The Back Muscle Surface Electromyography-Based Fatigue Index: A Digital Biomarker of Human Neuromuscular Aging?
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Participants
2.3. Experimental Protocol
2.3.1. Schedule of Assessments and Tasks
2.3.2. Equipment and Tests
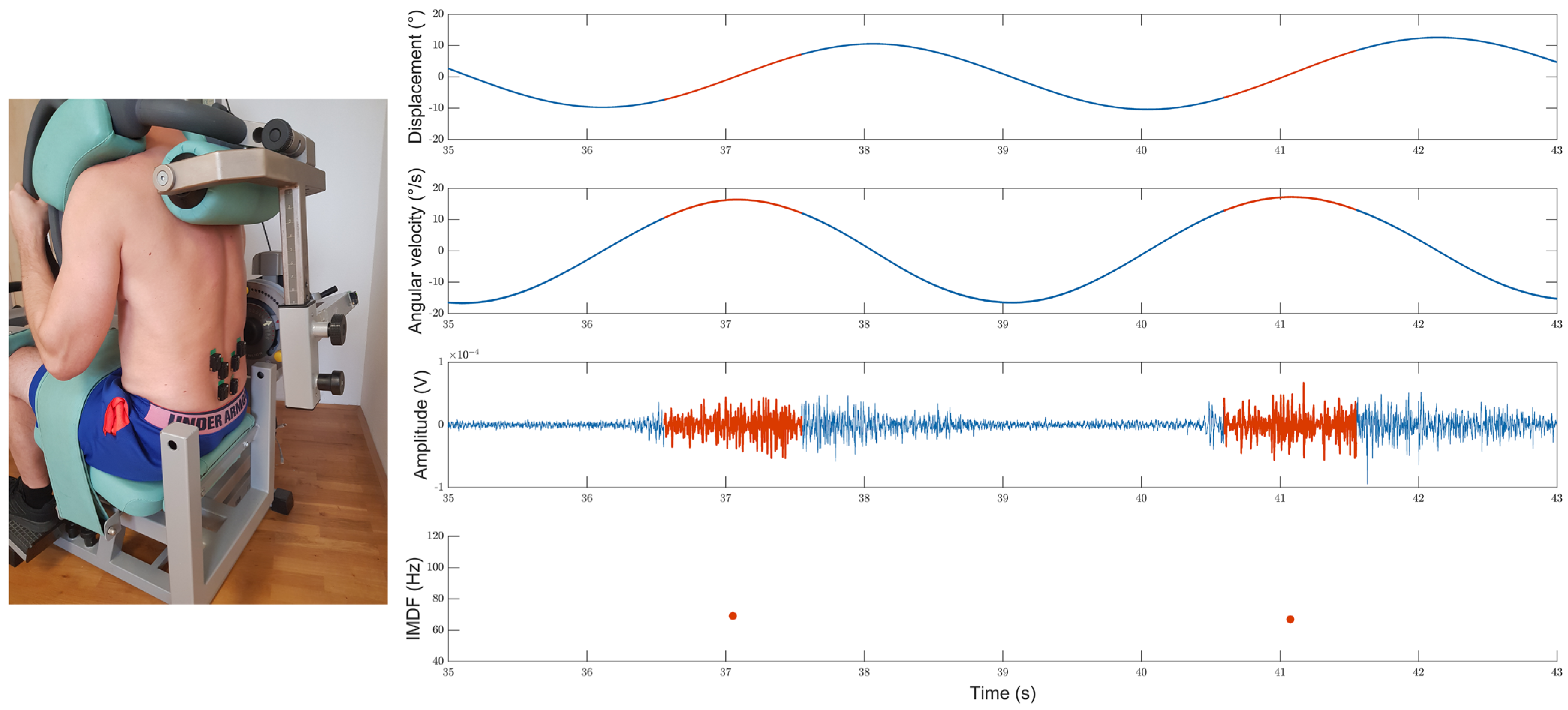
Back Extension Dynamometer
Surface EMG
Additional Accelerometer Data
Other Tests
2.3.3. Test Procedures
Maximum Back Extension Test
Cyclic Back Extension Exercise
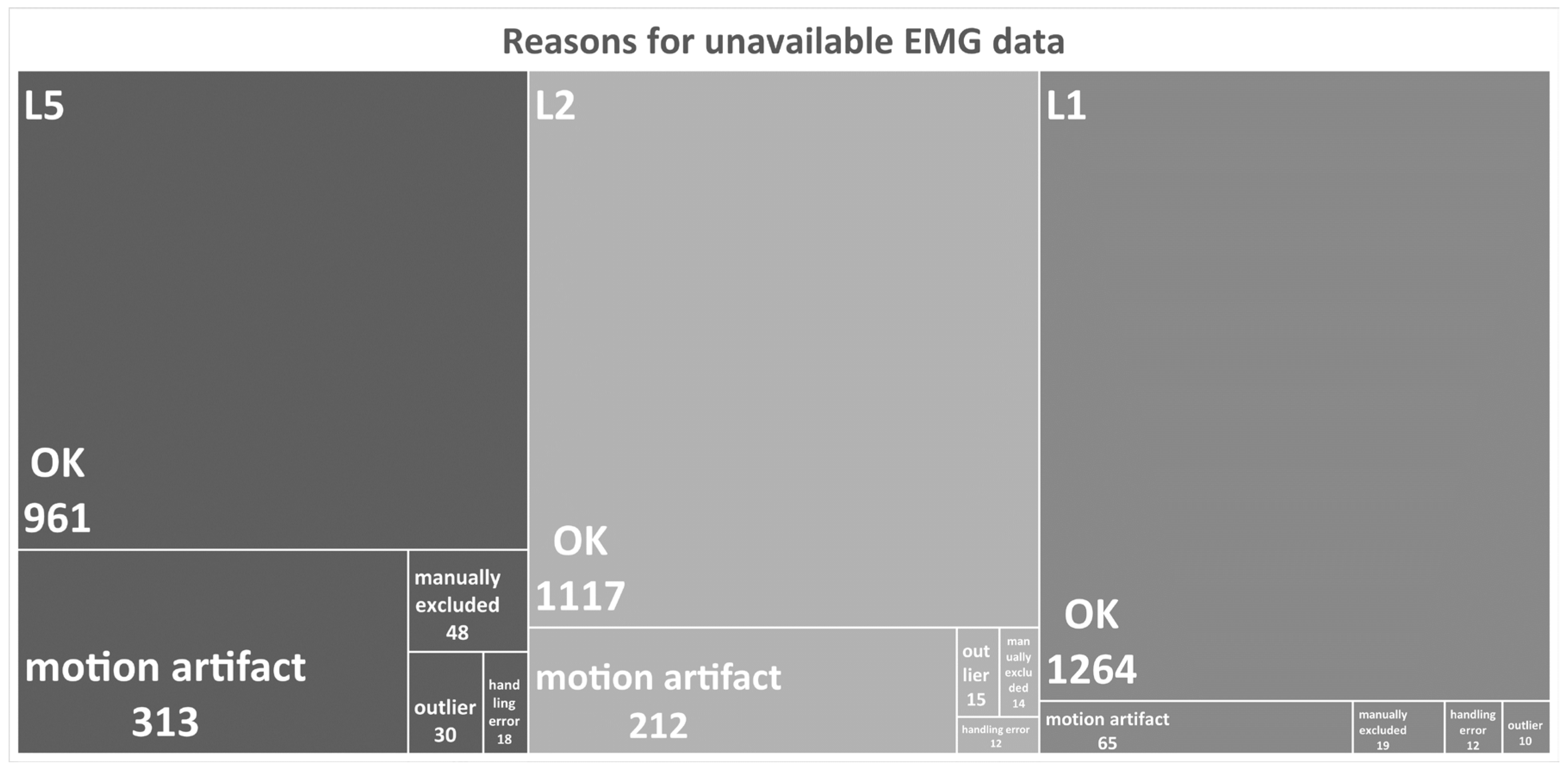
2.4. Signal Processing
2.4.1. Accelerometer Data Analysis
2.5. Definition of Variables
2.6. Sample Size Estimation
2.7. Age- and Sex-Specific Subgroups
2.8. Reliability of SEMG Measures
3. Results
3.1. Age-Dependent IMDF-SEMG Changes during the Cyclic Exercise
3.2. Retest Reliability of the IMDF-SEMG Estimates in Older and Younger Individuals
4. Discussion
- Normalized IMDF-SEMG fatigue slope values were significantly smaller in older than younger individuals with cLBP, with differences that were more prominent in males than in females;
- Normalized IMDF-SEMG fatigue values were greater in younger male participants with cLBP vs. female participants with cLBP;
- Neither pain intensity nor health-related disablement had a major effect on the IMDF-SEMG slope;
- The relative reliability was overall moderate-to-good for the IMDF-SEMG slope values (derived from data normalized by the IMDF-SEMG onset value); the absolute reliability indicated a small test–retest variability for both the IMDF-SEMG slope values and the IMDF-SEMG onset values.
