Strontium Ranelate Inhibits Osteoclastogenesis through NF-κB-Pathway-Dependent Autophagy
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Osteoclast Culture
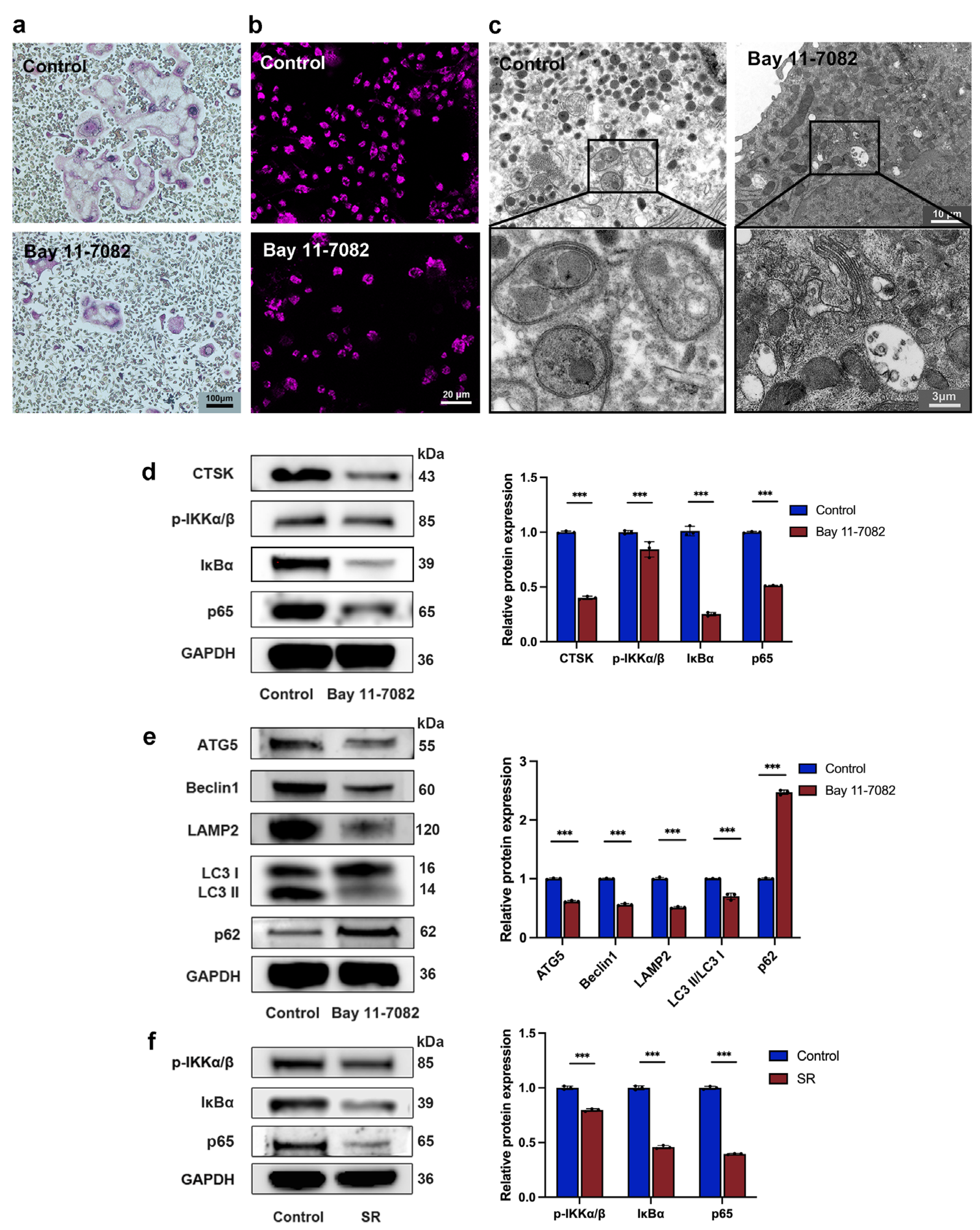
2.3. In Vitro TRAP Staining
2.4. Western Blot
2.5. Monodansylcadaverine Staining
2.6. Transmission Electron Microscopy
2.7. Animal Maintenance
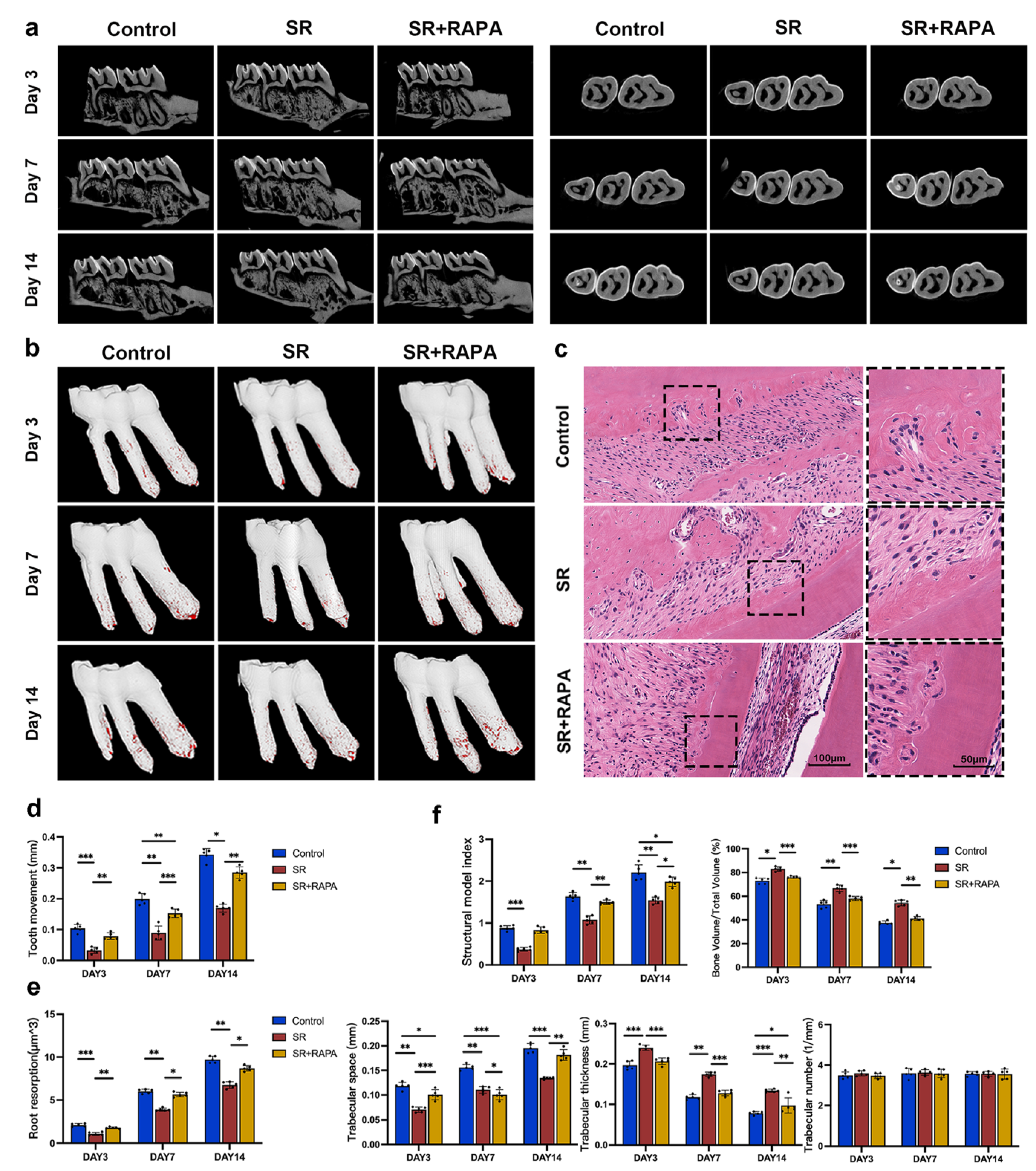
2.8. Orthodontic Tooth Movement Model
2.9. Drug Treatment in Rats
2.10. Sample Collection and Treatment
2.11. Analysis of Data Obtained with Micro-CT
2.12. Hematoxylin-Eosin Staining
2.13. In Vivo Tartrate-Resistant Acid Phosphatase Staining
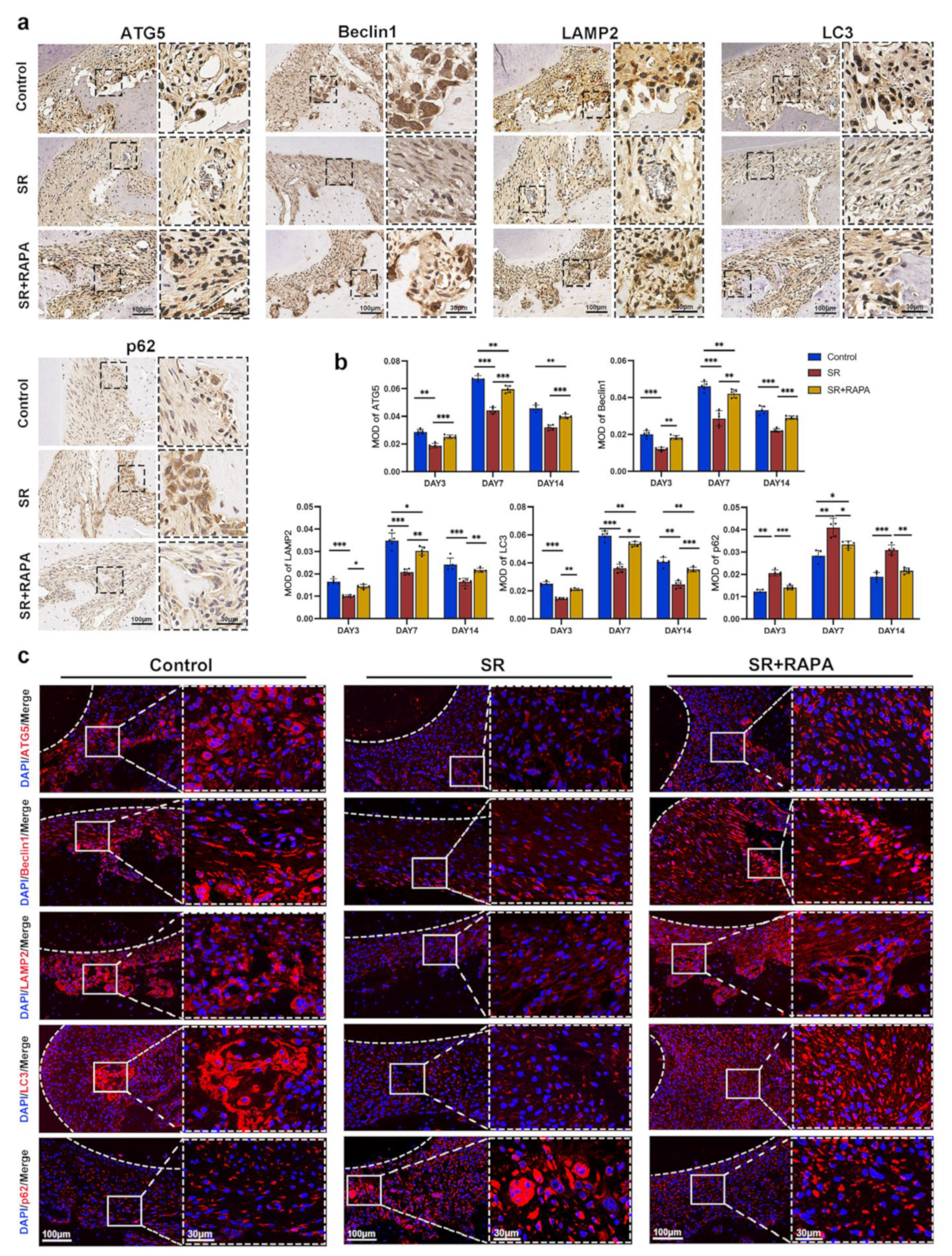
2.14. Immunohistochemistry
2.15. Immunofluorescence
2.16. Statistics
3. Results
3.1. Strontium Ranelate Inhibited Osteoclastogenesis through Autophagy
3.2. Strontium Ranelate Inhibited Autophagy through the NF-κB Pathway
3.3. Strontium Ranelate Reduced Orthodontic Tooth Movement and Root Resorption in Rats through Autophagy
3.4. Strontium Ranelate Might Inhibit Osteoclastogenesis through NF-κB-Mediated Autophagy in Sprague–Dawley Rats
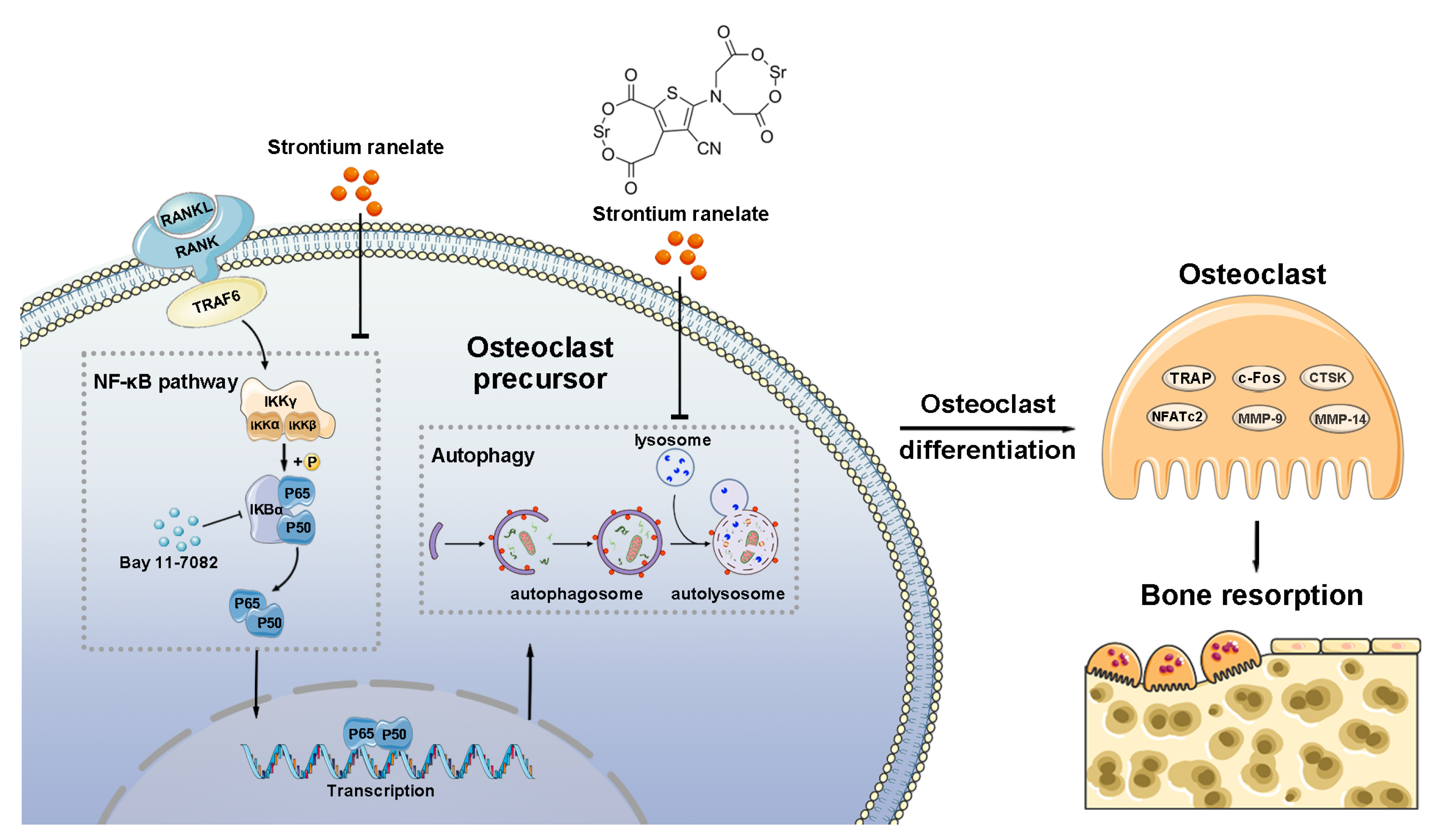
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meyer-Marcotty, P.; Klenke, D.; Knocks, L.; Santander, P.; Hrasky, V.; Quast, A. The adult orthodontic patient over 40 years of age: Association between periodontal bone loss, incisor irregularity, and increased orthodontic treatment need. Clin. Oral Investig. 2021, 25, 6357–6364. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, J.; Reichardt, E.; Sharma, P.; Hilber, A.; Meyer-Marcotty, P.; Stellzig-Eisenhauer, A.; Schlagenhauf, U.; Sickel, F.E. Interest in orthodontic tooth alignment in adult patients affected by periodontitis: A questionnaire-based cross-sectional pilot study. J. Periodontol. 2019, 90, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Falgayrac, G.; Farlay, D.; Ponçon, C.; Béhal, H.; Gardegaront, M.; Ammann, P.; Boivin, G.; Cortet, B. Bone matrix quality in paired iliac bone biopsies from postmenopausal women treated for 12 months with strontium ranelate or alendronate. Bone 2021, 153, 116107. [Google Scholar] [CrossRef] [PubMed]
