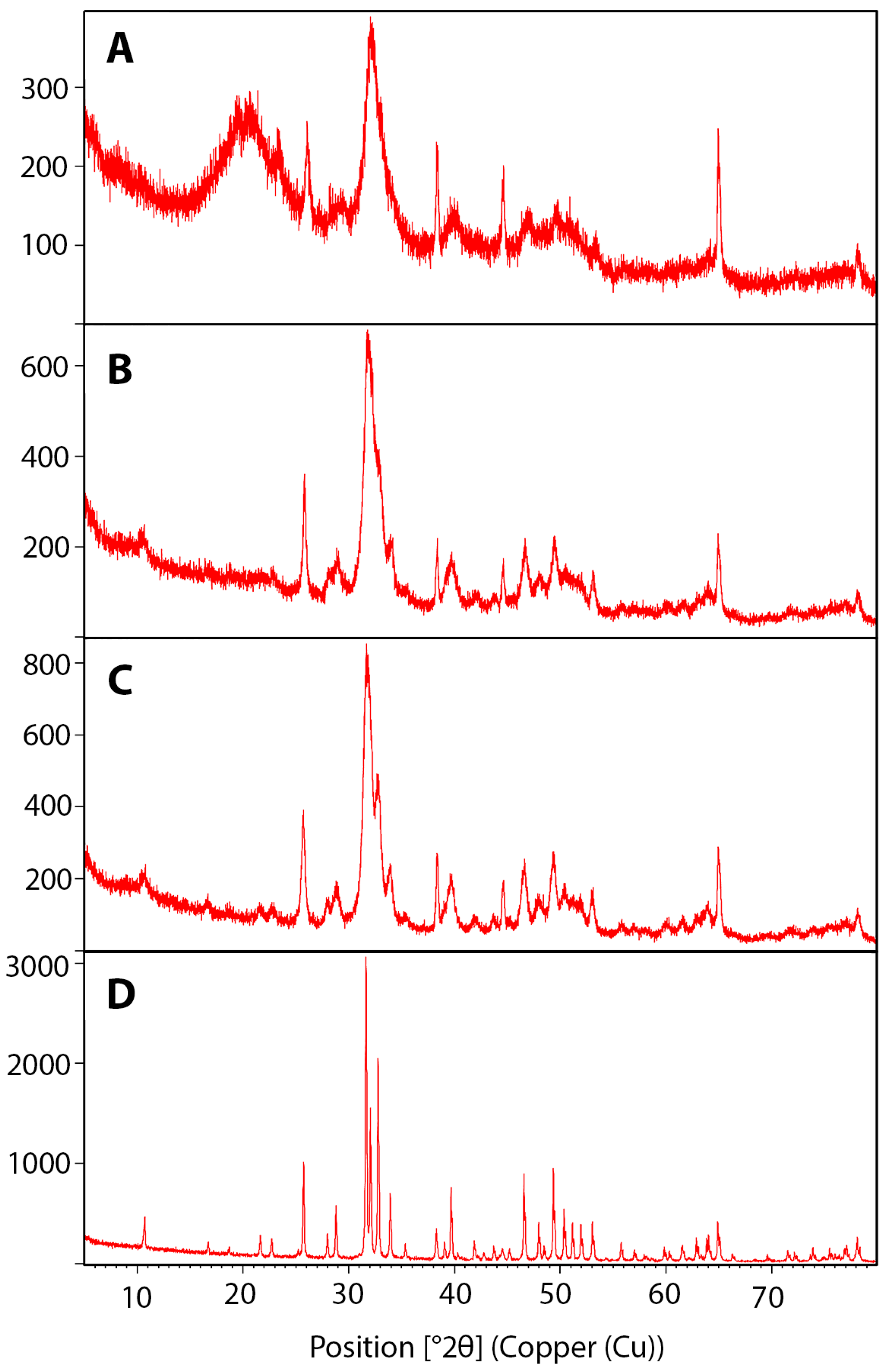
2.4. Crystallinity Analysis of Bone Blocks
2.5. Bone Microstructure Using Scanning Electron Microscopy and Chemical Characterization Using Energy Dispersive Spectroscopy
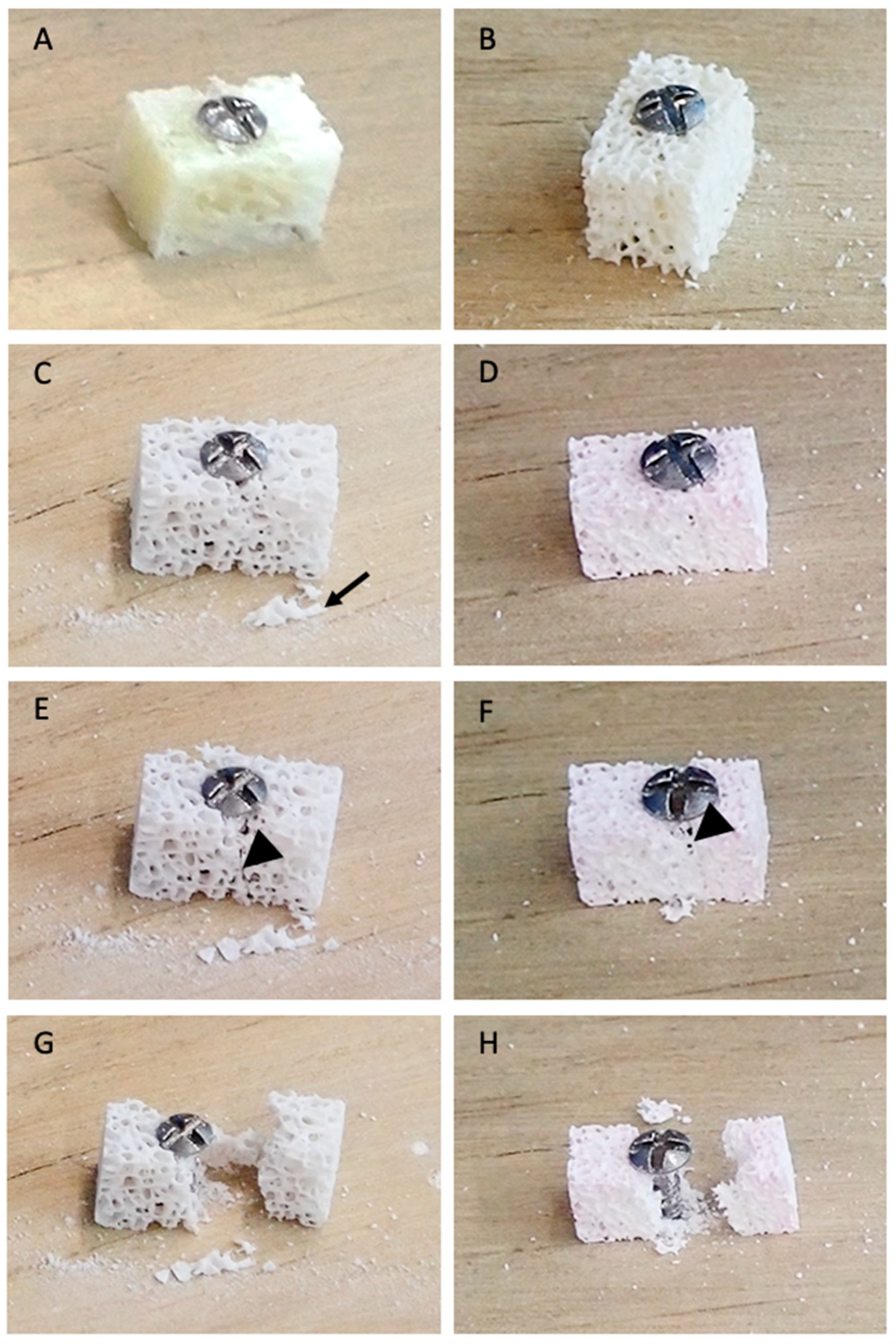
2.6. Qualitative Mechanical Assessment of Blocks Using a Drill Test
2.7. Biological Validation
2.7.1. Human Calvarial Osteoblast (HCO) Cell Culture
2.7.2. HCO Metabolic Activity Assessment (PrestoBlue™)
2.7.3. Observation of Cellular Adhesion by Actin Filaments’ Staining Using Phalloidin and Nuclei Staining Using DAPI
2.8. Statistical Analysis
3. Results
3.1. Thermogravimetric Analysis to Assess Organic Content and Carbonate
3.2. Compression Strength Was Increased with High Temperature Sintering Compared to Lower Temperature Sintering
3.3. Crystallinity Increased with Higher Temperature Sintering
3.4. Microcracks Were Detected by Scanning Electron Microscopy after Sintering
3.5. Chemical Characterization Using Energy Dispersive X-ray Spectroscopy
3.6. Drill Test
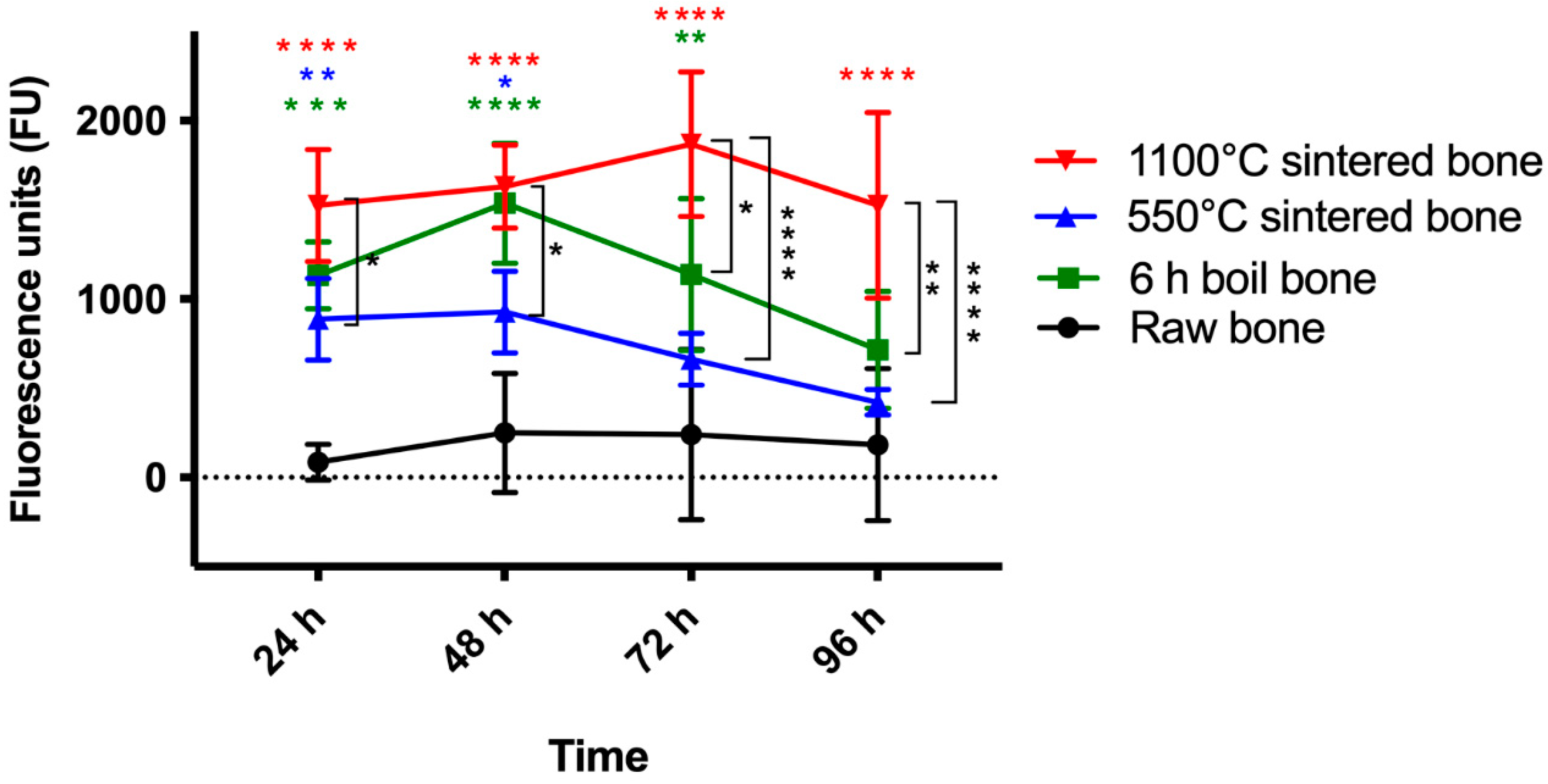
3.7. Metabolic Activity
3.8. Observing Cellular Adhesion by Phalloidin Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone Substitutes: An Update. Injury 2005, 36, S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Elsalanty, M.E.; Genecov, D.G. Bone Grafts in Craniofacial Surgery. Craniomaxillofac. Trauma Reconstr. 2009, 2, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Cosyn, J.; Cleymaet, R.; Hanselaer, L.; De Bruyn, H. Regenerative Periodontal Therapy of Infrabony Defects Using Minimally Invasive Surgery and a Collagen-Enriched Bovine-Derived Xenograft: A 1-Year Prospective Study on Clinical and Aesthetic Outcome. J. Clin. Periodontol. 2012, 39, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Gottlow, J.; Nyman, S.; Lindhe, J.; Karring, T.; Wennström, J. New Attachment Formation in the Human Periodontium by Guided Tissue Regeneration. Case Reports. J. Clin. Periodontol. 1986, 13, 604–616. [Google Scholar] [CrossRef]
