Exploratory Study on Chemosensory Event-Related Potentials in Long COVID-19 and Mild Cognitive Impairment: A Common Pathway?
Abstract
1. Introduction
1.1. Long Covid and MCI: Is There Overlapping Symptomatology?
1.2. Olfactory, Psychophysical, and Psychophysiological Assessments
1.2.1. Psychophysical Assessment
1.2.2. Psychophysiological Assessment: CSERP as Biomarkers of Olfactory Impairment
2. Materials and Methods
2.1. Subjects
2.2. OERP Recording
2.3. Olfactometer and Task Methodology
2.4. Statistical Analysis:
3. Results
Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tavares-Júnior, J.W.L.; de Souza, A.C.C.; Borges, J.W.P.; Oliveira, D.N.; Siqueira-Neto, J.I.; Sobreira-Neto, M.A.; Braga-Neto, P. COVID-19 Associated Cognitive Impairment: A Systematic Review. Cortex 2022, 152, 77–97. [Google Scholar] [CrossRef]
- Hartung, T.J.; Neumann, C.; Bahmer, T.; Chaplinskaya-Sobol, I.; Endres, M.; Geritz, J.; Haeusler, K.G.; Heuschmann, P.U.; Hildesheim, H.; Hinz, A.; et al. Fatigue and Cognitive Impairment after COVID-19: A Prospective Multicentre Study. EClinicalMedicine 2022, 53, 101651. [Google Scholar] [CrossRef]
- Taquet, M.; Sillett, R.; Zhu, L.; Mendel, J.; Camplisson, I.; Dercon, Q.; Harrison, P.J. Neurological and Psychiatric Risk Trajectories after SARS-CoV-2 Infection: An Analysis of 2-Year Retrospective Cohort Studies Including 1 284 437 Patients. Lancet Psychiatry 2022, 9, 815–827. [Google Scholar] [CrossRef]
- Desforges, M.; Le Coupanec, A.; Dubeau, P.; Bourgouin, A.; Lajoie, L.; Dubé, M.; Talbot, P.J. Human Coronaviruses and Other Respiratory Viruses: Underestimated Opportunistic Pathogens of the Central Nervous System? Viruses 2020, 12, 14. [Google Scholar] [CrossRef]
- Ercoli, T.; Masala, C.; Pinna, I.; Orofino, G.; Solla, P.; Rocchi, L.; Defazio, G. Qualitative Smell/Taste Disorders as Sequelae of Acute COVID-19. Neurol. Sci. 2021, 42, 4921–4926. [Google Scholar] [CrossRef]
- Ciaccio, M.; Lo Sasso, B.; Scazzone, C.; Gambino, C.M.; Ciaccio, A.M.; Bivona, G.; Piccoli, T.; Giglio, R.V.; Agnello, L. COVID-19 and Alzheimer’s Disease. Brain Sci. 2021, 11, 305. [Google Scholar] [CrossRef]
- Ding, Q.; Shults, N.V.; Gychka, S.G.; Harris, B.T.; Suzuki, Y.J. Protein Expression of Angiotensin-Converting Enzyme 2 (ACE2) Is Upregulated in Brains with Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 1687. [Google Scholar] [CrossRef]
- Baazaoui, N.; Iqbal, K. COVID-19 and Neurodegenerative Diseases: Prion-Like Spread and Long-Term Consequences. J. Alzheimer’s Dis. 2022, 88, 399–416. [Google Scholar] [CrossRef]
- Lingor, P.; Demleitner, A.F.; Wolff, A.W.; Feneberg, E. SARS-CoV-2 and Neurodegenerative Diseases: What We Know and What We Don’t. J. Neural Transm. 2022, 129, 1155–1167. [Google Scholar] [CrossRef]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 Is Associated with Changes in Brain Structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef]
- Frosolini, A.; Parrino, D.; Fabbris, C.; Fantin, F.; Inches, I.; Invitto, S.; Spinato, G.; Filippis, C. Magnetic Resonance Imaging Confirmed Olfactory Bulb Reduction in Long COVID-19: Literature Review and Case Series. Brain Sci. 2022, 12, 430. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The Diagnosis of Mild Cognitive Impairment Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Martin, T.; Giordani, B.; Kavcic, V. EEG Asymmetry and Cognitive Testing in MCI Identification. Int. J. Psychophysiol. 2022, 177, 213–219. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, Z. Exploring Potential Electrophysiological Biomarkers in Mild Cognitive Impairment: A Systematic Review and Meta-Analysis of Event-Related Potential Studies. J. Alzheimer’s Dis. 2017, 58, 1283–1292. [Google Scholar] [CrossRef]
