Abstract
Aeromonas caviae CHZ306, a marine-derived bacterium isolated from zooplankton, can use chitin (a polymer of a β-(1,4)-linked N-acetyl-D-glucosamine) as a carbon source. The chitin is hydrolyzed by chitinolytic enzymes, namely endochitinases and exochitinases (chitobiosidase and N-acetyl-glucosaminidase). Indeed, the chitinolytic pathway is initiated by the coexpression of the enzymes endochitinase (EnCh) and chitobiosidase (ChB); however, few studies, including biotechnological production of these enzymes, have been reported, although chitosaccharide are helpful in several industries, such as cosmetics. This study demonstrates the potential to maximize the simultaneous EnCh and ChB production by nitrogen supplementation on culture media. Twelve different nitrogen supplementation sources (inorganic and organic) previously analyzed in elemental composition (carbon and nitrogen) were tested and evaluated in the Erlenmeyer flask culture of A. caviae CHZ306 for EnCh and ChB expression. None of the nutrients inhibited bacterial growth, and the maximum activity in both EnCh and ChB was observed at 12 h, using corn-steep solids and peptone A. Corn-steep solids and peptone A were then combined at three ratios (1:1, 1:2, and 2:1) to maximize the production. The high activities for EnCh (30.1 U.L−1) and ChB (21.3 U.L−1) were obtained with 2:1 corn-steep solids and peptone A, corresponding to more than 5- and 3-fold enhancement, respectively, compared to the control condition.
1. Introduction
Chitin, the second most abundant natural polymer, is a nitrogen-containing polysaccharide composed of a β-(1,4)-linked N-acetyl-D-glucosamine (GlcNac) [1,2]. Chitin is widely distributed as a structural component of crustaceans, insects, and other arthropods and as a component of most fungi and some algae cell walls. Approximately 75% of the total weight of shellfish, such as shrimp, crabs, and krill, is considered waste, and chitin comprises 20–58% of the dry weight of the said waste [3]. Annually, more than 1000 metric tons of chitin are synthesized in the aquatic biosphere and deposited in marine sediment; thus, chitinolytic bacteria efficiently degrade this material to maintain the balance in the marine ecosystem [1,4]. Bacteria can synthesize chitinases to hydrolyze chitin as a carbon, nitrogen, and energy source, consequently making possible the recycling of chitin in the environment [2,5]. The chitinases (EC 3.2.11.14) are an efficient group of multiple enzymes classified into glycoside hydrolase families [6,7]. The chitin degradation by chitinases proceeds via well-characterized consecutive steps; these enzymes are classified into two main categories: endochitinases—EnCh (EC 3.2.1.14), which produce small oligosaccharides and exochitinases—ExCh (EC 3.2.1.29), which are further subdivided into two subcategories: (i): chitobiosidase—ChB (EC 3.2.1.29), which catalyzes the release of N,N’-diacetylchitobiose [(GlcNAc)2], and (ii): N-acetyl-D-glucosaminidase—NAG (EC 3.2.1.30), which cleaves the previous oligomeric products into monomers of GlcNAc [1,4,7]. In bacteria, chitinases play a role in nutrition and parasitism [8]. In contrast, chitinases have been used for chitosaccharide production (e.g., oligomers and monomers of GlcNAc) in food, cosmetic, pharmaceutical, and biomaterial fields [1,9]. Actually, (GlcNAc)2 (N,N’-diacetyl-chitobiose) has significant relevance to the biotechnological, pharmaceutical, cosmetic, food, and agriculture industries due to its wide range of biological activities [7,10]. In this sense, (GlcNAc)2 can be used as a cosmetic ingredient due to its high solubility and as a building block for polysaccharide synthesis [7,11]. From an economic perspective, the market price of (GlcNAc)2 is approximately US $2000 per g, thus representing about 10,000 times more than GlcNAc [12].
Heterotrophic bacteria from aquatic environments related to chitin decomposition include the genera Aeromonas, Enterobacter, Chromobacterium, Arthrobacter, Flavobacterium, Serratia, Bacillus, Erwinia, and Vibrio [13]. Members of the Aeromonas species are widely distributed in nature, and strains isolated from soil and seawater are capable of secreting chitinases [2,4,6]. Aeromonas species, including 36 members, are described as Gram-negative, motile, facultatively anaerobic, rod-shaped, and oxidase-positive bacteria [14,15]. However, some Aeromonas species have been reported to induce pathogenicity (i.e., A. hydrophila, A. caviae, and A. veronii producing systemic infections in humans) [16]. The pathogenic potential of A. caviae does not represent an obstacle to applying their crude enzyme extracts to GlcNAc production since they are just intermediaries in the process [9]. Thus, Aeromonas members have been attractive novel candidates for technological areas such as biocatalysts, bioremediation, and syntesis of polyester [17] and chitin oligosaccharide production [5,9,18,19].
On the other hand, nitrogen is an essential nutrient for all life forms and is used to synthesize many macromolecules such as proteins, nucleic acids, lipids, and carbohydrates [20,21,22]. This element supports growth rate, and yield can play a vital role in the economic efficiency of the bioprocess [23,24,25]. Still, nitrogen limitation causes growth retardation due to the expansion of the G1 phase of the cell cycle through a reduction in ribosomal biogenesis and translation [26]. Therefore, obtaining nitrogen from the external environment is essential for all living organisms [27]. Assimilable inorganic nitrogen sources include nitrates, ammonia, and ammonium salts. Organic nitrogen is mainly obtained from urea and complex sources such as yeast extract, meat extract, tryptone or peptone, and whey, providing growth factors [28,29,30]. Therefore, culture media optimization is critical to maximizing yield and productivity, [3]. Although some microorganisms can use around 30 distinct nitrogen-containing compounds [21], previous studies included the evaluation of eleven sources for Aeromonas sp. [31] but not for specific A. caviae; therefore, screening twelve nitrogen sources, including elemental composition, in this bacteria is the goal of our work. In the current study, we demonstrate the influence of nitrogen supplementation on culture media for chitinase production using marine-derived A. caviae CHZ306. For this, we evaluated the EnCh and ChB production in culture media supplemented with different inorganic and organic nitrogen sources to maximize the production of chitinases in industrial bioprocesses.