4.1. Methodological Considerations, Individuals’ Maximum Back Extension Strength, and Mechanical Performance Parameters during the Cyclic Exercise
4.2. IMDF-SEMG Characteristics
4.3. Reliability of SEMG Variables
4.4. Limitations
4.5. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gustafsson, T.; Ulfhake, B. Sarcopenia: What Is the Origin of This Aging-Induced Disorder? Front. Genet. 2021, 12, 688526. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Fortin, M.; Videman, T.; Gibbons, L.E.; Battie, M.C. Paraspinal muscle morphology and composition: A 15-yr longitudinal magnetic resonance imaging study. Med. Sci. Sport. Exerc. 2014, 46, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Dahlqvist, J.R.; Vissing, C.R.; Hedermann, G.; Thomsen, C.; Vissing, J. Fat Replacement of Paraspinal Muscles with Aging in Healthy Adults. Med. Sci. Sport. Exerc. 2017, 49, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Dallaway, A.; Kite, C.; Griffen, C.; Duncan, M.; Tallis, J.; Renshaw, D.; Hattersley, J. Age-related degeneration of the lumbar paravertebral muscles: Systematic review and three-level meta-regression. Exp. Gerontol. 2020, 133, 110856. [Google Scholar] [CrossRef]
- Alcazar, J.; Alegre, L.M.; Suetta, C.; Judice, P.B.; van Roie, E.; Gonzalez-Gross, M.; Rodriguez-Manas, L.; Casajus, J.A.; JP, M.A.; Nielsen, B.R.; et al. Threshold of Relative Muscle Power Required to Rise from a Chair and Mobility Limitations and Disability in Older Adults. Med. Sci. Sport. Exerc. 2021, 53, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.F.; Fielding, R.A. Skeletal muscle power: A critical determinant of physical functioning in older adults. Exerc. Sport Sci. Rev. 2012, 40, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Ranger, T.A.; Cicuttini, F.M.; Jensen, T.S.; Peiris, W.L.; Hussain, S.M.; Fairley, J.; Urquhart, D.M. Are the size and composition of the paraspinal muscles associated with low back pain? A systematic review. Spine J. 2017, 17, 1729–1748. [Google Scholar] [CrossRef]
- Seyedhoseinpoor, T.; Taghipour, M.; Dadgoo, M.; Sanjari, M.A.; Takamjani, I.E.; Kazemnejad, A.; Khoshamooz, Y.; Hides, J. Alteration of lumbar muscle morphology and composition in relation to low back pain: A systematic review and meta-analysis. Spine J. 2021, 22, 660–676. [Google Scholar] [CrossRef]
- Hodges, P.W.; Bailey, J.F.; Fortin, M.; Battie, M.C. Paraspinal muscle imaging measurements for common spinal disorders: Review and consensus-based recommendations from the ISSLS degenerative spinal phenotypes group. Eur. Spine J. 2021, 30, 3428–3441. [Google Scholar] [CrossRef]
- Brinjikji, W.; Luetmer, P.H.; Comstock, B.; Bresnahan, B.W.; Chen, L.E.; Deyo, R.A.; Halabi, S.; Turner, J.A.; Avins, A.L.; James, K.; et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am. J. Neuroradiol. 2015, 36, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Wakao, N.; Matsui, H.; Watanabe, T.; Iida, H.; Watanabe, K. Clinical characteristics of geriatric patients with non-specific chronic low back pain. Sci. Rep. 2022, 12, 1286. [Google Scholar] [CrossRef] [PubMed]
- GBD Ageing Collaborators. Global, regional, and national burden of diseases and injuries for adults 70 years and older: Systematic analysis for the Global Burden of Disease 2019 Study. BMJ 2022, 376, e068208. [Google Scholar]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Lamb, S.E.; Bruce, J.; Hossain, A.; Ji, C.; Longo, R.; Lall, R.; Bojke, C.; Hulme, C.; Withers, E.; Finnegan, S.; et al. Screening and Intervention to Prevent Falls and Fractures in Older People. N. Engl. J. Med. 2020, 383, 1848–1859. [Google Scholar] [CrossRef]
- Lim, J.Y.; Frontera, W.R. Single skeletal muscle fiber mechanical properties: A muscle quality biomarker of human aging. Eur. J. Appl. Physiol. 2022, 122, 1383–1395. [Google Scholar] [CrossRef]
- Birkbeck, M.G.; Blamire, A.M.; Whittaker, R.G.; Sayer, A.A.; Dodds, R.M. The role of novel motor unit magnetic resonance imaging to investigate motor unit activity in ageing skeletal muscle. J. Cachexia Sarcopenia Muscle 2021, 12, 17–29. [Google Scholar] [CrossRef]