- Ferreri, F.; Guerra, A.; Vollero, L.; Ponzo, D.; Määtta, S.; Könönen, M.; Vecchio, F.; Pasqualetti, P.; Miraglia, F.; Simonelli, I.; et al. TMS-EEG Biomarkers of Amnestic Mild Cognitive Impairment Due to Alzheimer’s Disease: A Proof-of-Concept Six Years Prospective Study. Front. Aging Neurosci. 2021, 13, 737281. [Google Scholar] [CrossRef]
- Invitto, S.; Grasso, A. Chemosensory Perception: A Review on Electrophysiological Methods in “Cognitive Neuro-Olfactometry”. Chemosensors 2019, 7, 45. [Google Scholar] [CrossRef]
- Invitto, S.; Piraino, G.; Ciccarese, V.; Carmillo, L.; Caggiula, M.; Trianni, G.; Nicolardi, G.; Di Nuovo, S.; Balconi, M. Potential Role of OERP as Early Marker of Mild Cognitive Impairment. Front. Aging Neurosci. 2018, 10, 272. [Google Scholar] [CrossRef]
- Walla, P.; Duregger, C.; Deecke, L.; Dal-Bianco, P. Dysfunctional Incidental Olfaction in Mild Cognitive Impairment (MCI): An Electroencephalography (EEG) Study. Brain Sci. 2011, 1, 3–15. [Google Scholar] [CrossRef]
- Bahar-Fuchs, A.; Chételat, G.; Villemagne, V.L.; Moss, S.; Pike, K.; Masters, C.L.; Rowe, C.; Savage, G. Olfactory Deficits and Amyloid-β Burden in Alzheimer’s Disease, Mild Cognitive Impairment, and Healthy Aging: A PiB PET Study. J. Alzheimer’s Dis. 2010, 22, 1081–1087. [Google Scholar] [CrossRef]
- Blomkvist, A.; Hofer, M. Olfactory Impairment and Close Social Relationships. A Narrative Review. Chem. Senses 2021, 46, bjab037. [Google Scholar] [CrossRef]
- Jung, H.J.; Shin, I.-S.; Lee, J.-E. Olfactory Function in Mild Cognitive Impairment and Alzheimer’s Disease: A Meta-Analysis. Laryngoscope 2019, 129, 362–369. [Google Scholar] [CrossRef]
- Chen, B.; Espin, M.; Haussmann, R.; Matthes, C.; Donix, M.; Hummel, T.; Haehner, A. The Effect of Olfactory Training on Olfaction, Cognition, and Brain Function in Patients with Mild Cognitive Impairment. J. Alzheimer’s Dis. 2022, 85, 745–754. [Google Scholar] [CrossRef]
- Hatami, M.; Tohidi, M.; Mohebi, R.; Khalili, D.; Azizi, F.; Hadaegh, F. Adolescent Lipoprotein Classifications According to National Health and Nutrition Examination Survey (NHANES) vs. National Cholesterol Education Program (NCEP) for Predicting Abnormal Lipid Levels in Adulthood in a Middle East Population. Lipids Health Dis. 2012, 11, 107. [Google Scholar] [CrossRef]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. “Sniffin” Sticks’: Olfactory Performance Assessed by the Combined Testing of Odor Identification, Odor Discrimination and Olfactory Threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef]
- Trecca, E.M.C.; Fortunato, F.; Gelardi, M.; Petrone, P.; Cassano, M. Development of a Questionnaire to Investigate Socio-Cultural Differences in the Perception of Smell, Taste and Flavour. Acta. Otorhinolaryngol. Ital. 2021, 41, 336–347. [Google Scholar] [CrossRef]
- Hura, N.; Yi, J.S.; Lin, S.Y.; Roxbury, C.R. Magnetic Resonance Imaging as a Diagnostic and Research Tool in Patients with Olfactory Dysfunction: A Systematic Review. Am. J. Rhinol. Allergy 2022, 36, 668–683. [Google Scholar] [CrossRef]
- Kandemirli, S.G.; Altundag, A.; Yildirim, D.; Tekcan Sanli, D.E.; Saatci, O. Olfactory Bulb MRI and Paranasal Sinus CT Findings in Persistent COVID-19 Anosmia. Acad. Radiol. 2021, 28, 28–35. [Google Scholar] [CrossRef]
- Invitto, S.; Calcagnì, A.; Piraino, G.; Ciccarese, V.; Balconi, M.; De Tommaso, M.; Toraldo, D.M. Obstructive Sleep Apnea Syndrome and Olfactory Perception: An OERP Study. Respir. Physiol. Neurobiol. 2019, 259, 37–44. [Google Scholar] [CrossRef]
- Harada, H.; Eura, Y.; Shiraishi, K.; Kato, T.; Soda, T. Coherence Analysis of EEG Changes during Olfactory Stimulation. Clin. EEG Neurosci. 1998, 29, 96–100. [Google Scholar] [CrossRef]
- Hanson-Vaux, G.; Crisinel, A.S.; Spence, C. Smelling Shapes: Crossmodal Correspondences between Odors and Shapes. Chem. Senses 2013, 38, 161–166. [Google Scholar] [CrossRef]
