Process Optimization and Efficacy Assessment of Standardized PRP for Tendinopathies in Sports Medicine: Retrospective Study of Clinical Files and GMP Manufacturing Records in a Swiss University Hospital
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics Committee Approval of the Retrospective Study
- (i)
- Discriminate the success rate according to the age of the patients at the time of the PRP treatment.
- (ii)
- Highlight whether PRP could help in resuming physical activity faster in younger versus older patients.
- (iii)
- Determine how many PRP injections were necessary on average.
- (iv)
- Highlight the nature and volume of the resumed physical activity in the studied patient population.
- (i)
- Number of PRP applications required for healing.
- (ii)
- Ratio of platelet concentration in the PRP injection to platelet concentration in native blood (i.e., platelet concentration factor) for each patient.
- (iii)
- Possible relationship between the number of PRP injections required and the platelet concentration factor.
- (iv)
- Potential treatment-related adverse events as detected.
- (v)
- Patient clinical evolution following the PRP treatment.
2.2. Clinical Data Gathering and Processing
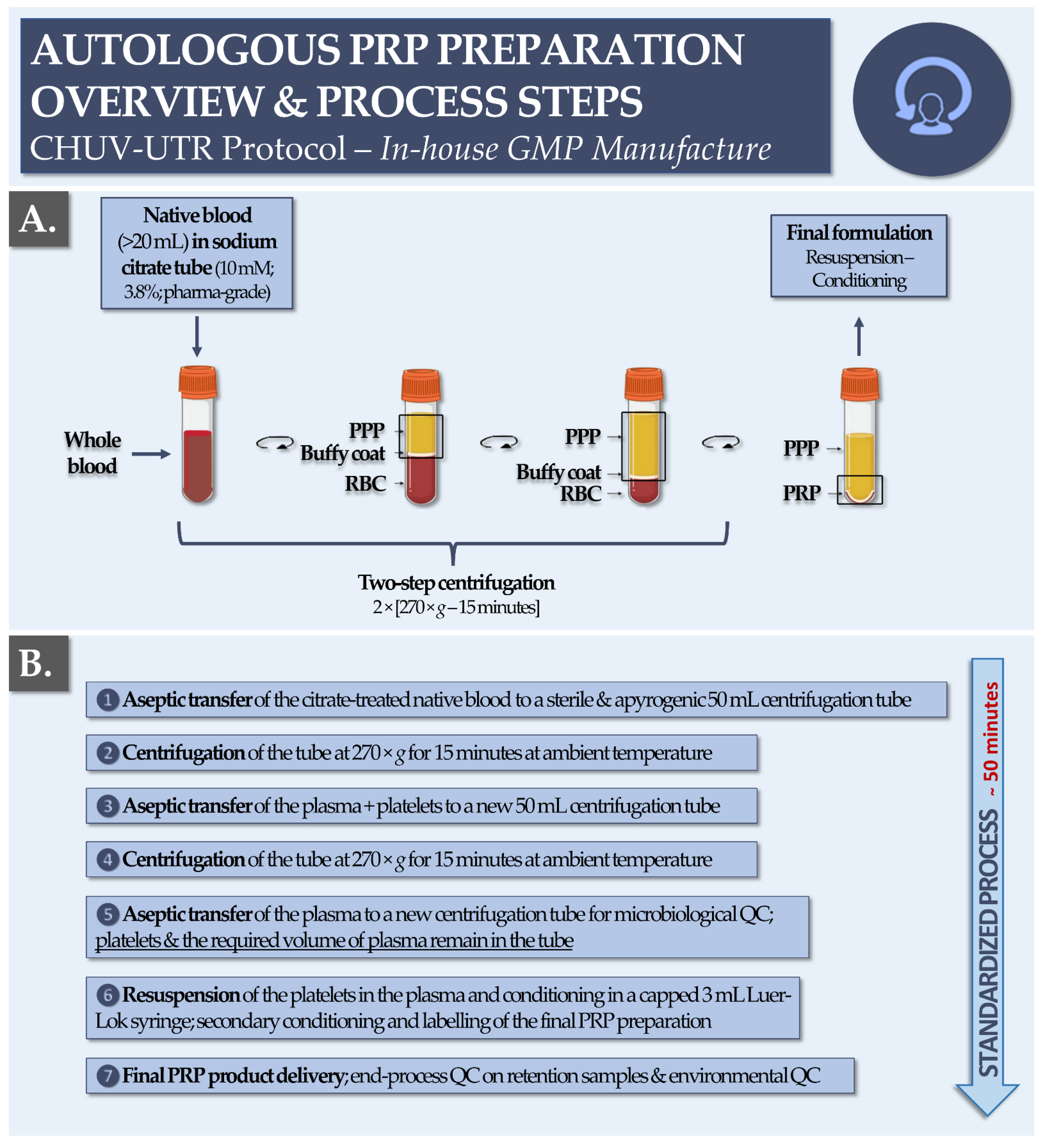
2.3. GMP Manufacturing Process for Autologous PRP at the CHUV
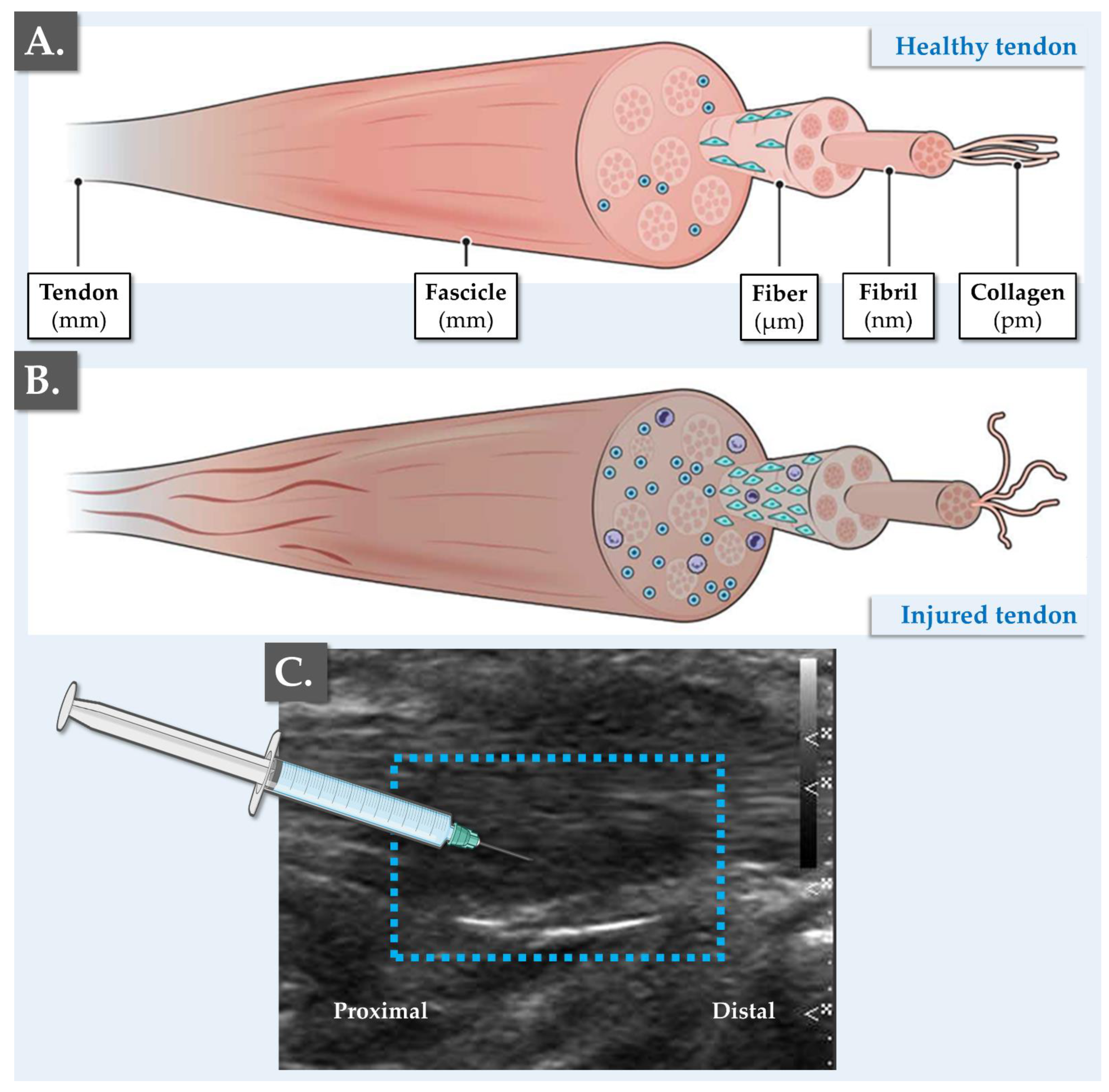