2. Materials and Methods
2.1. Microorganisms and Growth Conditions
Aeromonas caviae CHZ306, a marine chitinolytic bacterium isolated from zooplankton samples from the coast of São Paulo state, Brazil (23°59′13” S, 46°22′26” W) [32], was investigated in this study. Aeromonas caviae CHZ306 was characterized at the genus and species levels by complete 16S rRNA gene sequencing and multilocus sequence analysis (MLSA) [9]. To better understand the chitinase diversity and select specific enzymes for chitin derivative production, the genome of Aeromonas caviae CHZ306 was sequenced, and its draft genome sequence was deposited at DDBJ/ENA/GenBank under the accession number MDSC01000000 [33]. The strain was grown on colloidal α-chitin agar plates at 28 °C for 96 h, according to the method described by Souza et al. (2009) [32]. To prepare the pre-inoculums, approximately five colonies were added to a 250 mL Erlenmeyer flask containing 100 mL of sterile colloidal chitin broth and incubated in an orbital incubator at 180 rpm and 28 °C for 24 h to obtain a cellular concentration of approximately 5.0 × 105 CFU.mL−1.
2.2. Evaluation of Nitrogen Supplementation in Bacterial Growth and Chitinase Production
The ability of A. caviae CHZ306 to utilize inorganic and organic compounds as a supplementary nitrogen source was tested using the mineral-salt medium described below, in which either inorganic or organic compounds were present at a concentration of 0.2 g.L−1. Colloid α-chitin (10 g.L−1) was used as the substrate. The broth medium base was composed of minerals and salts prepared to 1 L with: 0.2 g KH2PO4, 1.6 g K2HPO4, 0.2 g MgSO4.7H2O, 0.1 g NaCl, 0.01 g FeSO4.7H2O, and 0.02 g CaCl2.2H2O [32]. This mineral-based broth media was supplemented with 10 g.L−1 colloidal α-chitin and twelve different nitrogen sources listed in Table 1. The final pH medium was adjusted to 7.0. The cultures were carried out using a 250 mL Erlenmeyer flask containing 100 mL of medium with 1 mL pre-inoculum grown. The cultures were placed in an orbital incubator at 180 rpm, 28 °C, and samples (500 μL) were periodically collected for up to 96 h and evaluated for cell growth and chitinase activity.

Table 1.
Characteristics of nutrients used for Aeromonas caviae CHZ306 growth and chitinase production.
2.3. Improvement of Endochitinase and Chitobiosidase Production by Nitrogen Source Mixture
The mineral-based medium described in the previous item included 10 g.L−1 of colloidal α-chitin, which was supplemented with mixtures of corn-steep solids and peptone A in 1:1, 1:2, and 2:1 proportions to reach a supplementation of 0.2 g.L−1 nitrogen (specific quantities are shown in Table 2). The cultures’ conditions and analysis were carried out according to item 2.2.

Table 2.
Mixtures of corn-steep solids (M11) and peptone A (M9) in a chitin-containing medium were used for Aeromonas caviae CHZ306 cultivation.
2.4. Analytical Methods
2.4.1. Elemental Composition
Initially, the colloid chitin and complex nitrogenous sources were digested with concentrated HNO3 and H2O2 (30% w/w) with heating at 925 °C. Elemental composition for carbon (C) and nitrogen (N) was determined using an Optical Atomic Emission Spectrometer with Inductively Coupled Plasma (ICP-OES, Radial) model Arcos-Spectro® (Ametek, Berwyn, PA, USA). Meanwhile, the C and N contents of chemically defined nutrients were calculated based on chemical composition and molecular mass.
2.4.2. Bacterial Growth
The drop plate method determined the cellular growth, expressed by colony-forming units per liter (CFU.L−) [34]. Serially diluted samples (10 μL) were plated (in triplicate) on colloidal α-chitin agar plates at 34 °C for 96 h.
2.4.3. Chitinase Activity
Culture samples were centrifuged to collect the supernatant at 3000× g for 5 min at 4 °C. EnCh and ChB activities from the supernatant were determined by using the Chitinase Assay Kit (Sigma-Aldrich, Saint Louis, MO, USA), using 10 μL of supernatant and 90 μL of a specific substrate solution (1 mg.mL−1 of p-nitrophenol-triacylchitotriose [pNP-(GlcNAc)3] or p-nitrophenol-diacetylchitobiose [pNP-(GlcNAc)2]). The mixtures were incubated at 37 °C for 30 min; optical density was read at 405 nm using a microplate reader, model Spectramax® plus 384 (Molecular Devices, Sunnyvale, CA, USA). The substrates [pNP-(GlcNAc)3] and [pNP-(GlcNAc)2] were used as substrates for EnCh and ChB, respectively. One unit (U) of EnCh and ChB was defined as the amount of each enzyme required to release 1.0 μmol of p-nitrophenol (pNP) from each substrate per minute.
2.5. Statistical Analysis
Experimental data for bacterial growth and enzymatic activity were presented as the average of three biological assays and expressed as the mean ± standard deviation (sd). Statistically significant differences (p < 0.05, 5% significance level) were determined by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison post-test using the GraphPad Prism® 7 software.
3. Results
3.1. Analysis of the Elemental Composition of Chitin and Nitrogenous Sources
Firstly, we determined the C and N content (%) in colloid α-chitin and twelve nitrogen sources, namely ammonium sulfate (M1), ammonium acetate (M2), ammonium nitrate (M3), urea (M4), casaminoacids (M5), meat extract (M6), yeast extract (M7), peptone Bacto (M8), peptone A (M9), peptone G (M10), corn-steep solids (M11), and tryptone (M12), presented in Table 1. The colloid α-chitin contained 44% carbon and 6% nitrogen. Among the chemically defined nutrients (viz. M1–M4), urea (CH4N2O) had the highest nitrogen content (46.6%). Only ammonium sulfate (NH4)2SO4 (M1) and ammonium nitrate NH4NO3; (M3) do not have carbon in their chemical composition. Regarding complex nutrients (viz. M5–M12), all of them simultaneously provided carbon (from 33.3% to 44.3%) and nitrogen (from 7.5 to 16%). Then, we estimated the required amount of each nutrient to obtain ~0.21 g.L−1 nitrogen in the medium, as proposed by Cardozo et al. [18]. Finally, all these media were tested for bacterial growth and chitinase production.