- Van de Pol, G.J.; Bonar, F.; Salmon, L.J.; Roe, J.P.; Pinczewski, L.A. Supercritical Carbon Dioxide–Sterilized Bone Allograft in the Treatment of Tunnel Defects in 2-Stage Revision Anterior Cruciate Ligament Reconstruction: A Histologic Evaluation. Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 706–713. [Google Scholar] [CrossRef]
- von Recum, J.; Schwaab, J.; Guehring, T.; Grützner, P.-A.; Schnetzke, M. Bone Incorporation of Silicate-Substituted Calcium Phosphate in 2-Stage Revision Anterior Cruciate Ligament Reconstruction: A Histologic and Radiographic Study. Arthrosc. J. Arthrosc. Relat. Surg. 2017, 33, 819–827. [Google Scholar] [CrossRef]
- Russell, T.A.; Leighton, R.K. Comparison of Autogenous Bone Graft and Endothermic Calcium Phosphate Cement for Defect Augmentation in Tibial Plateau Fractures. A Multicenter, Prospective, Randomized Study. J. Bone Jt. Surg. Ser. A 2008, 90, 2057–2061. [Google Scholar] [CrossRef]
- Bloemers, F.W.; Blokhuis, T.J.; Patka, P.; Bakker, F.C.; Wippermann, B.W.; Haarman, H.J.T.M. Autologous Bone versus Calcium-Phosphate Ceramics in Treatment of Experimental Bone Defects. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 66, 526–531. [Google Scholar] [CrossRef]
- Mertens, C.; Steveling, H.G.; Seeberger, R.; Hoffmann, J.; Freier, K. Reconstruction of Severely Atrophied Alveolar Ridges with Calvarial Onlay Bone Grafts and Dental Implants. Clin. Implant Dent. Relat. Res. 2013, 15, 673–683. [Google Scholar] [CrossRef]
- Fretwurst, T.; Spanou, A.; Nelson, K.; Wein, M.; Steinberg, T.; Stricker, A. Comparison of Four Different Allogeneic Bone Grafts for Alveolar Ridge Reconstruction: A Preliminary Histologic and Biochemical Analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 424–431. [Google Scholar] [CrossRef]
- Campbell, D.G.; Li, P. Sterilization of HIV with Irradiation: Relevance to Infected Bone Allografts. Aust. N. Z. J. Surg. 1999, 69, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Eagan, M.J.; McAllister, D.R. Biology of Allograft Incorporation. Clin. Sport. Med. 2009, 28, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Todo, M. Effects of Sintering Temperature on the Compressive Mechanical Properties of Collagen/Hydroxyapatite Composite Scaffolds for Bone Tissue Engineering. Mater. Lett. 2016, 173, 231–234. [Google Scholar] [CrossRef]
