Rapid and Stable Formation Method of Human Astrocyte Spheroid in a High Viscous Methylcellulose Medium and Its Functional Advantages
Abstract
1. Introduction
2. Materials and Methods
2.1. HASTR/ci35 Cells
2.2. MC Medium
2.3. Spheroid Formation
2.4. Spheroid Histological Staining
2.5. Real-Time PCR Analysis
2.6. Stimulation with Inflammatory Cytokines
2.7. Neurite Growth Assay
3. Results and Discussion
3.1. Rapid Formation of Astrocyte Spheroid in the MC Medium
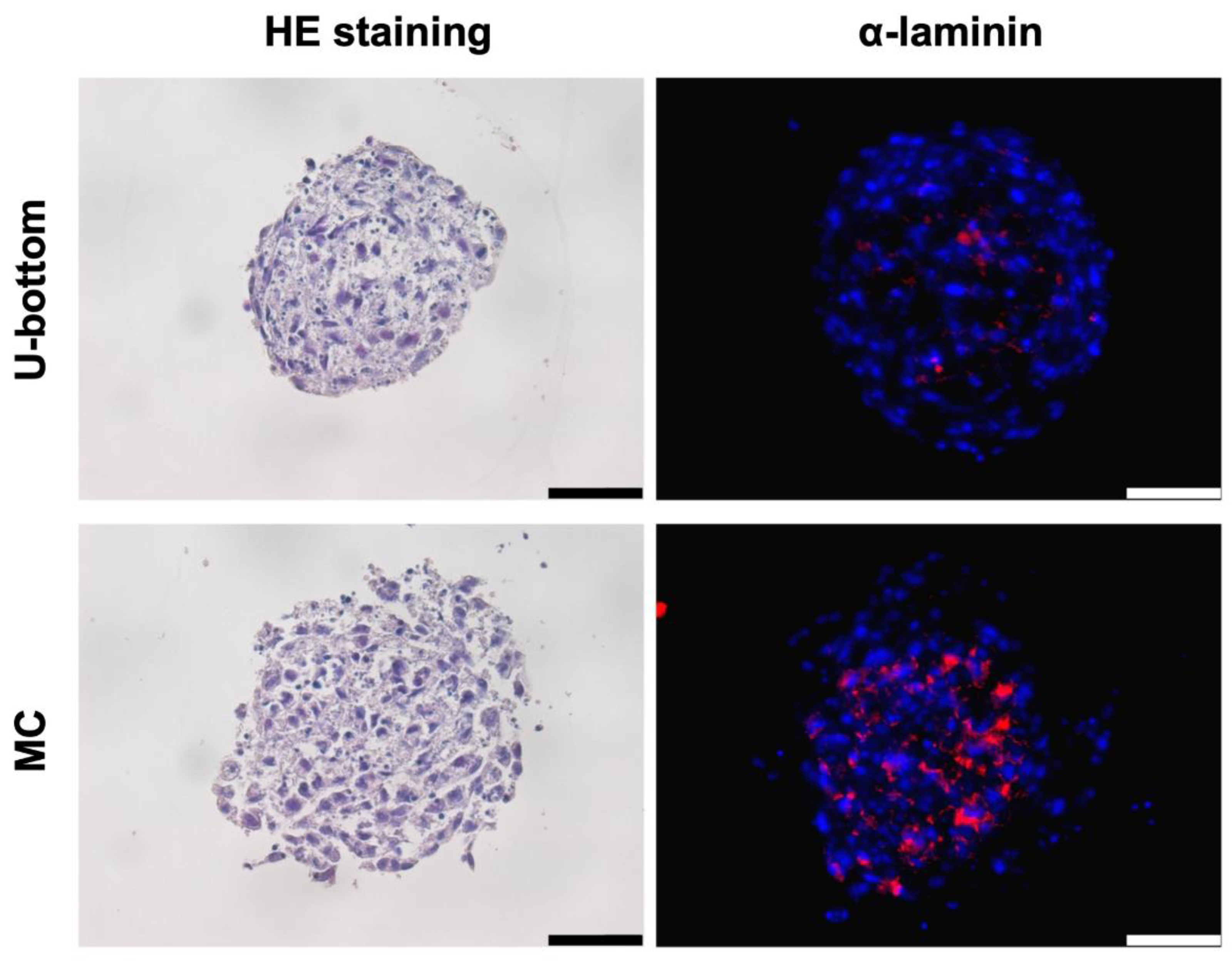
3.2. Histological Analysis of HLC Spheroid
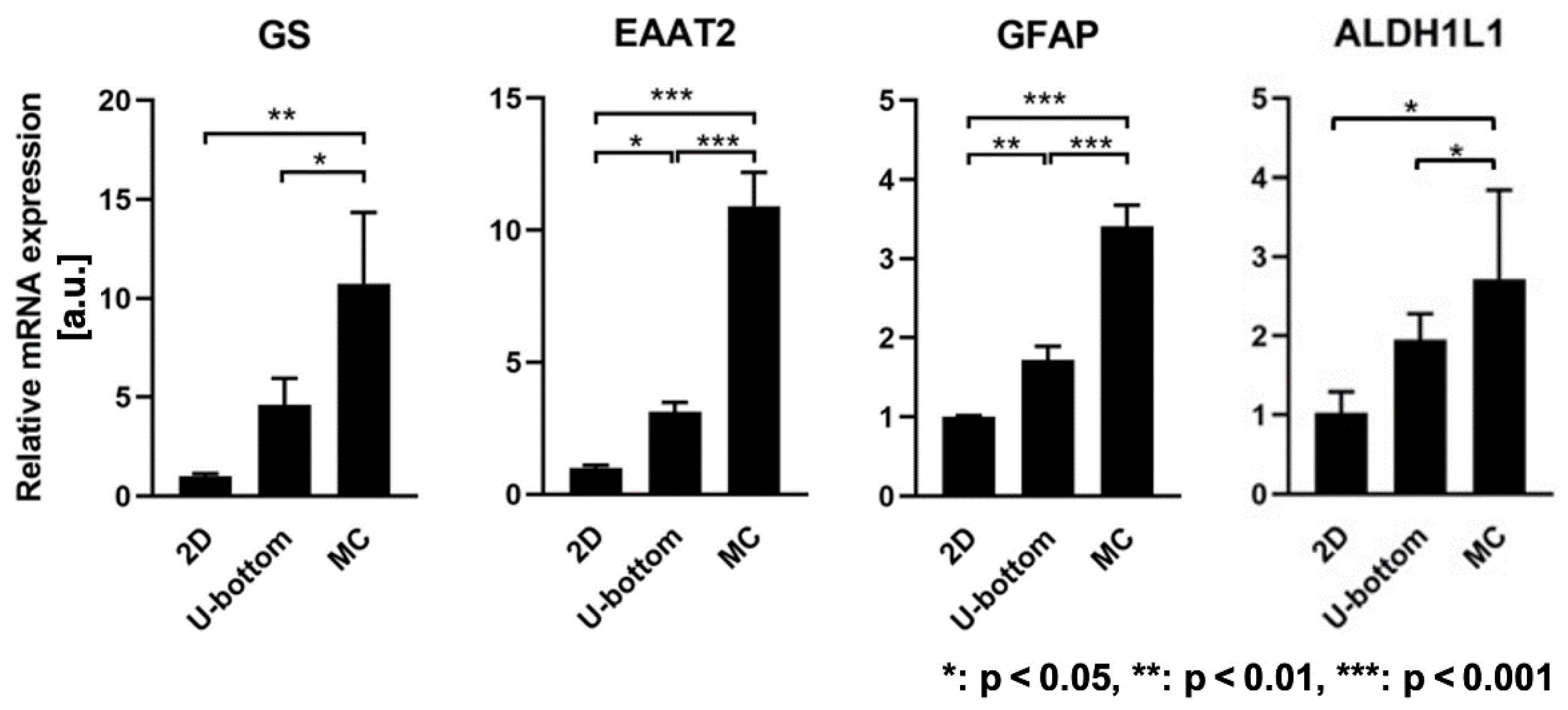
3.3. Gene Expression Enhancement of 3D HASTR/ci35 Spheroids
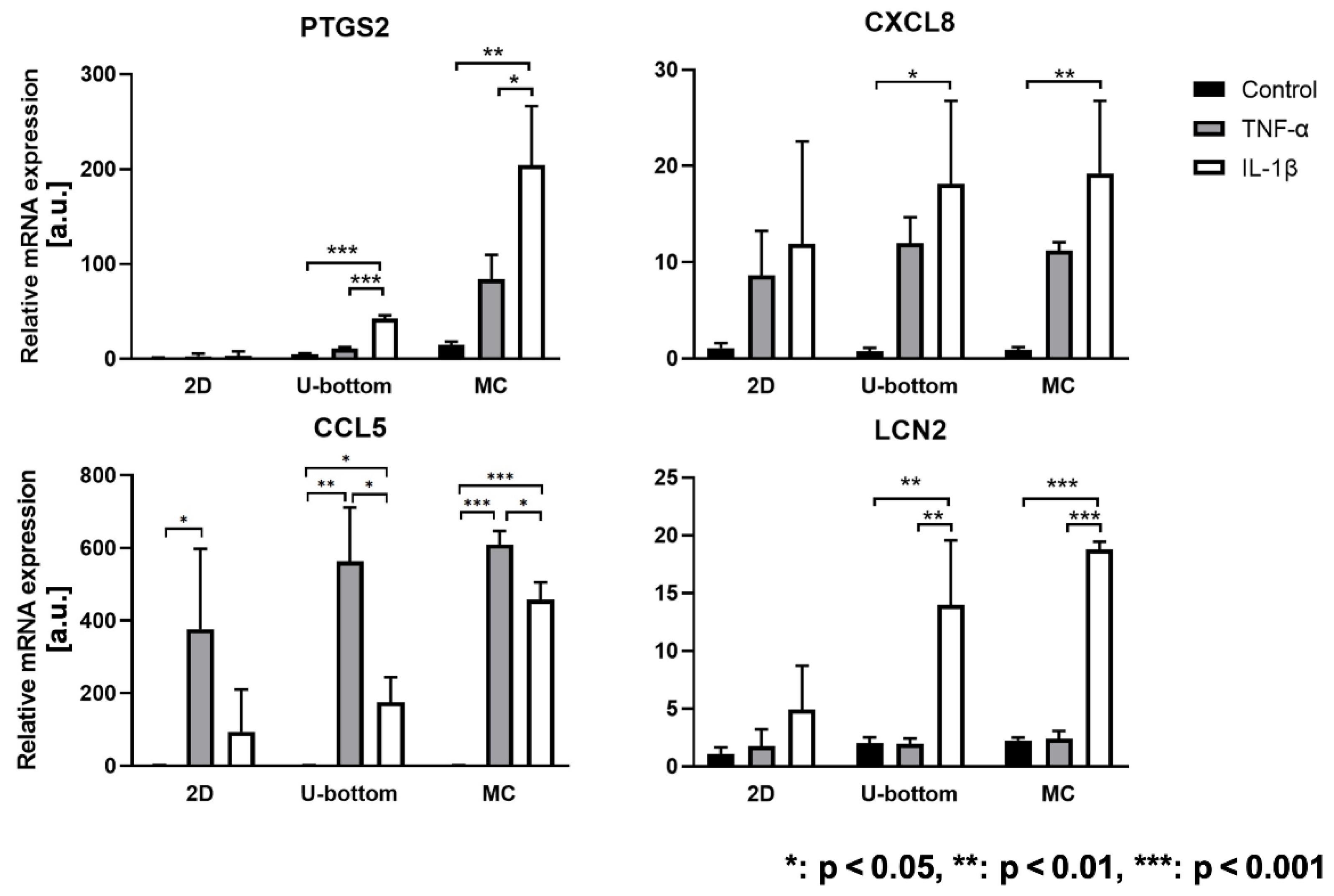
3.4. Inflammatory Reaction of 3D HASTR/ci35 Spheroids
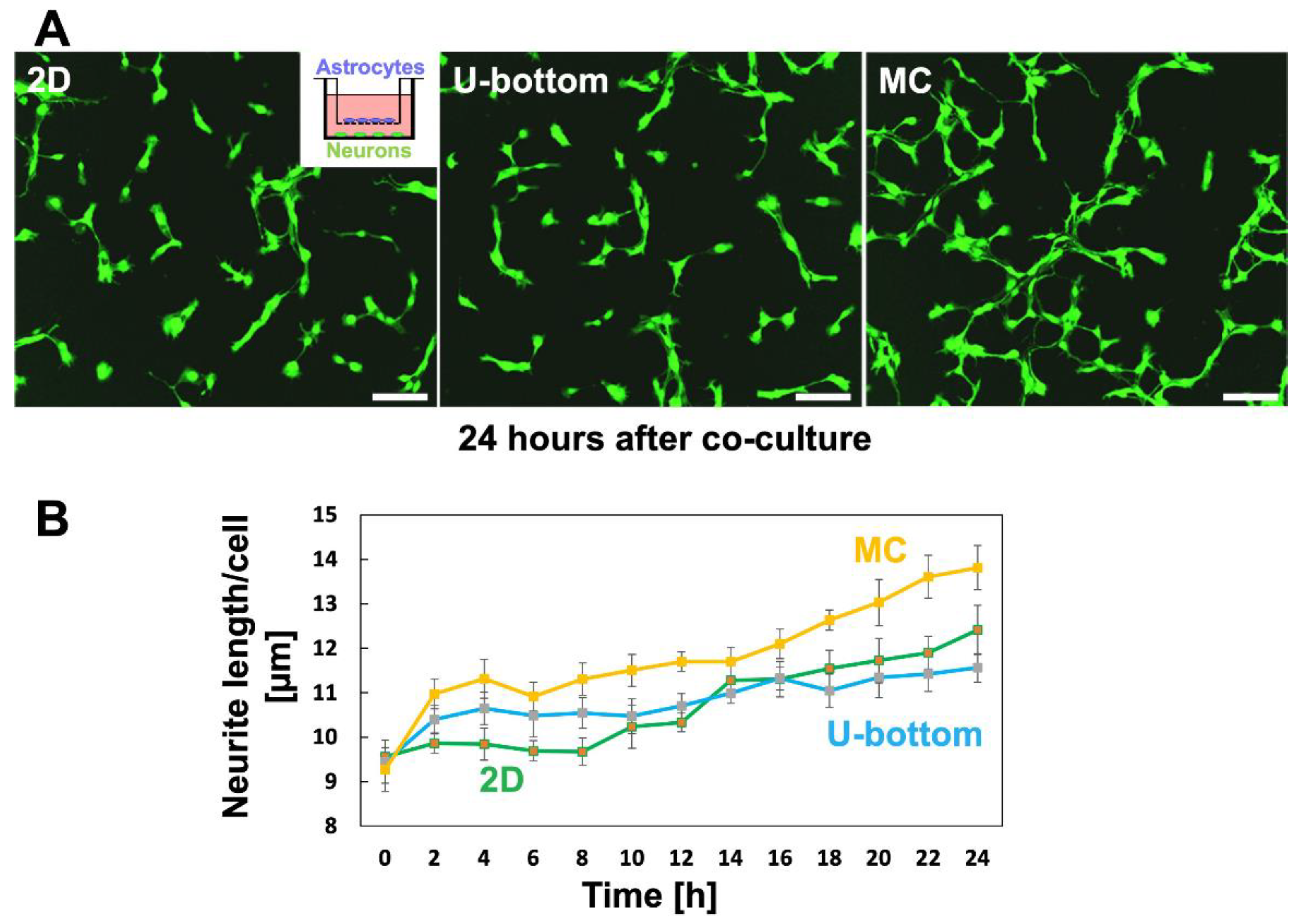
3.5. Effect of 3D Astrocytes on Neurite Growth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khakh, B.S.; Sofroniew, M.V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 2015, 18, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Chandrasekar, V.; Laux, P.; Luch, A.; Dakua, S.P.; Zamboni, P.; Shelar, A.; Yang, Y.; Pandit, V.; Tisato, V.; et al. Micropatterned Neurovascular Interface to Mimic the Blood-Brain Barrier’s Neurophysiology and Micromechanical Function: A BBB-on-CHIP Model. Cells 2022, 11, 2801. [Google Scholar] [CrossRef] [PubMed]
- Goubard, V.; Fino, E.; Venance, L. Contribution of astrocytic glutamate and GABA uptake to corticostriatal information processing. J. Physiol. 2011, 589, 2301–2319. [Google Scholar] [CrossRef]