- Wirsig, K.; Kilian, D.; von Witzleben, M.; Gelinsky, M.; Bernhardt, A. Impact of Sr and hypoxia on 3D triple cultures of primary human osteoblasts, osteocytes and osteoclasts. Eur. J. Cell Biol. 2022, 101, 151256. [Google Scholar] [CrossRef]
- Bonnelye, E.; Chabadel, A.; Saltel, F.; Jurdic, P. Dual effect of strontium ranelate: Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone 2008, 42, 129–138. [Google Scholar] [CrossRef]
- Kirschneck, C.; Wolf, M.; Reicheneder, C.; Wahlmann, U.; Proff, P.; Roemer, P. Strontium ranelate improved tooth anchorage and reduced root resorption in orthodontic treatment of rats. Eur. J. Pharmacol. 2014, 744, 67–75. [Google Scholar] [CrossRef]
- Gusman, D.-J.-R.; Matheus, H.-R.; Alves, B.-E.-S.; Ervolino, E.; de Araujo, N.-J.; Piovezan, B.-R.; Fiorin, L.-G.; de Almeida, J.-M. Influence of systemic strontium ranelate on the progression and as adjunctive therapy for the nonsurgical treatment of experimental periodontitis. J. Clin. Exp. Dent. 2021, 13, e1239–e1248. [Google Scholar] [CrossRef]
- Christensen, T.E.K.; Berglund Davidsen, M.; Van Malderen, S.; Garrevoet, J.; Offermanns, V.; Andersen, O.Z.; Foss, M.; Birkedal, H. Local Release of Strontium from Sputter-Deposited Coatings at Implants Increases the Strontium-to-Calcium Ratio in Peri-implant Bone. ACS Biomater. Sci. Eng. 2022, 8, 620–625. [Google Scholar] [CrossRef]
- Song, T.; Yang, J.; Liu, P.; Liu, M.; Li, D.; Xiao, Y.; Wang, Y.; Zhang, X. Icariin self-crosslinked network functionalized strontium-doped bioceramic scaffolds synergistically enhanced the healing of osteoporotic bone defects. Compos. Part B Eng. 2022, 235, 109759. [Google Scholar] [CrossRef]