2.4. Medical Method for Orthobiological Management of Tendinopathies in Sports Medicine at the CHUV
2.5. Statistical Assessment of Data
3. Results
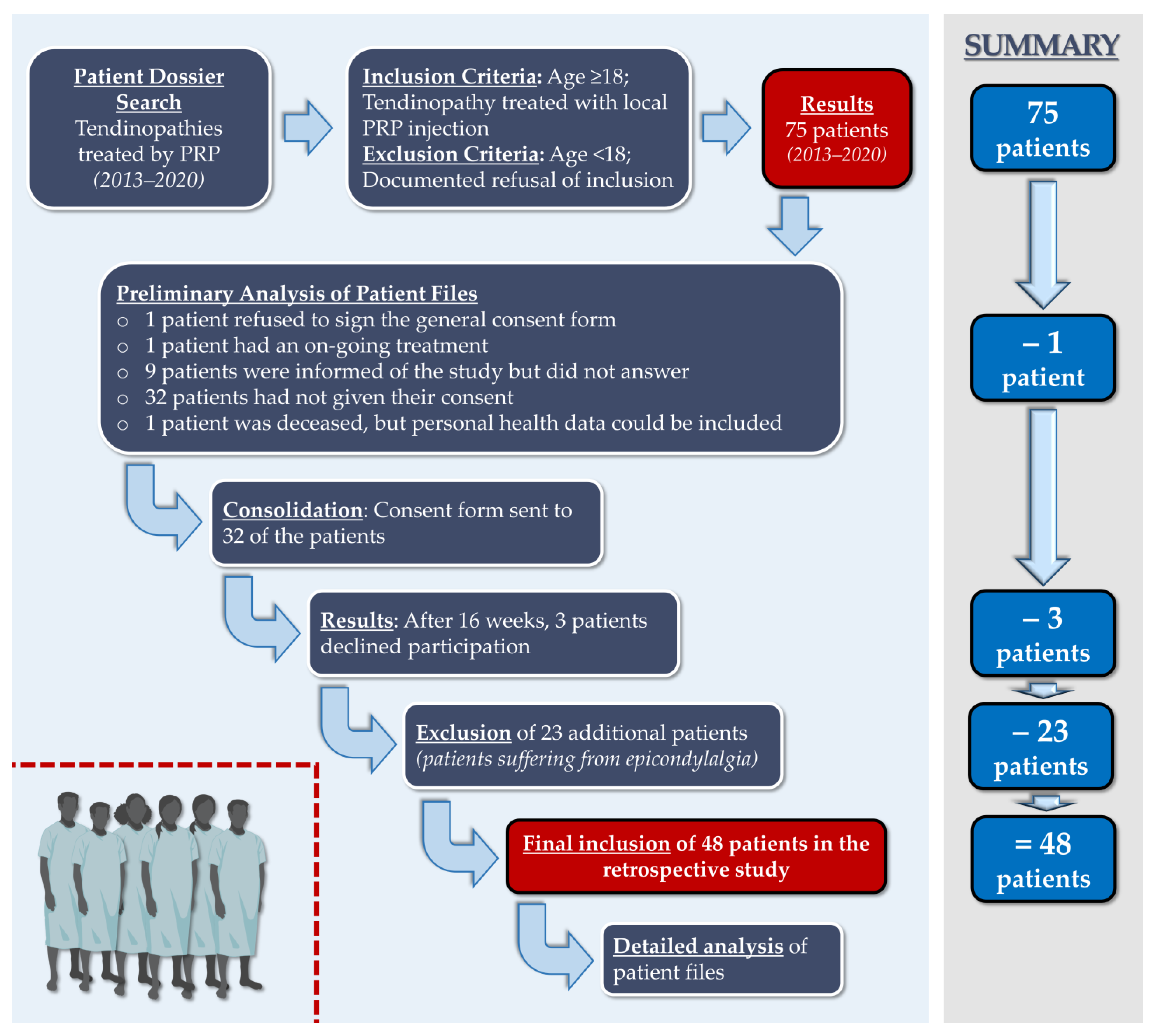
3.1. Retrospective Study Workflow and PRP GMP Manufacture
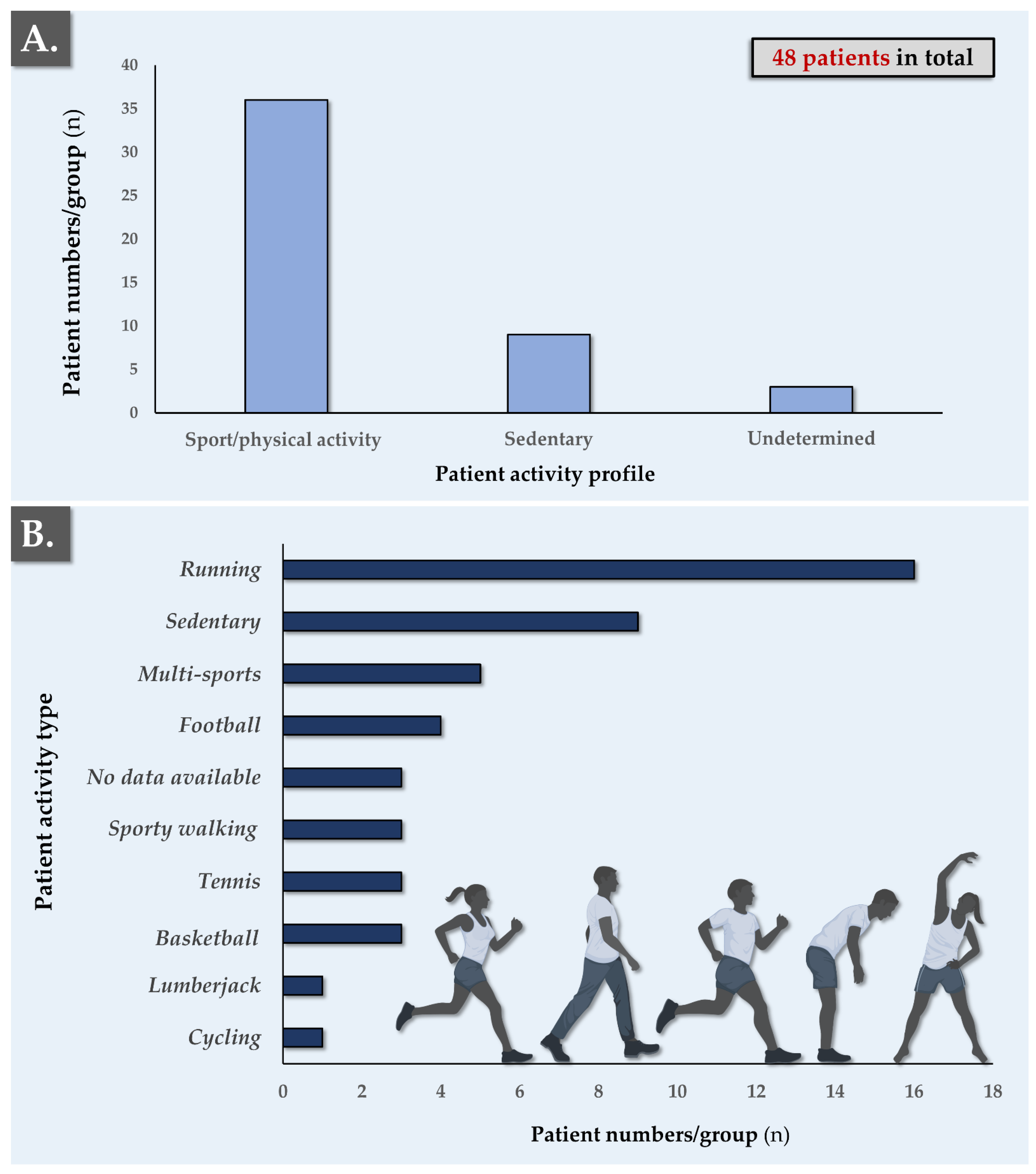
3.2. Patient-Related Parameters and PRP Clinical Administration for Tendinopathies
3.3. PRP GMP Manufacturing Data Analysis and Clinical Efficacy Evaluation
3.4. Standardized PRP GMP Manufacturing Process Statistical Evaluation
4. Discussion
4.1. Applicable Legal Bases and Advantages of GMP Autologous PRP Manufacture
- (i)
- Training and qualification of personnel.
- (ii)
- Validation of premises, equipment, testing procedures, and computerized systems.