- Karacayli, U.; Gunduz, O.; Salman, S.; Ozyegin, L.S.; Agathopoulos, S.; Oktar, F.N. Effect of Sintering Temperature on Mechanical Properties and Microstructure of Sheep-Bone Derived Hydroxyapatite (SHA). In Proceedings of the 13th International Conference on Biomedical Engineering: ICBME, Singapore, 3–6 December 2008; Springer: Berlin/Heidelberg, Germany, 2009; Volume 23, pp. 1271–1274. [Google Scholar]
- Xu, A.T.; Qi, W.T.; Lin, M.N.; Zhu, Y.H.; He, F.M. The Optimization of Sintering Treatment on Bovine-Derived Bone Grafts for Bone Regeneration: In Vitro and in Vivo Evaluation. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 272–281. [Google Scholar] [CrossRef]
- Ghanaati, S.; Barbeck, M.; Booms, P.; Lorenz, J.; Kirkpatrick, C.J.; Sader, R.A. Potential Lack of “Standardized” Processing Techniques for Production of Allogeneic and Xenogeneic Bone Blocks for Application in Humans. Acta Biomater. 2014, 10, 3557–3562. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone Regenerative Medicine: Classic Options, Novel Strategies, and Future Directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y. Sintered Bone: A New Type of Bone Graft. In Bone Transplantation; Springer: Berlin/Heidelberg, Germany, 1989; pp. 316–317. [Google Scholar]
- Ueno, Y. Experimental Studies of Sintered Bone Implantation. Orthop. Surg. Suppl. 1985, 8, 85–88. [Google Scholar]
- Ueno, Y.; Sasaki, S.; Shima, Y.; Ueyoshi, A.; Harada, M.; Terao, K.; Akiyama, T. Studies of Sintered Bone as a Bone Substitute. Orthop Ceram. Implant. 1983, 3, 11–16. [Google Scholar]
- Ginebra, M.P.; Espanol, M.; Maazouz, Y.; Bergez, V.; Pastorino, D. Bioceramics and Bone Healing. EFORT Open Rev. 2018, 3, 173–183. [Google Scholar] [CrossRef]
- Piedad, M.; Fernández, R.; Gehrke, S.A.; Pérez, C.; Martinez, A.; Calvo Guirado, J.L.; De Aza, P.N. Materials SEM-EDX Study of the Degradation Process of Two Xenograft Materials Used in Sinus Lift Procedures. Materials 2017, 10, 542. [Google Scholar] [CrossRef]