- Caudrillier, A.; Hurtel-Lemaire, A.-S.; Wattel, A.; Cournarie, F.; Godin, C.; Petit, L.; Petit, J.-P.; Terwilliger, E.; Kamel, S.; Brown, E.M.; et al. Strontium ranelate decreases receptor activator of nuclear factor-ΚB ligand-induced osteoclastic differentiation in vitro: Involvement of the calcium-sensing receptor. Mol. Pharmacol. 2010, 78, 569–576. [Google Scholar] [CrossRef]
- Jin, K.; Zheng, L.; Ye, L.; Xie, Z.; Gao, J.; Lou, C.; Pan, W.; Pan, B.; Liu, S.; Chen, Z.; et al. Chicago sky blue 6B (CSB6B), an allosteric inhibitor of macrophage migration inhibitory factor (MIF), suppresses osteoclastogenesis and promotes osteogenesis through the inhibition of the NF-κB signaling pathway. Biochem. Pharmacol. 2021, 192, 114734. [Google Scholar] [CrossRef]
- Cossu, F.; Camelliti, S.; Lecis, D.; Sorrentino, L.; Majorini, M.T.; Milani, M.; Mastrangelo, E. Structure-based identification of a new IAP-targeting compound that induces cancer cell death inducing NF-κB pathway. Comput. Struct. Biotechnol. J. 2021, 19, 6366–6374. [Google Scholar] [CrossRef]
- Müller, D.; Donath, S.; Brückner, E.G.; Biswanath Devadas, S.; Daniel, F.; Gentemann, L.; Zweigerdt, R.; Heisterkamp, A.; Kalies, S.M.K. How Localized Z-Disc Damage Affects Force Generation and Gene Expression in Cardiomyocytes. Bioengineering 2021, 8, 213. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Weitzmann, M.N. The intact strontium ranelate complex stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-κB activation. Mol. Cell. Biochem. 2012, 359, 399–407. [Google Scholar] [CrossRef]
- Chu, B.; Chen, S.; Zheng, X.; Ye, J.; Cheng, X.; Zhang, L.; Guo, D.; Wang, P.; Hong, D.; Hong, Z. Nepetin inhibits osteoclastogenesis by inhibiting RANKL-induced activation of NF-κB and MAPK signalling pathway, and autophagy. J. Cell. Mol. Med. 2020, 24, 14366–14380. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Emr, S.D. Autophagy as a regulated pathway of cellular degradation. Science 2000, 290, 1717–1721. [Google Scholar] [CrossRef]