3.2. Evaluation of Nitrogen Supplementation in Bacterial Growth and Chitinase Production
A. caviae CHZ306 cultures were grown in an orbital shaker at 28 °C for 96 h to evaluate inorganic and organic nutrients that could potentially serve as growth substrates. A total of twelve nutrients were tested as nitrogen supplementation (Table 1). As shown in Figure 1, none of the nutrients inhibited A. caviae CHZ306 growth, as the bacteria proliferated in all media tested, either with nitrogen supplementation from chemically defined (viz. M1–M4) or complex (viz. M5–M12) sources. Among all the media tested, corn-steep (M11) was far more favorable for bacterial growth and survival. This positive effect of corn-steep solids on bacterial growth was observed early, in the initial 12 h (3.37 × 109 CFU.L−1), remaining superior to all other media up to 96 h (3.4 × 1010 CFU.L−1), as shown in Figure 2A,B. At 96 h, Figure 2B, corn-steep solids (M11) were the only source that achieved ~1010 CFU.L−1 growth, followed by M2 (ammonium acetate), M6 (meat extract), M7 (yeast extract), and M9 (peptone A), achieving ~109 CFU.L−1. Thus, comparing only the chemically defined media (M1 to M4), ammonium acetate supplementation (M2) enabled more remarkable microbial growth (1.42 × 109 CFU.L−1), presenting a similar profile of complex sources (viz. M7, M6, and M9). Besides, all nutrients tested, except ammonium nitrate (M3), provide superior bacterial growth than ammonium sulfate (M1, 1.32 × 108 CFU.L−1), commonly used in A. caviae cultivation [9,34,35]. The data obtained show that A. caviae CHZ306 utilized a variety of inorganic and organic compounds, particularly corn-steep solids (2.8 g.L−1), as a supplementary source of nitrogen, carbon, and energy for its growth.
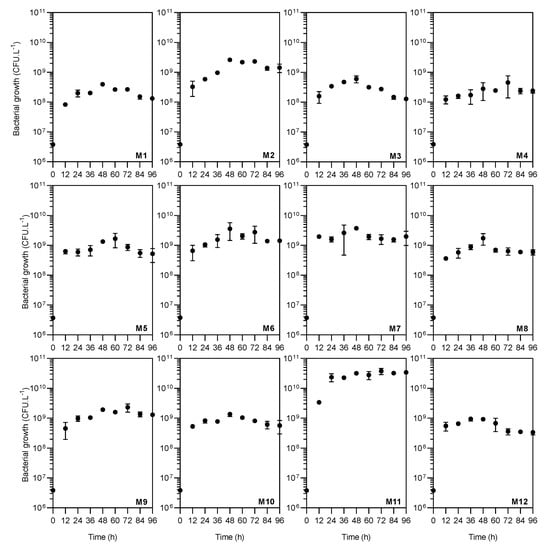
Figure 1.
Growth kinetic profiles of Aeromonas caviae CHZ306 in the chitin-containing medium under different nitrogen supplementations (viz. M1–M12). M1 (ammonium sulfate), M2 (ammonium acetate), M3 (ammonium nitrate), M4 (urea), M5 (casaminoacids), M6 (meat extract), M7 (yeast extract), M8 (peptone Bacto), M9 (peptone A), M10 (peptone G), M11 (corn-steep solids), and M12 (tryptone). All data represent the mean ± standard deviation (sd).
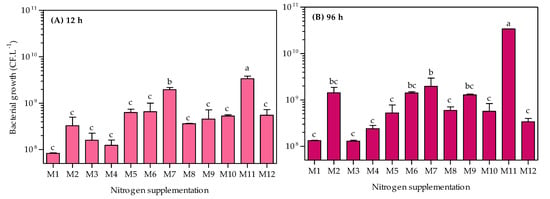
Figure 2.
Comparative effect of nitrogen supplementation (viz. M1–M12) on the growth of Aeromonas caviae CHZ306. M1 (ammonium sulfate), M2 (ammonium acetate), M3 (ammonium nitrate), M4 (urea), M5 (casaminoacids), M6 (meat extract), M7 (yeast extract), M8 (peptone Bacto), M9 (peptone A), M10 (peptone G), M11 (corn-steep solids), and M12 (tryptone). All data represent the mean ± standard deviation (sd). Different letters indicate statistical differences among columns at the same time (p < 0.05).
Simultaneously, the ability of the A. caviae CHZ306 strain to produce both EnCh and ChB enzymes in a chitin-containing medium supplemented with nitrogen sources was tested throughout the 96 h cultivation. The effects of nitrogen supplementation on chitinase production are shown in Figure 3. Overall, the maximum activity in both enzymes was observed at 12 h (Figure 3A and Figure 4), then activity gradually decreased in all tested media (Figure 3B–H). The medium supplemented with ammonium sulfate (M1), previously used in our studies, showed maximum activity of 5.6 and 5.9 U.L−1 at 12 h for EnCh (Figure 4A) and ChB (Figure 4B) enzymes, respectively. The highest activity for EnCh and ChB at 12 h was obtained using peptone A-M9 (23.1 and 21.2 U.L−1, respectively) and corn-steep solids-M11 (32.1 and 23.0 U.L−1, respectively), followed by M6 (meat extract), M8 (peptone Bacto), M10 (peptone G), and M12 (tryptone) that showed similar enzymatic activity. Compared to the ammonium sulfate (M1), the basal medium, peptone A (M9), and corn-steep solids (M11) showed 4.1- and 5.8-fold enhancements in EnCh production and 3.6- and 3.8-fold in ChB production, respectively. Notably, although M2 (ammonium acetate) and M7 (yeast extract) promoted bacterial growth, they were inefficient for EnCh and ChB production. In contrast, A. caviae CHZ306 grew less on M8, M10, and M12 media; however, it could express EnCh and ChB on these media.
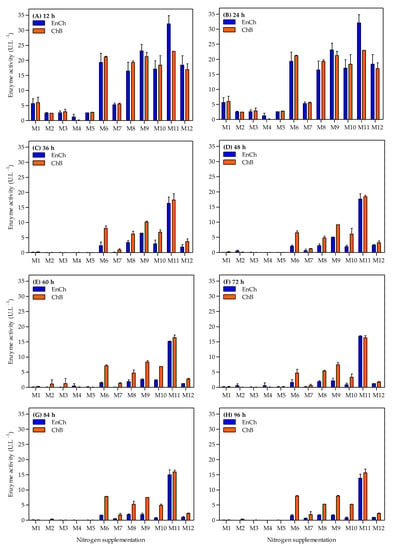
Figure 3.