- Pazzaglia, U.E.; Congiu, T.; Basso, P.; Alessandri, I.; Cucca, L.; Raspanti, M. The Application of Heat-Deproteinization to the Morphological Study of Cortical Bone: A Contribution to the Knowledge of the Osteonal Structure. Microsc. Res. Tech. 2016, 79, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Mazón, P.; Pérez-Díaz, L.; Calvo-Guirado, J.L.; Velásquez, P.; Aragoneses, J.M.; Fernández-Domínguez, M.; De Aza, P.N. Study of Two Bovine Bone Blocks (Sintered and Non-Sintered) Used for Bone Grafts: Physico-Chemical Characterization and in Vitro Bioactivity and Cellular Analysis. Materials 2019, 12, 452. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Mazón, P.; Del Fabbro, M.; Tumedei, M.; Aramburú, J.; Pérez-Díaz, L.; De Aza, P.N. Histological and Histomorphometric Analyses of Two Bovine Bone Blocks Implanted in Rabbit Calvaria. Symmetry 2019, 11, 641. [Google Scholar] [CrossRef]
- Ellingham, S.T.D.; Thompson, T.J.U.; Islam, M. Thermogravimetric Analysis of Property Changes and Weight Loss in Incinerated Bone. Palaeogeogr. Palaeoclim. Palaeoecol. 2015, 438, 239–244. [Google Scholar] [CrossRef]
- Murugan, R.; Rao, K.P.; Sampath Kumar, T.S. Heat-Deproteinated Xenogeneic Bone from Slaughterhouse Waste: Physico-Chemical Properties. Bull. Mater. Sci. 2003, 26, 523–528. [Google Scholar] [CrossRef]
- Esmaeilkhanian, A.; Sharifianjazi, F.; Abouchenari, A.; Rouhani, A.; Parvin, N.; Irani, M. Synthesis and Characterization of Natural Nano-Hydroxyapatite Derived from Turkey Femur-Bone Waste. Appl. Biochem. Biotechnol. 2019, 189, 919–932. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, W.L.; Wang, X.Y.; Gong, Y.D.; Tian, J.M.; Zhao, N.M.; Zhang, X.F. Characterization and Osteoblast-like Cell Compatibility of Porous Scaffolds: Bovine Hydroxyapatite and Novel Hydroxyapatite Artificial Bone. J. Mater. Sci. Mater. Med. 2006, 17, 815–823. [Google Scholar] [CrossRef]