- Kienbacher, T.; Habenicht, R.; Starek, C.; Mair, P.; Wolf, M.; Paul, B.; Riegler, S.; Kollmitzer, J.; Ebenbichler, G. The potential use of spectral electromyographic fatigue as a screening and outcome monitoring tool of sarcopenic back muscle alterations. J. Neuroeng. Rehabil. 2014, 11, 106. [Google Scholar] [CrossRef]
- Habenicht, R.; Ebenbichler, G.; Bonato, P.; Kollmitzer, J.; Ziegelbecker, S.; Unterlerchner, L.; Mair, P.; Kienbacher, T. Age-specific differences in the time-frequency representation of surface electromyographic data recorded during a submaximal cyclic back extension exercise: A promising biomarker to detect early signs of sarcopenia. J. Neuroeng. Rehabil. 2020, 17, 8. [Google Scholar] [CrossRef]
- Kupa, E.J.; Roy, S.H.; Kandarian, S.C.; De Luca, C.J. Effects of muscle fiber type and size on EMG median frequency and conduction velocity. J. Appl. Physiol. 1995, 79, 23–32. [Google Scholar] [CrossRef]
- Brody, L.R.; Pollock, M.T.; Roy, S.H.; De Luca, C.J.; Celli, B. pH-induced effects on median frequency and conduction velocity of the myoelectric signal. J. Appl. Physiol. 1991, 71, 1878–1885. [Google Scholar] [CrossRef] [PubMed]
- Greenhaff, P.L.; Soderlund, K.; Ren, J.M.; Hultman, E. Energy metabolism in single human muscle fibres during intermittent contraction with occluded circulation. J. Physiol. 1993, 460, 443–453. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.J. The use of surface electromyography in biomechanics. J. Appl. Biomech. 1997, 13, 135–163. [Google Scholar] [CrossRef]
- Roy, S.H.; De Luca, C.J.; Emley, M.; Oddsson, L.I.; Buijs, R.J.; Levins, J.A.; Newcombe, D.S.; Jabre, J.F. Classification of back muscle impairment based on the surface electromyographic signal. J. Rehabil. Res. Dev. 1997, 34, 405–414. [Google Scholar]
- Oddsson, L.I.; De Luca, C.J. Activation imbalances in lumbar spine muscles in the presence of chronic low back pain. J. Appl. Physiol. 2003, 94, 1410–1420. [Google Scholar] [CrossRef]
- Beauchamp, M.K.; Jette, A.M.; Ni, P.; Latham, N.K.; Ward, R.E.; Kurlinski, L.A.; Percac-Lima, S.; Leveille, S.G.; Bean, J.F. Leg and Trunk Impairments Predict Participation in Life Roles in Older Adults: Results From Boston RISE. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 663–669. [Google Scholar] [CrossRef]
- Lariviere, C.; Arsenault, A.B.; Gravel, D.; Gagnon, D.; Loisel, P.; Vadeboncoeur, R. Electromyographic assessment of back muscle weakness and muscle composition: Reliability and validity issues. Arch. Phys. Med. Rehabil. 2002, 83, 1206–1214. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.S.; Crombez, G.; Linton, S.J. The fear-avoidance model of pain. Pain 2016, 157, 1588–1589. [Google Scholar] [CrossRef]
- Hasenbring, M.I.; Fehrmann, E.; Ebenbichler, G. Embodied Pain: There is a Need to Reflect Interactions Between Cognitions, Behavior, and Neuromuscular Activity in Chronic Pain. Clin. J. Pain 2020, 36, 178–180. [Google Scholar] [CrossRef]
- Mazzeo, R.S.; Cavanagh, P.; Evans, W.J.; Fiatarone, M.; Hagberg, J.; McAuley, E.; Startzell, J. ACSM Position Stand: Exercise and Physical Activity for Older Adults. Med. Sci. Sport. Exerc. 1998, 30, 992–1008. [Google Scholar] [CrossRef]
- American College of Sports Medicine; Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med. Sci. Sport. Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- Bonato, P.; Roy, S.H.; Knaflitz, M.; De Luca, C.J. Time-frequency parameters of the surface myoelectric signal for assessing muscle fatigue during cyclic dynamic contractions. IEEE Trans. Biomed. Eng. 2001, 48, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Ebenbichler, G.R.; Bonato, P.; Roy, S.H.; Lehr, S.; Posch, M.; Kollmitzer, J.; Della Croce, U. Reliability of EMG time-frequency measures of fatigue during repetitive lifting. Med. Sci. Sport. Exerc. 2002, 34, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- van Dieen, J.H.; Reeves, N.P.; Kawchuk, G.; van Dillen, L.R.; Hodges, P.W. Motor Control Changes in Low Back Pain: Divergence in Presentations and Mechanisms. J. Orthop. Sport. Phys. Ther. 2019, 49, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Mannion, A.F.; Connolly, B.; Wood, K.; Dolan, P. The use of surface EMG power spectral analysis in the evaluation of back muscle function. J. Rehabil. Res. Dev. 1997, 34, 427–439. [Google Scholar] [PubMed]