- Zhang, S.; He, W.; Li, A.; Zhao, C.; Chen, Y.; Xu, C.; Zhang, Q.; Zheng, D.; Chen, M.; Miao, H.; et al. Involvement of the TNF-α/SATB2 axis in the induced apoptosis and inhibited autophagy of osteoblasts by the antipsychotic Risperidone. Mol. Med. 2022, 28, 46. [Google Scholar] [CrossRef]
- Xu, H.; Xia, M.; Sun, L.; Wang, H.; Zhang, W.-B. Osteocytes Enhance Osteogenesis by Autophagy-Mediated FGF23 Secretion Under Mechanical Tension. Front. Cell Dev. Biol. 2021, 9, 782736. [Google Scholar] [CrossRef]
- Park, H.-J.; Son, H.-J.; Sul, O.-J.; Suh, J.-H.; Choi, H.-S. 4-Phenylbutyric acid protects against lipopolysaccharide-induced bone loss by modulating autophagy in osteoclasts. Biochem. Pharmacol. 2018, 151, 9–17. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, W.; Wang, B.; Yi, Z.; Gong, P.; Xiong, Y. 1α,25-Dihydroxyvitamin D3 ameliorates diabetes-induced bone loss by attenuating FoxO1-mediated autophagy. J. Biol. Chem. 2021, 296, 100287. [Google Scholar] [CrossRef]
- Na, W.; Lee, E.-J.; Kang, M.-K.; Kim, Y.-H.; Kim, D.Y.; Oh, H.; Kim, S.-I.; Oh, S.Y.; Kang, Y.-H. Aesculetin Inhibits Osteoclastic Bone Resorption through Blocking Ruffled Border Formation and Lysosomal Trafficking. Int. J. Mol. Sci. 2020, 21, 8581. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Yu, Y.; Li, C.; Han, J.; Xu, J. Phosphorylation of BCL2 at the Ser70 site mediates RANKL-induced osteoclast precursor autophagy and osteoclastogenesis. Mol. Med. 2022, 28, 22. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Chen, Q.; Zhao, S.; Wen, S.; Chen, W.; Ye, W.; Gong, T.; Jiang, M.; Liu, X. IKKα contributes to ischemia-induced autophagy after acute cerebral ischemic injury. Ann. Transl. Med. 2022, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, L.; Zhang, J.; Yu, J.; Cheng, X.; Peng, B. Anti-osteoclastogenic activity of isoliquiritigenin via inhibition of NF-κB-dependent autophagic pathway. Biochem. Pharmacol. 2016, 106, 82–93. [Google Scholar] [CrossRef]