- Kaeppler, K. Crossmodal Associations Between Olfaction and Vision: Color and Shape Visualizations of Odors. Chemosens. Percept. 2018, 11, 95–111. [Google Scholar] [CrossRef]
- Tsushima, Y.; Nishino, Y.; Ando, H. Olfactory Stimulation Modulates Visual Perception Without Training. Front. Neurosci. 2021, 15, 642584. [Google Scholar] [CrossRef] [PubMed]
- Invitto, S.; Montinaro, R.; Ciccarese, V.; Venturella, I.; Fronda, G.; Balconi, M. Smell and 3D Haptic Representation: A Common Pathway to Understand Brain Dynamics in a Cross-Modal Task. A Pilot OERP and FNIRS Study. Front. Behav. Neurosci. 2019, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Schriever, V.A.; Peters, P.; Olze, H.; Uecker, F.C.; Hummel, T. Influence of Airflow Rate and Stimulus Concentration on Olfactory Event-Related Potentials (OERP) in Humans. Chem. Senses 2018, 43, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Pause, B.M.; Krauel, K. Chemosensory Event-Related Potentials (CSERP) as a Key to the Psychology of Odors. Int. J. Psychophysiol. 2000, 36, 105–122. [Google Scholar] [CrossRef]
- Gudziol, H.; Fischer, J.; Bitter, T.; Guntinas-Lichius, O. Chemosensory Event-Related Brain Potentials (CSERP) after Strictly Monorhinal Stimulation. Int. J. Psychophysiol. 2014, 93, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Crowley, K.E.; Colrain, I.M. A Review of the Evidence for P2 Being an Independent Component Process: Age, Sleep and Modality. Clin. Neurophysiol. 2004, 115, 732–744. [Google Scholar] [CrossRef]
- Flohr, E.L.R.; Boesveldt, S.; Haehner, A.; Iannilli, E.; Sinding, C.; Hummel, T. Time-Course of Trigeminal versus Olfactory Stimulation: Evidence from Chemosensory Evoked Potentials. Int. J. Psychophysiol. 2015, 95, 388–394. [Google Scholar] [CrossRef]
- Invitto, S.; Mazzatenta, A. Olfactory Event-Related Potentials and Exhaled Organic Volatile Compounds: The Slow Link Between Olfactory Perception and Breath Metabolic Response. A Pilot Study on Phenylethyl Alcohol and Vaseline Oil. Brain Sci. 2019, 9, 84. [Google Scholar] [CrossRef]
- Morgan, C.D.; Murphy, C. Differential Effects of Active Attention and Age on Event-Related Potentials to Visual and Olfactory Stimuli. Int. J. Psychophysiol. 2010, 78, 190–199. [Google Scholar] [CrossRef]
- Invitto, S.; Grasso, A.; Lofrumento, D.D.; Ciccarese, V.; Paladini, A.; Paladini, P.; Marulli, R.; De Pascalis, V.; Polsinelli, M.; Placidi, G. Chemosensory Event-Related Potentials and Power Spectrum Could Be a Possible Biomarker in 3M Syndrome Infants? Brain Sci. 2020, 10, 201. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Kim, S. Influence of Fragrances on Human Psychophysiological Activity: With Special Reference to Human Electroencephalographic Response. Sci. Pharm. 2016, 84, 724–752. [Google Scholar] [CrossRef]
- Hummel, T.; Barz, S.; Pauli, E.; Kobal, G. Chemosensory Event-Related Potentials Change with Age. Electroencephalogr. Clin. Neurophysiol. /Evoked Potentials Sect. 1998, 108, 208–217. [Google Scholar] [CrossRef]
- Cespón, J.; Galdo-Álvarez, S.; Díaz, F. Age-Related Changes in ERP Correlates of Visuospatial and Motor Processes. Psychophysiology 2013, 50, 743–757. [Google Scholar] [CrossRef]
- Murphy, C.; Morgan, C.D.; Geisler, M.W.; Wetter, S.; Covington, J.W.; Madowitz, M.D.; Nordin, S.; Polich, J.M. Olfactory Event-Related Potentials and Aging: Normative Data. Int. J. Psychophysiol. 2000, 36, 133–145. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; Hummel, T.; Hopkins, C.; Dibattista, M.; Menini, A.; Spinato, G.; Fabbris, C.; Emanuelli, E.; D’Alessandro, A.; Marzolino, R.; et al. High Prevalence of Long-Term Olfactory, Gustatory, and Chemesthesis Dysfunction in Post-COVID-19 Patients: A Matched Case-Control Study with One-Year Follow-up Using a Comprehensive Psychophysical Evaluation. Rhinology 2021, 59, 517–527. [Google Scholar] [CrossRef]
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013; ISBN 0-89042-554-X.