- Mannion, A.F.; Dumas, G.A.; Stevenson, J.M.; Cooper, R.G. The influence of muscle fiber size and type distribution on electromyographic measures of back muscle fatigability. Spine 1998, 23, 576–584. [Google Scholar] [CrossRef]
- Ng, J.K.; Richardson, C.A.; Kippers, V.; Parnianpour, M. Relationship between muscle fiber composition and functional capacity of back muscles in healthy subjects and patients with back pain. J. Orthop. Sport. Phys. Ther. 1998, 27, 389–402. [Google Scholar] [CrossRef]
- Hodges, P.W.; van den Hoorn, W.; Wrigley, T.V.; Hinman, R.S.; Bowles, K.A.; Cicuttini, F.; Wang, Y.; Bennell, K. Increased duration of co-contraction of medial knee muscles is associated with greater progression of knee osteoarthritis. Man. Ther. 2016, 21, 151–158. [Google Scholar] [CrossRef]
- Piasecki, J.; Inns, T.B.; Bass, J.J.; Scott, R.; Stashuk, D.W.; Phillips, B.E.; Atherton, P.J.; Piasecki, M. Influence of sex on the age-related adaptations of neuromuscular function and motor unit properties in elite masters athletes. J. Physiol. 2021, 599, 193–205. [Google Scholar] [CrossRef]
- Clark, B.C.; Manini, T.M.; The, D.J.; Doldo, N.A.; Ploutz-Snyder, L.L. Gender differences in skeletal muscle fatigability are related to contraction type and EMG spectral compression. J. Appl. Physiol. 2003, 94, 2263–2272. [Google Scholar] [CrossRef]
- Sions, J.M.; Elliott, J.M.; Pohlig, R.T.; Hicks, G.E. Trunk Muscle Characteristics of the Multifidi, Erector Spinae, Psoas, and Quadratus Lumborum in Older Adults With and Without Chronic Low Back Pain. J. Orthop. Sport. Phys. Ther. 2017, 47, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Ansdell, P.; Brownstein, C.G.; Skarabot, J.; Hicks, K.M.; Howatson, G.; Thomas, K.; Hunter, S.K.; Goodall, S. Sex differences in fatigability and recovery relative to the intensity-duration relationship. J. Physiol. 2019, 597, 5577–5595. [Google Scholar] [CrossRef] [PubMed]
- Kienbacher, T.; Kollmitzer, J.; Anders, P.; Habenicht, R.; Starek, C.; Wolf, M.; Paul, B.; Mair, P.; Ebenbichler, G. Age-related test-retest reliability of isometric trunk torque measurements in patiens with chronic low back pain. J. Rehabil. Med. 2016, 48, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hägg, G. European recommendations for surface electromyography. Roessingh. Res. Dev. 1999, 8, 13–54. [Google Scholar]
- Craig, C.L.; Marshall, A.L.; Sjostrom, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sport. Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Wiesinger, G.F.; Nuhr, M.; Quittan, M.; Ebenbichler, G.; Wolfl, G.; Fialka-Moser, V. Cross-cultural adaptation of the Roland-Morris questionnaire for German-speaking patients with low back pain. Spine 1999, 24, 1099–1103. [Google Scholar] [CrossRef]
- Hasenbring, M.I.; Hallner, D.; Rusu, A.C. Fear-avoidance- and endurance-related responses to pain: Development and validation of the Avoidance-Endurance Questionnaire (AEQ). Eur. J. Pain 2009, 13, 620–628. [Google Scholar] [CrossRef]
- Cohen, L. Time-Frequency Analysis; Prentice Hall: Hoboken, NJ, USA, 1995; Volume 778. [Google Scholar]
- Lariviere, C.; Bilodeau, M.; Forget, R.; Vadeboncoeur, R.; Mecheri, H. Poor back muscle endurance is related to pain catastrophizing in patients with chronic low back pain. Spine 2010, 35, E1178–E1186. [Google Scholar] [CrossRef]
- Ebenbichler, G.R.; Unterlerchner, L.; Habenicht, R.; Bonato, P.; Kollmitzer, J.; Mair, P.; Riegler, S.; Kienbacher, T. Estimating Neural Control from Concentric vs. Eccentric Surface Electromyographic Representations during Fatiguing, Cyclic Submaximal Back Extension Exercises. Front. Physiol. 2017, 8, 299. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 16 August 2022).