- Pramanik, S.; Hanif, A.S.M.; Pingguan-Murphy, B.; Osman, N.A.A. Morphological Change of Heat Treated Bovine Bone: A Comparative Study. Materials 2013, 6, 65–75. [Google Scholar] [CrossRef]
- Doumeng, M.; Makhlouf, L.; Berthet, F.; Marsan, O.; Delbé, K.; Denape, J.; Chabert, F. A Comparative Study of the Crystallinity of Polyetheretherketone by Using Density, DSC, XRD, and Raman Spectroscopy Techniques. Polym. Test. 2021, 93, 106878. [Google Scholar] [CrossRef]
- Porter, G.C.; Abdelmoneim, D.; Li, K.C.; Duncan, W.J.; Coates, D.E. The Effect of Low-Temperature Thermal Processing on Bovine Hydroxyapatite Bone Substitutes, toward Bone Cell Interaction and Differentiation. Materials 2022, 15, 2504. [Google Scholar] [CrossRef]
- Accorsi-Mendonça, T.; Conz, M.B.; Barros, T.C.; de Sena, L.Á.; de Soares, G.A.; Granjeiro, J.M. Physicochemical Characterization of Two Deproteinized Bovine Xenografts. Braz. Oral Res. 2008, 22, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Hoang, G.; Manivasagan, P.; Moorthy, M.S.; Nguyen, T.P.; Vy Phan, T.T.; Kim, H.H.; Kim, M.H.; Nam, S.Y.; Oh, J. Nano-Hydroxyapatite Bioactive Glass Composite Scaffold with Enhanced Mechanical and Biological Performance for Tissue Engineering Application. Ceram. Int. 2018, 44, 15735–15746. [Google Scholar] [CrossRef]
- Fages, J.; Marty, A.; Delga, C.; Condoret, J.S.; Combes, D.; Frayssinet, P. Use of Supercritical CO2 for Bone Delipidation. Biomaterials 1994, 15, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Li, D.; Liu, M.; Jin, J.; Lv, R.; Huang, Z.; Wang, J. The Influence of Approaches for the Purification of Natural Cancellous Bone Grafts: Morphology, Microstructure, Composition, Strength and Biocompatibility Study. Mater. Lett. 2010, 64, 2056–2059. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone Grafts and Biomaterials Substitutes for Bone Defect Repair: A Review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Pripatnanont, P.; Nuntanaranont, T.; Vongvatcharanon, S.; Limlertmongkol, S. Osteoconductive Effects of 3 Heat-Treated Hydroxyapatites in Rabbit Calvarial Defects. J. Oral. Maxillofac. Surg. 2007, 65, 2418–2424. [Google Scholar] [CrossRef]