Time-course profiles of endochitinase (EnCh) and chitobiosidase (ChB) activity during Aeromonas caviae CHZ306 cultivation in the chitin-containing medium under different nitrogen supplementations (viz. M1–M12). M1 (ammonium sulfate), M2 (ammonium acetate), M3 (ammonium nitrate), M4 (urea), M5 (casaminoacids), M6 (meat extract), M7 (yeast extract), M8 (peptone Bacto), M9 (peptone A), M10 (peptone G), M11 (corn-steep solids), and M12 (tryptone). All data represent the mean ± standard deviation (sd).
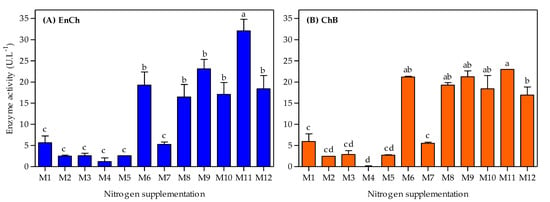
Figure 4.
Endochitinase (EnCh) (A) and chitobiosidase (ChB) (B) activity at 12 h Aeromonas caviae CHZ306 is cultivated in the chitin-containing medium under different nitrogen supplementations (viz. M1–M12). M1 (ammonium sulfate), M2 (ammonium acetate), M3 (ammonium nitrate), M4 (urea), M5 (casaminoacids), M6 (meat extract), M7 (yeast extract), M8 (peptone Bacto), M9 (peptone A), M10 (peptone G), M11 (corn-steep solids), and M12 (tryptone). All data represent the mean ± standard deviation (sd). Different letters indicate statistical differences among columns for each enzyme (p < 0.05).
3.3. Improvement of Selected Nitrogen Sources in Endochitinase and Chitobiosidase Production
Table 2 presents three different mixtures of corn-steep solids (M11), and peptone A (M9) formulated to verify the interactions between these two selected nitrogen sources on A. caviae CHZ306 growth and chitinase production during 96 h, keeping constant the initial nitrogen supplementation of ~0.2 g.L−1.
As shown in Figure 5A, the A. caviae CHZ306 grew similarly in the three mixtures of corn-steep solids (M11), and peptone A (M9) studied, achieving ~109 CFU.L−1 after 96 h of cultivation. Similar to the previous assay, the maximum activity for both enzymes was observed at 12 h, and their activities gradually reduced; notably, the EnCh activity decreased more than ChB over 96 h of cultivation. The EnCh production (Figure 5B) was favored in mediums M11–M9 (2:1) with maximum activity at 12 h (30 U.L−1) followed by 23 U.L−1 in M11–M9 (1:2) and 16.6 U.L−1 in M11–M9 (1:1). Compared to the ammonium sulfate (M1), the control medium, M11–M9 (2:1), M11–M9 (1:2), and M11–M9 (1:1) showed 5.3-, 4.1-, and 2.9-fold enhancements in EnCh production, respectively. The ChB production (Figure 5C) was similar in the three mixtures, also recording a maximum activity at 12 h of 21.4 U.L−1 for M11–M9 at a ratio of 2:1, 21.2 U.L−1 for M11–M9 (1:2), and 21.5 U.L−1 for M11–M9 (1:1). Compared to the control medium (M1), these media showed 3.6-fold enhancement in EnCh production.
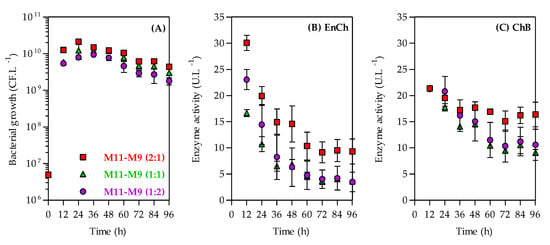
Figure 5.
Time-course kinetics profiles of bacterial growth (A), endochitinase (EnCh) activity (B), and chitobiosidase (ChB) activity (C) of Aeromonas caviae CHZ306 in the chitin-containing medium under corn-steep solids (M11) and peptone A (M9) mixtures at ratios of 1:1 (green triangles), 1:2 (purple circles), and 2:1 (red squares). All data represent the mean ± standard deviation (sd).
4. Discussion
In this study, we determined the nitrogenous compounds as potential substrates for the growth of A. caviae CHZ 306, particularly in a chitin-containing mineral-based medium. A total of twelve different inorganic and organic nutrients, representing chemically defined and complex sources, serve as substrates for bacterial growth. In general, the composition of the culture medium (including nitrogen sources) significantly influences on cell growth, physiology, and biochemical pathways of microorganisms [36,37]. Thus, the enzyme production varies depending on the nitrogen source; in some cases, there may be a preference for a particular type: nitrogen-rich sources are more rapidly assimilated than nitrogen-poor sources due to the efficiency of transport systems [24,29,38]. In addition, the microorganisms can extract nitrogen from a broad spectrum of organic and inorganic substrates [27]. Therefore, screening and selecting the most suitable nitrogen source for cultivation is essential to maximize product formation.
Ammonium sulfate (M1) and ammonium nitrate (M3) repressed the growth and chitinase production, although ammonium ions could be directly assimilated and act as donors of amino acid synthesis [39]. In M1, the decrease in biomass production is commonly caused by acidification by anion sulfate in media culture [40]. However, ammonium sulfate was the most favorable nitrogen source to produce chitinase by Aeromonas sp. JK1 [31], and some authors also reported that Aeromonas species could degrade nitrates to nitrites [14]. Moreover, the efficient bacterial growth reached using ammonium acetate (M2) is possible since the salt dissolution in pure water produces a neutral pH solution due to the acidity of NH4+ (pKa 9.25), which exactly balances the basicity of acetate (pKb 9.25) [41]. In general, inorganic nitrogen sources have an inefficient impact compared to organic sources [42]. However, urea (M4) presents low chitinase production even though the uptake is not proton-coupled and does not cause medium acidification in contrast to ammonium salts [19,40]. However, other Aeromonas species, such as A. hydrophila, can assimilate significant amounts of urea and nitrogen from ammonium through oxidation by glutamate synthase, while nitrate is utilized through nitrate reductase [43].