- Liu, X.S. Statistical Power Analysis for the Social and Behavioral Sciences: Basic and Advanced Techniques; Routledge/Taylor & Francis Group: New York, NY, USA, 2013; p. xvi378. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. emmeans: Estimated Marginal Means, aka Least-Squares Means, R package version 1.4.5; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Brennan, R.L. Generalizability Theory; Springer: New York, NY, USA, 2001; p. 538. [Google Scholar]
- Cronbach, L.J.; Rajaratnam, N.; Gleser, G.C. Theory of generalizability: A liberalization of reliability theory. Br. J. Stat. Psychol. 1963, 16, 137–163. [Google Scholar] [CrossRef]
- Shavelson, R.J.; Webb, N.M. Generalizability Theory: A Primer; Sage Publications: Newbury Park, CA, USA, 1991. [Google Scholar]
- Lariviere, C.; Da Silva, R.A.; Arsenault, A.B.; Nadeau, S.; Plamondon, A.; Vadeboncoeur, R. Specificity of a back muscle roman chair exercise in healthy and back pain subjects. Med. Sci. Sport. Exerc. 2011, 43, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.K.; Pereira, H.M.; Keenan, K.G. The aging neuromuscular system and motor performance. J. Appl. Physiol. 2016, 121, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Christie, A.; Snook, E.M.; Kent-Braun, J.A. Systematic review and meta-analysis of skeletal muscle fatigue in old age. Med. Sci. Sport. Exerc. 2011, 43, 568–577. [Google Scholar] [CrossRef]
- Deering, R.E.; Senefeld, J.W.; Pashibin, T.; Neumann, D.A.; Hunter, S.K. Muscle function and fatigability of trunk flexors in males and females. Biol. Sex Differ. 2017, 8, 12. [Google Scholar] [CrossRef]
- De Luca, C.J.; Roy, A.M.; Erim, Z. Synchronization of motor-unit firings in several human muscles. J. Neurophysiol. 1993, 70, 2010–2023. [Google Scholar] [CrossRef]
- Erim, Z.; Beg, M.F.; Burke, D.T.; de Luca, C.J. Effects of aging on motor-unit control properties. J. Neurophysiol. 1999, 82, 2081–2091. [Google Scholar] [CrossRef]
- Merletti, R.; Farina, D.; Gazzoni, M.; Schieroni, M.P. Effect of age on muscle functions investigated with surface electromyography. Muscle Nerve 2002, 25, 65–76. [Google Scholar] [CrossRef]
- Merletti, R.; Lo Conte, L.R.; Cisari, C.; Actis, M.V. Age related changes in surface myoelectric signals. Scand. J. Rehabil. Med. 1992, 24, 25–36. [Google Scholar]
- Ebenbichler, G.; Habenicht, R.; Ziegelbecker, S.; Kollmitzer, J.; Mair, P.; Kienbacher, T. Age- and sex-specific effects in paravertebral surface electromyographic back extensor muscle fatigue in chronic low back pain. Geroscience 2020, 42, 251–269. [Google Scholar] [CrossRef]
- Vleeming, A.; Pool-Goudzwaard, A.L.; Stoeckart, R.; van Wingerden, J.P.; Snijders, C.J. The posterior layer of the thoracolumbar fascia. Its function in load transfer from spine to legs. Spine 1995, 20, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Willard, F.H.; Vleeming, A.; Schuenke, M.D.; Danneels, L.; Schleip, R. The thoracolumbar fascia: Anatomy, function and clinical considerations. J. Anat. 2012, 221, 507–536. [Google Scholar] [CrossRef] [PubMed]
- Broxterman, R.M.; Ade, C.J.; Craig, J.C.; Wilcox, S.L.; Schlup, S.J.; Barstow, T.J. Influence of blood flow occlusion on muscle oxygenation characteristics and the parameters of the power-duration relationship. J. Appl. Physiol. 2015, 118, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Porter, B.E.; Lund, I.V.; Varodayan, F.P.; Wallace, R.W.; Blendy, J.A. The role of transcription factors cyclic-AMP responsive element modulator (CREM) and inducible cyclic-AMP early repressor (ICER) in epileptogenesis. Neuroscience 2008, 152, 829–836. [Google Scholar] [CrossRef]
- Amonoo-Kuofi, H.S. The number and distribution of muscle spindles in human intrinsic postvertebral muscles. J. Anat. 1982, 135, 585–599. [Google Scholar]
- De Luca, C.J.; Kline, J.C. Influence of proprioceptive feedback on the firing rate and recruitment of motoneurons. J. Neural. Eng. 2012, 9, 016007. [Google Scholar] [CrossRef]
- Bogduk, N. Clinical Anatomy of the Lumbar Spine and Sacrum; Elsevier Health Sciences: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Contessa, P.; Adam, A.; De Luca, C.J. Motor unit control and force fluctuation during fatigue. J. Appl. Physiol. 2009, 107, 235–243. [Google Scholar] [CrossRef]