- Wu, D.; Meng, B.; Cheng, Y.; Gan, L.; Huang, P.; Cao, Y. The effect of risedronate on orthodontic tooth movement in ovariectomized rats. Arch. Oral Biol. 2019, 105, 59–64. [Google Scholar] [CrossRef]
- Zamai, R.S.; Corrêa, M.G.; Ribeiro, F.V.; Cirano, F.R.; Casati, M.Z.; Messora, M.R.; Pimentel, S.P. Does resveratrol favor peri-implant bone repair in rats with ovariectomy-induced osteoporosis? Gene expression, counter-torque and micro-CT analysis. Braz. Oral Res. 2023, 37, e003. [Google Scholar] [CrossRef]
- Farronato, M.; Baselli, G.; Baldini, B.; Favia, G.; Tartaglia, G.M. 3D Cephalometric Normality Range: Auto Contractive Maps (ACM) Analysis in Selected Caucasian Skeletal Class I Age Groups. Bioengineering 2022, 9, 216. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Li, Y.; Zhan, Q.; Bao, M.; Yi, J.; Li, Y. Biomechanical and biological responses of periodontium in orthodontic tooth movement: Up-date in a new decade. Int. J. Oral Sci. 2021, 13, 20. [Google Scholar] [CrossRef]
- Jacox, L.A.; Tang, N.; Li, Y.; Bocklage, C.; Graves, C.; Coats, S.; Miao, M.; Glesener, T.; Kwon, J.; Giduz, N.; et al. Orthodontic loading activates cell-specific autophagy in a force-dependent manner. Am. J. Orthod. Dentofac. Orthop. 2022, 161, 423–436.E1. [Google Scholar] [CrossRef]
- Li, Z.; Tian, X.; Ji, X.; Wang, J.; Chen, H.; Wang, D.; Zhang, X. ULK1-ATG13 and their mitotic phospho-regulation by CDK1 connect autophagy to cell cycle. PLoS Biol. 2020, 18, e3000288. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Huang, Y.; Bai, C.; Zhang, X.; Fang, M.; Ju, Z.; Liu, B. Membrane dynamics of ATG4B and LC3 in autophagosome formation. J. Mol. Cell Biol. 2022, 13, 853–863. [Google Scholar] [CrossRef]
- Ulakcsai, Z.; Bagaméry, F.; Szökő, É.; Tábi, T. The role of autophagy induction in the mechanism of cytoprotective effect of resveratrol. Eur. J. Pharm. Sci. 2018, 123, 135–142. [Google Scholar] [CrossRef]