- Gonda, Y.; Ioku, K.; Shibata, Y.; Okuda, T.; Kawachi, G.; Kamitakahara, M.; Murayama, H.; Hideshima, K.; Kamihira, S.; Yonezawa, I.; et al. Stimulatory Effect of Hydrothermally Synthesized Biodegradable Hydroxyapatite Granules on Osteogenesis and Direct Association with Osteoclasts. Biomaterials 2009, 30, 4390–4400. [Google Scholar] [CrossRef]
- Dumitrescu, C.R.; Neacsu, I.A.; Surdu, V.A.; Nicoara, A.I.; Iordache, F.; Trusca, R.; Ciocan, L.T.; Ficai, A.; Andronescu, E. Nano-Hydroxyapatite vs. Xenografts: Synthesis, Characterization, and in Vitro Behavior. Nanomaterials 2021, 11, 2289. [Google Scholar] [CrossRef]
- Scapin, M.A.; Guilhen, S.N.; Cotrim, M.E.; Pires, M.A.F. Determination of Ca/P Molar Ratio in Hydroxyapatite (HA) by X-Ray Fluorescence Technique. In Proceedings of the INAC 2015: International Nuclear Atlantic Conference, Sao Paulo, Brazil, 4–9 October 2015; Volume 47, pp. 8–10. [Google Scholar]
- Tebyanian, H.; Norahan, M.H.; Eyni, H.; Movahedin, M.; Mortazavi, S.J.; Karami, A.; Nourani, M.R.; Baheiraei, N. Effects of Collagen/β-Tricalcium Phosphate Bone Graft to Regenerate Bone in Critically Sized Rabbit Calvarial Defects. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800018820490. [Google Scholar] [CrossRef]
- Camargo, B.A.; Nunes, D.B.; Spazzin, A.O.; Federizzi, L.; Schuh, C.; Gomes, É.A. Combining the Effects of Undersized Drilling and Bone Density on Implant Insertion Torque. Braz. J. Oral Sci. 2016, 15, 201–204. [Google Scholar] [CrossRef]
- Pandey, R.K.; Panda, S.S. Drilling of Bone: A Comprehensive Review. J. Clin. Orthop. Trauma 2013, 4, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Matthews, L.S.; Green, C.A.; Goldstein, S.A. The Thermal Effects of Skeletal Fixation-Pin Insertion in Bone. J. Bone Jt. Surg. Ser. A 1984, 66, 1077–1083. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Wibowo, D.B.; Kurdi, O.; Tauviqirrahman, M.; Jamari, J. Minimizing Risk of Failure from Ceramic-on-Ceramic Total Hip Prosthesis by Selecting Ceramic Materials Based on Tresca Stress. Sustainability 2022, 14, 13413. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Permana, M.S.; Winarni, T.I.; van der Heide, E. Adopted Walking Condition for Computational Simulation Approach on Bearing of Hip Joint Prosthesis: Review over the Past 30 Years. Heliyon 2022, 8, e12050. [Google Scholar] [CrossRef] [PubMed]