- Schousboe, A.; Bak, L.K.; Waagepetersen, H.S. Astrocytic Control of Biosynthesis and Turnover of the Neurotransmitters Glutamate and GABA. Front. Endocrinol. 2013, 4, 102. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A. Lack of appropriate stoichiometry: Strong evidence against an energetically important astrocyte-neuron lactate shuttle in brain. J. Neurosci. Res. 2017, 95, 2103–2125. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Allaman, I. Lactate in the brain: From metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018, 19, 235–249. [Google Scholar] [CrossRef]
- Karagiannis, A.; Gallopin, T.; Lacroix, A.; Plaisier, F.; Piquet, J.; Geoffroy, H.; Hepp, R.; Naude, J.; Le Gac, B.; Egger, R.; et al. Lactate is an energy substrate for rodent cortical neurons and enhances their firing activity. eLife 2021, 10, e71424. [Google Scholar] [CrossRef]
- Sloan, S.A.; Darmanis, S.; Huber, N.; Khan, T.A.; Birey, F.; Caneda, C.; Reimer, R.; Quake, S.R.; Barres, B.A.; Pasca, S.P. Human Astrocyte Maturation Captured in 3D Cerebral Cortical Spheroids Derived from Pluripotent Stem Cells. Neuron 2017, 95, 779–790.e6. [Google Scholar] [CrossRef]
- Roybon, L.; Lamas, N.J.; Garcia, A.D.; Yang, E.J.; Sattler, R.; Lewis, V.J.; Kim, Y.A.; Kachel, C.A.; Rothstein, J.D.; Przedborski, S.; et al. Human stem cell-derived spinal cord astrocytes with defined mature or reactive phenotypes. Cell Rep. 2013, 4, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zuo, Y.X.; Jiang, R.T. Astrocyte morphology: Diversity, plasticity, and role in neurological diseases. CNS Neurosci. Ther. 2019, 25, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Khakh, B.S.; Deneen, B. The Emerging Nature of Astrocyte Diversity. Annu. Rev. Neurosci. 2019, 42, 187–207. [Google Scholar] [CrossRef]