- (iii)
- Traceability assurance (i.e., proper labeling of samples and materials).
- (iv)
- Storage and distribution of final products.
- (v)
- Performance of self-inspections/audits for any complaints, recalls, and notifications to hemovigilance, along with implementation of appropriate corrective and preventive actions.
4.2. PRP Manufacturing Process Standardization for Enhanced Therapeutic Quality
4.3. High Interest in Orthobiologics for Tendinopathy Management in Aging Populations
- (i)
- (ii)
- (iii)
4.4. Study Significance and Limitations
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CHUV | centre hospitalier universitaire vaudois |
| EC | European Commission |
| ECM | extracellular matrix |
| EDTA | ethylenediaminetetraacetic acid |
| EGF | epidermal growth factor |
| EU | European Union |
| GAG | glycosaminoglycan |
| GH | growth hormone |
| GMP | Good Manufacturing Practices |
| GPG | Good Practice Guidelines |
| HA | hyaluronic acid |
| IGF | insulin-like growth factor |
| MD | medical device |
| NSAID | non-steroidal anti-inflammatory drugs |
| PDGF | platelet-derived growth factor |
| PPP | platelet-poor plasma |
| PRP | platelet-rich plasma |
| QC | quality control |
| RBC | red blood cells |
| SD | standard deviation |
| TGF | transforming growth factor |
| TPA | Therapeutic Products Act |
| USA | United States of America |
| UTR | regenerative therapy unit |
| VEGF | vascular endothelial growth factor |
References
- Scott, A.; Ashe, M.C. Common tendinopathies in the upper and lower extremities. Curr. Sport Med. Rep. 2006, 5, 233–241. [Google Scholar] [CrossRef]
- Seinmann, S.; Pfeifer, C.G.; Brochhausen, C.; Docheva, D. Spectrum of tendon pathologies: Triggers, trails and end-state. Int. J. Mol. Sci. 2020, 21, 844. [Google Scholar] [CrossRef] [PubMed]
- Clayton, R.A.; Court-Brown, C.M. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury 2008, 39, 1338–1344. [Google Scholar] [CrossRef]
- Hopkins, C.; Fu, S.C.; Chua, E.; Hu, X.; Rolf, C.; Mattila, V.M.; Qin, L.; Yung, P.S.; Chan, K.M. Critical review on the socio-economic impact of tendinopathy. Asia Pac. J. Sport. Med. Arthrosc. Rehabil. Technol. 2016, 4, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Maffulli, N. Tendon injury and tendinopathy: Healing and repair. J. Bone Jt. Surg. Am. 2005, 87, 187–202. [Google Scholar] [CrossRef]
- Abate, M.; Schiavone, C.; Salini, V. The use of hyaluronic acid after tendon surgery and in tendinopathies. BioMed Res. Int. 2014, 2014, 783632. [Google Scholar] [CrossRef]
- Ho, J.O.; Sawadkar, P.; Mudera, V. A review on the use of cell therapy in the treatment of tendon disease and injuries. J. Tissue Eng. 2014, 5, 2041731414549678. [Google Scholar] [CrossRef] [PubMed]
- Lamplot, J.D.; Rodeo, S.A.; Brophy, R.H. A practical guide for the current use of biologic therapies in sports medicine. Am. J. Sport. Med. 2019, 48, 488–503. [Google Scholar] [CrossRef]
- Costa-Almeida, R.; Calejo, I.; Gomes, M.E. Mesenchymal stem cells empowering tendon regenerative therapies. Int. J. Mol. Sci. 2019, 20, 3002. [Google Scholar] [CrossRef]
- Romero, A.; Barrachina, L.; Ranera, B.; Remacha, A.R.; Moreno, B.; de Blas, I.; Sanz, A.; Vasquez, F.J.; Vitoria, A.; Junquera, C.; et al. Comparison of autologous bone marrow and adipose tissue derived mesenchymal stem cells, and platelet-rich plasma, for treating surgically induced lesions of the equine superficial digital flexor tendon. Vet. J. 2017, 224, 76–84. [Google Scholar] [CrossRef]
- Van den Boom, N.A.C.; Winters, M.; Haisma, H.J.; Moen, M.H. Efficacy of stem cell therapy for tendon disorders: A systematic review. Orthop. J. Sport. Med. 2020, 8, 2325967120915857. [Google Scholar] [CrossRef]
- Van der Vlist, A.C.; Winters, M.; Weir, A.; Ardern, C.L.; Welton, N.J.; Caldwell, D.M.; Verhaar, J.A.; de Vos, R.J. Which treatment is most effective for patients with Achilles tendinopathy? A living systematic review with network meta-analysis of 29 randomised controlled trials. Br. J. Sport. Med. 2021, 55, 249–256. [Google Scholar] [CrossRef]
- Laurent, A.; Abdel-Sayed, P.; Grognuz, A.; Scaletta, C.; Hirt-Burri, N.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; Kronen, P.; Nuss, K.; et al. Industrial development of standardized fetal progenitor cell therapy for tendon regenerative medicine: Preliminary safety in xenogeneic transplantation. Biomedicines 2021, 9, 380. [Google Scholar] [CrossRef] [PubMed]
- Cash, C.; Scott, L.; Lane Walden, R.; Kuhn, A.; Bowman, E. Bibliometric analysis of the top 50 highly cited articles on platelet-rich plasma in osteoarthritis and tendinopathy. Regen. Med. 2022, 17, 491–506. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, H.; Cui, Q.; Han, P.; Yang, S.; Shi, M.; Zhang, T.; Zhang, Z.; Li, Z. Tendon stem cell-derived exosomes regulate inflammation and promote the high-quality healing of injured tendon. Stem Cell Res. Ther. 2020, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Zhang, T.; Shi, M.; Song, X.; Yang, S.; Liu, H.; Zhang, M.; Cui, Q.; Li, Z. Hepatocyte growth factor-induced tendon stem cell conditioned medium promotes healing of injured Achilles tendon. Front. Cell Dev. Biol. 2021, 9, 654084. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.; Porcello, A.; Fernandez, P.G.; Jeannerat, A.; Peneveyre, C.; Abdel-Sayed, P.; Scaletta, C.; Hirt-Burri, N.; Michetti, M.; de Buys Roessingh, A.; et al. Combination of hyaluronan and lyophilized progenitor cell derivatives: Stabilization of functional hydrogel products for therapeutic management of tendinous tissue disorders. Pharmaceutics 2021, 13, 2196. [Google Scholar] [CrossRef]