In the case of complex nutrients (viz. M5–M12), they could be assimilated by two independent pathways, such as a deamination reaction releasing ammonia or a transamination reaction, where the nitrogen is transferred to the acceptor substrate (α-ketoacid) [27]. Casaminoacid (M5) and yeast extract (M7) had a repressive effect on chitinase production. M5 is an acid hydrolysate of casein containing amino acids and short peptides, although it is adequate for media culture with minimal nitrogen requirements [42,43]. While M7 contains essential nutrients to satisfy nutritional requirements (i.e., carbohydrates, proteins, amino acids, vitamins, and trace elements) [44,45,46], some reports indicated no effect on chitinase production by A. hydrophila HS4 [3] and Aeromonas sp. JK1 [31]. M7 showed high cell growth and is considered, along with M11, the best source of organic nitrogen for biomass production. However, it is worth mentioning that the metabolic pathways of enzyme synthesis are much slower than the biomass production routes. [20,36]. On the other hand, meat extract (M6), peptone Bacto (M8), peptone G (M10), and tryptone (M12) showed significant chitinase production and growth at 12 h of culture. These results are possible due to composition; for example, M7 is a mixture of peptides and amino acids, nucleotide fractions, organic acids, minerals, and some vitamins [44,47,48]; M8 contains essential amino acids, low molecular peptides, trace elements, and vitamins [44,49]; M10, a pancreatic digest of gelatin, presents higher proline residues [44,50]; and M11, a pancreatic digest (i.e., trypsin hydrolysate) of casein, contains oligopeptides in different lengths and smaller amounts of amino acids and carbohydrates [44,45].
In our findings, peptone A (M9) and corn-steep solids (M11) were the most favorable nitrogen sources for simultaneous EnCh and ChB production, probably because these nutrients provide elements necessary for synthesizing chitinase enzymes. In this sense, both nitrogen sources were selected to continue evaluating chitinase production. M9 is a pancreatic digest of animal tissue and contains a mixture of small and large peptide sizes [44,49,50]. Thus, the nutritional support of peptones and derivates highly depends on the specific peptide (i.e., length and sequence) and free amino acid composition [20]. Moreover, M11 is a soluble solids by-product of the corn milling industry used for microbial growth, providing amino acids, vitamins, minerals, and growth factors [51,52]. The presence of corn-steep solids was also essential to increase the production of the L-asparaginase enzyme by Leucosporidium scottii L115 [37].
Comparative data from chitinase production by Aeromonas species are presented in Table 3. Some authors indicated that culture medium containing complex nutrients, e.g., peptones, tryptone, yeast extract, and malt extract, despite unknown composition, are cost-effective for processes due to their ability to support maximum product yield and performance, including for large-scale fermentations [36,49].
It is known that the composition of the culture medium affects the final cost of a bioprocess, directly affectingmicrobial growth and product formation [37]. The organic nitrogen from complex sources could be metabolized by cells directly, promoting chitinase production due to its high concentration of most amino acids and growth factors [53]. In this sense, manipulating medium components and concentrations is essential in microbial development because the range of nutrients required depends upon their metabolism [51,54]. Most microorganisms can sense qualitative and quantitative nutrient changes, improving their utilization in competitive environments [21].
Here, maximum chitinase (EnCh and ChB) yields were obtained in the mixture medium M11–M9 (2:1). Indeed, the enzyme production using the mixture of nitrogen sources could be higher than a single nitrogen source [36]. Furthermore, because some organic nitrogen sources are expensive in industrial bioprocess, medium costs can be reduced by replacing them entirely or partially with a less expensive component [50,51]. Medium M11–M9 (2:1) also presents a high C:N ratio, although Arous et al. (2016) [55] indicated that high C:N ratios are favorable for the accumulation of carbohydrates and intracellular lipids instead of enzyme production. This result might be the consequence of channeling more carbon for cell growth and chitinase production.
Moreover, it is suggested that enzyme production was obtained by using quickly metabolized nitrogen sources. Although no significant differences were observed in bacterial growth and chitinolytic activity between medium M11 and the mixture M11–M9 (2:1), the availability of commercial corn-steep solids is reduced and could hinder their acquisition at a large scale. In this sense, partial replacement with peptone A is a beneficial strategy.
Finally, A. caviae CHZ306 represents an exciting source of enzymes because it contains different genes encoding chitinases [33]. Moreover, in opposition to the chemical process for chitin degradation, enzymatic hydrolysis is a more sustainable alternative because it does not require the presence of toxic compounds or excessive wastewater generation [56]. The chitin derivatives such as chitosan and chitosaccharides present biological and physicochemical properties interesting for several applications, including biotechnology, biomaterials, food, medicine, cosmetics, wastewater treatments, etc. [56,57]. In this sense, using purified EnCh or ChB in a specific chitin-degradation step and controlling the length of the chitosaccharide would allow the definition of molecules and the evaluation of their biological activity. Additionally, a deep study of the biochemical properties of both enzymes is recommended to obtain a detailed understanding of reaction mechanisms. It is also possible to take advantage of the synthetic capacity of hydrolases in unconventional media (i.e., the synthesis of complex oligosaccharides and polysaccharides).

Table 3.
Comparative reports of chitinase production by Aeromonas species.
Table 3.
Comparative reports of chitinase production by Aeromonas species.
| Strain | Carbon Source (g.L−1) | Nitrogen Source (g.L−1) | Time Culture (h) | Chitinase Activity | Ref | |
|---|---|---|---|---|---|---|
| (U.L−1) | (U.mg−1) | |||||
| Aeromonas caviae CHZ306 | Colloidal chitin (10.0) | Corn-steep solids (1.77) Peptone A (0.51) | 12 | 30 (EnCh) 21.4 (ChB) | nr | This study |
| Aeromonas hydrophila H-2330 | Colloidal chitin (5.0) | Polypepton (5.0) Yeast extract (3.0) | 24 | 140 | 1.7 | [58] |
| Aeromonas schubertii | Colloidal chitin (10.0) | Tryptone (1.0) Yeast extract (1.0) | 96 | 155 | 0.47 | [59] |
| Aeromonas sp. GJ-18 | Swollen chitin (10.0) | Tryptone (10.0) | 120 | 1,440 | 14.4 | [19] |
| Aeromonas sp. JK1 | Colloidal chitin (7.5) | Ammonium sulfate (1.5) | 48 | 9000 * | nr | [31] |
| Aeromonas sp. ZD_05 | Colloidal chitin (10.0) | Peptone (7.0) | 72 | 10,000 * | nr | [53] |
| Aeromonas punctata HS6 | Colloidal chitin (10.0) Starch (10) | Yeast extract (10.0) | 48 | 82,640 | nr | [3] |
| Aeromonas hydrophila HS4 | Colloidal chitin (10.0) Starch (10) | Malt extract (10.0) | 24–48 | 86,010 | nr | [3] |
| Aeromonas sp. PTCC 1691 | Colloidal chitin (7.5) | Ammonium sulfate (1.5) | 48 | 92,000 | nr | [35] |
* The values were estimated based on the graphics reported in the works; nr = not reported. Ench: endochitinase, and ChB: chitobiosidase.