- Endo, F.; Kasai, A.; Soto, J.S.; Yu, X.; Qu, Z.; Hashimoto, H.; Gradinaru, V.; Kawaguchi, R.; Khakh, B.S. Molecular basis of astrocyte diversity and morphology across the CNS in health and disease. Science 2022, 378, eadc9020. [Google Scholar] [CrossRef] [PubMed]
- Furihata, T.; Ito, R.; Kamiichi, A.; Saito, K.; Chiba, K. Establishment and characterization of a new conditionally immortalized human astrocyte cell line. J. Neurochem. 2016, 136, 92–105. [Google Scholar] [CrossRef]
- Kitamura, K.; Ito, R.; Umehara, K.; Morio, H.; Saito, K.; Suzuki, S.; Hashimoto, M.; Saito, Y.; Anzai, N.; Akita, H.; et al. Differentiated HASTR/ci35 cells: A promising in vitro human astrocyte model for facilitating CNS drug development studies. J. Pharmacol. Sci. 2018, 137, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Urich, E.; Patsch, C.; Aigner, S.; Graf, M.; Iacone, R.; Freskgard, P.O. Multicellular self-assembled spheroidal model of the blood brain barrier. Sci. Rep. 2013, 3, 1500. [Google Scholar] [CrossRef]
- Kojima, N.; Takeuchi, S.; Sakai, Y. Rapid aggregation of heterogeneous cells and multiple-sized microspheres in methylcellulose medium. Biomaterials 2012, 33, 4508–4514. [Google Scholar] [CrossRef]
- Tao, F.; Mihara, H.; Kojima, N. Generation of Hepatic Tissue Structures Using Multicellular Spheroid Culture. Methods Mol. Biol. 2019, 1905, 157–165. [Google Scholar] [CrossRef]