- Farina, D.; Gazzoni, M.; Merletti, R. Assessment of low back muscle fatigue by surface EMG signal analysis: Methodological aspects. J. Electromyogr. Kinesiol. 2003, 13, 319–332. [Google Scholar] [CrossRef]
- Brouwer, N.P.; Tabasi, A.; Kingma, I.; Stegeman, D.F.; van Dijk, W.; Moya-Esteban, A.; Sartori, M.; van Dieen, J.H. Low back muscle action potential conduction velocity estimated using high-density electromyography. J. Electromyogr. Kinesiol. 2022, 66, 102679. [Google Scholar] [CrossRef]
- McGregor, R.A.; Cameron-Smith, D.; Poppitt, S.D. It is not just muscle mass: A review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev. Health 2014, 3, 9. [Google Scholar] [CrossRef]
- Haig, A.J.; London, Z.; Sandella, D.E. Symmetry of paraspinal muscle denervation in clinical lumbar spinal stenosis: Support for a hypothesis of posterior primary ramus stretching? Muscle Nerve 2013, 48, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.C.; Haig, A.J.; Yamakawa, K.S.; Miner, J.A. Paraspinal electromyography: Age-correlated normative values in asymptomatic subjects. Spine 2005, 30, E499–E502. [Google Scholar] [CrossRef] [PubMed]
| <50 Years Mean (SE) | >50 Years Mean (SE) | <50 vs. >50 * | Males Mean (SE) | Females Mean (SE) | Males vs. Females * | |
|---|---|---|---|---|---|---|
| Age (years) | 34.0 (1.3) | 64.1 (1.1) | <0.001 | 47.6 (2.3) | 50.5 (2.2) | 0.37 |
| BMI (kg/m²) | 24.48 (0.42) | 26.80 (0.41) | <0.001 | 26.33 (0.35) | 24.72 (0.53) | 0.012 |
| Back Extension Strength (Nm) | 209.1 (9.2) | 192.6 (9.9) | 0.22 | 265.3 (7.1) | 148.3 (4.8) | <0.001 |
| Grip Strength (kg) | 39.3 (1.6) | 31.3 (1.5) | <0.001 | 46.9 (1.0) | 25.9 (0.6) | <0.001 |
| Back Muscle Fatigue (VAS 0–10 (strongest)) | 5.6 (0.3) | 4.5 (0.3) | 0.004 | 5.7 (0.3) | 4.5 (0.3) | 0.001 |
| Pain (last month) (VAS 0–10 (worst)) | 48.9 (1.3) | 52.1 (1.9) | 0.15 | 49.9 (1.5) | 50.3 (1.3) | 0.84 |
| Pain (test day) (VAS 0–10 (worst)) | 37.2 (1.1) | 39.9 (1.5) | 0.15 | 37.8 (1.2) | 39.1 (1.3) | 0.45 |
| RMDQ (0–24 (worst)) | 5.54 (0.37) | 8.37 (0.43) | <0.001 | 7.25 (0.31) | 6.22 (0.46) | <0.001 |
| IPAQ (kcal/wk) | 4141 (484) | 4306 (330) | 0.78 | 4049 (364) | 4417 (414) | 0.50 |
| AEQ-FAR (0–6 (strongest)) | 1.53 (0.09) | 1.70 (0.09) | 0.16 | 1.69 (0.08) | 1.52 (0.10) | 0.19 |
| AEQ-ER (0–6 (strongest)) | 3.18 (0.08) | 3.54 (0.07) | 0.002 | 3.30 (0.08) | 3.44 (0.09) | 0.24 |
| Exercise Velocity (con °/s) | 33.2 (1.2) | 33.2 (0.6) | 0.95 | 33.2 (1.2) | 33.2 (1.2) | 0.99 |
| Exercise Velocity (con changes 1 °/s) | 0.02 (0.002) | 0.01 (0.001) | 0.14 | 0.02 (0.002) | 0.02 (0.002) | 0.23 |
| ROM (°) | 33.8 (0.6) | 33.2 (0.6) | 0.60 | 32.7 (0.6) | 33.8 (0.6) | 0.20 |
| ROM changes 1 (10−3)° | 0.26 (0.02) | 0.22 (0.02) | 0.045 | 0.22 (0.02) | 0.25 (0.02) | 0.22 |
| Mean (SE) | Linear Mixed Effects Model | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Electrode Level | <50 Years (n = 114) | >50 Years (n = 108) | Males (n = 110) | Females (n = 112) | Age t; p | Age d | Sex t; p | Sex d | Age × Sex t; p | Age × Sex d | Day 1 vs. 2 t; p | Day 1 vs. 3 t; p |
| Onsets | ||||||||||||
| All | 63.1 (0.8) | 62.6 (0.8) | 61.0 (0.8) | 64.6 (0.8) | −2.78; 0.003 | 0.37 | −0.88; 0.38 | 0.12 | 3.37; 0.001 | 0.45 | 0.16; 0.86 | −0.46; 0.67 |
| L5 | 72.7 (1.2) | 74.9 (1.3) | 71.0 (1.2) | 76.3 (1.2) | −1.32; 0.16 | 0.18 | −0.46; 0.65 | 0.06 | 2.58; 0.008 | 0.35 | −0.38; 0.69 | −1.44; 0.17 |
| L2 | 62.0 (0.9) | 60.5 (1.0) | 59.1 (0.9) | 63.4 (1.0) | −3.52; <0.001 | 0.47 | −0.91; 0.37 | 0.12 | 3.76; <0.001 | 0.50 | −0.90; 0.35 | 1.19; 0.25 |
| L1 | 54.6 (0.6) | 52.5 (0.6) | 53.0 (0.7) | 54.2 (0.6) | −3.36; 0.001 | 0.45 | −1.26; 0.20 | 0.17 | 2.86; 0.003 | 0.39 | −0.22; 0.83 | −0.68; 0.50 |
| m.n | 49.4 (0.6) | 48.2 (0.6) | 48.0 (0.6) | 49.6 (0.6) | −3.23; 0.001 | 0.43 | −1.13; 0.26 | 0.15 | 3.24; 0.001 | 0.43 | −0.70; 0.47 | −0.58; 0.56 |
| Changes normalized to Onsets | ||||||||||||