5. Conclusions
Medium composition improvement is relevant in developing a commercial fermentation process affecting microbial growth and product yield. Based on our study, complex nitrogen sources play an essential role in chitinase production. The supplementation of peptone A (M9) and corn-steep solids (M11) as sole nitrogen sources increased EnCh and ChB production by at least 4.1- and 3.6-fold, respectively. In this sense, the organic nitrogen sources, corn-steep solids, and peptone A (at a ratio of 2:1), have been shown to stimulate the cell growth of A. caviae CHZ306 and to promote the high synthesis of EnCh (30.1 U.L−1) and ChB (21.3 U.L−1), in comparison with basal medium, corresponding to more than 5- and 3-fold enhancement, respectively. Further assays should focus on optimizing culture conditions and process implementation in automated bioreactor systems.
Author Contributions
Conceptualization, F.C., V.F. and A.P.; methodology, F.C.; software, V.F.; validation F.C.; formal analysis, F.C., V.F. and O.P.-P.; investigation, F.C.; resources, A.P.; data curation, F.C.; writing—original draft preparation, F.C. and O.P.-P.; writing—review and editing, O.P.-P. and V.F.; visualization, A.P.; supervision, A.P.; project administration, A.P.; funding acquisition O.P.-P. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge the financial support of the São Paulo Research Foundation (FAPESP) and grants #2012/16824-0, #2013/18773-6, and #2016/20970-2. The APC was funded by Universidad Privada Norbert Wiener, Lima, Perú.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We want to thank Paula Rachel Louro Leite for her assistance with the statistical analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Halder, S.K.; Maity, C.; Jana, A.; Ghosh, K.; Das, A.; Paul, T.; Das Mohapatra, P.K.; Pati, B.R.; Mondal, K.C. Chitinases biosynthesis by immobilized Aeromonas hydrophila SBK1 by prawn shells valorization and application of enzyme cocktail for fungal protoplast preparation. J. Biosci. Bioeng. 2014, 117, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, M.A.; Gai, Y.; Zhuang, Q.; Wang, F.; Xiao, X.; Wang, F. Aeromonas caviae CB101 Contains Four chitinases encoded by a single gene chi1. Mol. Biotechnol. 2010, 44, 213–220. [Google Scholar] [CrossRef]
- Saima; Kuddus, M.; Roohi; Ahmad, I. Isolation of novel chitinolytic bacteria and production optimization of extracellular chitinase. J. Genet. Eng. Biotechnol. 2013, 11, 39–46. [Google Scholar] [CrossRef]
- Lan, X.; Zhang, X.; Hu, J.; Shimosaka, M. Cloning, expression, and characterization of a chitinase from the chitinolytic bacterium Aeromonas hydrophila strain SUWA-9. Biosci. Biotechnol. Biochem. 2006, 70, 2437–2442. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, A.K.; Vortmann, M.; E Dirks-Hofmeister, M.; Moerschbacher, B.M.; Philipp, B. Identification of a novel chitinase from Aeromonas hydrophila AH-1N for the degradation of chitin within fungal mycelium. FEMS Microbiol. Lett. 2019, 366, fny294. [Google Scholar] [CrossRef]
- Pentekhina, I.; Hattori, T.; Tran, D.M.; Shima, M.; Watanabe, T.; Sugimoto, H.; Suzuki, K. Chitinase system of Aeromonas salmonicida, and characterization of enzymes involved in chitin degradation. Biosci. Biotechnol. Biochem. 2020, 84, 1936–1947. [Google Scholar] [CrossRef]
- Jeong, H.C.; Ju, W.-T.; Jo, K.-H.; Park, R.D. Purification and characterization of a 34-kDa chitobiosidase from Aeromonas sp. GJ-18. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 7–12. [Google Scholar] [CrossRef]
- Dahiya, N.; Tewari, R.; Hoondal, G.S. Biotechnological aspects of chitinolytic enzymes: A review. Appl. Microbiol. Biotechnol. 2006, 71, 773–782. [Google Scholar] [CrossRef]
- Cardozo, F.A.; Gonzalez, J.M.; Feitosa, V.A.; Pessoa, A.; Rivera, I.N.G. Bioconversion of α-chitin into N-acetyl-glucosamine using chitinases produced by marine-derived Aeromonas caviae isolates. World J. Microbiol. Biotechnol. 2017, 33, 201. [Google Scholar] [CrossRef]
- Kumar, M.; Rajput, M.; Soni, T.; Vivekanand, V.; Pareek, N. Chemoenzymatic Production and Engineering of Chitooligosaccharides and N-acetyl Glucosamine for Refining Biological Activities. Front. Chem. 2020, 8, 469. [Google Scholar] [CrossRef]
- Ren, X.-B.; Dang, Y.-R.; Liu, S.-S.; Huang, K.-X.; Qin, Q.-L.; Chen, X.-L.; Zhang, Y.-Z.; Wang, Y.-J.; Li, P.-Y. Identification and Characterization of Three Chitinases with Potential in Direct Conversion of Crystalline Chitin into N,N′-diacetylchitobiose. Mar. Drugs 2022, 20, 165. [Google Scholar] [CrossRef]
- Osada, M.; Kikuta, K.; Yoshida, K.; Totani, K.; Ogata, M.; Usui, T. Non-catalytic dehydration of N,N′-diacetylchitobiose in high-temperature water. RSC Adv. 2014, 4, 33651–33657. [Google Scholar] [CrossRef]
- Brzezinska, M.S.; Jankiewicz, U.M.; Burkowska-But, A.; Walczak, M. Chitinolytic microorganisms and their possible application in environmental protection. Curr. Microbiol. 2014, 68, 71–81. [Google Scholar] [CrossRef]
- Fernández-Bravo, A.; Figueras, M.J. An update on the genus Aeromonas: Taxonomy, epidemiology, and pathogenicity. Microorganisms 2020, 8, 129. [Google Scholar] [CrossRef]