- Lu, V.; Tennyson, M.; Zhang, J.; Khan, W. Mesenchymal stem cell-derived extracellular vesicles in tendon and ligament repair-A systematic review of in vivo studies. Cells 2021, 10, 2553. [Google Scholar] [CrossRef]
- Yao, Z.; Li, J.; Xiong, H.; Cui, H.; Ning, J.; Wang, S.; Ouyang, X.; Qian, Y.; Fan, C. MicroRNA engineered umbilical cord stem cell-derived exosomes direct tendon regeneration by mTOR signaling. J. Nanobiotech. 2021, 19, 169. [Google Scholar] [CrossRef]
- Song, K.; Jiang, T.; Pan, P.; Yao, Y.; Jiang, Q. Exosomes from tendon derived stem cells promote tendon repair through miR-144-3p-regulated tenocyte proliferation and migration. Stem Cell Res. Ther. 2022, 13, 80. [Google Scholar] [CrossRef]
- Xue, Z.; Chen, Z.; Wu, T.; Li, R.; Chen, C.; Liu, J.; Hou, H.; Zheng, X.; Wang, H. VEGFA-enriched exosomes from tendon-derived stem cells facilitate tenocyte differentiation, migration, and transition to a fibroblastic phenotype. BioMed Res. Int. 2022, 2022, 8537959. [Google Scholar] [CrossRef]
- Laurent, A.; Porcello, A.; Jeannerat, A.; Peneveyre, C.; Coeur, A.; Abdel-Sayed, P.; Scaletta, C.; Michetti, M.; de Buys Roessingh, A.; Jordan, O.; et al. Lyophilized progenitor tenocyte extracts: Sterilizable cytotherapeutic derivatives with antioxidant properties and hyaluronan hydrogel functionalization effects. Antioxidants 2023, 12, 163. [Google Scholar] [CrossRef]
- Alsousou, J.; Thompson, M.; Hulley, P.; Noble, A.; Willett, K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery. J. Bone Joint Surg. 2009, 91, 987–996. [Google Scholar] [CrossRef]
- Hsu, W.K.; Mishra, A.; Rodeo, S.R.; Fu, F.; Terry, M.A.; Randelli, P.; Canale, S.T.; Kelly, F.B. Platelet-rich plasma in orthopaedic applications: Evidence-based recommendations for treatment. J. Am. Acad. Orthop. Surg. 2013, 21, 739–748. [Google Scholar] [CrossRef]
- Samadi, P.; Sheykhhasan, M.; Khoshinani, H.M. The use of platelet-rich plasma in aesthetic and regenerative medicine: A comprehensive review. Aesth. Plast. Surg. 2019, 43, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Sun, Y.; Qi, Y.; Zhu, Y.; Wei, H.; Wu, D.; Li, C. Effect of platelet-rich plasma injection on mild or moderate carpal tunnel syndrome: An updated systematic review and meta-analysis of randomized controlled trials. BioMed Res. Int. 2020, 2020, 5089378. [Google Scholar] [CrossRef]
- Coulange Zavarro, A.; De Girolamo, L.; Laver, L.; Sánchez, M.; Tischer, T.; Filardo, G.; Sabatier, F.; Magalon, J. The top 100 most cited articles on platelet-rich plasma use in regenerative medicine–A bibliometric analysis–from the ESSKA orthobiologic initiative. Bioengineering 2022, 9, 580. [Google Scholar] [CrossRef]
- Akhundov, K.; Pietramaggiori, G.; Waselle, L.; Darwiche, S.; Guerid, S.; Scaletta, C.; Hirt-Burri, N.; Applegate, L.A.; Raffoul, W. Development of a cost-effective method for platelet-rich plasma (PRP) preparation for topical wound healing. Ann. Burns Fire Dis. 2012, 4, 207–213. [Google Scholar]
- Fiorentino, S.; Roffi, A.A.L.A.; Filardo, B.G.; Marcacci, M.; Kon, E. European definitions, current use, and EMA stance of platelet-rich plasma in sports medicine. J. Knee Surg. 2015, 1, 51–54. [Google Scholar] [CrossRef]
- Yaman, R.; Kinard, T.N. Platelet rich plasma: Hope or hype? Ann. Blood 2022, 7, 1–7. [Google Scholar] [CrossRef]
- Chemali, M.; Laurent, A.; Scaletta, C.; Waselle, L.; Simon, J.-P.; Michetti, M.; Brunet, J.-F.; Flahaut, M.; Hirt-Burri, N.; Raffoul, W.; et al. Burn center organization and cellular therapy integration: Managing risks and costs. J. Burn Care Res. 2021, 42, 911–924. [Google Scholar] [CrossRef]
- Martinez, C.E.; Smith, P.C.; Palma Alvarado, V.A. The influence of platelet-derived products on angiogenesis and tissue repair: A concise update. Front. Physiol. 2015, 6, 290. [Google Scholar] [CrossRef] [PubMed]
- Andriolo, L.; Altamura, S.A.; Reale, D.; Candrian, C.; Zaffagnini, S.; Filardo, G. Nonsurgical treatments of patellar tendinopathy: Multiple injections of platelet-rich plasma are a suitable option: A systematic review and meta-analysis. Am. J. Sport. Med. 2019, 47, 1001–1018. [Google Scholar] [CrossRef] [PubMed]
- Senna, M.K.; Shaat, R.M.; Alaa Ali Awad, A. Platelet-rich plasma in treatment of patients with idiopathic carpal tunnel syndrome. Clin. Rheumatol. 2019, 38, 3643–3654. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Zheng, Y.; Rai, S.; Din, Y.; Zhou, Y.; Liu, R.; Li, J. Efficacy and safety of platelet-rich plasma in the treatment of carpal tunnel syndrome: A network meta-analysis of different injection treatments. Front. Pharmacol. 2022, 13, 906075. [Google Scholar] [CrossRef]