- Ammarullah, M.I.; Hartono, R.; Supriyono, T.; Santoso, G.; Sugiharto, S.; Permana, M.S. Polycrystalline Diamond as a Potential Material for the Hard-on-Hard Bearing of Total Hip Prosthesis: Von Mises Stress Analysis. Biomedicines 2023, 11, 951. [Google Scholar] [CrossRef]
- Aarthy, S.; Thenmuhil, D.; Dharunya, G.; Manohar, P. Exploring the Effect of Sintering Temperature on Naturally Derived Hydroxyapatite for Bio-Medical Applications. J. Mater. Sci. Mater. Med. 2019, 30, 21. [Google Scholar] [CrossRef] [PubMed]
- Barbeck, M.; Udeabor, S.; Lorenz, J.; Schlee, M.; Holthaus, M.G.; Raetscho, N.; Choukroun, J.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. High-Temperature Sintering of Xenogeneic Bone Substitutes Leads to Increased Multinucleated Giant Cell Formation: In Vivo and Preliminary Clinical Results. J. Oral Implantol. 2015, 41, e212–e222. [Google Scholar] [CrossRef]
- De Carvalho, B.; Rompen, E.; Lecloux, G.; Schupbach, P.; Dory, E.; Art, J.-F.; Lambert, F. Effect of Sintering on In Vivo Biological Performance of Chemically Deproteinized Bovine Hydroxyapatite. Materials 2019, 12, 3946. [Google Scholar] [CrossRef]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
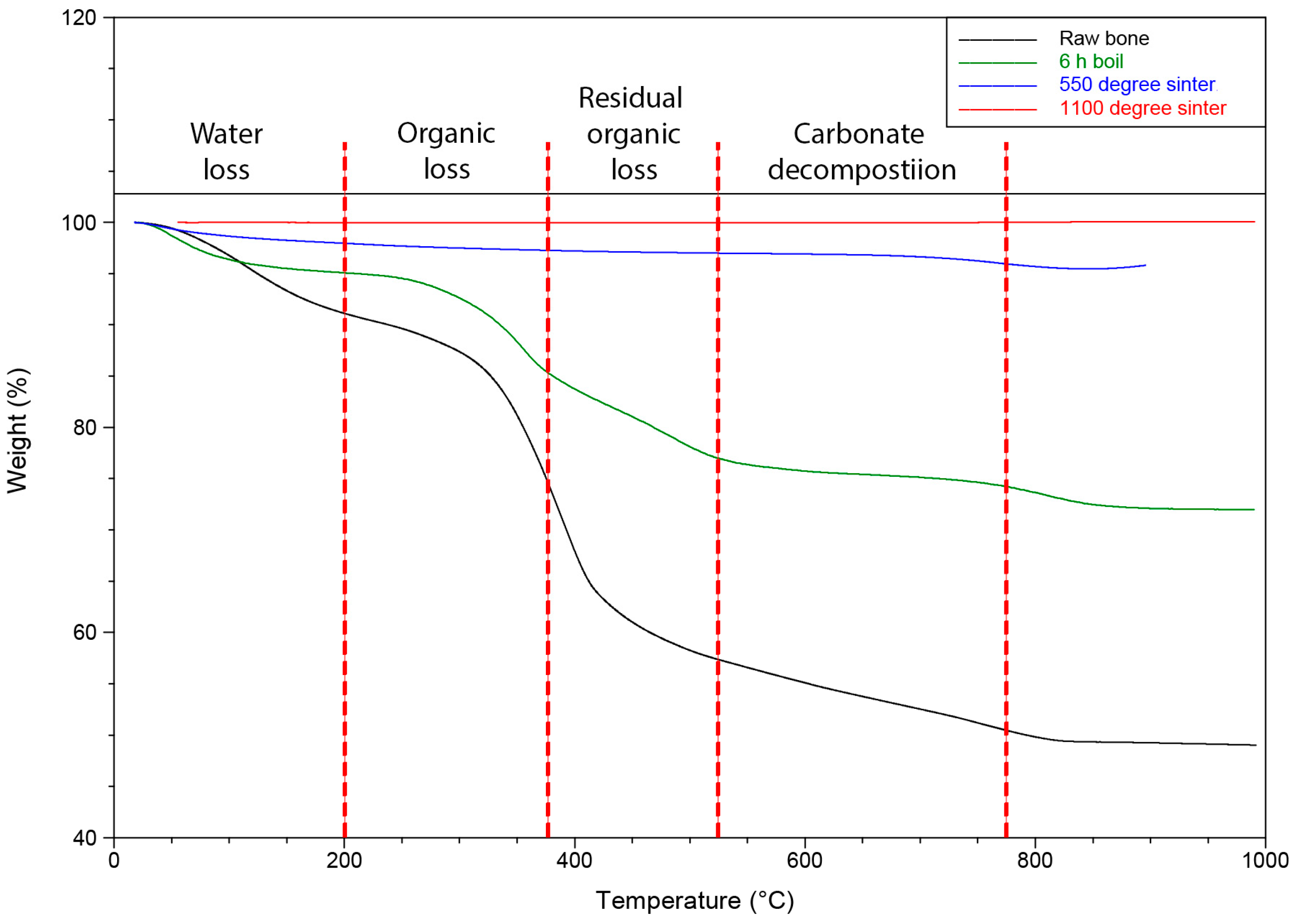



| Groups | Treatments | Water | Organic Content | Residual Organic Content | Carbonate Decomposition |
|---|---|---|---|---|---|
| % | % | % | % | ||
| 1 | Raw bone | 8.11 | 17.55 | 11.87 | 3.03 |
| 2 | Boil (6 h) | 4.88 | 9.65 | 9.09 | 2.16 |
| 3 | Boil (6 h), sintering 550 °C (6 h) | 2.13 | 0.66 | 0.28 | 0.96 |
| 4 | Boil (6 h), sintering 1100 °C (6 h) | 0.02 | 0.02 | 0.02 | 0.000 |
| Groups | Treatments | Crystallinity (%) |
|---|---|---|
| 1 | Raw bone | 23.31 |
| 2 | Boil (6 h) | 48.20 |