- Sayo, K.; Aoki, S.; Kojima, N. Fabrication of bone marrow-like tissue in vitro from dispersed-state bone marrow cells. Regen. Ther. 2016, 3, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Hanada, S.; Matsushima, K.; Arakawa, H.; Ishida, N.; Kato, Y.; Okimura, S.; Watanabe, T.; Kojima, N. Enhancement and maintenance of hepatic metabolic functions by controlling 3D aggregation of cryopreserved human iPS cell-derived hepatocyte-like cells. J. Biosci. Bioeng. 2023, 135, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa-Tomikawa, N.; Ogawa, J.; Douet, V.; Xu, Z.; Kamikubo, Y.; Sakurai, T.; Kohsaka, S.; Chiba, H.; Hattori, N.; Yamada, Y.; et al. Laminin alpha1 is essential for mouse cerebellar development. Matrix Biol. 2012, 31, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.J.; McClenahan, F.K.; Leiton, C.V.; Aranmolate, A.; Shan, X.; Colognato, H. The extracellular matrix protein laminin alpha2 regulates the maturation and function of the blood-brain barrier. J. Neurosci. 2014, 34, 15260–15280. [Google Scholar] [CrossRef]
- Zapata-Acevedo, J.F.; Garcia-Perez, V.; Cabezas-Perez, R.; Losada-Barragan, M.; Vargas-Sanchez, K.; Gonzalez-Reyes, R.E. Laminin as a Biomarker of Blood-Brain Barrier Disruption under Neuroinflammation: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 6788. [Google Scholar] [CrossRef]
- Sato, J.; Horibe, S.; Kawauchi, S.; Sasaki, N.; Hirata, K.I.; Rikitake, Y. Involvement of aquaporin-4 in laminin-enhanced process formation of mouse astrocytes in 2D culture: Roles of dystroglycan and alpha-syntrophin in aquaporin-4 expression. J. Neurochem. 2018, 147, 495–513. [Google Scholar] [CrossRef]