- Dwivedi, M.; Mishra, A.; Prasad, A.; Azim, A.; Singh, R.; Baronia, A.; Prasad, K.; Dwivedi, U. Aeromonas caviae septicemia in immunocompetent gastrointestinal carriers. Braz. J. Infect. Dis. 2008, 12, 47–548. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [PubMed]
- Canellas, A.L.B.; Laport, M.S. The biotechnological potential of Aeromonas: A bird’s eye view. Crit. Rev. Biotechnol. 2022, 1–13. [Google Scholar] [CrossRef]
- Cardozo, F.A.; Facchinatto, W.M.; Colnago, L.A.; Campana-Filho, S.P.; Pessoa, A. Bioproduction of N-acetyl-glucosamine from colloidal α-chitin using an enzyme cocktail produced by Aeromonas caviae CHZ306. World J. Microbiol. Biotechnol. 2019, 35, 114. [Google Scholar] [CrossRef]
- Kuk, J.H.; Jung, W.J.; Jo, G.H.; Ahn, J.S.; Kim, K.Y.; Park, R.D. Selective preparation of N-acetyl-D-glucosamine and N,N′- diacetylchitobiose from chitin using a crude enzyme preparation from Aeromonas sp. Biotechnol. Lett. 2005, 27, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Pillaca-Pullo, O.S.; Lopes, A.M.; Rodriguez-Portilla, L.M.; Estela-Escalante, W. Optimizing medium composition with wastewater from Coffea arabica processing to produce single-cell protein using Candida sorboxylosa. J. Chem. Technol. Biotechnol. 2023, 98, 106–116. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Huang, J.; Man, D.; Guo, M. Comparisons of urea or ammonium on growth and fermentative metabolism of Saccharomyces cerevisiae in ethanol fermentation. World J. Microbiol. Biotechnol. 2021, 37, 71. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, P.; Chen, Y.; Cao, Q.; Liu, X.; Li, D. The production of single cell protein from biogas slurry with high ammonia-nitrogen content by screened Nectaromyces rattus. Poult. Sci. 2021, 100, 101334. [Google Scholar] [CrossRef]
- Marius, K.S.; Mahamadi, N.; Ibrahim, K.; Iliassou, M.; Sonagnon, H.S.K.; Yerobessor, D.; Wahauwouele, H.C.; Essodolom, T.; Alfred, S. Production of single cell protein (SCP) and essentials amino acids from Candida utilis FMJ12 by solid state fermentation using mango waste supplemented with nitrogen sources. Afr. J. Biotechnol. 2018, 17, 716–723. [Google Scholar] [CrossRef]
- Su, Y.; Seguinot, P.; Sanchez, I.; Ortiz-Julien, A.; Heras, J.M.; Querol, A.; Camarasa, C.; Guillamón, J.M. Nitrogen sources preferences of non-Saccharomyces yeasts to sustain growth and fermentation under winemaking conditions. Food Microbiol. 2020, 85, 103287. [Google Scholar] [CrossRef]
- Roca-Mesa, H.; Sendra, S.; Mas, A.; Beltran, G.; Torija, M.-J. Nitrogen preferences during alcoholic fermentation of different non-saccharomyces yeasts of oenological interest. Microorganisms 2020, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- Broach, J.R. Nutritional control of growth and development in yeast. Genetics 2012, 192, 73–105. [Google Scholar] [CrossRef]
- Linder, T. Nitrogen assimilation pathways in budding yeasts. In Non-Conventional Yeasts: From Basic Research to Application; Springer: Cham, Switzerland, 2019; pp. 197–236. [Google Scholar]
- Djellouli, M.; Martínez-Álvarez, O.; Arancibia, M.Y.; Florez-Cuadrado, D.; Ugarte-Ruíz, M.; Domínguez, L.; Zadi-Karam, H.; Karam, N.; Roudj, S.; López-Caballero, M.E. Effect of seafood peptones on biomass and metabolic activity by Enterococcus faecalis DM19. LWT Food Sci. Technol. 2017, 81, 94–100. [Google Scholar] [CrossRef]
- Jiménez-Martí, E.; Del Olmo, M. Addition of ammonia or amino acids to a nitrogen-depleted medium affects gene expression patterns in yeast cells during alcoholic fermentation. FEMS Yeast Res. 2008, 8, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.G. Microbiology. In Bioprocess Engineering: An Introductory Engineering and Life Science Approach; Woodhead Publishing: Cambridge, UK, 2013; pp. 7–24. [Google Scholar]
- Al-Ahmadi, K.J.; Yazdi, M.T.; Najafi, M.F.; Shahverdi, A.R.; Faramarzi, M.A.; Zarrini, G.; Behravan, J. Optimization of medium and cultivation conditions for chitinase production by newly isolated: Aeromonas sp. Biotechnology 2008, 7, 266–272. [Google Scholar]
- Souza, C.P.; Burbano-Rosero, E.M.; Almeida, B.C.; Martins, G.G.; Albertini, L.S.; Rivera, I.N.G. Culture medium for isolating chitinolytic bacteria from seawater and plankton. World J. Microbiol. Biotechnol. 2009, 25, 2079–2082. [Google Scholar] [CrossRef]
- Cardozo, F.A.; Zimpel, C.K.; Guimaraes, A.; Pessoa, A.; Rivera, I.N.G. Draft genome sequence of marine-derived Aeromonas caviae CHZ306, a potential chitinase producer strain. Genome Announc. 2016, 4, e01293-16. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, H.; Rodrigues, M.; Campos, C.; Chaves, M.; Nunes, I.; Juliano, Y.; Novo, N. Counting of viable cluster-forming and non cluster-forming bacteria: A comparison between the drop and the spread methods. J. Microbiol. Methods 1995, 22, 39–50. [Google Scholar] [CrossRef]
- Jamialahma, K.; Behravan, J.; Najafi, M.F.; Yazdi, M.T.; Shahverdi, A.; Faramarzi, M.A. Enzymatic production of N-acetyl-D-glucosamine from chitin using crude enzyme preparation of Aeromonas sp. PTCC1691. Biotechnology 2011, 10, 292–297. [Google Scholar] [CrossRef]
- Dinarvand, M.; Rezaee, M.; Masomian, M.; Jazayeri, S.D.; Zareian, M.; Abbasi, S.; Ariff, A.B. Effect of C/N ratio and media optimization through response surface methodology on simultaneous productions of intra- and extracellular inulinase and invertase from aspergillus Niger ATCC 20611. Biomed Res. Int. 2013, 2013, 508968. [Google Scholar] [CrossRef]