- Galán, V.; Iñigo-Dendariarena, I.; Galán, I.; Prado, R.; Padilla, S.; Anitua, E. The effectiveness of plasma rich in growth factors (PRGF) in the treatment of nerve compression syndromes of the upper extremity: A retrospective observational clinical study. J. Clin. Med. 2022, 11, 4789. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Ortiz-Babilonia, C.; Xu, A.L.; Rogers, D.; Vulcano, E.; Aiyer, A.A. The statistical fragility of platelet-rich plasma as treatment for plantar fasciitis: A systematic review and simulated fragility analysis. Foot Ankle Orthop. 2022, 7, 24730114221144049. [Google Scholar] [CrossRef]
- Gupta, A.K.; Cole, J.; Deutsch, D.P.; Everts, P.A.; Niedbalski, R.P.; Panchaprateep, R.; Rinaldi, F.; Rose, P.T.; Sinclair, R.; Vogel, J.E.; et al. Platelet-rich plasma as a treatment for androgenetic alopecia. Dermatol. Surg. 2019, 45, 1262–1273. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Systematic review: Adipose-derived mesenchymal stem cells, platelet-rich plasma and biomaterials as new regenerative strategies in chronic skin wounds and soft tissue defects. Int. J. Mol. Sci. 2021, 22, 1538. [Google Scholar] [CrossRef]
- Bigby, M.; Grimalt, R. Platelet-rich plasma lacks evidence of clinically significant improvement in androgenetic alopecia. J. Am. Acad. Dermatol. 2021, 84, 1183–1185. [Google Scholar] [CrossRef]
- Chan, G.K.L.; Guo, M.S.; Dai, D.K.; Lai, Q.W.S.; Fung, K.W.C.F.; Zheng, B.Z.; Wu, K.Q.; Man, B.K.K.; Dong, T.T.; Tsim, K.W.K. An optimized extract, named self-growth colony, from platelet-rich plasma shows robust skin rejuvenation and anti-ageing properties: A novel technology in development of cosmetics. Skin Pharmacol. Physiol. 2021, 34, 74–85. [Google Scholar] [CrossRef]
- Chamata, E.S.; Bartlett, E.L.; Weir, D.; Rohrich, R.J. Platelet-rich plasma: Evolving role in plastic surgery. Plast. Reconstr. Surg. 2021, 147, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Alves, R.; Cole, J.; Andjelkov, K. AIRMESS—Academy of International Regenerative Medicine & Surgery Societies: Recommendations in the use of platelet-rich plasma (PRP), autologous stem cell-based therapy (ASC-BT) in androgenetic alopecia and wound healing. Exp. Opin. Biol. Ther. 2021, 21, 1443–1449. [Google Scholar] [CrossRef]
- Fadadu, P.P.; Mazzola, A.J.; Hunter, C.W.; Davis, T.T. Review of concentration yields in commercially available platelet-rich plasma (PRP) systems: A call for PRP standardization. Reg. Anesth. Pain Med. 2019, 44, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.R.; Mooney, D.J.; Weber, E. Advances toward transformative therapies for tendon diseases. Sci. Transl. Med. 2022, 14, eabl8814. [Google Scholar] [CrossRef]
- Dhurat, R.; Sukesh, M.S. Principles and methods of preparation of platelet-rich plasma: A review and author’s perspective. J. Cutan. Aesthet. Surg. 2014, 7, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Klatte-Schulz, F.; Schmidt, T.; Uckert, M.; Scheffler, S.; Kalus, U.; Rojewski, M.; Schrezenmeier, H.; Pruss, A.; Wildemann, B. Comparative analysis of different platelet lysates and platelet rich preparations to stimulate tendon cell biology: An in vitro study. Int. J. Mol. Sci. 2018, 19, 212. [Google Scholar] [CrossRef]
- European Parliament and Council. Directive 2002/98/EC setting standards of quality and safety for the collection, testing, processing, storage and distribution of human blood and blood components and amending Directive 2001/83/EC. Off. J. Eur. Union 2003, 33, 30–40. [Google Scholar]
- European Parliament and Council. Directive 2004/33/EC implementing Directive 2002/98/EC of the European Parliament and of the Council as regards certain technical requirements for blood and blood components. Off. J. Eur. Union 2004, 91, 25–39. [Google Scholar]
- European Parliament and Council. Directive 2005/62/EC implementing Directive 2002/98/EC of the European Parliament and of the Council as regards Community standards and specifications relating to a quality system for blood establishments. Off. J. Eur. Union. 2005, 256, 41–48. [Google Scholar]
- Anitua, E.; Prado, R.; Orive, G. Closing regulatory gaps: New ground rules for platelet-rich plasma. Trends Biotech. 2015, 33, 492–495. [Google Scholar] [CrossRef]
- Anitur, E.; Prado, R.; Orive, G. A new regulatory framework for platelet-rich plasma in Spain. J. Knee Surg. 2015, 28, 355–356. [Google Scholar] [CrossRef]
- European Parliament and Council. Directive 2016/1214 amending Directive 2005/62/EC as regards quality system standards and specifications for blood establishments. Off. J. Eur. Union 2016, 199, 14–15. [Google Scholar]
- European Parliament and Council. Regulation (EU) 2017/745 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC. Off. J. Eur. Union 2017, 117, 1–175. [Google Scholar]
- European Parliament and Council. Regulation (EU) 2017/746 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU. Off. J. Eur. Union 2017, 117, 176–332. [Google Scholar]
- Sussman, W.I.; Mautner, K.; Malanga, G. The role of rehabilitation after regenerative and orthobiologic procedures for the treatment of tendinopathy: A systematic review. Regen. Med. 2018, 13, 249–263. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Federal Assembly of the Swiss Confederation. Federal Law on Medication and Medical Devices (Law on Therapeutic Products) SR 812.21; Switzerland’s Federal Council: Bern, Switzerland, 2000; Available online: https://fedlex.data.admin.ch/eli/cc/2001/422 (accessed on 1 February 2023).