- Tao, F.; Sayo, K.; Sugimoto, K.; Aoki, S.; Kojima, N. Development of a tunable method to generate various three-dimensional microstructures by replenishing macromolecules such as extracellular matrix components and polysaccharides. Sci. Rep. 2020, 10, 6567. [Google Scholar] [CrossRef] [PubMed]
- Aumailley, M.; Bruckner-Tuderman, L.; Carter, W.G.; Deutzmann, R.; Edgar, D.; Ekblom, P.; Engel, J.; Engvall, E.; Hohenester, E.; Jones, J.C.; et al. A simplified laminin nomenclature. Matrix Biol. 2005, 24, 326–332. [Google Scholar] [CrossRef]
- Aumailley, M. The laminin family. Cell Adhes. Migr. 2013, 7, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, Z.L.; Norris, E.H.; Strickland, S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat. Commun. 2014, 5, 3413. [Google Scholar] [CrossRef]
- Allnoch, L.; Leitzen, E.; Zdora, I.; Baumgartner, W.; Hansmann, F. Astrocyte depletion alters extracellular matrix composition in the demyelinating phase of Theiler’s murine encephalomyelitis. PLoS ONE 2022, 17, e0270239. [Google Scholar] [CrossRef] [PubMed]
- Jurga, A.M.; Paleczna, M.; Kadluczka, J.; Kuter, K.Z. Beyond the GFAP-Astrocyte Protein Markers in the Brain. Biomolecules 2021, 11, 1361. [Google Scholar] [CrossRef]
- Pekny, M.; Wilhelmsson, U.; Tatlisumak, T.; Pekna, M. Astrocyte activation and reactive gliosis-A new target in stroke? Neurosci. Lett. 2019, 689, 45–55. [Google Scholar] [CrossRef]