- Moguel, I.S.; Yamakawa, C.K.; Brumano, L.P.; Pessoa, A.; Mussatto, S.I. Selection and Optimization of Medium Components for the Efficient Production of L-Asparaginase by Leucosporidium scottii L115—A Psychrotolerant Yeast. Fermentation 2022, 8, 398. [Google Scholar] [CrossRef]
- Da Cruz, S.H.; Cilli, E.M.; Ernandes, J.R. Structural complexity of the nitrogen source and influence on yeast growth and fermentation. J. Inst. Brew. 2002, 108, 54–61. [Google Scholar] [CrossRef]
- Raita, S.; Kusnere, Z.; Spalvins, K.; Blumberga, D. Optimization of Yeast Cultivation Factors for Improved SCP Production. Environ. Clim. Technol. 2022, 26, 848–861. [Google Scholar] [CrossRef]
- Prins, R.C.; Billerbeck, S. A buffered media system for yeast batch culture growth. BMC Microbiol. 2021, 21, 127. [Google Scholar] [CrossRef] [PubMed]
- Konermann, L. Addressing a Common Misconception: Ammonium Acetate as Neutral pH ‘Buffer’ for Native Electrospray Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2017, 28, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Hosseany, E.N.; Elhamid, S.M.A.; Khair, A.G.A.-E.; Azzaz, H.; Zahran, M.O. Utilization of hydrolyzed UF-permeate Supplemented with different nitrogen sources and vitamins for production of Baker’s yeast. Biotechnology 2019, 18, 55–63. [Google Scholar] [CrossRef]
- Taabodi, M.; May, E.B.; Bryant, R.B.; Saporito, L.S.; Skeen, O.K.; Hashem, F.M.; Allen, A.L. Aeromonas hydrophila, Bacillus thuringiensis, Escherichia coli and Pseudomonas aeruginosa utilization of Ammonium-N, Nitrate-N and Urea-N in culture. Heliyon 2020, 6, e03711. [Google Scholar] [CrossRef] [PubMed]
- Becton, D.A.C. BD BionutrientsTM Technical Manual—Advanced Bioprocessing. Available online: http://static.bdbiosciences.com/eu/documents/bionutrients_tech_manual.pdf (accessed on 20 December 2022).
- Puhm, M.; Ainelo, H.; Kivisaar, M.; Teras, R. Tryptone in Growth Media Enhances Pseudomonas putida Biofilm. Microorganisms 2022, 10, 618. [Google Scholar] [CrossRef] [PubMed]
- Malairuang, K.; Krajang, M.; Sukna, J.; Rattanapradit, K.; Chamsart, S. High cell density cultivation of Saccharomyces cerevisiae with intensive multiple sequential batches together with a novel technique of fed-batch at cell level (FBC). Processes 2020, 8, 1321. [Google Scholar] [CrossRef]
- Tanguler, H.; Erten, H. Utilisation of spent brewer’s yeast for yeast extract production by autolysis: The effect of temperature. Food Bioprod. Process. 2008, 86, 317–321. [Google Scholar] [CrossRef]
- Hahn-Hägerdal, B.; Karhumaa, K.; Larsson, C.U.; Gorwa-Grauslund, M.; Görgens, J.; van Zyl, W.H. Role of cultivation media in the development of yeast strains for large scale industrial use. Microb. Cell Factories 2005, 4, 31. [Google Scholar] [CrossRef]
- Jan, D.C.-H.; Jones, S.J.; Emery, A.N.; Al-Rubeai, M. Peptone, a low-cost growth-promoting nutrient for intensive animal cell culture. Cytotechnology 1994, 16, 17–26. [Google Scholar] [CrossRef]
- Gray, V.; Müller, C.; Watkins, I.; Lloyd, D. Peptones from diverse sources: Pivotal determinants of bacterial growth dynamics. J. Appl. Microbiol. 2008, 104, 554–565. [Google Scholar] [CrossRef]
- Saxena, J.; Tanner, R.S. Optimization of a corn steep medium for production of ethanol from synthesis gas fermentation by Clostridium ragsdalei. World J. Microbiol. Biotechnol. 2012, 28, 1553–1561. [Google Scholar] [CrossRef]
- Wang, G.; Shi, B.; Zhang, P.; Zhao, T.; Yin, H.; Qiao, C. Effects of corn steep liquor on β-poly(l-malic acid) production in Aureobasidium melanogenum. AMB Express 2020, 10, 211. [Google Scholar] [CrossRef]
- Ghasemi, Y.; Dehdari, Z.; Mohkam, M.; Kargar, M. Isolation and optimization of cultivation conditions for production of chitinase by Aeromonas sp. ZD-05 from the Persian Gulf. J. Pure Appl. Microbiol. 2013, 7, 913–918. [Google Scholar]
- Kennedy, M.; Krouse, D. Strategies for improving fermentation medium performance: A review. J. Ind. Microbiol. Biotechnol. 1999, 23, 456–475. [Google Scholar] [CrossRef]
- Arous, F.; Azabou, S.; Jaouani, A.; Zouari-Mechichi, H.; Nasri, M.; Mechichi, T. Biosynthesis of single-cell biomass from olive mill wastewater by newly isolated yeasts. Environ. Sci. Pollut. Res. 2016, 23, 6783–6792. [Google Scholar] [CrossRef]
- Jung, W.-J.; Park, R.-D. Bioproduction of chitooligosaccharides: Present and perspectives. Mar. Drugs 2014, 12, 5328–5356. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.; Antelo, L.T.; Franco-Uría, A.; Alonso, A.A.; Pérez-Martín, R. Chitin production from crustacean biomass: Sustainability assessment of chemical and enzymatic processes. J. Clean. Prod. 2018, 172, 4140–4151. [Google Scholar] [CrossRef]
- Hiraga, K.; Shou, L.; Kitazawa, M.; Takahashi, S.; Shimada, M.; Sato, R.; Oda, K. Isolation and characterization of chitinase from a flake-chitin degrading marine bacterium, Aeromonas hydrophila H-2330. Biosci. Biotechnol. Biochem. 1997, 61, 174–176. [Google Scholar] [CrossRef]
- Guo, S.-H.; Chen, J.-K.; Lee, W.-C. Purification and characterization of extracellular chitinase from Aeromonas schubertii. Enzym. Microb. Technol. 2004, 35, 550–556. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).