- Alcalai, R.; Arad, M.; Wakimoto, H.; Yadin, D.; Gorham, J.; Wang, L.; Burns, E.; Maron, B.J.; Roberts, W.C.; Konno, T.; et al. Cardiomyopathy: Consequences of Impaired Autophagy in the Heart. J. Am. Heart Assoc. 2021, 10, e018829. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kang, N.; Kang, J.Y.; Kim, K.W.; Choi, J.-H.; Yang, Y.-M.; Shin, D.M. Sestrin2 Regulates Osteoclastogenesis via the p62-TRAF6 Interaction. Front. Cell Dev. Biol. 2021, 9, 646803. [Google Scholar] [CrossRef]
- Hsu, L.-C.; Reddy, S.V.; Yilmaz, Ö.; Yu, H. Sphingosine-1-Phosphate Receptor 2 Controls Podosome Components Induced by RANKL Affecting Osteoclastogenesis and Bone Resorption. Cells 2019, 8, 17. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y.; Wang, L.; Han, J. Autophagy promotes osteoclast podosome disassembly and cell motility athrough the interaction of kindlin3 with LC3. Cell Signal. 2020, 67, 109505. [Google Scholar] [CrossRef]
- Zhu, G.; Chen, W.; Tang, C.-Y.; McVicar, A.; Edwards, D.; Wang, J.; McConnell, M.; Yang, S.; Li, Y.; Chang, Z.; et al. Knockout and Double Knockout of Cathepsin K and Mmp9 reveals a novel function of Cathepsin K as a regulator of osteoclast gene expression and bone homeostasis. Int. J. Biol. Sci. 2022, 18, 5522–5538. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, M.; Zhang, S.; Yang, Z.; Zhu, Y.; Wang, Y.; Chen, Z.; Lv, X.; Huang, Z.; Xie, Y.; et al. BHLHE40 promotes osteoclastogenesis and abnormal bone resorption via c-Fos/NFATc1. Cell Biosci. 2022, 12, 70. [Google Scholar] [CrossRef]
- Kim, I.; Kim, J.H.; Kim, K.; Seong, S.; Lee, K.-B.; Kim, N. IRF2 enhances RANKL-induced osteoclast differentiation via regulating NF-κB/NFATc1 signaling. BMB Rep. 2021, 54, 482–487. [Google Scholar] [CrossRef]
- Ji, L.; Gao, J.; Kong, R.; Gao, Y.; Ji, X.; Zhao, D. Autophagy exerts pivotal roles in regulatory effects of 1α,25-(OH)D on the osteoclastogenesis. Biochem. Biophys. Res. Commun. 2019, 511, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Zhang, C.; Wang, D.; Song, R.; Ma, Y.; Cao, Y.; Zhao, H.; Bian, J.; Gu, J.; Liu, Z. Suppression of AMP-activated protein kinase reverses osteoprotegerin-induced inhibition of osteoclast differentiation by reducing autophagy. Cell Prolif. 