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef]
- Brenner, M.; Messing, A. Regulation of GFAP Expression. ASN Neuro 2021, 13, 1759091420981206. [Google Scholar] [CrossRef] [PubMed]
- Placone, A.L.; McGuiggan, P.M.; Bergles, D.E.; Guerrero-Cazares, H.; Quinones-Hinojosa, A.; Searson, P.C. Human astrocytes develop physiological morphology and remain quiescent in a novel 3D matrix. Biomaterials 2015, 42, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist 2014, 20, 160–172. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 2015, 16, 249–263. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Shiratori-Hayashi, M.; Tsuda, M. Role of reactive astrocytes in the spinal dorsal horn under chronic itch conditions. J. Pharmacol. Sci. 2020, 144, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zhou, S.; Yin, S.; Jian, L.; Luo, P.; Dong, J.; Liu, E. Lipocalin-2 and Cerebral Stroke. Front. Mol. Neurosci. 2022, 15, 850849. [Google Scholar] [CrossRef]
- Zhao, R.Y.; Wei, P.J.; Sun, X.; Zhang, D.H.; He, Q.Y.; Liu, J.; Chang, J.L.; Yang, Y.; Guo, Z.N. Role of lipocalin 2 in stroke. Neurobiol. Dis. 2023, 179, 106044. [Google Scholar] [CrossRef]
- Bi, F.; Huang, C.; Tong, J.; Qiu, G.; Huang, B.; Wu, Q.; Li, F.; Xu, Z.; Bowser, R.; Xia, X.G.; et al. Reactive astrocytes secrete lcn2 to promote neuron death. Proc. Natl. Acad. Sci. USA 2013, 110, 4069–4074. [Google Scholar] [CrossRef]