- Musicco, M.; Sorbi, S.; Bonavita, V.; Caltagirone, C. Validation of the Guidelines for the Diagnosis of Dementia and Alzheimer’s Disease of the Italian Neurological Society. Study in 72 Italian Neurological Centres and 1549 Patients. Neurol. Sci. 2004, 25, 289–295. [Google Scholar] [CrossRef]
- Tremblay, C.; Frasnelli, J. Olfactory and Trigeminal Systems Interact in the Periphery. Chem. Senses 2018, 43, 611–616. [Google Scholar] [CrossRef]
- Afonso, P. The Impact of the COVID-19 Pandemic on Mental Health. Acta Med. Port. 2020, 33, 356–357. [Google Scholar] [CrossRef]
- Najafloo, R.; Majidi, J.; Asghari, A.; Aleemardani, M.; Kamrava, S.K.; Simorgh, S.; Seifalian, A.; Bagher, Z.; Seifalian, A.M. Mechanism of Anosmia Caused by Symptoms of COVID-19 and Emerging Treatments. ACS Chem. Neurosci. 2021, 12, 3795–3805. [Google Scholar] [CrossRef]
- Hariyanto, T.I.; Putri, C.; Arisa, J.; Situmeang, R.F.V.; Kurniawan, A. Dementia and Outcomes from Coronavirus Disease 2019 (COVID-19) Pneumonia: A Systematic Review and Meta-Analysis. Arch. Gerontol. Geriatr. 2021, 93, 104299. [Google Scholar] [CrossRef]
- Fawaz, M.; Samaha, A. COVID-19 Quarantine: Post-Traumatic Stress Symptomatology among Lebanese Citizens. Int. J. Soc. Psychiatry 2020, 66, 666–674. [Google Scholar] [CrossRef]
- Aghagoli, G.; Gallo Marin, B.; Katchur, N.J.; Chaves-Sell, F.; Asaad, W.F.; Murphy, S.A. Neurological Involvement in COVID-19 and Potential Mechanisms: A Review. Neurocritical Care 2021, 34, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Invitto, S.; Romano, D.; Garbarini, F.; Bruno, V.; Urgesi, C.; Curcio, G.; Grasso, A.; Pellicciari, M.C.; Kock, G.; Betti, V.; et al. Major Stress-Related Symptoms During the Lockdown: A Study by the Italian Society of Psychophysiology and Cognitive Neuroscience. Front. Public Health 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Altundag, A.; Cayonu, M.; Kayabasoglu, G.; Salihoglu, M.; Tekeli, H.; Saglam, O.; Hummel, T. Modified Olfactory Training in Patients with Postinfectious Olfactory Loss. Laryngoscope 2015, 125. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L.; Kamath, V. The Influences of Age on Olfaction: A Review. Front. Psychol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Attems, J.; Walker, L.; Jellinger, K.A. Olfaction and Aging: A Mini-Review. Gerontology 2015, 61. [Google Scholar] [CrossRef]
- Costumero, V.; Barrós-Loscertales, A. The Left Frontoparietal Brain Network in Addictions. In Handbook of Substance Misuse and Addictions: From Biology to Public Health; Patel, V.B., Preedy, V.R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–24. ISBN 978-3-030-67928-6. [Google Scholar]
- Costumero, V.; Rosell-Negre, P.; Bustamante, J.C.; Fuentes-Claramonte, P.; Llopis, J.J.; Ávila, C.; Barrós-Loscertales, A. Left Frontoparietal Network Activity Is Modulated by Drug Stimuli in Cocaine Addiction. Brain Imaging Behav. 2018, 12, 1259–1270. [Google Scholar] [CrossRef]
- Uehara, S.; Mizuguchi, N.; Hirose, S.; Yamamoto, S.; Naito, E. Involvement of Human Left Frontoparietal Cortices in Neural Processes Associated with Task-Switching between Two Sequences of Skilled Finger Movements. Brain Res. 2019, 1722, 146365. [Google Scholar] [CrossRef]
- Vettore, M.; De Marco, M.; Pallucca, C.; Bendini, M.; Gallucci, M.; Venneri, A. White-Matter Hyperintensity Load and Differences in Resting-State Network Connectivity Based on Mild Cognitive Impairment Subtype. Front. Aging Neurosci. 2021, 13, 737359. [Google Scholar] [CrossRef]
- Azamat, S.; Betul Arslan, D.; Erdogdu, E.; Kicik, A.; Cengiz, S.; Eryürek, K.; Tufekcioglu, Z.; Bilgic, B.; Hanagasi, H.; Demiralp, T.; et al. Detection of Visual and Frontoparietal Network Perfusion Deficits in Parkinson’s Disease Dementia. Eur. J. Radiol. 2021, 144, 109985. [Google Scholar] [CrossRef] [PubMed]
- The Cognitive Impact of Long COVID: What Can Psychologists Do. Available online: https://www.apa.org/monitor/2022/11/psychologists-long-covid (accessed on 10 February 2023).