| 3 | Boil (6 h), sintering 550 °C for 6 h | 63.15 |
| 4 | Boil (6 h), sintering 1100 °C for 6 h | 95.33 |
| Groups | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Elements | % | SD | % | SD | % | SD | % | SD |
| C | 68.00 | 3.5 | 39.70 | 1.2 | 15.70 | 1.2 | 11.70 | 2.1 |
| O | 13.30 | 1.5 | 24.30 | 0.6 | 34.00 | 0.0 | 35.70 | 0.6 |
| Na | 0.40 | 0.1 | 0.30 | 0.0 | 0.20 | 0.2 | 0.00 | 0.0 |
| Mg | 0.20 | 0.1 | 0.40 | 0.1 | 0.60 | 0.2 | 0.40 | 0.3 |
| Al | 0.03 | 0.0 | 0.00 | 0.0 | 0.00 | 0.0 | 0.00 | 0.0 |
| Ca | 12.00 | 1.7 | 24.30 | 0.6 | 33.70 | 1.5 | 35.00 | 2.0 |
| P | 6.30 | 0.6 | 11.00 | 0.0 | 16.00 | 0.0 | 17.00 | 0.0 |
| Ca/P ratio | 1.90 | 0.1 | 2.20 | 0.1 | 2.10 | 0.1 | 2.10 | 0.1 |
| P2O5 | 14.30 | 1.5 | 25.30 | 1.2 | 36.00 | 0.0 | 38.30 | 0.6 |
| CaO | 16.70 | 2.1 | 34.30 | 0.6 | 47.00 | 1.7 | 49.00 | 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elahi, A.; Duncan, W.; Li, K.-C.; Waddell, J.N.; Coates, D. Comparison of Low and High Temperature Sintering for Processing of Bovine Bone as Block Grafts for Oral Use: A Biological and Mechanical In Vitro Study. Bioengineering 2023, 10, 473. https://doi.org/10.3390/bioengineering10040473
Elahi A, Duncan W, Li K-C, Waddell JN, Coates D. Comparison of Low and High Temperature Sintering for Processing of Bovine Bone as Block Grafts for Oral Use: A Biological and Mechanical In Vitro Study. Bioengineering. 2023; 10(4):473. https://doi.org/10.3390/bioengineering10040473
Chicago/Turabian StyleElahi, Asrar, Warwick Duncan, Kai-Chun Li, John Neil Waddell, and Dawn Coates. 2023. "Comparison of Low and High Temperature Sintering for Processing of Bovine Bone as Block Grafts for Oral Use: A Biological and Mechanical In Vitro Study" Bioengineering 10, no. 4: 473. https://doi.org/10.3390/bioengineering10040473
APA StyleElahi, A., Duncan, W., Li, K.-C., Waddell, J. N., & Coates, D. (2023). Comparison of Low and High Temperature Sintering for Processing of Bovine Bone as Block Grafts for Oral Use: A Biological and Mechanical In Vitro Study. Bioengineering, 10(4), 473. https://doi.org/10.3390/bioengineering10040473