- Oh, H.N.; Park, S.; Lee, S.; Chun, H.S.; Shin, W.H.; Kim, W.K. In vitro neurotoxicity evaluation of biocidal disinfectants in a human neuron-astrocyte co-culture model. Toxicol. Vitr. 2022, 84, 105449. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gonsalvez, G.B.; Mysona, B.A.; Smith, S.B.; Bollinger, K.E. Sigma 1 Receptor Contributes to Astrocyte-Mediated Retinal Ganglion Cell Protection. Investig. Investig. Opthalmol. Vis. Sci. 2022, 63, 1. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, F.; Kitamura, K.; Hanada, S.; Sugimoto, K.; Furihata, T.; Kojima, N. Rapid and Stable Formation Method of Human Astrocyte Spheroid in a High Viscous Methylcellulose Medium and Its Functional Advantages. Bioengineering 2023, 10, 349. https://doi.org/10.3390/bioengineering10030349
Tao F, Kitamura K, Hanada S, Sugimoto K, Furihata T, Kojima N. Rapid and Stable Formation Method of Human Astrocyte Spheroid in a High Viscous Methylcellulose Medium and Its Functional Advantages. Bioengineering. 2023; 10(3):349. https://doi.org/10.3390/bioengineering10030349
Chicago/Turabian StyleTao, Fumiya, Keita Kitamura, Sanshiro Hanada, Kazuyuki Sugimoto, Tomomi Furihata, and Nobuhiko Kojima. 2023. "Rapid and Stable Formation Method of Human Astrocyte Spheroid in a High Viscous Methylcellulose Medium and Its Functional Advantages" Bioengineering 10, no. 3: 349. https://doi.org/10.3390/bioengineering10030349
APA StyleTao, F., Kitamura, K., Hanada, S., Sugimoto, K., Furihata, T., & Kojima, N. (2023). Rapid and Stable Formation Method of Human Astrocyte Spheroid in a High Viscous Methylcellulose Medium and Its Functional Advantages. Bioengineering, 10(3), 349. https://doi.org/10.3390/bioengineering10030349