2020, 53, e12714. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, G.; Kawabata, T.; Takamatsu, H.; Saita, S.; Nakamura, S.; Nishikawa, K.; Fujiwara, M.; Enokidani, Y.; Yamamuro, T.; Tabata, K.; et al. Degradation of the NOTCH intracellular domain by elevated autophagy in osteoblasts promotes osteoblast differentiation and alleviates osteoporosis. Autophagy 2022, 18, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wei, Y.; Lei, L.; Zhong, J.; Shen, Y.; Tan, J.; Xia, M.; Wu, Y.; Sun, W.; Chen, L. RANKL expression of primary osteoblasts is enhanced by an IL-17-mediated JAK2/STAT3 pathway through autophagy suppression. Connect. Tissue Res. 2021, 62, 411–426. [Google Scholar] [CrossRef]
- Onal, M.; Piemontese, M.; Xiong, J.; Wang, Y.; Han, L.; Ye, S.; Komatsu, M.; Selig, M.; Weinstein, R.S.; Zhao, H.; et al. Suppression of autophagy in osteocytes mimics skeletal aging. J. Biol. Chem. 2013, 288, 17432–17440. [Google Scholar] [CrossRef]
- Kim, S.-J.; Piao, Y.; Lee, M.G.; Han, A.R.; Kim, K.; Hwang, C.-J.; Seo, J.T.; Moon, S.J. Loss of Sirtuin 6 in osteoblast lineage cells activates osteoclasts, resulting in osteopenia. Bone 2020, 138, 115497. [Google Scholar] [CrossRef]
- Andreev, D.; Liu, M.; Weidner, D.; Kachler, K.; Faas, M.; Grüneboom, A.; Schlötzer-Schrehardt, U.; Muñoz, L.E.; Steffen, U.; Grötsch, B.; et al. Osteocyte necrosis triggers osteoclast-mediated bone loss through macrophage-inducible C-type lectin. J. Clin. Investig. 2020, 130, 4811–4830. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, Y.; Wu, Q.; Ji, Y.C.; Feng, Z.F.; Kang, F.W. HIF-1α facilitates osteocyte-mediated osteoclastogenesis by activating JAK2/STAT3 pathway in vitro. J. Cell. Physiol. 2019, 234, 21182–21192. [Google Scholar] [CrossRef]
- Huynh, H.; Wan, Y. mTORC1 impedes osteoclast differentiation via calcineurin and NFATc1. Commun. Biol. 2018, 1, 29. [Google Scholar] [CrossRef]