- Invitto, S.; Leucci, M.; Accogli, G.; Schito, A.; Nestola, C.; Ciccarese, V.; Rinaldi, R.; Boscolo Rizzo, P.; Spinato, G.; Leo, S. Chemobrain, Olfactory and Lifestyle Assessment in Onco-Geriatrics: Sex-Mediated Differences between Chemotherapy and Immunotherapy. Brain Sci. 2022, 12, 1390. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Miyata, J.; Mori, Y.; Isobe, M.; Urayama, S.-I.; Aso, T.; Fukuyama, H.; Murai, T.; Takahashi, H. Lateralization of Intrinsic Frontoparietal Network Connectivity and Symptoms in Schizophrenia. Psychiatry Res. Neuroimaging 2017, 260, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhang, Y.; Shi, Y.; Yin, X.; Wang, S.; Li, Y.; Zhao, T.; Liu, W.; Zhou, A.; Jia, L. A 19-Year-Old Adolescent with Probable Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 91, 915–922. [Google Scholar] [CrossRef]

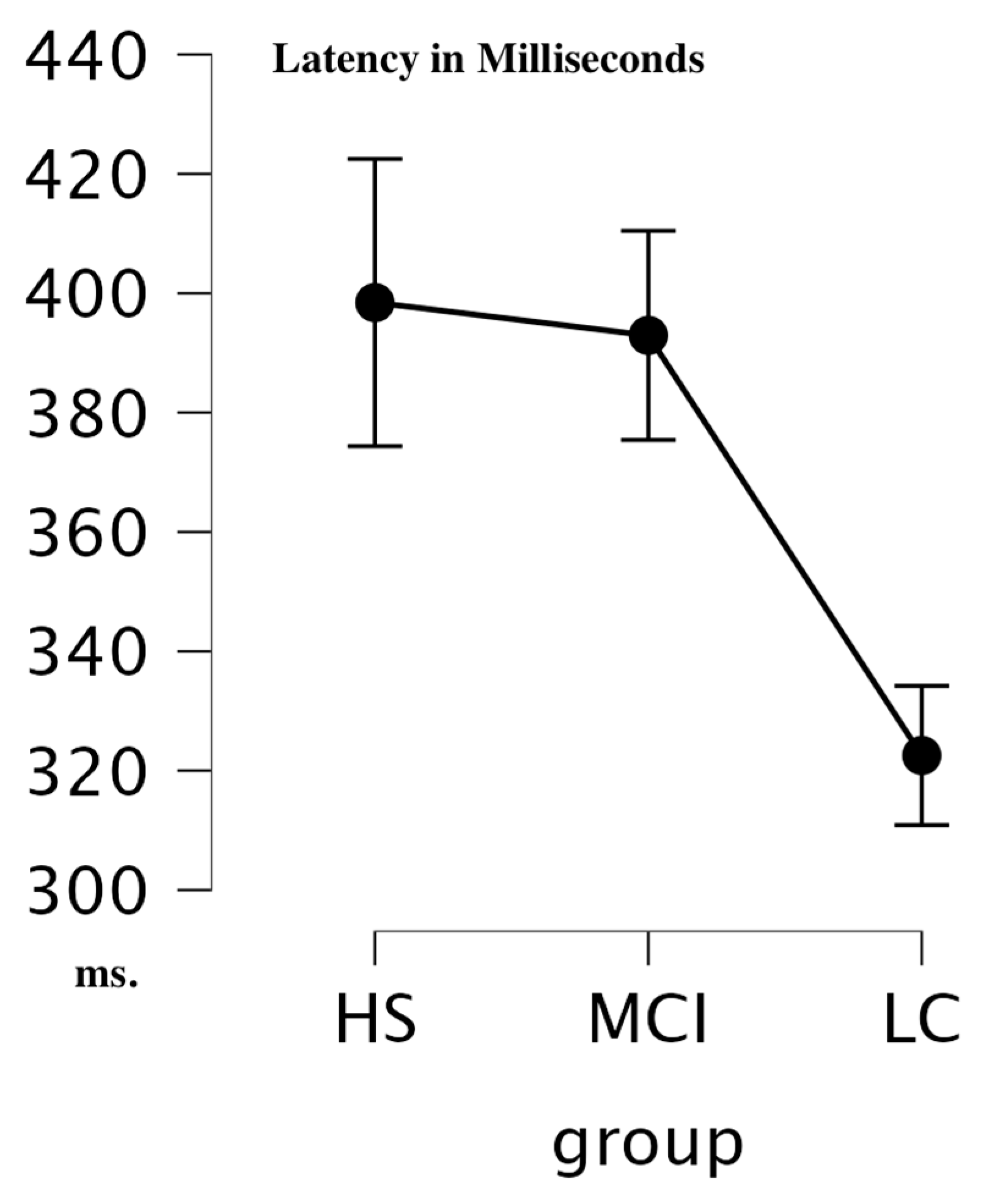
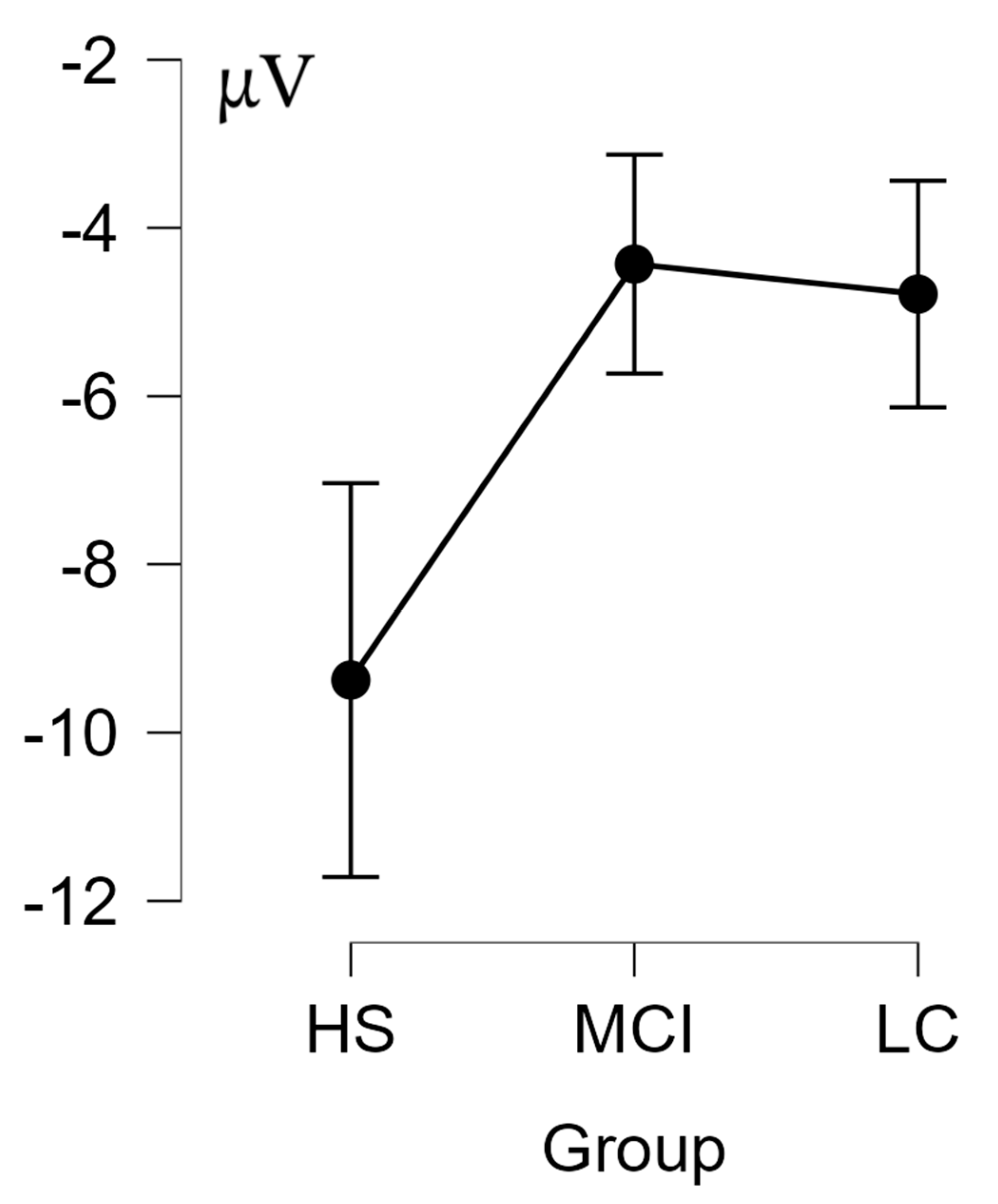
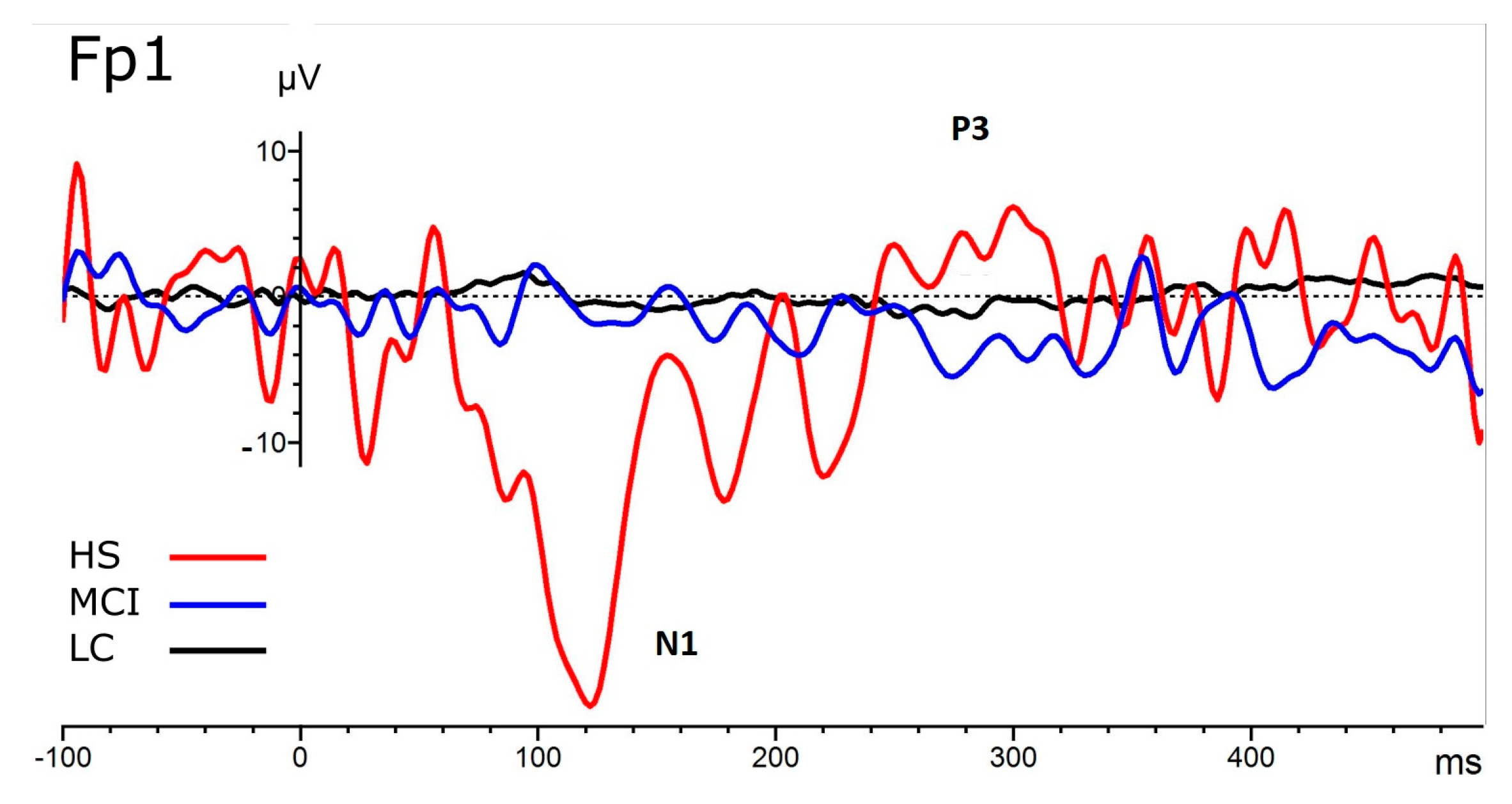

| CSERP | Condition | Cases | F | p | η2 |