- Laurent, A.; Hirt-Burri, N.; Scaletta, C.; Michetti, M.; de Buys Roessingh, A.S.; Raffoul, W.; Applegate, L.A. Holistic approach of Swiss fetal progenitor cell banking: Optimizing safe and sustainable substrates for regenerative medicine and biotechnology. Front. Bioeng. Biotechnol. 2020, 8, 557758. [Google Scholar] [CrossRef] [PubMed]
- Philippe, V.; Laurent, A.; Hirt-Burri, N.; Abdel-Sayed, P.; Scaletta, C.; Schneebeli, V.; Michetti, M.; Brunet, J.-F.; Applegate, L.A.; Martin, R. Retrospective analysis of autologous chondrocyte-based cytotherapy production for clinical use: GMP process-based manufacturing optimization in a Swiss university hospital. Cells 2022, 11, 1016. [Google Scholar] [CrossRef]
- Fink, G.; Summer, B.E.H.; McQueen, J.K.; Wilson, H.; Rosie, R. Sex hormones, mood, mental state and memory. Clin. Exp. Pharm. Physiol. 1998, 25, 764–775. [Google Scholar] [CrossRef]
- Sniekers, Y.H.; Weinans, H.; van Osch, G.J.V.M.; van Leeuwen, J.P.T.M. Estrogen is important for maintenance of cartilage and subchondral bone in a murine model of knee osteoarthritis. Arthritis Res. Ther. 2010, 12, R182. [Google Scholar] [CrossRef]
- Dixit, M.; Bahadur Poudel, S.; Yakar, S. Effects of GH/IGF axis on bone and cartilage. Mol. Cell. Endocrinol. 2020, 519, 111052. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, T.A.H. Neovascularization in tendinopathy: From eradication to stabilization. Br. J. Sport. Med. 2020, 54, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Petrou, I.G.; Grognuz, A.; Hirt-Burri, N.; Raffoul, W.; Applegate, L.A. Cell therapies for tendons: Old cell choice for modern innovation. Swiss Med. Wkly 2014, 144, w13989. [Google Scholar] [CrossRef]
- Grognuz, A.; Aeberhard, P.-A.; Michetti, M.; Hirt-Burri, N.; Scaletta, C.; de Buys Roessingh, A.; Raffoul, W.; Laurent-Applegate, L.A. Cell therapies for tendon: Treatments and regenerative medicine. In Regenerative Medicine and Plastic Surgery; Duscher, D., Shiffman, M.A., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Stojanovic, P. Platelet rich plasma: A short overview of certain bioactive components. Open Med. 2016, 11, 242–247. [Google Scholar] [CrossRef]
- Boswell, S.G.; Cole, B.J.; Sundman, E.A.; Karas, V.; Fortier, L.A. Platelet-rich plasma: A milieu of bioactive factors. Arthroscopy 2012, 28, 429–439. [Google Scholar] [CrossRef]
- Gupta, A.K.; Renaud, H.J.; Bamimore, M. Platelet-rich plasma for androgenetic alopecia: Efficacy differences between men and women. Dermat. Ther. 2020, 33, e14143. [Google Scholar] [CrossRef]
- Bansal, H.; Leon, J.; Pont, J.L.; Wilson, D.A.; Bansal, A.; Agarwal, D.; Preoteasa, I. Platelet-rich plasma (PRP) in osteoarthritis (OA) knee: Correct dose critical for long term clinical efficacy. Sci. Rep. 2021, 11, 3971. [Google Scholar] [CrossRef]
- Popescu, M.N.; Iliescu, M.G.; Beiu, C.; Popa, L.G.; Mihai, M.M.; Berteanu, M.; Ionescu, A.M. Autologous platelet-rich plasma efficacy in the field of regenerative medicine: Product and quality control. BioMed Res. Int. 2021, 2021, 4672959. [Google Scholar] [CrossRef]
- Wasterlain, A.S.; Braun, H.J.; Harris, A.H.S.; Kim, H.J.; Dragoo, J.L. The systemic effects of platelet-rich plasma injection. Am. J. Sport. Med. 2013, 41, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Agostini, F.; Chieregato, K.; Amati, E.; Durante, C.; Rassu, M.; Ruggeri, M.; Sella, S.; Lombardi, E.; Mazzucato, M.; et al. The production method affects the efficacy of platelet derivatives to expand mesenchymal stromal cells in vitro. J. Transl. Med. 2017, 15, 90. [Google Scholar] [CrossRef] [PubMed]
- Harrison, T.E.; Bowler, J.; Levins, T.N.; Reeves, K.D.; Cheng, A.L. Platelet-rich plasma centrifugation changes leukocyte ratios. Cureus 2021, 13, e14470. [Google Scholar] [CrossRef]
- Machado, E.S.; Leite, R.; Dos Santos, C.C.; Artuso, G.L.; Gluszczak, F.; de Jesus, L.G.; Caldas, J.M.P.; Bredemeier, M. Turn down—Turn up: A simple and low-cost protocol for preparing platelet-rich plasma. Clinics 2019, 74, e1132. [Google Scholar] [CrossRef] [PubMed]
- Beitzel, K.; Allen, D.; Apostolakos, J.; Russell, R.P.; McCarthy, M.B.; Gallo, G.J.; Cote, M.P.; Mazzocca, A.D. US definitions, current use, and FDA stance on use of platelet-rich plasma in sports medicine. J. Knee Surg. 2015, 28, 29–34. [Google Scholar] [CrossRef]