| All | −0.13 (0.01) * | −0.07 (0.01) * | −0.13 (0.01) * | −0.08 (0.01) * | 4.67; <0.001 | 0.62 | 3.96; <0.001 | 0.54 | −1.98; 0.043 | 0.27 | −2.10; 0.025 | −3.03; 0.004 |
| L5 | −0.18 (0.01) * | −0.10 (0.01) * | −0.18 (0.01) * | −0.11 (0.01) * | 3.43; <0.001 | 0.46 | 3.48; <0.001 | 0.48 | −0.79; 0.42 | 0.11 | −1.05; 0.29 | −1.21; 0.25 |
| L2 | −0.14 (0.01) * | −0.06 (0.01) * | −0.13 (0.01) * | −0.08 (0.01) * | 5.73; <0.001 | 0.78 | 4.47; <0.001 | 0.62 | −3.44; <0.001 | 0.47 | −1.44; 0.14 | −3.76; 0.001 |
| L1 | −0.08 (0.01) * | −0.05 (0.01) * | −0.08 (0.01) * | −0.05 (0.01) * | 2.42; 0.013 | 0.33 | 1.74; 0.11 | 0.24 | −0.70; 0.47 | 0.10 | −2.53; 0.008 | −2.14; 0.040 |
| m.n. | −0.27 (0.01) * | −0.21 (0.01) * | −0.28 (0.01) * | −0.21 (0.01) * | 4.34; <0.001 | 0.58 | 4.57; <0.001 | 0.62 | −1.84; 0.062 | 0.25 | −1.56; 0.11 | −2.00; 0.051 |
| un.imb. | 10.6 (0.51) | 11.4 (0.8) | 10.42 (0.48) | 11.48 (0.79) | 0.03; 0.98 | 0.00 | 0.24; 0.78 | 0.03 | 0.52; 0.59 | 0.07 | 1.07; 0.34 | 0.26; 0.73 |
| c.imb. | −5.2 (0.63) | −4.36 (1.0) | −4.45 (0.66) | −5.12 (0.9) | 0.34; 0.72 | 0.05 | −0.65; 0.41 | 0.09 | 0.32; 0.75 | 0.05 | 0.27; 0.80 | −0.24; 0.77 |
| Electrode Level | Sex | Difference Mean (SE) | <50 vs. >50 p-Value | Age | Difference Mean (SE) | Males vs. Females p-Value |
|---|---|---|---|---|---|---|
| All electrodes | Male | −0.08 (0.02) | <0.001 | <50 years | −0.07 (0.02) | <0.001 |
| Female | −0.03 (0.02) | 0.05 | >50 years | −0.02 (0.02) | 0.3 | |
| L5 multifidus | Male | −0.08 (0.02) | 0.001 | <50 years | −0.08 (0.02) | 0.001 |
| Female | −0.06 (0.02) | 0.002 | >50 years | −0.06 (0.03) | 0.03 | |
| L2 longissimus | Male | −0.12 (0.02) | <0.001 | <50 years | −0.09 (0.02) | <0.001 |
| Female | −0.02 (0.02) | 0.3 | >50 years | 0.01 (0.02) | 0.6 | |
| L1 iliocostalis | Male | −0.04 (0.02) | 0.016 | <50 years | −0.03 (0.02) | 0.083 |
| Female | −0.02 (0.02) | 0.1 | >50 years | −0.01 (0.02) | 0.5 | |
| Most negative IDMF fatigue | Male | −0.09 (0.02) | <0.001 | <50 years | −0.09 (0.02) | <0.001 |
| Female | −0.04 (0.02) | 0.07 | >50 years | −0.04 (0.02) | 0.07 |
| IMDF-SEMG | ||||
|---|---|---|---|---|
| Electrode Level/Age Group | Onsets | Changes Normalized to Onsets | ||
| D1 | SEM | D 1 | SEM | |
| all electrodes | 0.77 | 5.45 | 0.62 | 0.06 |
| <50 years old | 0.80 | 5.14 | 0.66 | 0.06 |
| >50 years old | 0.75 | 5.76 | 0.46 | 0.06 |
| L5 | 0.84 | 6.31 | 0.67 | 0.08 |
| <50 years old | 0.85 | 6.12 | 0.71 | 0.07 |
| >50 years old | 0.82 | 6.51 | 0.53 | 0.08 |
| L2 | 0.84 | 5.07 | 0.47 | 0.09 |
| <50 years old | 0.82 | 5.21 | 0.54 | 0.09 |
| >50 years old | 0.85 | 4.92 | 0.19 | 0.09 |
| L1 | 0.76 | 4.15 | 0.38 | 0.07 |
| <50 years old | 0.74 | 4.40 | 0.43 | 0.08 |
| >50 years old | 0.78 | 3.88 | 0.16 | 0.07 |
| most negative | 0.72 | 4.36 | 0.71 | 0.06 |
| <50 years old | 0.74 | 4.20 | 0.75 | 0.06 |
| >50 years old | 0.70 | 4.54 | 0.53 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebenbichler, G.; Habenicht, R.; Blohm, P.; Bonato, P.; Kollmitzer, J.; Mair, P.; Kienbacher, T. The Back Muscle Surface Electromyography-Based Fatigue Index: A Digital Biomarker of Human Neuromuscular Aging? Bioengineering 2023, 10, 300. https://doi.org/10.3390/bioengineering10030300
Ebenbichler G, Habenicht R, Blohm P, Bonato P, Kollmitzer J, Mair P, Kienbacher T. The Back Muscle Surface Electromyography-Based Fatigue Index: A Digital Biomarker of Human Neuromuscular Aging? Bioengineering. 2023; 10(3):300. https://doi.org/10.3390/bioengineering10030300
Chicago/Turabian StyleEbenbichler, Gerold, Richard Habenicht, Peter Blohm, Paolo Bonato, Josef Kollmitzer, Patrick Mair, and Thomas Kienbacher. 2023. "The Back Muscle Surface Electromyography-Based Fatigue Index: A Digital Biomarker of Human Neuromuscular Aging?" Bioengineering 10, no. 3: 300. https://doi.org/10.3390/bioengineering10030300
APA StyleEbenbichler, G., Habenicht, R., Blohm, P., Bonato, P., Kollmitzer, J., Mair, P., & Kienbacher, T. (2023). The Back Muscle Surface Electromyography-Based Fatigue Index: A Digital Biomarker of Human Neuromuscular Aging? Bioengineering, 10(3), 300. https://doi.org/10.3390/bioengineering10030300