|---|---|---|---|---|---|
| N1 Latency | Within | Electrode | 0.513 | 0.907 | 0.010 |
| Electrode*Group | 0.818 | 0.715 | 0.032 | ||
| Between | Group | 0.888 | 0.420 | 0.032 | |
| P3 Latency | Within | Electrode | 2.444 | 0.004 | 0.046 |
| Electrode*Group | 0.636 | 0.910 | 0.024 | ||
| Between | Group | 10.119 | <0.001 | 0.074 |
| Group Comparison | Mean Diff | SE | t | Pholm | |
|---|---|---|---|---|---|
| HS | MCI | 8.309 | 20.338 | 0.409 | 0.685 |
| LC | 72.171 | 19.434 | 3.714 | 0.002 | |
| MCI | LC | 63.862 | 16.963 | 3.765 | 0.002 |
| OERP | Condition | Cases | F | p | η2 |
|---|---|---|---|---|---|
| N1 Amplitude | Within | Electrode | 3.602 | <0.001 | 0.063 |
| Electrode*Group | 2.172 | 0.001 | 0.076 | ||
| Between | Group | 4.170 | 0.023 | 0.035 | |
| P3 Amplitude | Within | Electrode | 2.159 | 0.013 | 0.045 |
| Electrode*Group | 0.526 | 0.979 | 0.022 | ||
| Between | Group | 2.193 | 0.125 | 0.015 |
| Group Comparison | Mean Diff | SE | t | Pholm | |
|---|---|---|---|---|---|
| HS | MCI | −4.947 | 1.903 | −2.599 | 0.040 |