- Graiet, H.; Lokchine, A.; Francois, P.; Velier, M.; Grimaud, F.; Loyens, M.; Berda-Haddad, Y.; Veran, J.; Dignat-George, F.; Sabatier, F.; et al. Use of platelet-rich plasma in regenerative medicine: Technical tools for correct quality control. BMJ Open Sport Exerc. Med. 2018, 4, e000442. [Google Scholar] [CrossRef] [PubMed]
- Tey, R.V.; Haldankar, P.; Joshi, V.R.; Raj, R.; Maradi, R. Variability in platelet-rich plasma preparations used in regenerative medicine: A comparative analysis. Stem Cells Int. 2022, 2022, 3852898. [Google Scholar] [CrossRef] [PubMed]
- Magalon, J.; Brandin, T.; Francois, P.; Degioanni, C.; De Maria, L.; Grimaud, F.; Veran, J.; Dignat-George, F.; Sabatier, F. Technical and biological review of authorized medical devices for platelets-rich plasma preparation in the field of regenerative medicine. Platelets 2021, 32, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Sebbagh, P.; Cannone, A.; Gremion, G.; Gremeaux, V.; Raffoul, W.; Hirt-Burri, N.; Michetti, M.; Abdel-Sayed, P.; Laurent, A.; Wardé, N.; et al. Current status of PRP manufacturing requirements & European regulatory frameworks: Practical tools for the appropriate implementation of PRP therapies in musculoskeletal regenerative medicine. Bioengineering 2023, 10, 292. [Google Scholar] [CrossRef]

| Year | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | Total |
|---|---|---|---|---|---|---|---|---|---|
| Patients (n) | 1 | 7 | 17 | 4 | 4 | 5 | 5 | 5 | 48 |
| Age of patients (years) | Average of 43.4 ± 16.6 years | ||||||||
| Patient age distribution (n) 1 | ≤45 years old: 26 patients (54%) 46–65 years old: 16 patients (33%) >65 years old: 6 patients (13%) | ||||||||
| Patient Follow-Up | Number of Patients | Patient Age (Mean ± SD) | Platelet Concentration Factors (Mean ± SD) |
|---|---|---|---|
| All patients | 48 | 43.3 ± 16.6 | 2.79 ± 1.34 |
| Positive evolution | 36 | 41.7 ± 22.9 | 2.92 ± 1.46 |
| Non-positive evolution | 12 | 47.5 ± 28.5 | 2.28 ± 0.28 |
| Patient Follow-Up | Male Patients (n) Percentage of Subgroup (%) Average Number of PRP Injections (n) | Female Patients (n) Percentage of Subgroup (%) Average Number of PRP Injections (n) |
|---|---|---|
| Positive evolution | 25 | 11 |
| 69.4% | 30.6% | |
| 1.6 | 1.6 | |
| Non-positive evolution | 6 | 6 |
| 50.0% | 50.0% | |
| 1.0 | 2.0 |
| Parameters | Experimental Results |
|---|---|
| Average gap value | 14.0% ± 6.3% |
| Average gap value in male patients | 18.7% |
| Average gap value in female patients | 9.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebbagh, P.; Hirt-Burri, N.; Scaletta, C.; Abdel-Sayed, P.; Raffoul, W.; Gremeaux, V.; Laurent, A.; Applegate, L.A.; Gremion, G. Process Optimization and Efficacy Assessment of Standardized PRP for Tendinopathies in Sports Medicine: Retrospective Study of Clinical Files and GMP Manufacturing Records in a Swiss University Hospital. Bioengineering 2023, 10, 409. https://doi.org/10.3390/bioengineering10040409
Sebbagh P, Hirt-Burri N, Scaletta C, Abdel-Sayed P, Raffoul W, Gremeaux V, Laurent A, Applegate LA, Gremion G. Process Optimization and Efficacy Assessment of Standardized PRP for Tendinopathies in Sports Medicine: Retrospective Study of Clinical Files and GMP Manufacturing Records in a Swiss University Hospital. Bioengineering. 2023; 10(4):409. https://doi.org/10.3390/bioengineering10040409
Chicago/Turabian StyleSebbagh, Patrick, Nathalie Hirt-Burri, Corinne Scaletta, Philippe Abdel-Sayed, Wassim Raffoul, Vincent Gremeaux, Alexis Laurent, Lee Ann Applegate, and Gerald Gremion. 2023. "Process Optimization and Efficacy Assessment of Standardized PRP for Tendinopathies in Sports Medicine: Retrospective Study of Clinical Files and GMP Manufacturing Records in a Swiss University Hospital" Bioengineering 10, no. 4: 409. https://doi.org/10.3390/bioengineering10040409
APA StyleSebbagh, P., Hirt-Burri, N., Scaletta, C., Abdel-Sayed, P., Raffoul, W., Gremeaux, V., Laurent, A., Applegate, L. A., & Gremion, G. (2023). Process Optimization and Efficacy Assessment of Standardized PRP for Tendinopathies in Sports Medicine: Retrospective Study of Clinical Files and GMP Manufacturing Records in a Swiss University Hospital. Bioengineering, 10(4), 409. https://doi.org/10.3390/bioengineering10040409