- Han, M.; Chen, X.-C.; Sun, M.-H.; Gai, M.-T.; Yang, Y.-N.; Gao, X.-M.; Ma, X.; Chen, B.-D.; Ma, Y.-T. Overexpression of IκBα in cardiomyocytes alleviates hydrogen peroxide-induced apoptosis and autophagy by inhibiting NF-κB activation. Lipids Health Dis. 2020, 19, 150. [Google Scholar] [CrossRef]
- Curtis, E.M.; Cooper, C.; Harvey, N.C. Cardiovascular safety of calcium, magnesium and strontium: What does the evidence say? Aging Clin. Exp. Res. 2021, 33, 479–494. [Google Scholar] [CrossRef]
- Berencsi, K.; Sami, A.; Ali, M.S.; Marinier, K.; Deltour, N.; Perez-Gutthann, S.; Pedersen, L.; Rijnbeek, P.; Van der Lei, J.; Lapi, F.; et al. Impact of risk minimisation measures on the use of strontium ranelate in Europe: A multi-national cohort study in 5 EU countries by the EU-ADR Alliance. Osteoporos. Int. 2020, 31, 721–755. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Sun, X.; Zhao, Y.; Liu, Y.; Gan, Z.; Zhang, Z.; Chen, X.; Cao, Y. Strontium Ranelate Inhibits Osteoclastogenesis through NF-κB-Pathway-Dependent Autophagy. Bioengineering 2023, 10, 365. https://doi.org/10.3390/bioengineering10030365
Wu D, Sun X, Zhao Y, Liu Y, Gan Z, Zhang Z, Chen X, Cao Y. Strontium Ranelate Inhibits Osteoclastogenesis through NF-κB-Pathway-Dependent Autophagy. Bioengineering. 2023; 10(3):365. https://doi.org/10.3390/bioengineering10030365
Chicago/Turabian StyleWu, Dongle, Xuan Sun, Yiwei Zhao, Yuanbo Liu, Ziqi Gan, Zhen Zhang, Xin Chen, and Yang Cao. 2023. "Strontium Ranelate Inhibits Osteoclastogenesis through NF-κB-Pathway-Dependent Autophagy" Bioengineering 10, no. 3: 365. https://doi.org/10.3390/bioengineering10030365
APA StyleWu, D., Sun, X., Zhao, Y., Liu, Y., Gan, Z., Zhang, Z., Chen, X., & Cao, Y. (2023). Strontium Ranelate Inhibits Osteoclastogenesis through NF-κB-Pathway-Dependent Autophagy. Bioengineering, 10(3), 365. https://doi.org/10.3390/bioengineering10030365