| HS | LC | −4.590 | 1.785 | −2.572 | 0.040 |
| MCI | LC | 0.357 | 1.647 | 0.217 | 0.830 |
| HS | MCI | −4.947 | 1.903 | −2.599 | 0.040 |
| COVID | −4.590 | 1.785 | −2.572 | 0.040 | |
| MCI | COVID | 0.357 | 1.647 | 0.217 | 0.830 |
| Electrode | Group | Mean μV | SD |
|---|---|---|---|
| Fp1 | HS | −20.232 | 25.64 |
| MCI | −4.403 | 3.115 | |
| LC | −3.689 | 5.488 | |
| F7 | HS | −10.288 | 11.55 |
| MCI | −1.961 | 3.452 | |
| LC | −4.892 | 5.821 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Invitto, S.; Boscolo-Rizzo, P.; Fantin, F.; Bonifati, D.M.; de Filippis, C.; Emanuelli, E.; Frezza, D.; Giopato, F.; Caggiula, M.; Schito, A.; et al. Exploratory Study on Chemosensory Event-Related Potentials in Long COVID-19 and Mild Cognitive Impairment: A Common Pathway? Bioengineering 2023, 10, 376. https://doi.org/10.3390/bioengineering10030376
Invitto S, Boscolo-Rizzo P, Fantin F, Bonifati DM, de Filippis C, Emanuelli E, Frezza D, Giopato F, Caggiula M, Schito A, et al. Exploratory Study on Chemosensory Event-Related Potentials in Long COVID-19 and Mild Cognitive Impairment: A Common Pathway? Bioengineering. 2023; 10(3):376. https://doi.org/10.3390/bioengineering10030376
Chicago/Turabian StyleInvitto, Sara, Paolo Boscolo-Rizzo, Francesco Fantin, Domenico Marco Bonifati, Cosimo de Filippis, Enzo Emanuelli, Daniele Frezza, Federico Giopato, Marcella Caggiula, Andrea Schito, and et al. 2023. "Exploratory Study on Chemosensory Event-Related Potentials in Long COVID-19 and Mild Cognitive Impairment: A Common Pathway?" Bioengineering 10, no. 3: 376. https://doi.org/10.3390/bioengineering10030376
APA StyleInvitto, S., Boscolo-Rizzo, P., Fantin, F., Bonifati, D. M., de Filippis, C., Emanuelli, E., Frezza, D., Giopato, F., Caggiula, M., Schito, A., Ciccarese, V., & Spinato, G. (2023). Exploratory Study on Chemosensory Event-Related Potentials in Long COVID-19 and Mild Cognitive Impairment: A Common Pathway? Bioengineering, 10(3), 376. https://doi.org/10.3390/bioengineering10030376








